Immune and microRNA responses to Helicobacter muridarum infection and indole-3-carbinol during colitis
Rasha Raheem Alkarkoushi, Yvonne Hui, Abbas S Tavakoli, Udai Singh, Prakash Nagarkatti, Mitzi Nagarkatti,Ioulia Chatzistamou, Marpe Bam, Traci L Testerman
Abstract
Key words: Helicobacter muridarum; MicroRNA; Immune; T regulatory cell; T helper 17 cell; Colitis; Cytokine
INTRODUCTION
Ulcerative colitis (UC) is a chronic, idiopathic inflammatory bowel disease (IBD) characterized by inflammation of the colon. In the past decade, inflammatory bowel disease has emerged as a public health challenge and a global disease with increasing incidence in newly industrialized and industrialized countries worldwide, especially in North America, and Europe[1]. In 2015, 3.1 million adults in the United States were living with IBD[2], with direct and indirect costs estimated to be between $14.6 and $31.6 billion in 2014 alone[3]. The pathogenesis of IBD is complex and influenced by genetic susceptibility, dysregulation of the innate and adaptive immune systems, environmental factors, and intestinal dysbiosis; however, a crucial feature of UC is the imbalance between T regulatory (Treg) cells and T helper 17 (Th17) cells[4].
The best-known member of theHelicobactergenus isHelicobacter pylori(H. pylori), which colonizes the stomach, causing gastritis, gastric cancer, and a range of extragastric diseases[5]. EnterohepaticHelicobacter(EHH) species colonize the colon and sometimes the biliary tree. Some of these poorly studied organisms commonly cause persistent, asymptomatic infections, but occasionally cause intestinal diseases or even cancer in species ranging from rodents to primates[6-8]. Several studies suggest that EHH species are associated with IBD in humans[9-11]. The prevalence of EHH species in human populations is not clearly known, but one study found 9% infection in healthy control patients[11].Helicobacter muridarum(H. muridarum) is an enterohepaticHelicobacter(EHH) species that was initially described as a member of the normal flora of conventional rodents[12]. Subsequent studies, however, showed thatH. muridarumcould induce colitis and gastritis in mice, suggesting a potential pathogenic role for the bacterium[13-15].
Dextran sodium sulfate (DSS)-induced colitis is the most widely used mouse colitis model for studying acute colitis and inflammation-associated colon cancer. DSS is a water-soluble, negatively charged, sulfated polysaccharide with a highly variable molecular weight. DSS-induced murine colitis, which most closely resembles human UC, employs DSS at a molecular weight of 40000 Da[16,17]. The mechanism by which DSS induces intestinal inflammation is disruption of tight junctions, allowing dissemination of proinflammatory intestinal contents[18]. In conjunction with colonic damage, DSS induces a range of proinflammatory cytokines and a Th1/Th17 response[17,19].
The aryl hydrocarbon receptor (AhR) regulates several signaling pathways relevant to intestinal health, including the balance between Tregs and Th17 cells[20,21]. AhR is needed for the survival of intraepithelial lymphocytes and also the organogenesis of lymphoid structures in the gastrointestinal tract[22]. Indole-3-carbinol (I3C), a dietary compound from cruciferous vegetables such as broccoli, activates AhR, as does its acidic condensation product, 3,3’-diindolylmethane (DIM)[23]. Both I3C and DIM have been investigated as treatments for a range of inflammatory diseases and cancers, including colitis[24-26], but the effects ofHelicobacterinfection on the response to I3C has not been studied. I3C and DIM are available for purchase as dietary supplements.
MicroRNAs (miRNAs) are being investigated as potential diagnostic and treatment tools. MiRNAs are highly conserved, noncoding, single-stranded, small ribonucleic acid molecules (17–27 nucleotides) that control gene expression post-transcriptionally. They typically bind at the 3’ untranslated region of the target gene messenger RNA (mRNA) leading to the degradation of the target RNA or inhibition of the translation of the RNA[27,28]. MiRNAs regulate genes involved in a wide range of cellular signaling pathways, including the Immune response. During the past ten years, much research has been done to uncover their roles in cellular proliferation, differentiation, maturation, and apoptosis[27]. Moreover, substantial scientific evidence underlines the functional roles and potential value of these tiny ribonucleic acid molecules for regulating autoimmunity and inflammation by affecting the differentiation, maturation, and functions of various immune cells in diseases including colitis[28,29]. Furthermore, many pieces of evidence show the participation of miRNAs in the regulation of T-cell development, differentiation, maturation, and activation[30]. Since the Treg/Th17 balance is crucial to intestinal health[31], understanding how miRNA expression is controlled by inflammatory and anti-inflammatory signals, such as I3C, could lead to identification of miRNAs capable of rebalancing the immune response in the inflamed colon.
The aims of this study were to examine the relative effects ofH. muridarumand I3C on mouse colon pathology, immune response, and miRNA expression. We used the standard mouse model of DSS-induced colitis in C57BL/6 mice. Some groups were infected withH. muridarumand treated with I3C. Treatment responses were monitored in the colon and mesenteric lymph nodes.
MATERIALS AND METHODS
Animals
The research described in this manuscript (including the acquisition of animals and all protocols for their use) was approved by the University of South Carolina Institutional Animal Care and Use Committee prior to commencement of studies. University of South Carolina is an AALAC accredited institution and all animal care procedures followed the NIH Guide for the Care and Use of Laboratory Animals. Female C57BL/6J mice (aged 8-10 wk) were purchased from The Jackson Laboratory, Bar Harbor, Maine, United States. Animals were housed in a controlled environment (12 h light/dark cycle) with food and water ad libitum. After one week of acclimation on a normal chow diet, the mice were randomly divided into groups. Groups of 5-7 animals were used in each experiment. The experimental groups included control (Ctrl),H. muridarum,H. muridarumplus DSS,H. muridarumplus DSS plus I3C, DSS, and DSS plus I3C (DSS/I3C). Each experiment included either all male or all female mice, as indicated in the text.
Bacterial strains, cultivation, and infection
H. muridarumstrain ATCC4982 was purchased from the American Type Culture Collection and was cultured in a humidified environment at 37 °C with 10% CO2, 5% O2in Ham’s F-12 medium containing 20 mL/L fetal calf serum in tissue culture flasks.H. muridarumbacteria were passaged every 2 to 3 d. After microscopically verifying appropriate morphology and motility, the culture was centrifuged at 25°C at 4500 rpm for 20 min, then the pellet was suspended in 9 g/L sodium chloride to produce a suspension containing approximately 28465 to 142072 adenosine triphosphate (ATP) relative luminescence units per 200 μL, as determined using the luminescent BacTiter-Glo ATP viability assay (Promega Corp.,Madison, Wisconsin, United States). Mice were inoculated by orogastric gavage (200 μL) every other day for a total of four inoculations. Viability of the remaining bacterial suspension was reconfirmed using the luminescent BacTiter-Glo ATP assay.
Infection was confirmed by polymerase chain reaction (PCR) of stool DNA usingH. muridarum-specific primers as follows. Fecal samples were collected and stored at -80°C until analysis. Fecal DNA was isolated using the EZNA stool DNA kit (Omega Bio-Tek, Inc., Norcross Georgia, United States) according to the manufacturer’s recommendations. Fecal PCR was performed usingH. muridarum16S rRNA genespecific primers (H. m. p30f, 5’-ATGGGTAAGAAAAAAAAAGATTGCAA-3’, and H. m. p30r, 5’-CTATTTCATATCCGCTCTTGAGAATC-3’), which amplify an 800 bp conserved region of the 16S rRNA, as previously described[32].
Induction of colitis with DSS
DSS (MW 40 000, Chem-Impex International, Inc, Wood Dale, Illinois, United States) at a concentration of 1-30 g/L was provided in drinking water for 10-13 d. The volume of DSS consumed, animal weight, diarrhea score, and stool blood score were recorded daily. The disease activity index was calculated from weight, diarrhea, and stool blood scores as previously described[33,34]. Stool blood was detected using a colorimetric fecal occult blood test (Helena Laboratories, ColoScreen catalog No. 5083). Briefly, we determined the disease activity index using the following variables: Stool blood (0, negative; 1, weakly positive; 2, strongly positive; 3, rusty-colored stool and 4, gross bleeding), changes in weight (0, < 1%; 1, 1%-5%; 2, 6%-10%; 3, 11%-15%; and 4, > 15%), and stool consistency (0-1, normal; 2-3, loose stools; and 4, diarrhea).
I3C preparation and dosage
For treatment groups, I3C purchased from Chem-Impex International, Inc. was suspended in DMSO prior to dilution in corn oil. I3C was administered orogastrically at a dose of 40 mg/kg in a total volume of 100 μL, as described previously[24]. Animals were treated with either I3C or vehicle (20 mL/L DMSO in corn oil) daily, beginning on the first day of the DSS cycle.
Histopathological colitis score
Formalin-fixed colon tissue was embedded in paraffin and cut into 5 μm thick sections. Next, the colon sections were stained using hematoxylin and eosin (H and E). Four randomly chosen, non-overlapping fields of each stained section were analyzed and assigned a colitis severity score by a pathologist using methods described previously[34,35]. In short, the degree of colitis was scored on the basis of the following parameters: Extent of the injury (0, none; 1, mucosa; 2, mucosa and submucosa; and 3, transmural), inflammation severity (0, none; 1, mild; 2, moderate; and 3, severe), and crypt damage (0, none; 1, basal one-third damaged; 2, basal two-thirds damaged; 3, crypt loss and the presence of surface epithelium; and 4, loss of the entire crypt and epithelium). Then the degrees for each of these aforementioned parameters were multiplied by an extent score that represented the percentage of each parameter that had a given feature as follows: 1, 0-25%; 2, 26%-50%; 3, 51%-75%; and 4, 76%-100%. We defined the total score as the sum of the three parameters. The bottom limit total colitis score was 0; the upper limit total
Characterization of CD4+ T cells in the mesenteric lymph node and spleen
For flow cytometry, the mesenteric lymph nodes (MLN) and spleens were pooled from each group of mice and placed in ice-cold medium. These tissues were mechanically disrupted, teased into single-cell suspensions, filtered through a cell strainer (70 μm), and placed in complete medium (RPMI-1640 containing 100 mL/L of heat-inactivated fetal bovine serum). The isolated cell suspension was stimulated with a cell stimulation cocktail (eBioscience?) plus protein transport inhibitors (Invitrogen, catalog 00-4975), for 4-6 h. Stimulated cells were incubated with anti-CD4 mAb and anti-CD25 mAb for 15 min on ice (Biolegend, United States). For intracellular cytokine staining, the cell suspension was incubated with anti-IFNγ, Interleukin-17 after treating the cells with Fixation/permeabilization kit (BD Biosciences catalog 554714). For Treg identification, we used FOXP3/Transcription Factor Staining Buffer set (eBioscience Invitrogen) before adding anti-FOXP3. Staining and washing were carried out in complete medium on ice. The stained cells were analyzed with a Beckman Coulter FC500 flow cytometer.
Enzyme linked immunosorbent assays
Interleukin-17 (IL-17), IL-6, IL-10, IL-4, IL-6, IL-21, IL-22, IL-23, IL-1β, transforming growth factor beta 1 (TGF-β1) , tumor necrosis factor-alpha (TNF-α) and interferon gamma (INF-γ), in the plasma and/or in colonic tissue lysates were quantified by ELISA kits (R and D Systems, Minneapolis, MN, United States) following the manufacturer’s recommendations. Colon tissues were prepared for ELISA as described previously[33]. Briefly, the mouse colons where washed immediately with cold phosphate buffered saline and frozen at -70°C until use. The samples were homogenized in 200 μL protein analysis buffer [10 mL of 1 mol/L Tris-hydrochloric acid (pH 8.0), 6 mL of 5 mol/L sodium chloride and 2 mL of Triton X-100 to 182 mL of sterilized distilled water][33]in 2 mL microcentrifuge tubes with a 0.9-2.0 mm stainless steel bead blend and homogenized with a tissue homogenizer (MP FastPrep-24) at a speed of 0.4 m/s for 20 s. Samples were frozen and thawed, and homogenized three times, then centrifuged at 30000gfor 30 min at 4°C. The supernatant was collected, and the pellet was re-suspended in phosphate buffer. Protein concentrations were determined using a bicinchoninic acid assay (Bio-Rad). Samples were frozen until the ELISA assays were performed and 0.5-1.0 mg/mL of protein was used for each run, depending on the interleukin type.
Sample collection and RNA isolation
Mesenteric lymph nodes were collected from the groups on the day of the sacrifice and immediately frozen at -70°C prior to use. Mesenteric lymph nodes were ground with mortar and pestle in liquid nitrogen. QIAzol Lysis Reagent (Qiagen, catalog 217004) was added to the samples and they were then homogenized by MP FastPrep-24 with 0.9-2.0 mm stainless steel beads (0.4 m/s for 10 s). Total RNA, including mRNA, miRNA and other small RNA molecules, were isolated from all the mesenteric lymph node with the miRNeasy Kit (Qiagen, Germany), following the manufacturer's procedure. The concentration and purity of the isolated RNA was determined using a Beckman Coulter DU800 UV/visible spectrophotometer. RNA quality was assessed by measuring the absorbance (A260/A280, A260/A230) of isolated samples and by agarose gel electrophoresis.
MicroRNA array analysis
The microarray was performed at the University of South Carolina School of Medicine following the protocol described by Bamet al[36,37]. Briefly, total RNA isolated as described above was hybridized to an Affymetrix miRNA-v3 gene chip (Affymetrix, Sunnyvale, CA, United States) as directed by the manufacturer. Raw data was processed in the Transcriptome Analysis Console (Affymetrix). The heat map was generated in Genesis[38]. The data from Transcriptome Analysis Console were used to calculate the linear fold-change of the expression of miRNAs to compare the miRNA expression differences among treatment groups. A linear fold-change of at least ± 1.5 was used as a cutoff value for the inclusion of a miRNA for further analysis. Moreover, only the miRNAs which were significant on the basis ofPvalue (< 0.05) calculated using student’st-test, were included in the analysis.
MiRNA-target gene prediction
Ingenuity Pathway Analysis (IPA, Qiagen, Redwood City, CA, United States) was used to predict the targets of the differentially expressed miRNAs. Networks relevant to regulatory T cells were generated to identify relevant miRNA species for testing. Other databases [TargetScan (http://www.targetscan.org/vert_72/), miRWalk (http://mirwalk.umm.uni-heidelberg.de/)] were also used to identify genes targeted by specific miRNAs.
Quantitative real-time PCR analysis of miRNA and gene expression
Total RNA from MLN was isolated and purified with the miRNeasy Kit (Qiagen, Valencia, CA, United States), following the manufacturer's procedure. The miScript II RT complementary DNA (cDNA) synthesis kit (Qiagen, Germany) was used according to the manufacturer's specifications to reverse-transcribe cDNA by taking 1 μg each of total RNA in a 20 μL total volume. The quantitative real-time (qRT-PCR) reactions were carried out using miScript Primer Assays or miScript Precursors (Qiagen, Germany) according to manufacturer instructions. U6, SnorD96, Snor68, Snor234, and Snor202 were evaluated for stability among groups and SnorD96 was chosen for normalization. SnorD96 has also been used by others[39,40]. Primers were purchased from Qiagen, Maryland.
For mRNA expression analysis, cDNA was made from total RNA as described. A two-step amplification qRT-PCR was carried out using SsoAdvanced? SYBR?green supermix from Bio-Rad (Hercules, CA, United States) with the mouse primers shown in Table 1. The real-time PCR conditions were as follows: Initial step at 95°C for 10 s followed by cycles (n= 40) consisting of 30 s at 95°C, followed by 30 s annealing/extension at 60°C and a final extension step for 30 s at 72°C. Data are normalized to expression of the reference gene encoding β-actin. Primers were purchased from Integrated Technologies and from Invitrogen. Melting temperatures ranged from 56.0°C to 64.5°C. Primer efficiency was measured for each primer set. All reactions were performed in triplicate. The qPCR experiments were carried out on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, United States). Fold changes were calculated using the 2?ΔΔCT(Livak) method.
Statistical analysis
Significance of differences between groups at single time points were determined using the Mann-WhitneyU-test using GraphPad Prism software (Version 8.2).Pvalues less than 0.05 were considered significant. Colitis symptom time course data were analyzed using a repeated measure analysis with 13 measures taken per animal (day one to day thirteen) randomly assigned to six groups (Ctrl,H. muridarum,H. muridarum/DSS,H. muridarum/DSS/I3C, DSS/I3C) and three experiments. Descriptive statistics were computed on the variables. For categorical variables, the univariate constructions will be included frequency distributions. For continuous variable statistics included measure of central tendency (mean and median) and measure of spread (standard deviation and range). Descriptive statistics for main variables were carried out for each group. In the analysis, expected mean squares were calculated and the appropriate combination used for hypothesis tests with specific functions of the repeated measures. General linear model analyses in SAS (MIXED procedure) were used to examine the effects of day, group, and day by group interaction. Post-hoc comparisons for the appropriate effects were examined. In addition, parameter estimates of the effects of covariate (experiment) and of the appropriate structure for the repeated observations was estimated. Adjusted Tukey-Kramer multiple comparison was used for significant effects. Significance levels are indicated asaP≤ 0.05;bP≤ 0.01;cP≤ 0.001;dP≤ 0.0001.
RESULTS
Exacerbation of colitis by H. muridarum is counteracted by I3C treatment
In three independent experiments, female wild-type C57BL/6 mice were infected with liveH. muridarumbacteria seven, five, three, and one days prior to commencement of DSS treatment (day zero). Pathology in mice treated with 30 g/L DSS was so severe that oneH. muridarum/DSS treated mouse required euthanasia. For this reason, 10 g/L DSS was used in subsequent experiments. Figure 1 shows average values from three independent experiments. Statistical analysis results are shown in Table 2 and Table S1. Overall disease activity was increased by each treatment exceptH. muridarumwhen compared to the control group.H. muridarumalone occasionally induced stool softening and a small amount of fecal occult blood, yetH. muridarumdecreased diarrhea scores in DSS-treated mice (Figure 1A and Table 2). On the other hand,H. muridarumincreased fecal occult blood and weight loss in DSS-treated mice. I3C was as effective in ameliorating colitis symptoms inH. muridarummice as it was in uninfected mice. Significant shortening of the colon, an indicator of inflammation, occurred inH. muridarum-infected mice compared with control mice (Figure 1B). DSS treatment ofH. muridarummice caused additional shortening and colon length was similar to DSS mice. I3C significantly increased colon length in both infected and uninfected mice. It is evident from the pathology scores that the infection withH. muridarumalone can induce pathology such as dilatation of glandular crypts, edema, and destruction of epithelium and glands (Figure 1B). In some cases, pathology caused byH. muridarumalone is comparable to that caused by DSS treatment, yet damage to the mucosa was not reflected in symptom scores in these mice. Treatment ofH. muridarum-infected mice with DSS further worsened pathology.
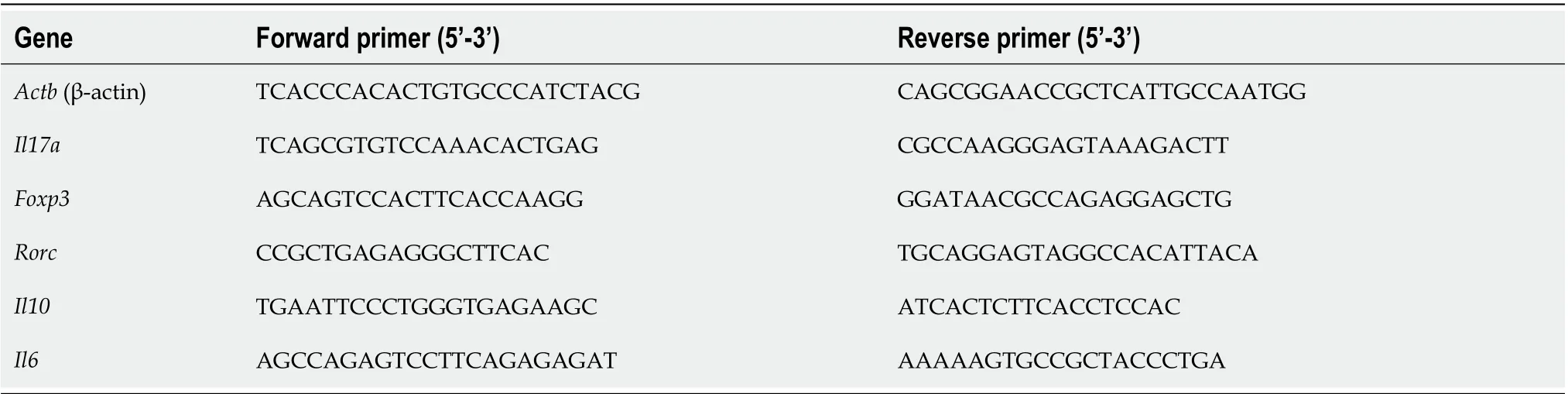
Table 1 Primers used for transcription analysis
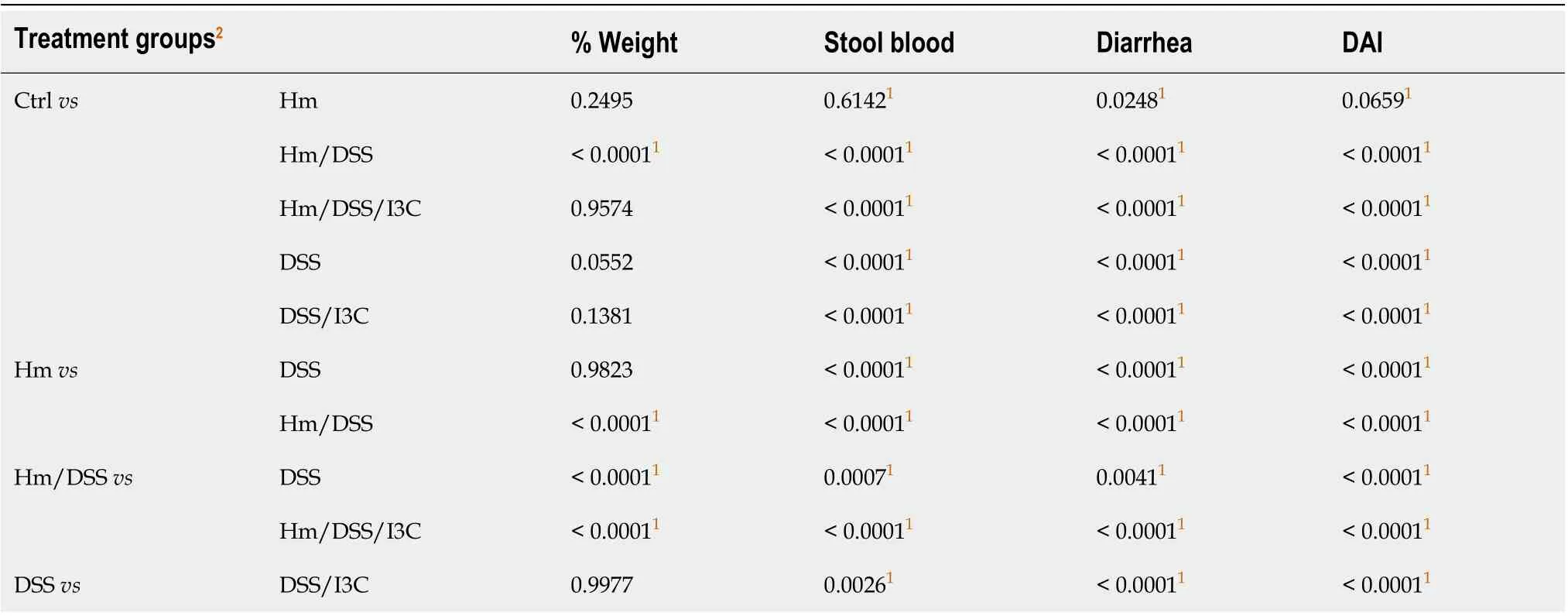
Table 2 Colitis symptom score P values
Effects of H. muridarum, DSS, and I3C on miRNA expression
Since miRNAs contribute to immune cell differentiation, we sought to determine which miRNAs were regulated by I3C, which were regulated byH. muridarum, and whetherH. muridarumaffected the I3C response. To accomplish this, we performed miRNA analysis on total RNA isolated from the mesenteric lymph nodes of all groups from one of the experiments. A heat map was constructed highlighting the differences in miRNA abundance among the groups (Figure 2A). We found that each group had a pattern that was distinct from all others. For example,H. muridaruminfection alone altered miRNA expression and miRNA expression in DSS, I3C treated mice was different depending on whether they were infected or uninfected.
We sought to determine whether I3C-regulated miRNAs are associated with regulation of the major Treg and Th17 transcriptional regulators, FOXP3 and RORC. To this end, we usedin silicoanalysis of predicted miRNA targets and pathways as well as online databases to search for miRNAs induced by I3C inH. muridarum/DSS mice that could targetFoxp3andRorcgenes. Among these potential miRNAs, we identified 3 candidates that had acceptable alignment scores and were highly predicted to targetFoxp3orRorc. These miRNAs included miR-let7a-5p and miR-29a-3p, which target RORC, and miR-874-5p and miR-6906-5p, which targetFoxP3. It should be noted that other members of the let-7 family also targetRorcand some were similarly regulated by I3C inH. muridarum-infected mice. We performed qRT-PCR on cDNA samples reverse transcribed from total MLN RNA. As predicted, we found increased expression of miR-23a-3p and let-7a-2, which targetRorc(Figure 2B). Differences between untreated and I3C-treated groups were only significant forH. muridarum-infected mice, but theRorc-targeted miRNAs miR-29a-3p and let-7a trended higher in uninfected mice. We also found that I3C decreased expression ofFoxp3-targeting miR-874-5p and miR-6906-5p inH. muridarum-infected mice, but only miR-6906-5p was reduced in uninfected mice. This is not surprising since miR-874-5p was not predicted to be elevated by DSS in uninfected mice, but it highlights the different miRNA responses seen among groups. The miRNAs miR-15b and miR-16 support Treg development by targeting a suppressor of Treg development and miR-15b/16 previously have been shown to be induced by DIM[41,42]. We also found these miRNAs to be increased by I3C inH. muridarum-infected, DSS-treated mice (Figure S1), but their expressions were predicted to be oppositely regulated in uninfected mice.
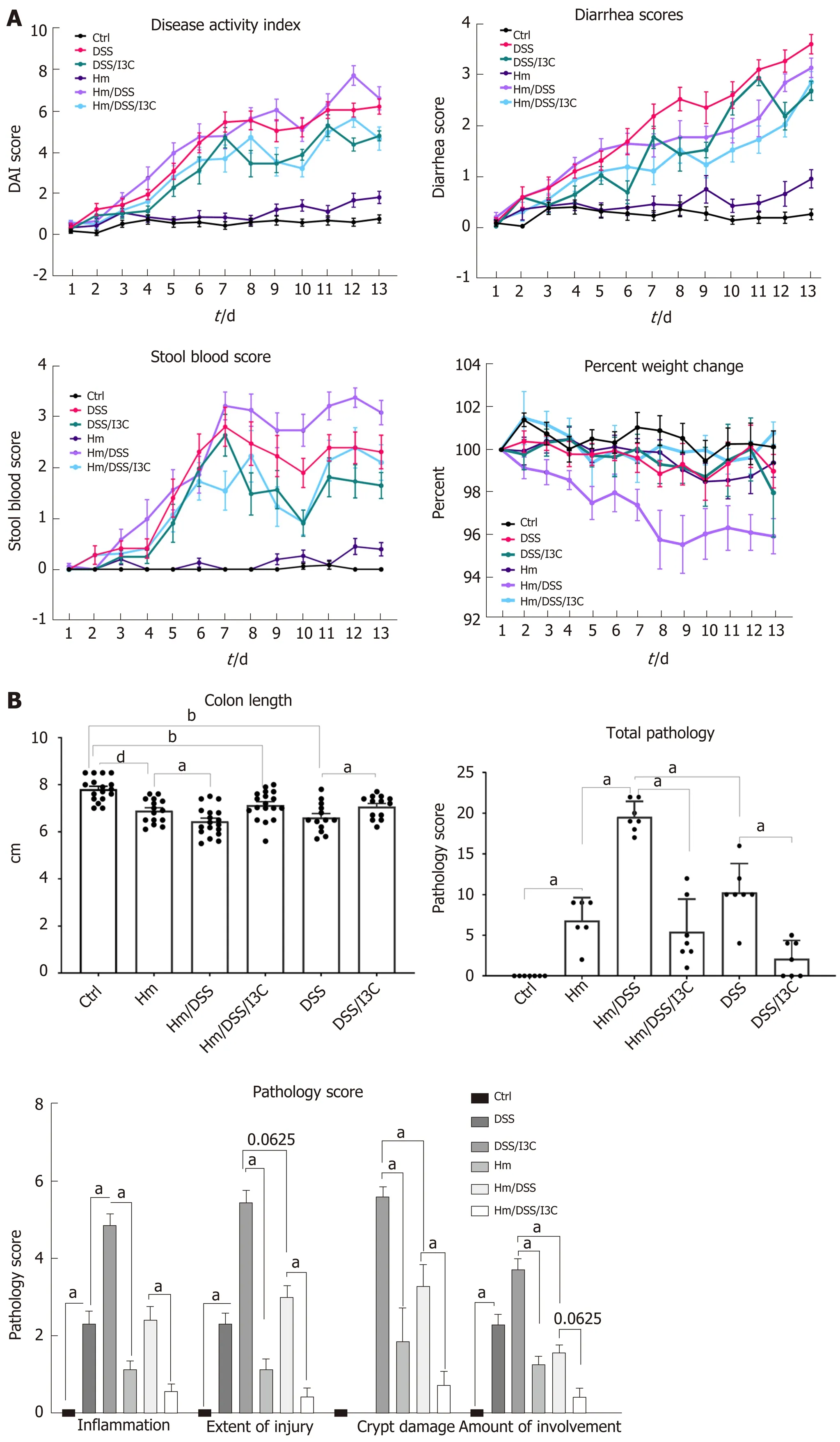
Figure 1 Symptom scores and histopathology. A: Colitis symptom scores (n = 17-21 per group); B: Colon length (n = 17-21 per group) and histopathology scores (n = 7 per group). aP ≤ 0.05; bP ≤ 0.01; cP ≤ 0.001; dP ≤ 0.0001. Ctrl: Control; Hm: Helicobacter muridarum; Hm/DSS: Helicobacter muridarum plus DSS; Hm/DSS/I3C: Helicobacter muridarum plus DSS plus I3C; DSS/I3C: DSS plus I3C.
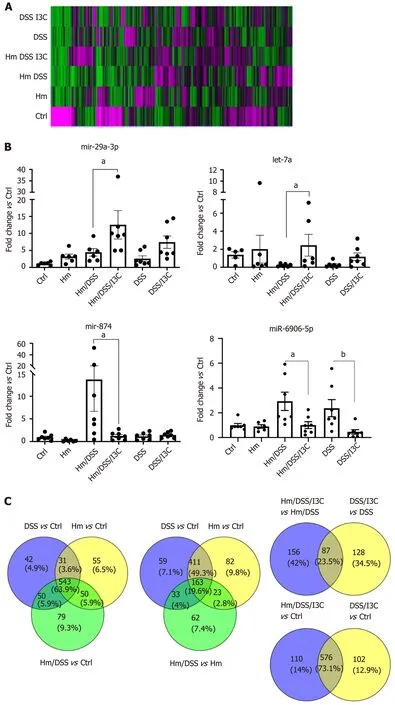
Figure 2 MicroRNA expression analysis. A: A heat map of expression intensities for each group was generated using Genesis software with red representing high expression and green representing low expression; B: Expression of microRNA as determined by quantitative real time PCR. All values are normalized to expression in the control group (n = 7); and C: Venn diagrams generated using Venny 2.1 demonstrate microRNA expression changes common to different treatment conditions. aP ≤ 0.05; bP ≤ 0.01. Ctrl: Control; Hm: Helicobacter muridarum; Hm/DSS: Helicobacter muridarum plus DSS; Hm/DSS/I3C: Helicobacter muridarum plus DSS plus I3C; DSS/I3C: DSS plus I3C.
A closer look at miRNA microarray data was illuminating. The first Venn diagram shown in Figure 2C highlights miRNAs that displayed a greater than 2-fold change in the colitis groups compared with the control group. TheH. muridarumgroup has 679 miRNAs up or down-regulated compared with the control,vs666 miRNAs in the DSS group, and 722 miRNAs in theH. muridarum/DSS group. Interestingly, the majority (n= 574) of the miRNA changes are shared between theH. muridarumgroup and the DSS group and most of these (n= 543) are also shared with theH. muridarum/DSS group as well. This demonstrates that the miRNA response toH. muridaruminfection is very similar to the response induced by DSS treatment. More importantly, miRNAs common between theH. muridarumgroup and the DSS group were almost all regulated in the same direction. All miRNA data are found in Table S2.
A second Venn diagram further highlights the effect of DSS treatment by substituting theH. muridarum/DSSvs H. muridarumcomparison for theH. muridarum vscontrol comparison. Not surprisingly, the number of miRNAs changed betweenH. muridarum/DSS andH. muridarum(n= 281) is much smaller than the effect ofH. muridarum/DSS compared to Ctrl (n= 722), but the majority of those miRNAs (69.8%) are also altered by DSS. It should be noted that 80.1% of the miRNAs altered by DSS treatment ofH. muridarummice are found within theH. muridarum/DSSvsCtrl comparison. 85.7% if miRNAs common between DSSvsCtrl andH. muridarum/DSSvs H. muridarumwere concordant in the direction of change. This recapitulates the findings shown in the first Venn diagram, indicating thatH. muridaruminfection and DSS treatment have similar effects.
A third Venn diagram was constructed to study the effects of I3C treatment. In I3Ctreated animals (Figure 2C), there was less overlap betweenH. muridarum-infected and –uninfected animals (87 miRNAs, or 23.5%). Oddly, most of the 87 common miRNAs (71.2%) were oppositely regulated inH. muridarum-infected and uninfected mice. In most cases, miRNAs were upregulated by I3C inH. muridarum-infected mice, but downregulated by I3C in uninfected mice. The predicted fold changes were also larger inH. muridarum-infected mice. The reason for this odd regulation pattern is discussed in the next paragraph. An alternative method for Identifying I3C effects is to compareH. muridarum/DSS/I3Cvscontrol with DSS/I3CvsCtrl (Figure 2C). These miRNA populations overlap heavily (73.1%). Most of the 87 previously identified miRNAs (56/87) are found in the overlap group. Since there is also heavy overlap between the putative I3C-regulated miRNAs and DSS-regulated miRNAs (529/666), it is not clear whether any of the miRNAs are strictly responsive to I3C; however, the overlap is consistent with the hypothesis that I3C normalizes miRNAs involved in colitis.
Examination of specific miRNAs provides a clearer demonstration of the effects ofH. muridarum, DSS, and I3C and an explanation for the differential regulation of miRNAs by I3C in infectedvsuninfected mice. We examined a list of 45 miRNAs that are altered in human IBD[43-45]. Almost all of the human IBD-associated miRNAs were altered byH. muridarumand/or DSS. Table 3 shows raw expression data and fold changes for the selected miRNAs. When compared with control values, these miRNAs were all downregulated, whereas many were upregulated compared to healthy controls in humans[43]. Possible reasons for this are discussed later. Expression decreases are mostly modest inH. muridarum vsCtrl, but extreme inH. muridarum/DSSvsCtrl- up to 3,646-fold decreased. The expression reductions were less extreme in theH. muridarum/DSS/I3C group compared to Ctrl. Expression of the selected miRNAs was lower in DSS/I3C group than the DSS group, but in many cases, the reductions were less than two-fold, which is why those miRNAs did not show up as common between infected and uninfected mice treated with I3C. It should be noted that there was not a global decrease in miRNA expression in any treated groupsvsCtrls; the decreases are specific to certain miRNAs. All 45 human IBD-associated miRNAs were among the 576 miRNAs in the putative I3C regulated group (Figure 2C) and all but two of the 45 were regulated byH. muridarumand/or DSS. Bianet al[46]also reported that many of these miRNAs are differentially regulated in DSS-treated mice, suggesting that a core set of miRNAs are relevant to colitis in both humans and mice[46].
H. muridarum infection alters T helper cell profiles.
Since miRNAs are pleotropic in their effects, we sought to confirm predicted effects on Treg and Th17 populations. MLN transcript analysis by qRT-PCR demonstrated that I3C decreased RORC and increased FOXP3 expression, consistent with a switch from Th17 to Treg (Figure 3). These results were mirrored by the decrease in IL17 and increase in IL10 expression. Expression ofIl6, which is involved in Th17 induction, is also shown. RORC and IL17 were more strongly induced by DSS inH. muridarum-infected mice than in uninfected mice, consistent with the increased pathology. Although FOXP3 was less strongly induced by I3C inH. muridarum-infected mice, IL10 expression was similar to that of uninfected mice treated with I3C. These results were corroborated by flow cytometry (Figure S2). The Th17 cell population increased sharply following DSS treatment ofH. muridarum-infected animals and decreased following I3C treatment while the Treg population increased.
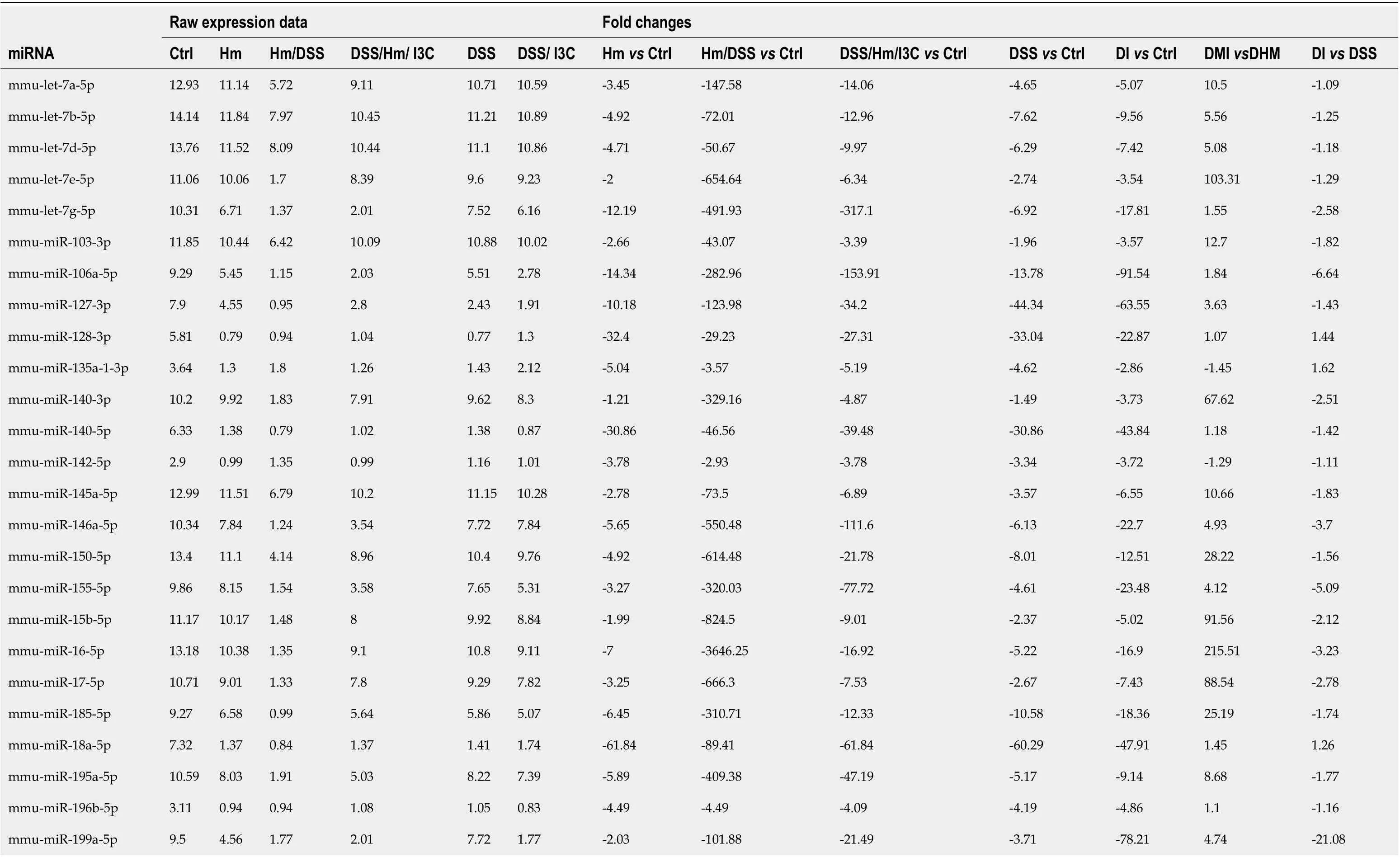
Table 3 MicroRNA expression
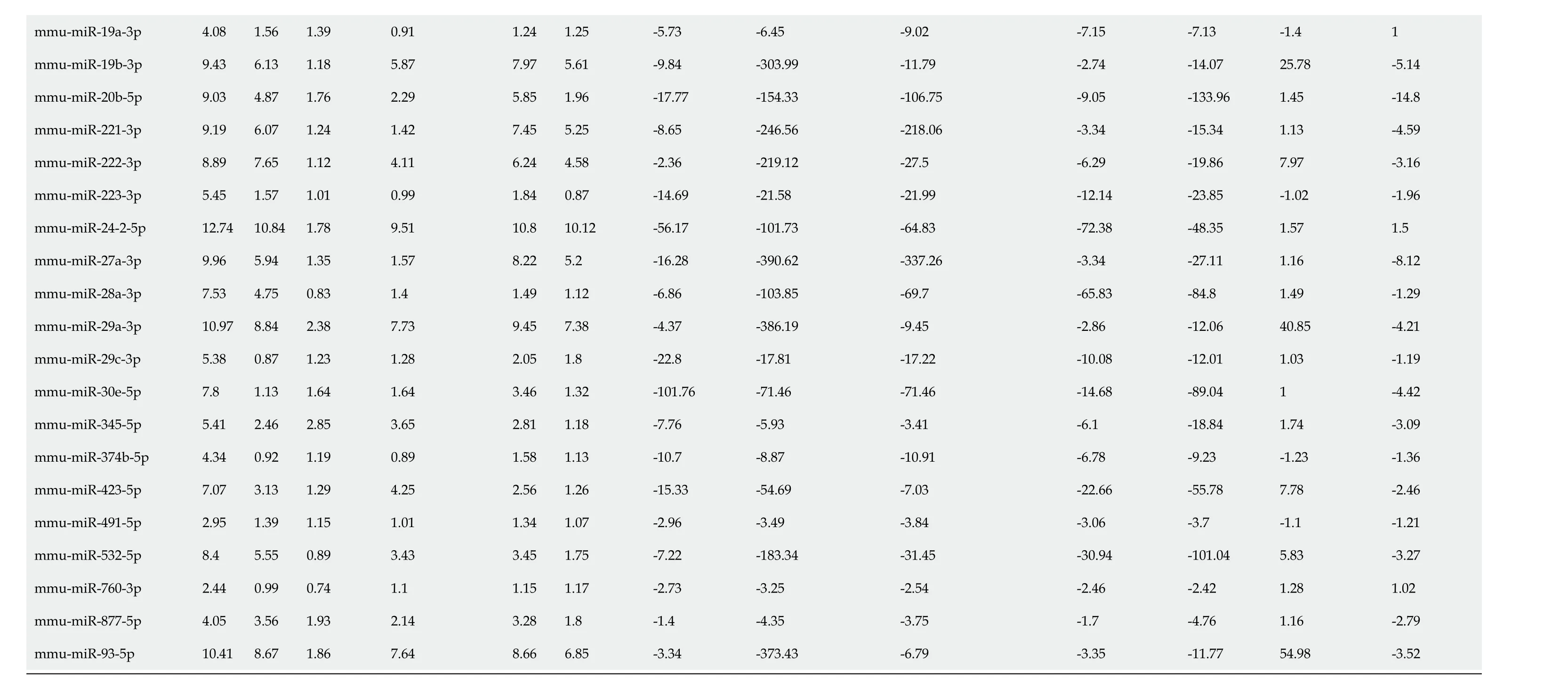
Ctrl: Control; Hm: Helicobacter muridarum; Hm/DSS: Helicobacter muridarum plus DSS; Hm/DSS/I3C: Helicobacter muridarum plus DSS plus I3C; DSS/I3C: DSS plus I3C.
Production of cytokines in colon tissue and plasma
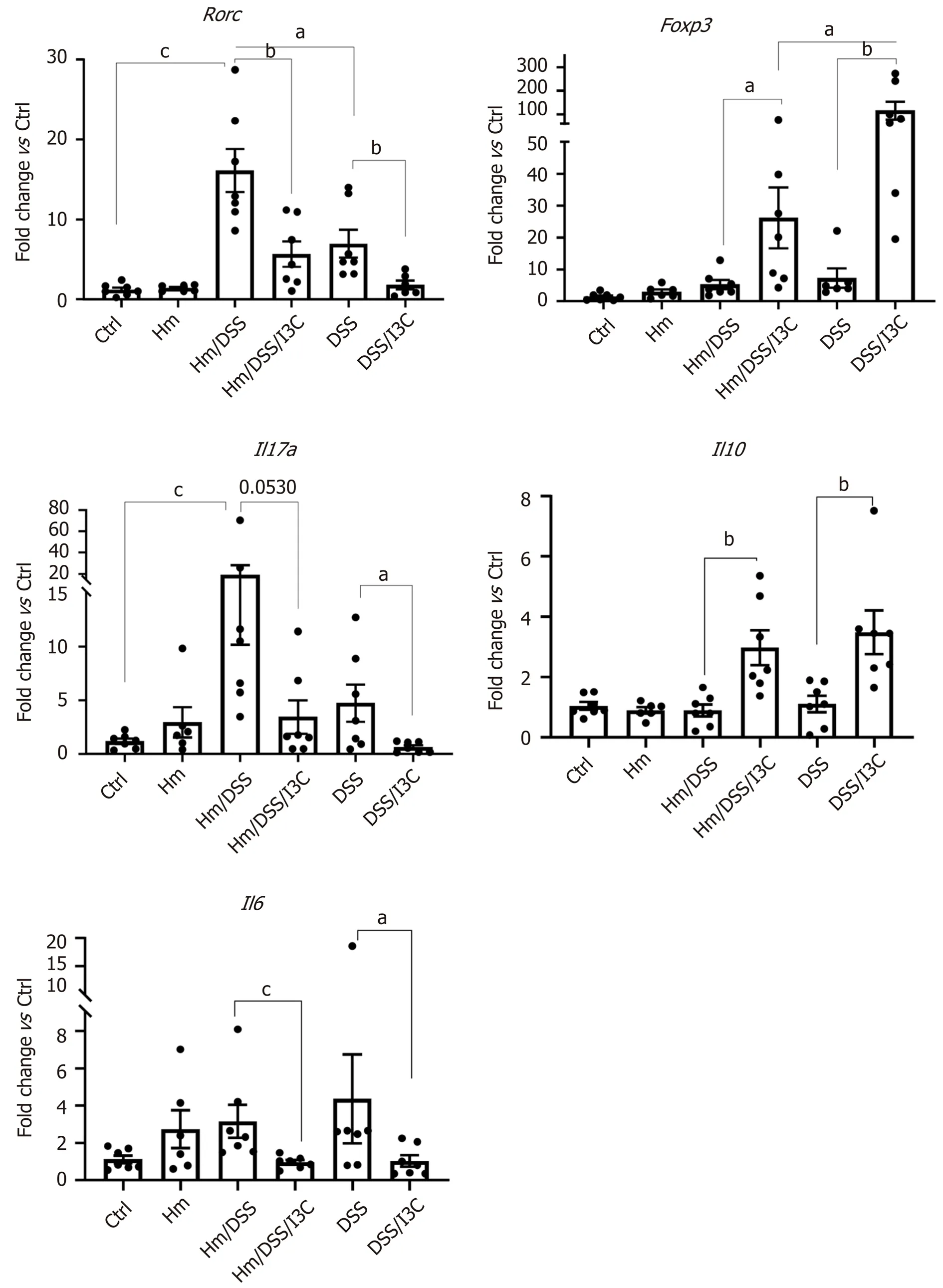
Figure 3 Expression of T helper 17 and Treg-associated genes in mesenteric lymph nodes. Gene expression was determined from total complementary DNA using qRT-PCR and values were normalized to the control group (n = 7). aP ≤ 0.05; bP ≤ 0.01; cP ≤ 0.001. Ctrl: Control; Hm: Helicobacter muridarum; Hm/DSS: Helicobacter muridarum plus DSS; Hm/DSS/I3C: Helicobacter muridarum plus DSS plus I3C; DSS/I3C: DSS plus I3C.
Our gene expression and flow cytometry data from the mesenteric lymph nodes clearly show that DSS andH. muridarumshift the T helper cell profile towards a Th17-dominated response, whereas I3C increases the Treg population. We next measured cytokine concentrations in colon homogenates to assess local immune cell and epithelial responses. These measurements encompass both epithelial cells and inflammatory cells. In most cases, production of pro-inflammatory cytokines was altered by multiple variables. Infection withH. muridarumalone increased all proinflammatory cytokines tested except IL-17 and IL-23 compared with control mice (Figure 4). In fact, cytokine levels inH. muridarum-infected mice were similar to those in uninfected, DSS-treated mice. Treatment ofH. muridarum-infected mice with DSS caused trends towards further increases in most cytokines, but this was only significant in the case of IL-17. I3C treatment reduced secretion of all proinflammatory cytokines in DSS-treated,H. muridarum-infected and/or uninfected mice.
Levels of the anti-inflammatory cytokines IL-10 and TGFβ were only significantly altered by I3C, though TGFβ levels trended lower in DSS-treated mice (Figure 4). There were trends towards decreased IL-4 levels in uninfected mice treated with I3C, but not inH. muridaruminfected mice treated with I3C. To summarize, I3C both decreases secretion of pro-inflammatory cytokines an increases secretion of antiinflammatory cytokines.
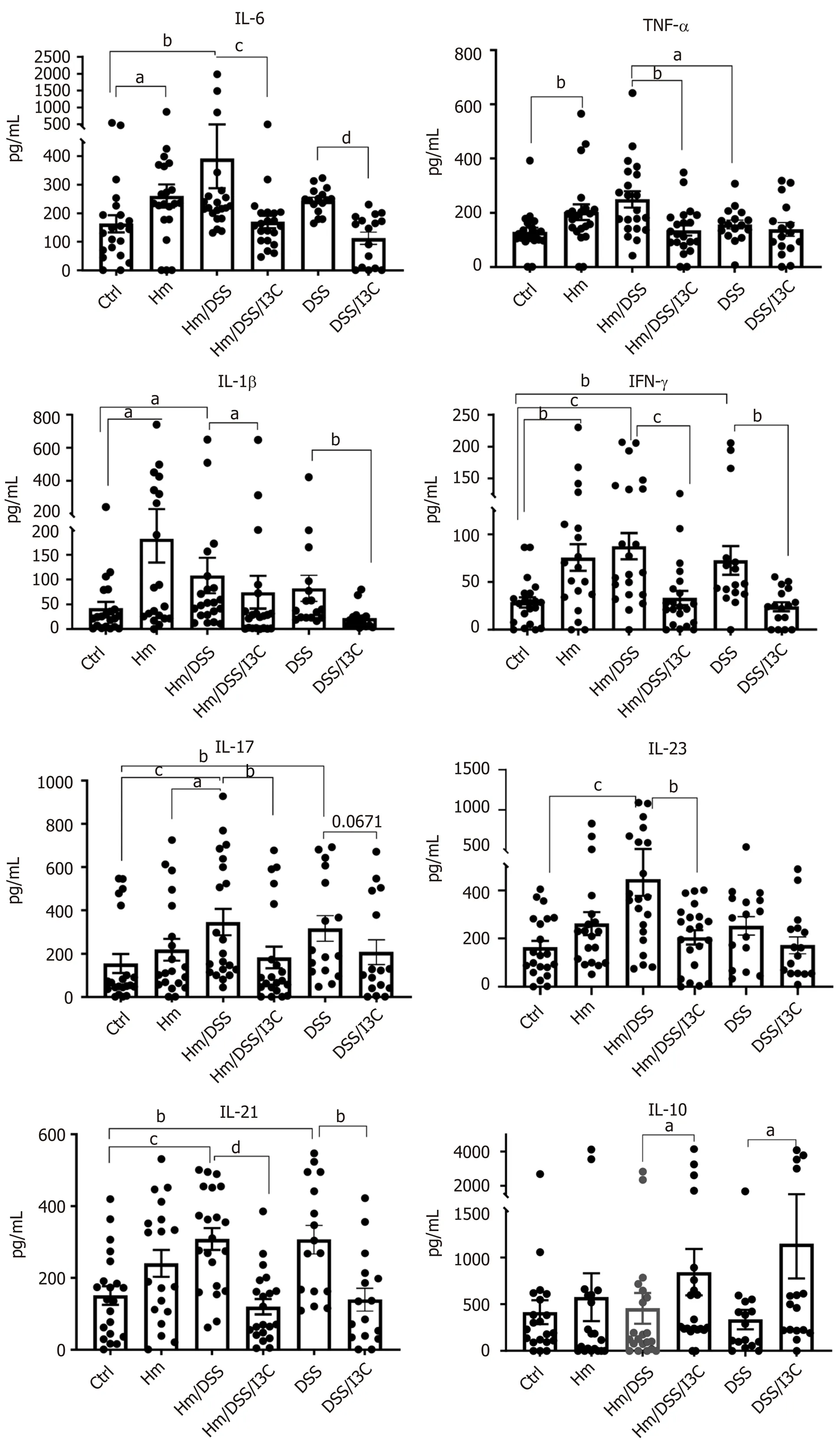
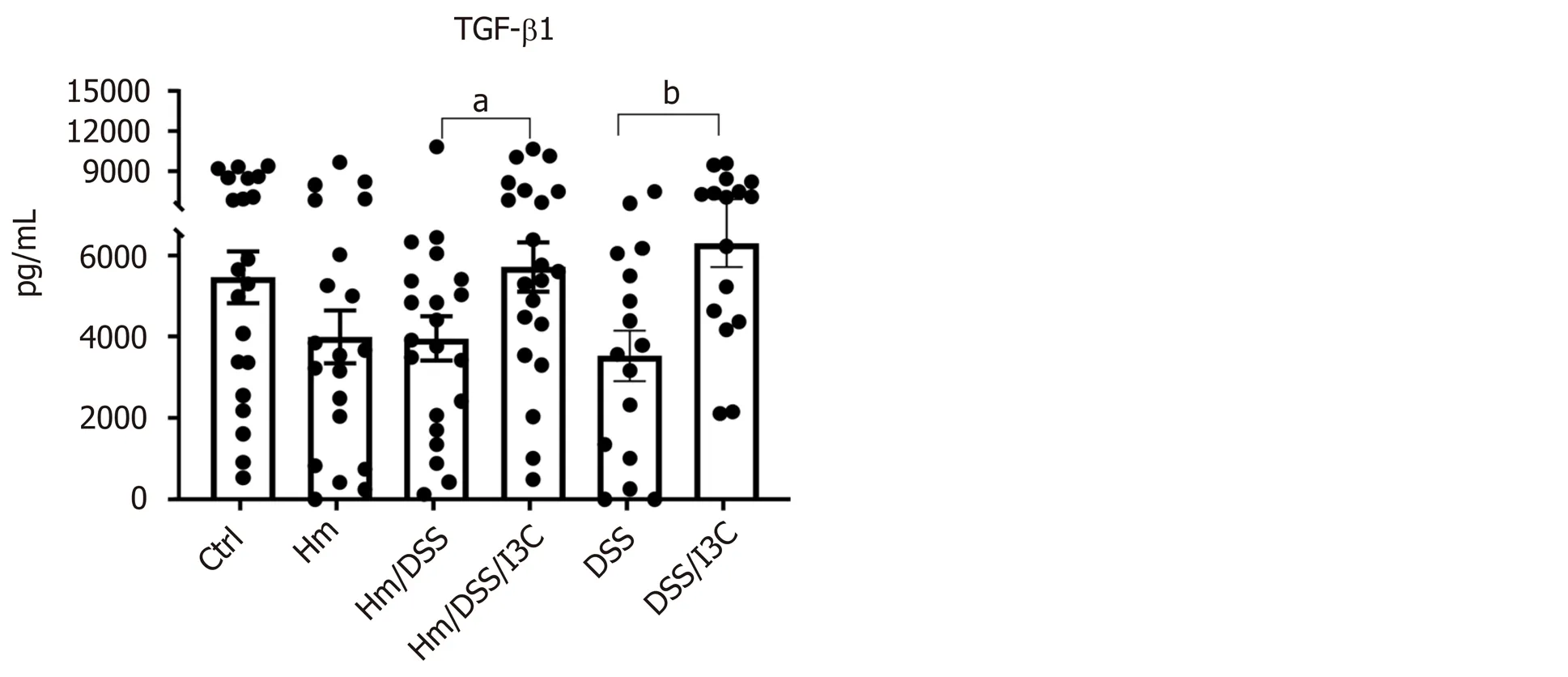
Figure 4 Colon cytokine production. Protein levels were determined by ELISA using colon homogenates (n = 17-21). aP ≤ 0.05; bP ≤ 0.01; cP ≤ 0.001; dP ≤ 0.0001. Ctrl: Control; Hm: Helicobacter muridarum; Hm/DSS: Helicobacter muridarum plus DSS; Hm/DSS/I3C: Helicobacter muridarum plus DSS plus I3C; DSS/I3C: DSS plus I3C.
Plasma cytokines showed less dramatic changes than colon cytokines, the proinflammatory IL-17 and IL-6 cytokine concentrations were elevated in theH. muridarum/DSS group and to a lesser extent in the DSS group. I3C treatment reduced both to control levels. IL-10 was elevated by I3C inH. muridarum-infected mice and trended higher in the DSS/I3C group (Figure 5). TGFβ was reduced only inH. muridarum-infected mice and did not respond to I3C treatment. IL-4 and IL-22 were not significantly altered under any condition (Figure S3). Serum amyloid a levels were significantly increased only inH. muridarum/DSS mice, consistent with more severe pathology in that group. The neutrophil marker myeloperoxidase was strongly increased byH. muridaruminfection, even in the absence of DSS treatment (Figure 5).
DISCUSSION
Effects of H. muridarum on susceptibility to DSS-induced colitis and treatment with I3C
Several models of inflammation and inflammatory bowel disease suggest that bacteria are necessary, but insufficient triggers of IBD[47]and several studies have reported that EHH species modulate IBD. As an example,H. macacaehas been connected with chronic idiopathic colitis in young rhesus macaques and a study of children with CD reported PCR evidence forHelicobacterinfection in 59% of patientsvs9% of healthy controls[11,48]. Similarly, Laharieet al[10]found thatH. pullorumorH. canadensisinfection was considerably related to CD in adults[10]. Finally,H. canis, another EHH species, has been detected in duodenal ulcerations associated with CD[49]. Therefore, certainHelicobacterspecies are almost certainly involved in IBD pathogenesis; however, the exact mechanism of EHH involvement remains undiscovered.
Th17 cells have a crucial role in colitis development in both humans and mouse models[50,51].H. pyloriis known to induce a Th17 response in the gastric mucosa[52-54], yetH. pyloriinfection is associated with a decreased risk of IBD[55].H. pylorionly colonizes the gastric mucosa, meaning that any effects ofH. pylorion the colon are likely due to systemic effects of infection. Furthermore, EHH species lack the majorH. pylorivirulence factorscagAandvacA. Thus, one cannot assume that mucosal or immune effects ofH. pyloriinfection will match those caused by EHH species. It is therefore necessary to use infection with EHH species to investigate potential mechanisms of EHH-mediated contributions to IBD.
The present study extends existing knowledge onH. muridarumpathogenesis.H. muridaruminfection has been previously shown to induce colitis in C57BL/6 mice treated with DSS and in monoassociated severe combined immunodeficiency mice following the transfer of certain T cell populations[13,35,56]. We found increased weight loss and stool blood inH. muridarum-infected mice treated with DSS compared with DSS treatment alone, but diarrhea was actually lessened. Increased stool blood suggests damage to the mucosal barrier, potentially increasing exposure to other members of the gut microbiota. ThoughH. muridarumalone did not cause appreciable colitis symptoms, it caused modest colon shortening and inflammatory infiltrates.

Figure 5 Plasma cytokine and inflammatory protein markers. Protein levels were determined by ELISA using plasma collected at the time of euthanasia (n = 5-7). aP ≤ 0.05; bP ≤ 0.01; cP ≤ 0.001. Ctrl: Control; Hm: Helicobacter muridarum; Hm/DSS: Helicobacter muridarum plus DSS; Hm/DSS/I3C: Helicobacter muridarum plus DSS plus I3C; DSS/I3C: DSS plus I3C.
Effects of H. muridarum, DSS, and I3C on microRNA expression
There is increasing interest in the use of miRNAs to diagnose and treat a wide variety of diseases and cancers. We examined mesenteric lymph node miRNA expression to determine whether miRNA signatures explained the effects ofH. muridarumand I3C. Many studies show that miRNAs participate in the regulation of crucial lymphocyte functions such as lymphocyte development, maturation, activation, and differentiation[19,28,57,58]. When considering the roles of miRNAs in Treg and Th17 function, it is necessary to distinguish between miRNAs involved in T cell differentiation and miRNAs involved in function. For example, miRNAs binding to the Treg transcriptional regulator gene FOXP3 prevent differentiation of Tregs and must be downregulated to allow Treg differentiation. Some miRNAs promote FoxP3 expression by downregulating expression of other genes, while others are induced by FoxP3 and influence Treg function. Still other miRNAs act in an autocrine manner, being both induced by FoxP3 and inducing FOXP3 expression[59]. Therefore, miRNA expression patterns can differ between na?ve and mature T cells and between highly active and anergic T cells. A further complication of miRNA interpretation is that the same miRNA can have different effects depending on the cell type in which it is expressed. For example, miR-155 reportedly induces both Treg and Th17 cell differentiation[60]making it impossible to predict whether increased miR-155 in the lymph node, or even within the T cell population, favors a Treg or Th17 phenotype. Relative concentrations of miRNAs within each cell most likely dictate the cell phenotype.
These nuances explain the often disparate and contradictory results published in the literature. For example, Iborraet al[43]sought to compare serum and mucosal miRNAs profiles in human ulcerative colitis and Crohn’s disease[43]. They found little overlap between serum and tissue miRNAs and many differences between their results and those published by others. Each miRNA has hundreds or thousands of targets[61]and each gene is likely controlled by dozens of miRNAs. In the context of IBD and colon cancer, miR-15b/16 is reported to regulate Tregs, macrophages, TP53, aquaporin 8, and the adenosine A2a receptor[41,62-65]. Surprisingly, miR-16 has even been suggested as a stable reference miRNA[66]. The multiple targets of miR-15b/16 may then explain why treatment of mice with miR-16 precursors ameliorates colitis, yet elevated miR-16 in human blood samples is associated with more severe IBD[62,67,68].
Interpretation of miRNA data in our study proved similarly challenging; however, there was remarkable overlap between the responses toH. muridarum, DSS, and I3C. Nonetheless, the direction of regulation was not always as expected. For example, when looking at a set of colitis-associated miRNAs, expression dropped following treatment with eitherH. muridarumor DSS, whereas human studies found increases in these miRNAs in IBD patientsvscontrols[43-45]. The human studies used either peripheral blood or colon biopsies as a source of miRNAs, whereas we used mesenteric lymph nodes. Lymph nodes differ in cellular composition compared to peripheral blood[69]and since the cell subsets change followingH. muridaruminfection or DSS treatment, the ratios of T cells to dendritic or other cell populations may change as well. Because colitis-associated miRNAs are likely expressed in multiple cell types, the meaning of a miRNA increase or decrease cannot be deciphered without knowing whether they rise or fall in each cell subtype. Presorting cells by flow cytometry would provide better data for understanding the effects of miRNA expression changes, but would not be feasible for most clinical samples due to the limited amounts of tissue available.
One would think that if DSS orH. muridarumdecreases miRNA expression, then I3C treatment should increase it back to the control value. This was not necessarily the case for the same reasons mentioned above. I3C increased the number of cells found in MLN, and likely the ratios of cell types. The fact that I3C altered roughly the same subset of miRNAs as DSS orH. muridarumis consistent with its known antiinflammatory effects, but does not shed light on the mechanism of I3C activity. Rather, the results are consistent with the hypothesis that miRNA changes due to I3C result from, rather than cause, immune response normalization.
We did not find measurable effects ofH. muridaruminfection alone on cytokine expression in the lymph nodes or on plasma cytokine levels; however, there were clear pro-inflammatory changes in the colon, which were further exacerbated by DSS administration. The cytokines induced byH. muridarum(TNFα, IL-1β, IL-6, and IFNγ) are typical of those induced by DSS treatment[17]. It is therefore not surprising thatH. muridarumfurther increased production of inflammatory cytokines and worsened pathology following DSS treatment. In humans with IBD, increases in mucosal TNFα, IL-1β, IL-6, IL-23 and IFNγ are due to lamina propria monocytes or macrophages[70-72]. IFNγ is also produced by Th1 cells or potentially a new intraepithelial lymphocyte subtype, IL-17+IFNγ+T cells[73]. Regardless of cell source, IFNγ plays an important role in IBD pathology in humans and mice[74,75]. Thus, the effects ofH. muridarumin our mouse model will likely be relevant to humans infected with enterohepatic species. In general,H. muridaruminfection more strongly influenced production of monocyte/dendritic cell-associated cytokines (TNFα, IL-1β, IL-6 , IL-23) compared to T cell-associated cytokines (IFNγ, IL-4, IL-10, IL-17, IL-21, IL-22), although IFNγ was increased byH. muridarumalone.
Cytokines secreted by monocytes or dendritic cells can drive T cell differentiation. TGFβ and IL-6 can drive several differentiation pathways depending upon which other cytokines are present[76]. The combination TGFβ, IL-6, and IL-23 efficiently induce Th17 differentiation[77]. In spite of local cytokine responses suggestive of a Th17-promoting milieu, we did not find evidence of a substantial T cell shift in mice infected withH. muridarumalone. DSS treatment was required for enhanced expression of RORC and IL17 in lymph nodes or increased plasma IL-17. In contrast, there was no apparent difference in Treg markers in lymph nodes or plasma between infected and uninfected mice. These data suggest that local effects ofH. muridarumare conducive to, but not sufficient for Th17 polarization. This is consistent with a “two hit” hypothesis, although in this case, the two hits areH. muridarumand an irritant rather than host genetics and the microbiome.
I3C shifts the immune balance
The use of alternative medicine to treat inflammatory disorders is appealing to many patients. Numerous studies demonstrate that dietary indoles possess anti-cancer properties such as anti-oxidant activity, regulation of cell cycle and apoptosis, and control of endocrine metabolism[78-83]. DIM is sold over the counter as BioResponse DIM?with claims that it promotes breast health, prostate health, and weight management. Before such products can be confidently recommended, their mechanisms of action must be uncovered. In the case of IBD, it is prudent to investigate the effect of gut microbiota on response to treatment.
Several natural compounds, including I3C, are AhR ligands[84]. AhR is now known to govern differentiation and function of both T cells and macrophages[85-87]. Several studies have shown that AhR plays a vital role in regulation of immune responses specifically promoting the generation of Tregs while suppressing Th17 cells[88,89]. Previous studies have provided convincing proof that Foxp3-positive Treg cells are essential for gastrointestinal immune homeostasis[90]and that increased Th17 differentiation promotes colitis[50,51]. Consistent with other reports from various disease models, we found that I3C increases the Treg population and decreases the Th17 population[91-93]. The shift from Th17 to Treg was not inhibited byH. muridaruminfection. Additionally, we found that I3C reduced production of every other proinflammatory cytokine tested, except for IL-22, which was not affected in any treatment group. Though we did not specifically analyze macrophages, others have shown that I3C or DIM suppresses IL-6, TNFα, IL-1β, IL-23 and IFNγ[94-96]. Therefore, I3C most likely inhibits colitis development via effects on both T cells and macrophages.
In summary, our studies suggest thatH. muridarumincreases susceptibility to DSSinduced colitis by inducing macrophage-associated cytokines and creating a mucosal milieu conducive to Th17 polarization. I3C ameliorates colitis via induction of Tregs, suppression of Th17 cells, and suppression of macrophage-associated proinflammatory cytokines. While no mouse model perfectly replicates human IBD, the identities of miRNAs altered byH. muridarumand DSS were similar to colitis studies in both mice and humans, although the direction of change was not always consistent. I3C is equally effective in the presence and absence ofH. muridarum. Further research is warranted on the roles of EHH species in human IBD and the use of I3C or similar AhR agonists for the treatment of inflammatory bowel disease.
ARTICLE HIGHLIGHTS

Research motivation
Given the limitations of performing IBD research in humans, an animal model of EHH-mediated pathology is needed. Such a model should reflect the biological changes seen during human IBD.Helicobacter muridarum(H. muridarum) has been referred to as a commensal in mice, yet we previously determined thatH. muridarumworsens colitis resulting from dextran sodium sulfate (DSS). This suggested that EHH species could represent environmental factors that cause or worsen IBD in genetically susceptible individuals. It is also important to determine whether phytochemicals being investigated as IBD treatments are influenced by infection with EHH species because there are no commercially available tests for EHH infection in humans.
Research objectives
We sought to determine how the immune and miRNA profiles ofH. muridaruminfected wild-type mice compared with DSS-treated mice and with published immune and miRNA profiles of IBD patients. We also determined whether efficacy of a broccoli-derived anti-inflammatory compound, indole-3-carbinol (I3C), was reduced byH. muridaruminfection.
Research methods
We measured changes in body weight, stool consistency, and stool blood followingH. muridaruminfection, DSS treatment, and/or I3C treatment. We then measured cytokine responses in the colon and plasma and histopathological changes in the colon. MiRNA changes and T cell population changes were measured in mesenteric lymph nodes.
Research results
WhileH. muridaruminfection alone did not cause clinical symptoms, it did cause colonic inflammation and induced proinflammatory cytokines. As expected,H. muridarumworsened colitis caused by DSS treatment, but it did not prevent amelioration of colitis by I3C treatment. Both the miRNA changes and cytokine responses toH. muridaruminfection were similar to those seen in human IBD and due to DSS treatment. Changes in cytokines and miRNA were consistent with a Th17 response.
Research conclusions
H. muridarumcauses subclinical colitis that increases vulnerability to DSS treatment. Since I3C is an aryl hydrocarbon receptor agonist, the efficacy of I3C in the presence ofH. muridarumsuggests thatH. muridarumdoes not influence the aryl hydrocarbon receptor agonist pathway. The strong similarities between cytokine and miRNA profiles induced by DSS and those induced byH. muridarumsuggest that similar mechanisms could be at play and that the mouse model is suitable for studying host interactions with EHH species.
Research perspectives
This research supports the hypothesis that EHH species could contribute to human IBD by exacerbating the response to other inflammatory stimuli. More research is needed on the prevalence of EHH species in humans and the mechanisms underlying EHH-mediated colonic damage.
 World Journal of Gastroenterology2020年32期
World Journal of Gastroenterology2020年32期
- World Journal of Gastroenterology的其它文章
- Association between human leukocyte antigen gene polymorphisms and multiple EPIYA-C repeats in gastrointestinal disorders
- Promising xenograft animal model recapitulating the features of human pancreatic cancer
- Etiology and management of liver injury in patients with COVID-19
- Novel virulence factor dupA of Helicobacter pylori as an important risk determinant for disease manifestation: An overview
- Inactive matrix Gla protein is elevated in patients with inflammatory bowel disease
- Development of a novel score for the diagnosis of bacterial infection in patients with acute-on-chronic liver failure
