Intracerebroventricularly-administered 1-methyl-4-phenylpyridinium ion and brain-derived neurotrophic factor affect catecholaminergic nerve terminals and neurogenesis in the hippocampus, striatum and substantia nigra
Jun-Fang Chen, Man Wang, Ying-Han Zhuang, Thomas Behnisch
The Institutes of Brain Science, the State Key Laboratory of Medical Neurobiology, and the Collaborative Innovation Center for Brain Science,Fudan University, Shanghai, China
Introduction
Parkinson’s disease is a neurodegenerative disorder characterized by the progressive degeneration of nigrostriatal dopaminergic neurons (Kong et al., 2008; Jung et al., 2014).To generate subchronic or chronic Parkinson’s disease-like mouse models, the intraperitoneal administration of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine(MPTP) has been widely used (Petroske et al., 2001; Zhu et al., 2011; Paul et al., 2017). MPTP induces neurodegeneration of dopaminergic neurons, resulting in motor impairments (Santos et al., 2017) that are similar to those in Parkinson’s disease patients. MPTP needs to be converted to 1-methyl-4-phenylpyridinium ion (MPP+) by glial monoamine oxidase B and be taken up into dopaminergic neurons by dopamine transporter (Espinosa-Oliva et al., 2014; Geed et al., 2014) to exert its cytotoxic effects (Schmidt and Ferger, 2001; Binukumar and Pant, 2016). Thus, administration of MPP+is widely used for the generation of Parkinson’s disease-like symptoms in mice (Petroske et al., 2001; Geed et al., 2014; Santos et al., 2017) and rats (Sonsalla et al., 2008).Because MPP+cannot freely traverse the blood-brain barrier, it has to be administrated into the brain by stereotaxic intracerebroventricular injection. In addition, this mode of MPP+administration avoids the detrimental peripheral effects of systemic intraperitoneal MPTP administration (Sonsalla et al., 2008). Depending on the dose and frequency of administration, different degrees of neuronal degeneration can be achieved (Petroske et al., 2001). The degeneration of dopaminergic neurons is also accompanied by the degeneration of dopaminergic projections from the substantia nigra(SN) and ventral tegmental area in the striatum (Bellucci et al., 2017; Kalia, 2018). Only recently have other brain areas,such as the hippocampus, been recognized as contributing to motor or non-motor deficits in Parkinson’s disease (Kohl et al., 2016; Liu et al., 2018; Medina, 2018). Many non-motor symptoms in Parkinson’s disease patients, such as fatigue,anxiety, depression and dementia, have been linked to altered catecholamine levels in the hippocampus (Pfeiffer,2016). Dopamine is released in the septotemporal axis of the hippocampus, from axon terminals originating from the ventral tegmental area and the SN (Gasbarri et al., 1997).Surprisingly, dopaminergic terminals and dopamine receptors in the hippocampus are anatomically distant from each other, leading to the notion that noradrenergic terminals from the locus coeruleus innervating the hippocampus and the dentate gyrus (DG) are capable of releasing dopamine under certain conditions (Smith and Greene, 2012). Anti-tyrosine hydroxylase (TH) staining labels nerve terminals of dopaminergic and noradrenergic neurons, revealing a dense network of catecholaminergic nerve terminals within the entire hippocampus, of which only a few are dopaminergic(Smith and Greene, 2012). A reduction in TH-positive nerve terminals in the striatum correlates with a reduction in dopamine content (Santiago et al., 2001); however, evaluation of norepinephrine levels after application of neurotoxins is rare. In addition, a change in TH-positive nerve terminals innervating the hippocampus after intracerebroventricular administration of the neurotoxin MPP+has not yet been documented.
We are interested in whether a single administration of MPP+evokes a compensatory increase in neurogenesis within the subgranular zone (SGZ) of the DG (Danielson et al., 2016) and SN (Mao and Wang, 2003). Previous studies have shown that adult neurogenesis in the DG is strongly associated with mood and cognitive functions, and that an imbalance in neurogenesis and cell death possibly leads to non-motor symptoms (Marxreiter et al., 2013; Salvi et al.,2016; Ermine et al., 2018; Farzanehfar, 2018). To resolve this misbalance, neurotrophic factors, such as fi broblast growth factor and brain-derived neurotrophic factor (BDNF), have been studied for their ability to enhance neuronal proliferation and differentiation (Lee et al., 2010; Hu et al., 2013).Indeed, endogenous neurogenesis in neuroproliferative zones in the adult brain is enhanced by administration or overexpression of growth factors, including BDNF (Pencea et al., 2001), epidermal growth factor and fibroblast growth factor 2 (Peng et al., 2008). However, it remains unclear whether cell death induced by single-dose MPP+alters neurogenesis or whether single-dose BDNF impacts the effect of the neurotoxin.
Here, we investigate the effects of a single intracerebroventricular MPP+administration on TH-positive nerve terminals in the hippocampus and striatum as well as on neurogenesis in the SGZ and substantia nigra 6 days later. In addition, we examine the impact of intracerebroventricular infusion of BDNF after the acute MPP+administration.
Materials and Methods
Animals
Forty male C57BL/6 mice, aged 6–7 weeks and weighing 20–24 g, were obtained from the Animal Center of the Chinese Academy of Sciences (Shanghai, China) and SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and maintained in accordance with the established standards of animal care and procedures of the Institutes of Brain Science and State Key Laboratory of Medical Neurobiology (approval No.31320103906; Fudan University, Shanghai, China). Efforts were made to minimize the number of animals used (Zhu et al., 2012).
Surgical procedure and treatments
Mice, intraperitoneally anesthetized with chloral hydrate(300 mg/kg; Sigma-Aldrich, St. Louis, MO, USA), were positioned in a stereotaxic apparatus (Stoelting, Wood Dale, IL,USA) (Wang et al., 2017). A guide cannula was implanted into the right lateral ventricle, 0 mm posterior to the bregma, 1 mm right of the midline and 2.25 mm deep, and fixed with jeweler’s acrylic cement. The coordinates were determined according to a mouse brain stereotaxic atlas (Pencea et al., 2001; Valvassori et al., 2015). After surgery, mice were allowed 2–3 days for recovery. On the day of administration,a smaller cannula was placed into the guide cannula so that its tip protruded 0.5 mm. The new cannula was connected to a microsyringe by a polyethylene tube. The compounds were applied at a rate of 0.3 μL/min. A schematic of the intracerebroventricular injection position within the brain and the cannula system is presented in Figure 1. Mice were randomly allocated to the saline, MPP+, MPP+/BDNF and BDNF groups. The saline group received ice-cold artificial cerebrospinal fluid (2 μL) in normal saline. The MPP+group received 0.6, 0.6 and 0.8 μL of a 50 mM MPP+iodide solution (100 nmol/2 μL; Sigma-Aldrich), intracerebroventricularly, at 2-hour intervals (Steffen et al., 1995). To determine if the intracerebroventricular administration of BDNF alters the effects of MPP+, the MPP+/BDNF group was given human recombinant BDNF (100 ng/μL in artificial cerebrospinal fluid; Invitrogen, Carlsbad, CA, USA) 1 day after MPP+treatment (Figure 1). The BDNF group was treated with BDNF 1 day after saline injection (Pencea et al., 2001; Benmansour et al., 2008; Deltheil et al., 2009; Valvassori et al.,2015).
To detect proliferating cells, bromodeoxyuridine (BrdU;Sigma-Aldrich) was used as a mitotic marker. Each animal received three intraperitoneal injections of BrdU (50 mg/kg body weight, for a total of 150 mg/kg) every 9 hours, and were transcardially perfused 9 hours after the last injection(Peng et al., 2008; Schlachetzki et al., 2016).
Preparation of brain sections
Mice were anesthetized with chloral hydrate and perfused transcardially with 4% (w/v) paraformaldehyde in 0.01 M phosphate-buffered saline (PBS) (pH 7.3), 7 days after saline or MPP+injection (Figure 1A). The brains were isolated and fixed in 4% paraformaldehyde at 4°C for 24 hours, and then rinsed with 0.01 M PBS and transferred to a 30% sucrose solution in 0.01 M PBS for 3 days. Sagittal sections, 30 μm in thickness, were prepared, and free- floating sections were stored in a cryopreservative solution (30% w/v sucrose, 30%v/v ethylene glycol in 0.01 M PBS) at ?20°C for 24 hours.
Immunohistochemistry
After 1 day of cryopreservation, sections were incubated in 1% H2O2in 0.01 M PBS for 15 minutes, followed by blocking solution (0.01 M PBS containing 5% goat serum, 0.3%Triton X-100 and 0.1% bovine serum albumin) for 2 hours at room temperature. After that, sections were incubated with a rabbit monoclonal anti-TH antibody (1:1000; AB152,Chemicon, China) at 4°C overnight. Sections were washed three times with PBS, and incubated with biotinylated goat anti-rabbit secondary antibody (1:500; Vector Laboratories,Burlingame, CA, USA) for 2 hours at 25°C. Immunoreactivity was visualized with the avidin-biotin peroxidase complex(Vectastain Elite ABC kit; Vector Laboratories) and 0.05%diaminobenzidine (Sigma) and 0.03% H2O2. Sections were washed, dehydrated, cleared in xylene, and coverslipped with permanent mounting medium (ProLong Gold Antifade Mountant with DAPI; Invitrogen), and images are shown in Figure 4. Briefly, to measure TH levels in the striatum, the mean staining intensity in an area of the striatum proximal to the ventricle was normalized to the mean intensity in an adjacent region.
Immuno fluorescence
Immunofluorescence was performed as described previously (Park and Enikolopov, 2010; Wang et al., 2017).Briefly, 1 day after cryosection, sections were incubated in 2 M HCl at 37°C for 30 minutes, and neutralized with 0.1 M boric acid, pH 8.5, at room temperature for 10 minutes.The sections were washed three times with 0.01 M PBS and incubated with blocking solution (0.01 M PBS containing 1% bovine serum albumin, 5% normal goat serum and 0.3%TritonX-100) for 2 hours. The sections were then treated with the following primary antibodies diluted in blocking solution at 4°C overnight: rat anti-BrdU (1:100; OBT0030S,Accurate Chemical) and rabbit anti-TH (1:500; AB152,Chemicon). After washing, the sections were incubated with secondary antibodies (1:500; Alexa Fluor 488, 546 or 555-conjugated; Thermo Fisher Scientific), diluted in 0.01 M PBS containing 5% normal goat serum, for 2 hours. The sections were post- fixed in 4% paraformaldehyde for 1 hour,washed, and incubated in DAPI (100 ng/mL, Roche) for 1 hour. Sections were then washed and mounted with Fluoromount Aqueous Mounting Medium (Sigma-Aldrich).
The sections were imaged by fluorescence microscopy(5× and 10× objectives; Olympus, Tokyo, Japan) and confocal microscopy (20× objective; Nikon A1 MP+/A1R MP+,Nikon, Tokyo, Japan). As described above, dopaminergic cell bodies in the SN and TH-positive nerve terminals in the striatum and hippocampus were labeled with antibodies against TH and BrdU. After determining the area of the DG and SN, the total number of labeled cells in every sixth coronal section of the region spanning bregma ?0.5 mm to ?2.0 mm was counted, then multiplied by six to obtain the estimated total number of positive cells per region. For the region of the striatum mainly containing TH-positive nerve terminals, the optical density of TH-immunoreactive fibers was determined by measuring 100 × 100 squares with ImageJ software (National Institutes of Health). The TH-positive fiber density in the striatum was corrected for the nonspecific background density, which was measured in the completely denervated parts surrounding the striatum and presented as the intensity ratio between the striatum in close proximity of the lateral ventricle and the rest of the striatum (Kim et al., 2005). The TH-positive fiber network in the hippocampus was analyzed by manually counting the number of crossings by TH-positive fibers of a line placed parallel to the cell body layer in the DG, CA3 and CA1 areas.The lines covered the dendritic areas of the CA3 and CA1 areas, and the hilus of the DG. The number of crossings was normalized to 100 μm.
Western blot assay
Mice were sacrificed 7 days after MPP+injection, as shown in Figure 1A. Western blot assay was performed as described previously (Dong et al., 2013; Huang et al., 2015;Wang et al., 2017). In brief, after anesthesia with isoflurane,brains were removed and immersed in pre-oxygenated (95%O2/5% CO2) ice-cold artificial cerebrospinal fluid (composition in mM: 119 NaCl, 2.5 KCl, 2.5 CaCl2?2H2O, 1.3 MgCl2?7H2O, 1.0 NaH2PO4, 11.0 glucose, 26.2 NaHCO3, pH 7.4). A piece of the entorhinal cortex was sliced off, and the two hemispheres were glued at the midline on the slicing platform of the sectioning system. Transverse hippocampal slices (350 μm) were cut and then snap frozen in liquid nitrogen and stored at ?80°C.
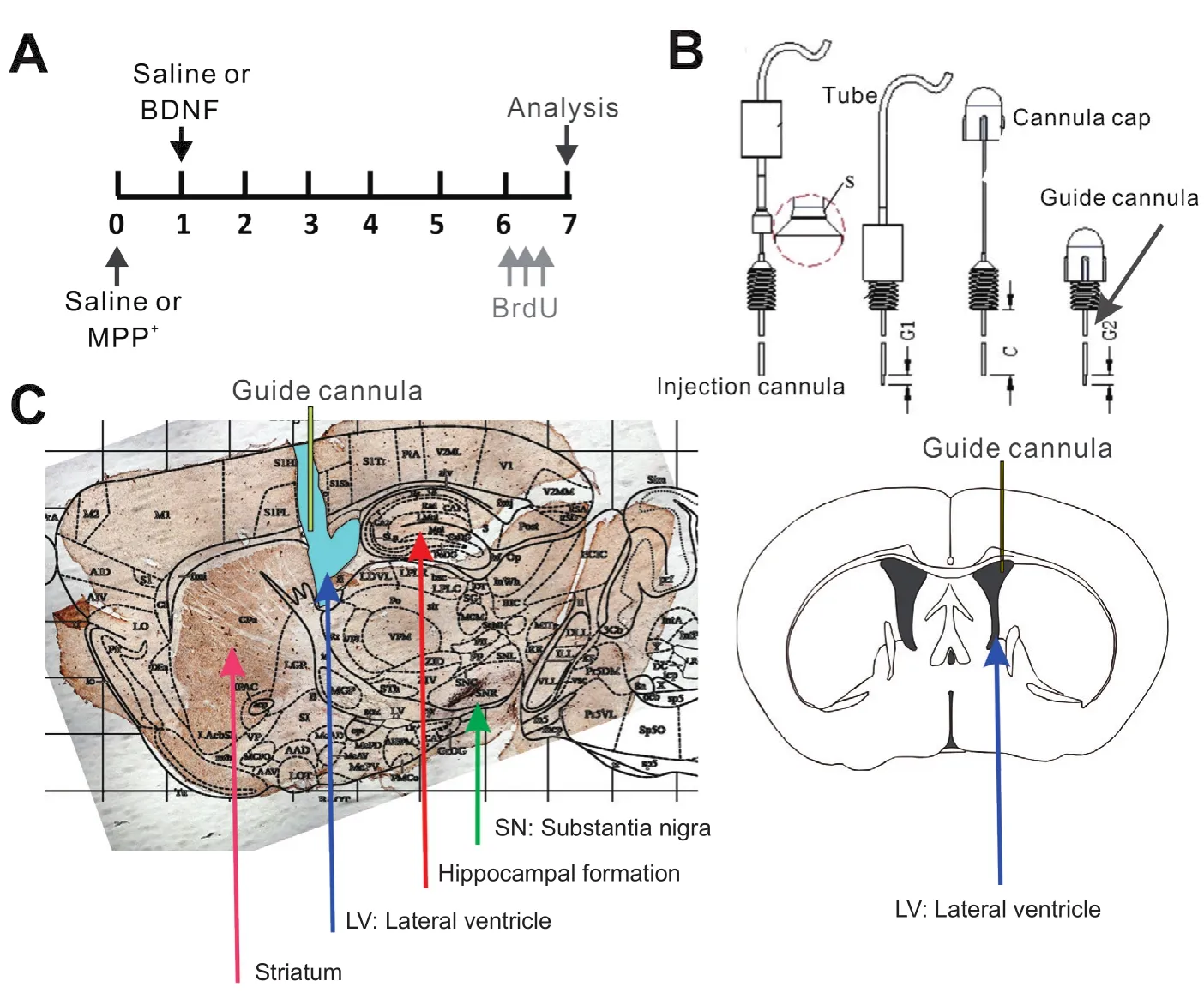
Figure 1 Illustration of the sequence of MPP+,BDNF and BrdU administration, the single-tube system for drug delivery, and the location of the cannula.

Figure 2 Distribution of TH-positive nerve terminals in the hippocampal formation.
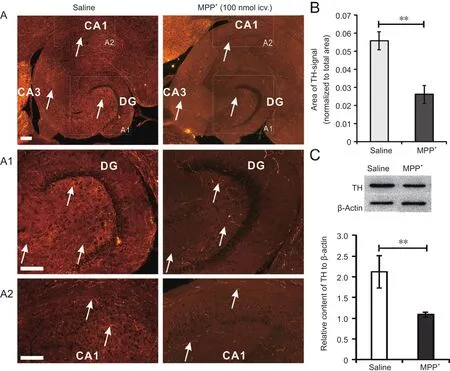
Figure 3 Intracerebroventricular administration of MPP+ significantly reduces TH levels in the hippocampal formation within 6 days.
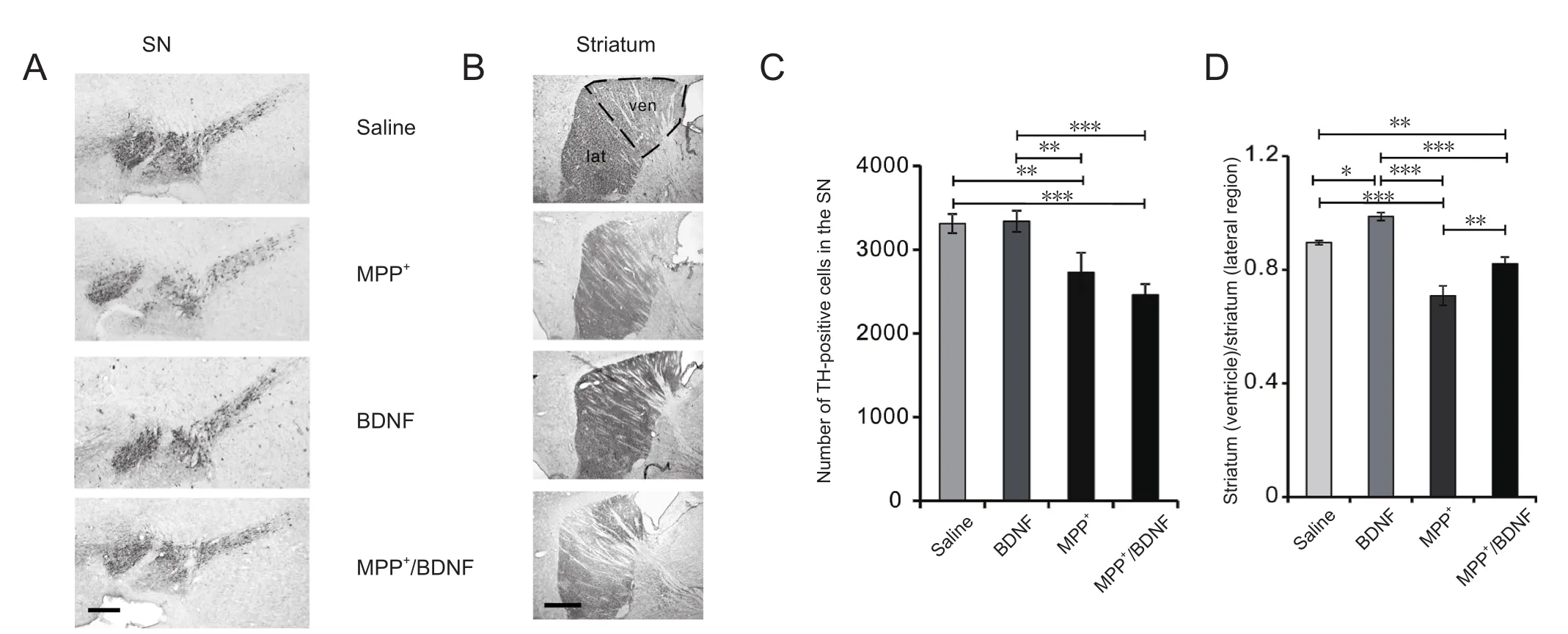
Figure 4 Effects of BDNF and MPP+ on TH levels in striatal nerve terminals and the number of dopaminergic cells in the SN.
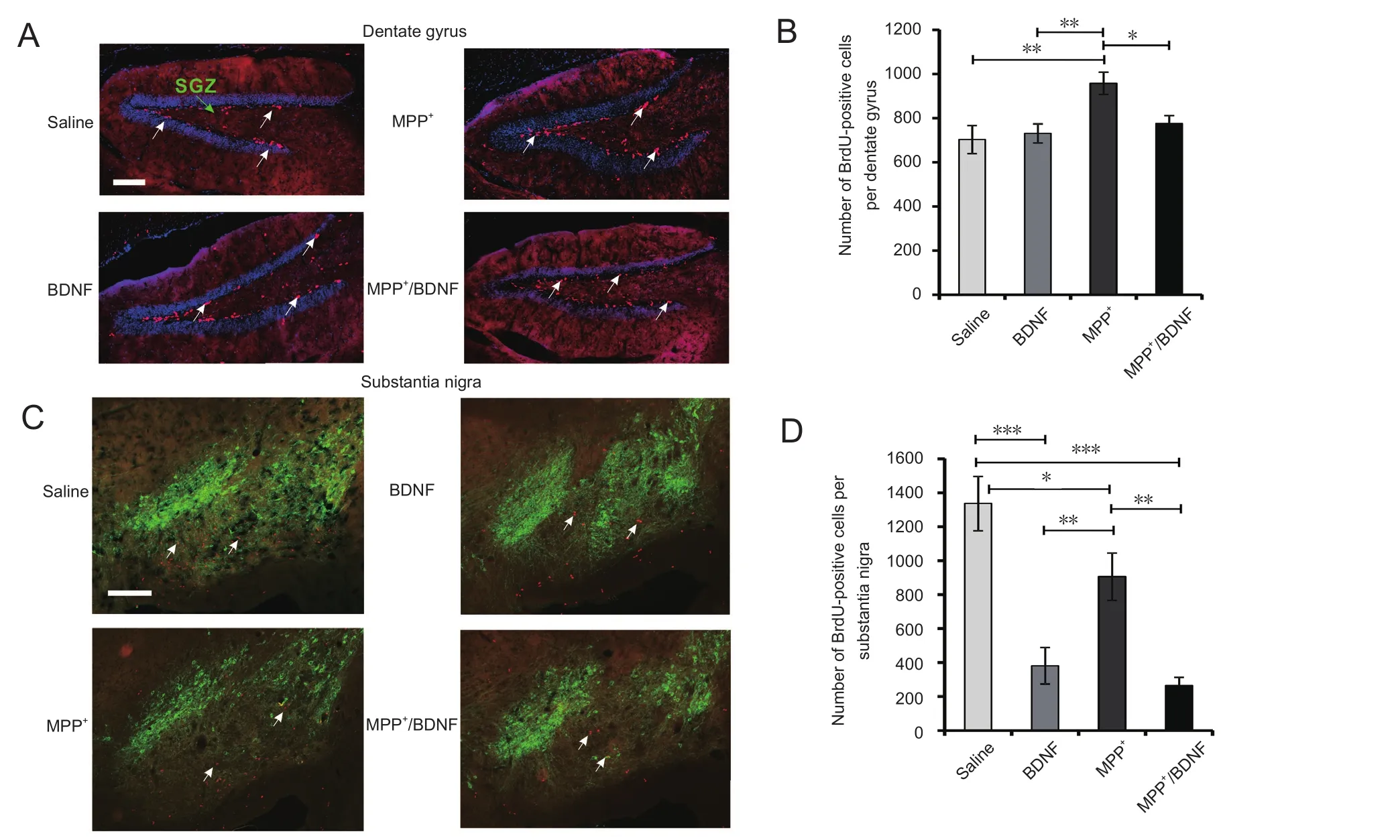
Figure 5 Confocal fluorescence images of BrdU-positive cells and their quantitative analysis in the SGZ, dentate gyrus and substantia nigra.
The frozen slices were thawed and then homogenized in radioimmunoprecipitation assay lysis buffer (50 mM Tris-Cl, 150 mM NaCl, 1% Triton X-100, 0.1% aprotinin, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate and 25 mM sodium fluoride, pH 7.4) supplemented with a protease and phosphatase inhibitor cocktail (Roche,Mannheim, Germany). The concentration of total proteins was measured using a bicinchoninic acid assay kit (Beyotime Institute of Biotechnology, Haimen, Jiangsu Province,China). The samples (30 μg per lane) were separated by 12%sodium dodecyl sulfate polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene fluoride membranes(Immobilon-P, Millipore, Billerica, MA, USA) using the Mini-PROTEAN Tetra System and the Mini Trans-Blot Electrophoretic Transfer System (BioRad, Hercules, CA,USA). The membranes were then incubated with primary antibodies overnight at 4°C after 2 hours of blocking in 5%nonfat milk at room temperature. The primary antibodies were anti-TH (1:1000; AB152, Chemicon) and anti-actin(1:1000; #4970S, Cell Signaling Technology). After washing in Tris-buffered saline-Tween 20, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:10,000; 111-035-003, Jackson ImmunoResearch, West Grove, PA, USA) at ambient temperature for 2 hours. The blots were thereafter incubated with enhanced chemiluminescence reagent (Thermo Scientific, Rockford, IL, USA), and luminescence was detected with the FluorChem E system (ProteinSimple). The experiments were performed in triplicate, and band intensities were quantified with AlphaView Analysis software (Cell Biosciences, Santa Clara, CA, USA).
Statistical analysis
All data are presented as the mean ± SEM. Statistical analyses were performed with SPSS 19.0 software (IBM China Company Limited, Beijing, China). A one-way analysis of variance with Fisher’s least significant differencepost hoctest or Student’st-test (unpaired) was used to compare group values. A value ofP< 0.05 was considered statistically significant (two-sided).
Results
Distribution of catecholaminergic nerve terminals in the hippocampal formation
We used immunofluorescence with an antibody against TH to characterize the distribution of catecholaminergic nerve terminals within the hippocampal formation. The catecholaminergic nerve terminals formed a dense fiber network that was not uniformly distributed within the various layers of the hippocampus or the DG (Figure 2A, B). However,it was difficult to correlate the location of the fibers with hippocampal subregions, such as the stratum pyramidale or stratum radiatum. The network was diffuse and did not colocalize with dendrites or cell bodies. TH is the rate limiting enzyme in the synthesis of catecholamines, hydroxylating L-tyrosine to L-3,4,-dihydroxyphenylalanine, which is then sequentially converted to dopamine and noradrenaline.Therefore, the TH immunoreactivity identifies either dopaminergic or noradrenergic fibers. Smith and Greene found that dopaminergic fibers were sparsely distributed within the hippocampus and that the majority of detectable fibers were actually noradrenergic nerve terminals primarily originating from the locus coeruleus (Collier et al., 2004; Smith and Greene, 2012; Takeuchi et al., 2016).
TH levels in catecholaminergic fibers of the hippocampal formation several days after MPP+ administration
The effects of intracerebroventricularly administered MPP+on TH levels in catecholaminergic nerve terminals in the hippocampal formation were analyzed by immunofluorescence and western blot assay 5 days after the MPP+injection(Figure 3). Fluorescent fibers in the images were isolated using threshold operators in ImageJ software, and the size of the labeled area was measured. The area was then normalized to the total area of the region of interest. The neurotoxin evoked a significant reduction in detectable fibers 6 days after intracerebroventricular MPP+administration (Figure 3B). The ratio of the TH-positive area to the area of the total hippocampal section was 0.0559 ± 0.0050 in the saline group and 0.0261 ± 0.0051 in the MPP+group. These results demonstrate that intracerebroventricular administration of MPP+significantly reduces TH levels in the hippocampus.Whereas it could not be found that specific areas within the hippocampus remained unaffected, intact TH-positive terminals were observed in the stratum radiatum, subiculum and entorhinal cortex (right, white arrows; Figure 3A). The immunofluorescence images in Figure 3A1 and 3A2 show TH-positive fibers and their distribution in the DG, CA1 and CA3 areas at high magnification. It is unknown whether these remaining fibers are dopaminergic or noradrenergic. It should be noted that the hilus in the DG, normally characterized by a dense network of catecholaminergic fibers, was nearly devoid of TH-positive fibers (Figure 3A1).
To further characterize the effects of MPP+on TH levels in the hippocampus, we performed western blot assay. This revealed a significant difference in TH levels between the saline and MPP+groups (Figure 3C). However, it is unclear whether this indicates axonopathy, leading to the degeneration and loss of nerve terminals, or just a reduction in TH levels.
Effects of BDNF and MPP+ on TH-positive terminals in the striatum and neurons in the SN
To examine if a single intracerebroventricular MPP+administration induces dopaminergic cell death in regions linked to Parkinson’s disease, an immunohistochemical analysis of TH-positive neurons in the SN and dopaminergic termi-nals in the striatum was performed 5 days after injection of MPP+. As illustrated in Figure 4, MPP+significantly reduced the number of dopaminergic neurons in the SN. The number of TH-immunoreactive neurons in the SN was 3310 ±114 in the saline group and 2600 ± 221 in the MPP+group(Figure 4A, C). We next examined whether intracerebroventricular BDNF injection 1 day after MPP+administration was neuroprotective. A single BDNF administration did not alter the number of TH-immunoreactive neurons in the SN(Figure 4A, C). The number of TH-immunoreactive neurons in the SN was 3337 ± 127 in the BDNF group and 2459± 128 in the MPP+/BDNF group. One-way analysis of variance revealed a significant difference in TH signal among the groups (F(3,18) = 9.57,P= 0.0005). Fisher’s least significant difference test indicated significant differences between the groups, except for the saline groupvs. the BNDF group and the MPP+groupvs. the MPP+/BDNF group (Figure 4C). These results indicate that intracerebroventricular administration of MPP+induced a significant reduction in TH-immunoreactive neurons in the SN and that BDNF did not attenuate this effect of MPP+.
In the striatum, TH levels were not uniformly reduced and were higher near the lateral ventricle. Therefore, the difference was expressed as the ratio of the signal intensity between the striatum near the ventricle and that in the lateral parts of the striatum. MPP+had a significant effect on the striatum (Figure 4B, D). This suggests that there is an MPP+concentration gradient from the lateral ventricle to the striatum, a concept that has been proposed previously for intracerebroventricular drug administration for the hippocampus (Valvassori et al., 2015). In addition, the findings indicate that the dose of MPP+used in the present study is sufficient to induce changes in TH levels and the degeneration of SN neurons.
In contrast to the SN, BDNF increased slightly, but significantly, the TH ratio and reduced the MPP+-induced loss of TH. The striatal TH signal intensity ratio was significantly higher in the MPP+/BDNF group compared with the MPP+group. The TH signal intensity ratio in the nerve terminals in the striatum was 0.89 ± 0.01 in the saline group (n= 4),0.98 ± 0.01 in the BDNF group (n= 4), 0.71 ± 0.03 in the MPP+group (n= 4), and 0.82 ± 0.02 in the MPP+/BDNF group (n= 5). One-way analysis of variance revealed a significant difference in TH signal among the groups (F(3,13)= 26.3,P< 0.0001), and Fisher’s least significant difference test indicated significant differences between the groups (P<0.0001), except for the saline groupvs. the BDNF group and the saline groupvs. the MPP+/BNDF group (Figure 4D).The findings indicate that BDNF significantly attenuates the effect of MPP+on striatal TH expression. Photomicrographs of TH-immunoreactive structures in the striatum and SN are presented in Figure 4A, B.
Neurogenesis
There is emerging evidence that dopamine levels regulate neurogenesis not only in the substantia nigra but also in other brain areas such as the hippocampus, subventricular area and striatum. In addition, it has been shown for intraperitoneally administered MPTP that degeneration of the dopaminergic system can enhance neurogenesis in a time-dependent manner. However, not much information is available on the effects of intraperitoneally administered MPP+on neurogenesis. Therefore, we next examined whether a change in the rate of neurogenesis occurs several days after saline, MPP+or MPP+/BDNF administration.As shown in Figure 1A, mitotic cells were labeled by intraperitoneal BrdU administration on day 6. On day 7,BrdU-positive cells in the SGZ and SN were counted. The number of BrdU-positive cells in the SGZ was not altered by the single-dose BDNF administration, but BDNF prevented the MPP+-mediated compensatory increase in neurogenesis(Figure 5B). Representative immunofluorescence images of BrdU-positive cells (red) in the SGZ of the DG are presented in Figure 5A for the different treatment conditions. The number of BrdU-positive cells in the SGZ was 703 ± 64 in the saline group, 730 ± 43 in the BDNF group, 958 ± 50 in the MPP+group, and 775 ± 37 in the MPP+/BDNF group.One-way analysis of variance revealed a significant difference in the number of BrdU-positive cells (F(3,15) = 5.6,P= 0.008), and Fisher’s least significant difference test indicated significant differences for the saline groupvs. the MPP+group, the BDNF groupvs. the MPP+group, and the MPP+groupvs. the MPP+/BDNF group (Figure 5B). The number of BrdU-positive cells in the MPP+group was substantially elevated in comparison with the other groups. While BDNF did not alter the number of BrdU-positive cells in the SGZ,it did so in the MPP+/BDNF group. The results show that intracerebroventricular MPP+strongly induces neurogenesis in the SGZ, and that BDNF diminishes this effect of MPP+.
The SN is also a site of neurogenesis in adult mice. BDNF substantially reduced the number of BrdU-positive cells 6 days after injection. A similar reduction in the number of mitotic cells was also observed in the MPP+/BDNF group,indicating that BDNF did not suppress the MPP+-induced reduction in neurogenesis in the SN (Figure 5D). The number of BrdU-positive cells in the SN was 1336 ± 159 in the saline group, 416 ± 65 in the BDNF group, 906 ± 139 in the MPP+group, and 266 ± 47 in the MPP+/BDNF group. Oneway analysis of variance revealed a significant difference in the number of BrdU-positive cells among groups (F(3, 13)=18.7,P< 0.0001), and Fisher’s least significant difference test indicated significant differences (P< 0.0001) for the saline groupvs. the BDNF group and the saline groupvs. the MPP+/BDNF group. There were also significant differences between the other groups, except for the BDNF groupvs. the MPP+/BDNF group (Figure 5D). Representative immunofluorescence images of BrdU-positive (red) and TH-immunoreactive cells (green) in the SN are presented in Figure 5C for the different conditions.
Discussion
In this study, the dense network of TH-immunoreactive fibers in the hippocampus and DG were sensitive to a single dose of intracerebroventricularly-administered MPP+. In addition, a loss of dopaminergic cells was observed in the SN, as well as a reduction in TH-immunoreactive fibers in the striatum adjacent to the lateral ventricle. MPP+altered the number of BrdU-positive cells in the SN and the SGZ,and intracerebroventricularly-injected BDNF blocked the increase in BrdU-positive cells in the SGZ but not the reduction in the SN. BDNF alone strongly reduced the number of BrdU-positive cells in the SN.
Very little is known of the dense network of TH-positive nerve terminals within the hippocampus and the DG (Smith and Greene, 2012). In many studies, TH has been used as a marker of dopaminergic fibers (Liang et al., 2016; Wu et al.,2016); however, specific retrograde or anterograde labeling of neurons in the SN or ventral tegmental area revealed low and non-uniform innervation within the septotemporal axis of the hippocampus (Gasbarri et al., 1997). In addition,dopamine receptors are anatomically separated from dopaminergic terminals, making it difficult to correlate function with anatomy. Nonetheless, the dense nerve terminal network revealed by TH immunofluorescence might represent the neural substrate of various hippocampal functions.Indeed, it has been shown that TH-positive nerve terminals from the locus coeruleus innervate the hippocampus and are capable of releasing dopamine under certain conditions(Smith and Greene, 2012). Because TH is the rate limiting enzyme in catecholamine synthesis, anti-TH staining will reveal not only dopaminergic fibers but also noradrenergic fibers (Smith and Greene, 2012). Here, we did not examine the distribution of dopamine transporter or noradrenaline transporter-containing fibers. However, Smith and Greene(2012) clearly identified the sparse distribution within the hippocampus, making it very likely that most detected fibers represent noradrenergic nerve terminals.
A single MPP+administration into the lateral ventricle was sufficient to induce cell death within the SN and a reduction in TH immunoreactivity in the parts of the striatum in close proximity to the lateral ventricle. Western blot assay confirmed that TH levels in the hippocampus and the DG were reduced. However, selectivity of MPP+for specific fibers within the hippocampus could not be detected, although it was previously hypothesized that dopaminergic fibers in the stratum pyramidale would primarily be affected (Smith and Greene, 2012). It remains unclear how MPP+evokes a reduction in TH levels in noradrenergic fibers, because they do not contain the dopamine transporter considered necessary for the detrimental effects of MPTP and MPP+to manifest in neurons. However, in our previous study on the acute effects of MPP+, a difference in the effects of MPTP and MPP+on hippocampal synaptic transmission was found. Specifically,the effect of MPTP, but not that of MPP+, on hippocampal synaptic transmission was dependent on the activation of dopamine receptors. Further study is needed to clarify the involvement of the noradrenergic system in the effects of MPP+on hippocampal synaptic transmission.
The DG is one of the most active areas of neurogenesis and neural differentiation in the adult mouse brain, and it is severely affected in Parkinson’s disease (Sairanen et al., 2005;Marxreiter et al., 2013; Schlachetzki et al., 2016). Within 7 days of MPP+administration, there was a substantial reduction in BrdU-positive cells in the SN, but notably, there was an increase in BrdU-positive cells in the SGZ. These findings are in agreement with studies on the effect of MPTP on adult neurogenesis in the SGZ and on the proliferation of newborn cells within the DG (Park and Enikolopov, 2010;Lesemann et al., 2012; Schlachetzki et al., 2016). Klein et al. (2014) found that the number of BrdU- and neuronal marker-positive cells increased by the sixth day after MPTP administration, although only small changes in the number of BrdU-positive cells were observed (Klein et al., 2014). All of these studies used intraperitoneal MPTP administration,and different MPTP doses and administration protocols were used. There are very few studies on the effects of intracerebroventricularly-administered MPP+on neurogenesis.Furthermore, it remains unclear how denervation of dopaminergic fibers impacts neurogenesis in the SGZ (Park and Enikolopov, 2010; Tapia-Bustos et al., 2017) or the subventricular zone (Freundlieb et al., 2006). The mechanisms by which dopamine affects the division of neural precursors are still insufficiently understood but may involve dopamine signaling in dividing cells and compensatory mechanisms that are activated during neurogenesis (Peng et al., 2008;Park and Enikolopov, 2010). Since the initial observation of a dense network of TH-positive nerve terminals in the hippocampus and the observation that the majority of these TH-positive fibers indicate that these are noradrenergic(Smith and Greene, 2012), investigators have sought to examine the effects of noradrenalin depletion on neurogenesis in the hippocampus, adding further complexity to standard Parkinson’s disease models (Borodovitsyna et al., 2017;Braun et al., 2017; Moreno-Castilla et al., 2017).
Based on the premise that the observed neurogenesis in the SGZ after MPP+administration might be enhanced by neurotrophic factors, we evaluated the effects of BDNF on neurogenesis in the SN and SGZ. BDNF can promote dopaminergic cell development, which might promote the restoration of dopaminergic function (Mao and Wang, 2003).Some drugs for treating depression and Parkinson’s disease act through the BDNF/TrkB signaling pathways (Nie et al.,2015; Zhu et al., 2015; Pollock et al., 2016). However, we found that BDNF administration 1 day after MPP+injection did not induce neurogenesis in the SGZ but prevented the increase in BrdU-positive cells after MPP+administration.Moreover, BDNF administration did not promote, but rather inhibited, neurogenesis in the SN. It is possible that the survival of newborn cells that migrated to the SN decreased after the administration of MPP+or BDNF or that a transient increase in the proliferation of precursors did not result in increased net neurogenesis (Lazarov and Hollands,2016). Other studies have shown that growth factors such as epidermal growth factor and fibroblast growth factor 2 promote the maintenance of adult neural stem cells and cell proliferation (Zhao et al., 2008; Usui et al., 2017). It remains unknown whether noradrenergic fibers innervating the hippocampus from the locus coeruleus have an impact on neurogenesis.
In summary, a dose of MPP+that causes cell death in the SN reduced TH levels in catecholaminergic nerve terminals in the striatum and hippocampus. In addition, it promoted neurogenesis in the hippocampus but not the SN. Because most TH-positive nerve terminals in the hippocampus are noradrenergic, our findings suggest that MPP+might also interfere with the noradrenergic system.
Author contributions:JFC and TB designed experiments and graphs and wrote the paper. JFC conducted and analyzed experiments. MW and YHZ analyzed brain sections. All authors approved the final version of this paper.
Conflicts of interest:We declare that we have no conflict of interest.
Financial support:This work was supported by the National Natural Science Foundation of China, No. 31320103906. The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Research ethics:All studies and individual protocols were conducted in accordance with the ethical standards of the Care, Welfare and Treatment of Laboratory Animals and were reviewed by the ethical review process at the Institutes of Brain Science and State Key Laboratory of Medical Neurobiology of Fudan University, Shanghai, China(approval No. 31320103906).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Bellucci A, Antonini A, Pizzi M, Spano P (2017) The end is the Beginning: Parkinson’s disease in the light of brain imaging. Front Aging Neurosci 9:330.
Benmansour S, Deltheil T, Piotrowski J, Nicolas L, Reperant C, Gardier AM, Frazer A, David DJ (2008) Influence of brain-derived neurotrophic factor (BDNF) on serotonin neurotransmission in the hippocampus of adult rodents. Eur J Pharmacol 587:90-98.
Binukumar BK, Pant HC (2016) TFP5/TP5 peptide provides neuroprotection in the MPTP model of Parkinson’s disease. Neural Regen Res 11:698-701.
Borodovitsyna O, Flamini M, Chandler D (2017) Noradrenergic modulation of cognition in health and disease. Neural Plast doi:10.1155/2017/603147.
Braun DJ, Kalinin S, Feinstein DL (2017) Conditional depletion of hippocampal brain-derived neurotrophic factor exacerbates neuropathology in a mouse model of Alzheimer’s disease. ASN Neuro doi: 10.1177/1759091417696161.
Collier TJ, Greene JG, Felten DL, Stevens SY, Collier KS (2004) Reduced cortical noradrenergic neurotransmission is associated with increased neophobia and impaired spatial memory in aged rats.Neurobiol Aging 25:209-221.
Danielson NB, Kaifosh P, Zaremba JD, Lovett-Barron M, Tsai J,Denny CA, Balough EM, Goldberg AR, Drew LJ, Hen R, Losonczy A, Kheirbek MA (2016) Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron 90:101-112.
Deltheil T, Tanaka K, Reperant C, Hen R, David DJ, Gardier AM(2009) Synergistic neurochemical and behavioural effects of acute intrahippocampal injection of brain-derived neurotrophic factor and antidepressants in adult mice. Int J Neuropsychopharmacol 12:905-915.
Dong LD, Chen J, Li F, Gao F, Wu J, Miao Y, Wang Z (2013) Enhanced expression of NR2B subunits of NMDA receptors in the inherited glaucomatous DBA/2J mouse retina. Neural Plast doi:10.1155/2013/670254.
Ermine CM, Wright JL, Frausin S, Kauhausen JA, Parish CL, Stanic D,Thompson LH (2018) Modelling the dopamine and noradrenergic cell loss that occurs in Parkinson’s disease and the impact on hippocampal neurogenesis. Hippocampus doi: 10.1002/hipo.22835.
Espinosa-Oliva AM, de Pablos RM, Santiago M (2014) In vivo effect of apomorphine and haloperidol on MPP neurotoxicity. Pharmacology 93:101-107.
Farzanehfar P (2018) Comparative review of adult midbrain and striatum neurogenesis with classical neurogenesis. Neurosci Res. doi:10.1016/j.neures.2018.01.002.
Freundlieb N, Francois C, Tande D, Oertel WH, Hirsch EC,Hoglinger GU (2006) Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J Neurosci 26:2321-2325.
Gasbarri A, Sulli A, Packard MG (1997) The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry 21:1-22.
Geed M, Garabadu D, Ahmad A, Krishnamurthy S (2014) Silibinin pretreatment attenuates biochemical and behavioral changes induced by intrastriatal MPP+ injection in rats. Pharmacol Biochem Behav 117:92-103.
Hu YS, Long N, Pigino G, Brady ST, Lazarov O (2013) Molecular mechanisms of environmental enrichment: impairments in Akt/GSK3beta, neurotrophin-3 and CREB signaling. PLoS One 8:e64460.
Huang Y, Chen J, Chen Y, Zhuang Y, Sun M, Behnisch T (2015) The neurotoxin 1-methyl-4-phenylpyridinium (MPP(+)) alters hippocampal excitatory synaptic transmission by modulation of the GABAergic system. Front Cell Neurosci 9:299.
Jung UJ, Jeon MT, Choi MS, Kim SR (2014) Silibinin attenuates MPP(+)-induced neurotoxicity in the substantia nigra in vivo. J Med Food 17:599-605.
Kalia LV (2018) Biomarkers for cognitive dysfunction in Parkinson’s disease. Parkinsonism Relat Disord 46 Suppl 1:S19-S23.
Kim YK, Lim HH, Song YK, Lee HH, Lim S, Han SM, Kim CJ (2005)Effect of acupuncture on 6-hydroxydopamine-induced nigrostratal dopaminergic neuronal cell death in rats. Neurosci Lett 384:133-138.
Klein C, Hain EG, Braun J, Riek K, Mueller S, Steiner B, Sack I (2014)Enhanced adult neurogenesis increases brain stiffness: in vivo magnetic resonance elastography in a mouse model of dopamine depletion. PLoS One 9:e92582.
Kohl Z, Ben Abdallah N, Vogelgsang J, Tischer L, Deusser J, Amato D, Anderson S, Muller CP, Riess O, Masliah E, Nuber S, Winkler J (2016) Severely impaired hippocampal neurogenesis associates with an early serotonergic deficit in a BAC alpha-synuclein transgenic rat model of Parkinson’s disease. Neurobiol Dis 85:206-217.
Kong XY, Cai Z, Pan L, Zhang L, Shu J, Dong YL, Yang N, Li Q,Huang XJ, Zuo PP (2008) Transplantation of human amniotic cells exerts neuroprotection in MPTP-induced Parkinson disease mice.Brain Res 1205:108-115.
Lazarov O, Hollands C (2016) Hippocampal neurogenesis: Learning to remember. Prog Neurobiol 138-140:1-18.
Lee HJ, Lim IJ, Lee MC, Kim SU (2010) Human neural stem cells genetically modified to overexpress brain-derived neurotrophic factor promote functional recovery and neuroprotection in a mouse stroke model. J Neurosci Res 88:3282-3294.
Lesemann A, Reinel C, Huhnchen P, Pilhatsch M, Hellweg R, Klaissle P, Winter C, Steiner B (2012) MPTP-induced hippocampal effects on serotonin, dopamine, neurotrophins, adult neurogenesis and depression-like behavior are partially influenced by fluoxetine in adult mice. Brain Res 1457:51-69.
Liang JQ, Wang L, He JC, Hua XD (2016) Verbascoside promotes the regeneration of tyrosine hydroxylase-immunoreactive neurons in the substantia nigra. Neural Regen Res 11:101-106.
Liu Q, Xu Y, Wan W, Ma Z (2018) An unexpected improvement in spatial learning and memory ability in alpha-synuclein A53T transgenic mice. J Neural Transm (Vienna) 125:203-210.
Mao L, Wang JQ (2003) Adult neural stem/progenitor cells in neurodegenerative repair. Sheng Li Xue Bao 55:233-244.
Marxreiter F, Regensburger M, Winkler J (2013) Adult neurogenesis in Parkinson’s disease. Cell Mol Life Sci 70:459-473.
Medina JH (2018) Neural, cellular and molecular mechanisms of active forgetting. Front Syst Neurosci doi: 10.3389/fnsys.2018.00003.eCollection 2018.
Moreno-Castilla P, Perez-Ortega R, Violante-Soria V, Balderas I,Bermudez-Rattoni F (2017) Hippocampal release of dopamine and norepinephrine encodes novel contextual information. Hippocampus 27:547-557.
Nie S, Xu Y, Chen G, Ma K, Han C, Guo Z, Zhang Z, Ye K, Cao X(2015) Small molecule TrkB agonist deoxygedunin protects nigrostriatal dopaminergic neurons from 6-OHDA and MPTP induced neurotoxicity in rodents. Neuropharmacology 99:448-458.
Park JH, Enikolopov G (2010) Transient elevation of adult hippocampal neurogenesis after dopamine depletion. Exp Neurol 222:267-276.
Paul R, Choudhury A, Kumar S, Giri A, Sandhir R, Borah A (2017)Cholesterol contributes to dopamine-neuronal loss in MPTP mouse model of Parkinson’s disease: Involvement of mitochondrial dysfunctions and oxidative stress. PLoS One 12:e0171285.
Pencea V, Bingaman KD, Wiegand SJ, Luskin MB (2001) Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum,septum, thalamus, and hypothalamus. J Neurosci 21:6706-6717.
Peng J, Xie L, Jin K, Greenberg DA, Andersen JK (2008) Fibroblast growth factor 2 enhances striatal and nigral neurogenesis in the acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neuroscience 153:664-670.
Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS (2001) Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience 106:589-601.
Pfeiffer RF (2016) Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 22:S119-S122.
Pollock K et al. (2016) Human mesenchymal stem cells genetically engineered to overexpress brain-derived neurotrophic factor improve outcomes in Huntington’s disease mouse models. Mol Ther 24:965-977.
Sairanen M, Lucas G, Ernfors P, Castren M, Castren E (2005)Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 25:1089-1094.
Salvi R, Steigleder T, Schlachetzki JC, Waldmann E, Schwab S, Winner B, Winkler J, Kohl Z (2016) Distinct effects of chronic dopaminergic stimulation on hippocampal neurogenesis and striatal doublecortin expression in adult mice. Front Neurosci 10:77.
Santiago M, Machado A, Cano J (2001) Validity of a quantitative technique to study striatal dopaminergic neurodegeneration by in vivo microdialysis. J Neurosci Methods 108:181-187.
Santos DB, Colle D, Moreira EL, Hort MA, Godoi M, Le Douaron G,Braga AL, Assreuy J, Michel PP, Prediger RD, Raisman-Vozari R,Farina M (2017) Succinobucol, a non-statin hypocholesterolemic drug, prevents premotor symptoms and nigrostriatal neurodegeneration in an experimental model of Parkinson’s disease. Mol Neurobiol 54:1513-1530.
Schlachetzki JC, Grimm T, Schlachetzki Z, Ben Abdallah NM, Ettle B,Vohringer P, Ferger B, Winner B, Nuber S, Winkler J (2016) Dopaminergic lesioning impairs adult hippocampal neurogenesis by distinct modification of alpha-synuclein. J Neurosci Res 94:62-73.
Schmidt N, Ferger B (2001) Neurochemical findings in the MPTP model of Parkinson’s disease. J Neural Transm (Vienna) 108:1263-1282.
Smith CC, Greene RW (2012) CNS dopamine transmission mediated by noradrenergic innervation. J Neurosci 32:6072-6080.
Sonsalla PK, Zeevalk GD, German DC (2008) Chronic intraventricular administration of 1-methyl-4-phenylpyridinium as a progressive model of Parkinson’s disease. Parkinsonism Relat Disord 14 Suppl 2:S116-118.
Steffen V, Santiago M, de la Cruz CP, Revilla E, Machado A, Cano J (1995) Effect of intraventricular injection of 1-methyl-4-phenylpyridinium: protection by acetyl-L-carnitine. Hum Exp Toxicol 14:865-871.
Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M,Watanabe M, Smith CC, Fernandez G, Deisseroth K, Greene RW,Morris RG (2016) Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537:357-362.
Tapia-Bustos A, Perez-Lobos R, Vio V, Lespay-Rebolledo C, Palacios E, Chiti-Morales A, Bustamante D, Herrera-Marschitz M, Morales P (2017) Modulation of postnatal neurogenesis by perinatal asphyxia: effect of D1 and D2 dopamine receptor agonists. Neurotox Res 31:109-121.
Usui T, Sakurai M, Kawasaki H, Ohama T, Yamawaki H, Sato K(2017) Establishment of a novel three-dimensional primary culture model for hippocampal neurogenesis. Physiol Rep doi: 10.14814/phy2.13318.
Valvassori SS, Arent CO, Steckert AV, Varela RB, Jornada LK, Tonin PT, Budni J, Mariot E, Kapczinski F, Quevedo J (2015) Intracerebral administration of BDNF protects rat brain against oxidative stress induced by ouabain in an animal model of mania. Mol Neurobiol 52:353-362.
Wang M, Li D, Yun D, Zhuang Y, Repunte-Canonigo V, Sanna PP,Behnisch T (2017) Translation of BDNF-gene transcripts with short 3’ UTR in hippocampal CA1 neurons improves memory formation and enhances synaptic plasticity-relevant signaling pathways. Neurobiol Learn Mem 138:121-134.
Wu YD, Liang PR, Long DY, Gao BM (2016) Effects of Ginkgo biloba Pingchan Recipe on loss and apoptosis of dopamine neurons in mouse models of Parkinson’s disease. Zhongguo Zuzhi Gongcheng Yanjiu 20:7327-7333.
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645-660.
Zhu G, Chen Y, Huang Y, Li Q, Behnisch T (2011) MPTP-meditated hippocampal dopamine deprivation modulates synaptic transmission and activity-dependent synaptic plasticity. Toxicol Appl Pharmacol 254:332-341.
Zhu G, Huang Y, Chen Y, Zhuang Y, Behnisch T (2012) MPTP modulates hippocampal synaptic transmission and activity-dependent synaptic plasticity via dopamine receptors. J Neurochem 122:582-593.
Zhu G, Li J, He L, Wang X, Hong X (2015) MPTP-induced changes in hippocampal synaptic plasticity and memory are prevented by memantine through the BDNF-TrkB pathway. Br J Pharmacol 172:2354-2368.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease
- Alpha-7 nicotinic acetylcholine receptor agonist treatment in a rat model of Huntington’s disease and involvement of heme oxygenase-1
- Compound of icariin, astragalus, and puerarin mitigates iron overload in the cerebral cortex of Alzheimer’s disease mice
- Structural neural connectivity of the vestibular nuclei in the human brain: a diffusion tensor imaging study
- Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer’s disease
- Brain remodeling after chronic median nerve compression in a rat model

