Preparation of 2D square-like Bi2S3-BiOCl heterostructures withenhanced visible light-driven photocatalytic performance for dye pollutant degradation
Jing-jing Xu*,Jing-wen YangPu ZhangQuan YuanYan-hong ZhuYu WangMiao-miao WuZheng-mei WangMin-dong Chen
aCollaborative Innovation Center of Atmospheric Environment and Equipment Technology,School of Environmental Science and Engineering,Nanjing University of Information Science and Technology,Nanjing 210044,China
bJiangsu Engineering Technology Research Center of Environmental Cleaning Materials,Nanjing University of Information Science and Technology,Nanjing 210044,China
cJiangsu Key Laboratory of Atmospheric Environment Monitoring and Pollution Control,Nanjing University of Information Science and Technology,Nanjing 210044,China
1.Introduction
Photocatalytic technology has been developing quickly since Fujishima and Honda(1972)found that titanium dioxide(TiO2)photoanodes can induce water splitting.Thefirst attempt at application of photocatalytic technology in thefield of organic pollutant degradation was the use of TiO2for the photodechlorination of polychlorobiphenyls(Carey et al.,1976).Because of their high reaction speed,stability,low toxicity,and many other advantages,semiconductor photocatalysts have attracted much attention over the past several decades.In recent years,photocatalysts such as TiO2(Bianchi et al.,2014;Wang et al.,2014),BiOX(X=Cl,Br,I)(Chen et al.,2013;Ao et al.,2016a,2016b,2016c;Qin et al.,2013;Zhang et al.,2013b),and Ag/AgX(X=Cl,Br)(Wang et al.,2012,2013c)have attracted much attention in thisfield.
Bismuth oxyhalide BiOCl has a lamellar structure and strong photocorrosion resistance.BiOCl has been put to many uses,such as pigments(Maile et al.,2005),photoluminescence(Deng et al.,2008),and photocatalysis(Shenawi-Khalil et al.,2011).Much progress has been made.Xiong et al.(2011)prepared square-like BiOCl nanosheets through an environmentally friendly hydrothermal process.At room temperature,Ye et al.(2013)synthesizedflower-like BiOCl composed of self-assembled hierarchical nanosheets,which performed well in the degradation of Rhodamine B(RhB).However,widebandgap BiOCl shows little response to visible light,which accounts for 45%of solar spectra.This means that BiOCl cannot utilize the solar energy efficiently.The fact that it can only be photo-excited under ultraviolet(UV)irradiation has limited its application to removal of organic pollutants.Major efforts have been made to obtain visible light-driven BiOClbased photocatalysts(Zhang et al.,2013a;Xia et al.,2013;Wang et al.,2013a;Cao et al.,2013).
Narrow-bandgap Bi2S3has been used to modify TiO2(Liu et al.,2017),Bi2O2CO3(Wang et al.,2013b),ZnS(Nawaz,2017),and some other wide-bandgap photocatalysts(Cheng et al.,2012)in order to improve their performance under visible light irradiation.Cao et al.(2012)synthesized a novel Bi2S3-sensitized BiOCl photocatalyst with a rose-like structure,which photodegraded 98.0%of RhB within 2 h under visible light irradiation,much more than BiOCl,Bi2S3,or TiO2alone.This showed that the combination of Bi2S3and BiOCl can turn BiOCl into a promising visible light-driven photocatalyst(Ferreira et al.,2016;Jiang et al.,2014).However,all the studies mentioned above focused on Bi2S3-modified three-dimensional(3D)flower-like structured BiOCl,and no study has focused on the preparation and activity of Bi2S3-modified two-dimensional(2D)plate-like structured BiOCl.2D Bi2S3-BiOCl would be highly active under visible light irradiation.
2.Experimental setup
2.1.Synthesis
Analytical-grade chemicals have often been used without further purification.In this study,Bi2S3-BiOCl composites with different Bi2S3contents were synthesized via a facile two-step anion exchange route at room temperature.First,we prepared white BiOCl nanosheets using the solvothermal method(Xiong et al.,2011).Second,thioacetamide(TAA)was used as the sulfur source to obtain the composites.In the experimental synthesizing of Bi2S3-BiOCl composites,0.26 g of BiOCl nanosheets were added into 25 mL of ultrapure water and sonicated for 10 min to form suspension A.Solution B was obtained after the dissolution of a certain amount of TAA in 25 mL of ultrapure water.Then,solution B was gradually poured into suspension A and stirred for 5 h at room temperature.Finally,the light gray products were obtained;they were washed with deionized water,and dried at 80°C for about 6 h.Four samples were prepared by changing the added amount of TAA.The obtained samples were defined as Bi2S3-BiOCl(8:1),Bi2S3-BiOCl(8:2),Bi2S3-BiOCl(8:4),and Bi2S3-BiOCl(8:8),as the added amounts of TAA were 0.009,0.019,0.038,and 0.075 g,respectively.
2.2.Characterization
We used X-ray diffraction(XRD)to examine the crystal form and crystallinity of the samples.Field emission scanning electron microscopy(SEM)and transmission electron microscopy(TEM)were utilized to observe the surface morphologies and microstructures of the samples,using a Hitachi S-4800 scanning electron microscope and a Hitachi H-7650 transmission electron microscope,respectively.The absorption ability of catalysts was measured through ultraviolet-visible(UV/Vis)diffuse reflectance spectra(DRS)on a Shimadzu UV3600 spectrometer.The Brunauer-Emmett-Teller(BET)surface area of the samples was obtained with a BET analyzer(ASAP 2020,Micromeritics Instrument Corporation)through N2adsorption-desorption isotherms.
2.3.Photocatalytic experiments
The photocatalytic activities of the as-prepared samples were measured through photodegradation of X-3B under visible light(λ≥ 400 nm,where λ is the wave length)irradiation,a 300 W Xe lamp was used as a light source,and a circulating cooling water system was used to keep the temperature at 12°C.In each experiment,a 0.01-g sample was added to 50 mL of X-3B solution with a concentration of 25 mg/L to form a suspension.In order to reach adsorptiondesorption equilibrium,the suspension was ultrasonic-treated for 2 min and further stirred for 30 min in the dark.Under light irradiation,about 1.5 mL of suspension was taken out for examination every 15 min.
3.Results and discussion
The as-prepared Bi2S3-BiOCl samples were analyzed through XRD characterization(Fig.1,where a.u.means arbitrary unit,and 2θ is the diffraction angle)to examine their phase structure,crystal form,and crystallinity.All the diffraction peaks of BiOCl and Bi2S3could be indexed according to the structures of tetragonal BiOCl(JCPDS No.06-0249)and bulk orthorhombic Bi2S3(JCPDS No.75-1306),respectively,indicating high crystal purity.No diffraction peaks of Bi2S3were observed in the curves of Bi2S3-BiOCl composites.This is probably due to the amorphous structure,high level of dispersity,and small crystallites of Bi2S3.The obtained results indicate that the addition of Bi2S3does not cause changes in the crystal phase of BiOCl.
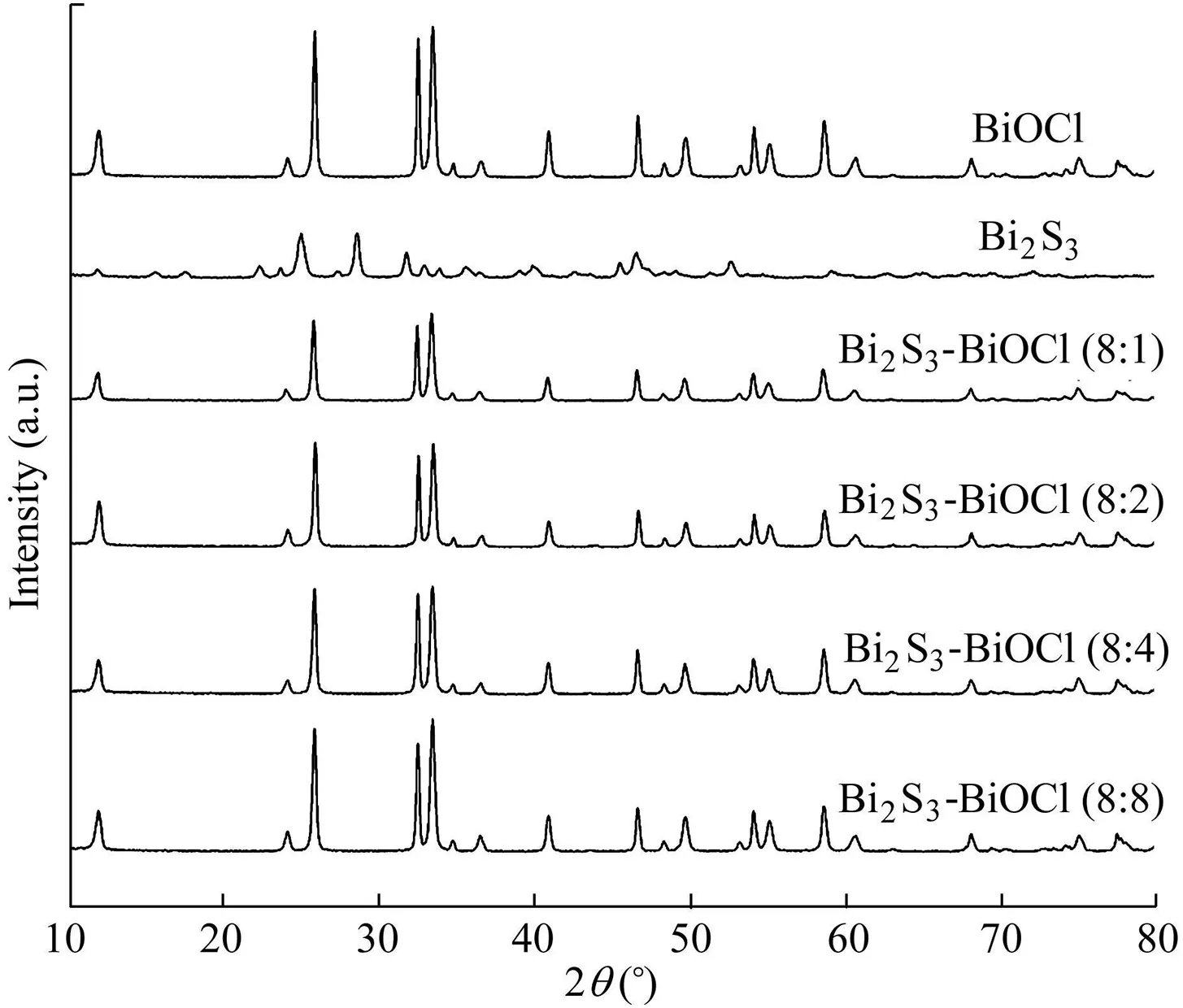
Fig.1.XRD patterns of BiOCl,Bi2S3,and Bi2S3-BiOCl composites.
During the preparation process of Bi2S3-BiOCl composites,thesquare-likeBiOClnanosheetswith athicknessof 10-25 nm(Fig.2(a))were dispersed in the ultrapure waterfirst.Then,through ion exchange,a reaction between Bi3+and TAA occurred over time(the stepwise equations were as follows:CH3CSNH2+H2O→CH3CONH2+H2S↑;2Bi3++3H2S→Bi2S3↓+6H+).Finally,Bi2S3was dispersed and anchored on the surface of BiOCl nanosheets,as shown clearly in Fig.3(b).Because of the rather lower solubility of Bi2S3(Ksp=1.0×10-97,where Kspis the solubility product),compared to BiOCl(Ksp=1.8×10-31)(Cheng et al.,2012),a partial anion ion exchange reaction induced the formation of Bi2S3on the surface of BiOCl.The microstructure and morphology of the samples were investigated by SEM and TEM.Compared to the morphology of Bi2S3nanorods with lengths of 100-180 nm(Fig.2(b)),Bi2S3-BiOCl composites were nanosheets(Fig.2(c)through(f))similar to pure BiOCl.Furthermore,it can be seen that the thickness of all the nanosheets were about 10-20 nm(marked by black arrows in Fig.2).Surface area and pore structures are crucial to the activity of photocatalysts.Therefore,the surface areas of the obtained samples were obtained through N2adsorptiondesorption isotherms.The surface areas of pure BiOCl,Bi2S3-BiOCl(8:1),Bi2S3-BiOCl(8:2),Bi2S3-BiOCl(8:4),Bi2S3-BiOCl(8:8),and Bi2S3were 2.91,5.12,5.63,5.90,6.24,and 15.62 m2/g,respectively.
The UV/Vis diffuse reflectance spectra of BiOCl,Bi2S3,and Bi2S3-BiOCl composites are shown in Fig.4.Pure BiOCl can only adsorb UV light with an absorption edge at about 360 nm.However,Bi2S3exhibits strong absorption intensity in the visible light region,even extending to the near-infrared region.As for Bi2S3-BiOCl composites,their absorption edges shift to about 420 nm and an enhancement in photoabsorption efficiency is observed.The results illustrate that Bi2S3has a photosensitization effect on BiOCl.Furthermore,the bandgap energy(Eg)of pure BiOCl,Bi2S3,and Bi2S3-BiOCl composites can be calculated to be about 3.49,1.27,and 2.95 eV,respectively.

Fig.2.SEM images of samples.
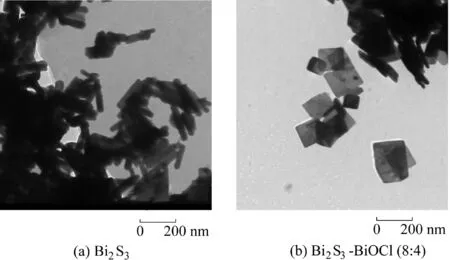
Fig.3.TEM images of samples.
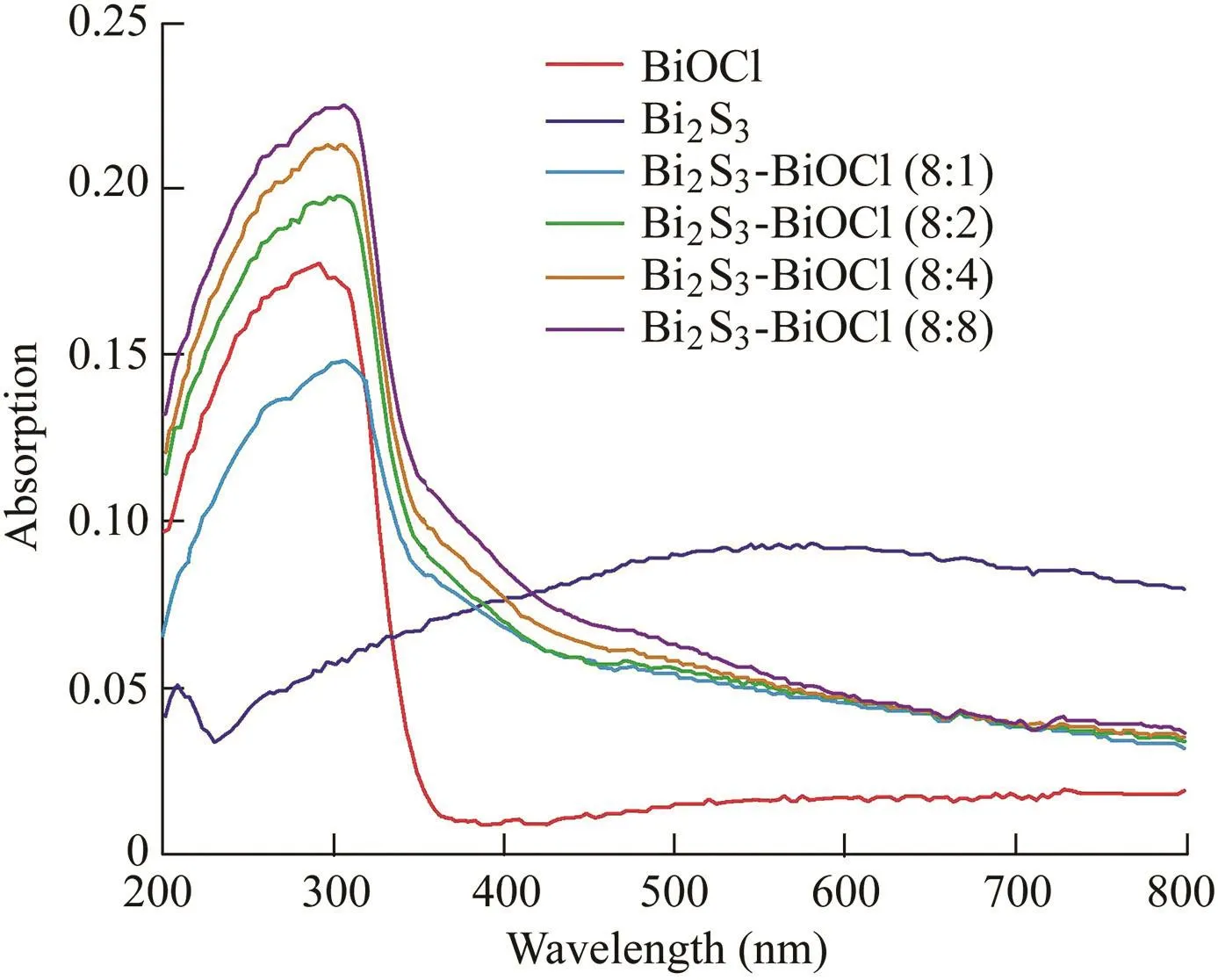
Fig.4.UV/Vis DRS of pure BiOCl,Bi2S3,and Bi2S3-BiOCl composites.
Fig.5 shows the concentration changes of X-3B dye in different samples with the visible light irradiation time in order to evaluate the photocatalytic activities,where C is the concentration of X-3B in the process and C0is the initial concentration of X-3B.After 30 min of stirring in the dark,a strong adsorption was observed on Bi2S3,while other samples exhibited relatively low levels of adsorption for X-3B.Furthermore,it can be seen that only 11.6%and 37.8%of X-3B were removed by BiOCl and Bi2S3after the adsorption and photocatalytic processes,respectively.The main reasons were that BiOCl has little response to visible light,and the photoexcited electron-hole pairs of Bi2S3would be recombined due to its narrow bandgap.Meanwhile,the degradation effi-ciency of Bi2S3-BiOCl(8:4)was 74.6%,which was the highest photodegradation efficiency of the samples.The apparent rate constants were 0.0015,0.014,0.02,0.028,0.018,and 0.096 min-1for pure BiOCl,Bi2S3-BiOCl(8:1),Bi2S3-BiOCl(8:2),Bi2S3-BiOCl(8:4),Bi2S3-BiOCl(8:8),and Bi2S3,respectively.Obviously,all Bi2S3-BiOCl composites performed better than both BiOCl and Bi2S3,and 8:4 was the optimal Bi/S ratio in this study.In order to determine the factor that caused the increase of the activity of Bi2S3-BiOCl composite photocatalysts,photocurrent was measured at open circuit potentials for all samples.The samples deposited onfluorine-doped tin oxide(FTO)conductive glass were used as anodes for the measurement.The results are shown in Fig.6.A uniform and apparent photocurrent response can be seen for all electrodes.Furthermore,all Bi2S3-BiOCl composites exhibited higher photocurrent intensity values than pure BiOCl.The photocurrent is determined by the recombination at the electrolyte interface and the transferring speed of excited electrons from the photocatalyst to FTO.Therefore,the increase of photocurrent for Bi2S3-BiOCl composites should be attributed to the higher separation efficiency of photogenerated charges.This results from the coupling of BiOCl and Bi2S3and the formation of heterojunctions between the two components.The formed heterojunctions help to transfer and separate the photogenerated electron-hole pairs,and thus increase the photocatalytic activity.It can also be seen that the photocatalytic performance and photocurrent of Bi2S3-BiOCl composites strengthen gradually with the increase of the Bi/S ratio from 8:1 to 8:4.However,Bi2S3-BiOCl(8:8)shows a weaker performance than Bi2S3-BiOCl(8:4),which may stem from the overload of Bi2S3.Therefore,there is an optimal value of the Bi/S ratio(8:4 in this study)that obtains the highest photocatalytic activity for Bi2S3-BiOCl composites.
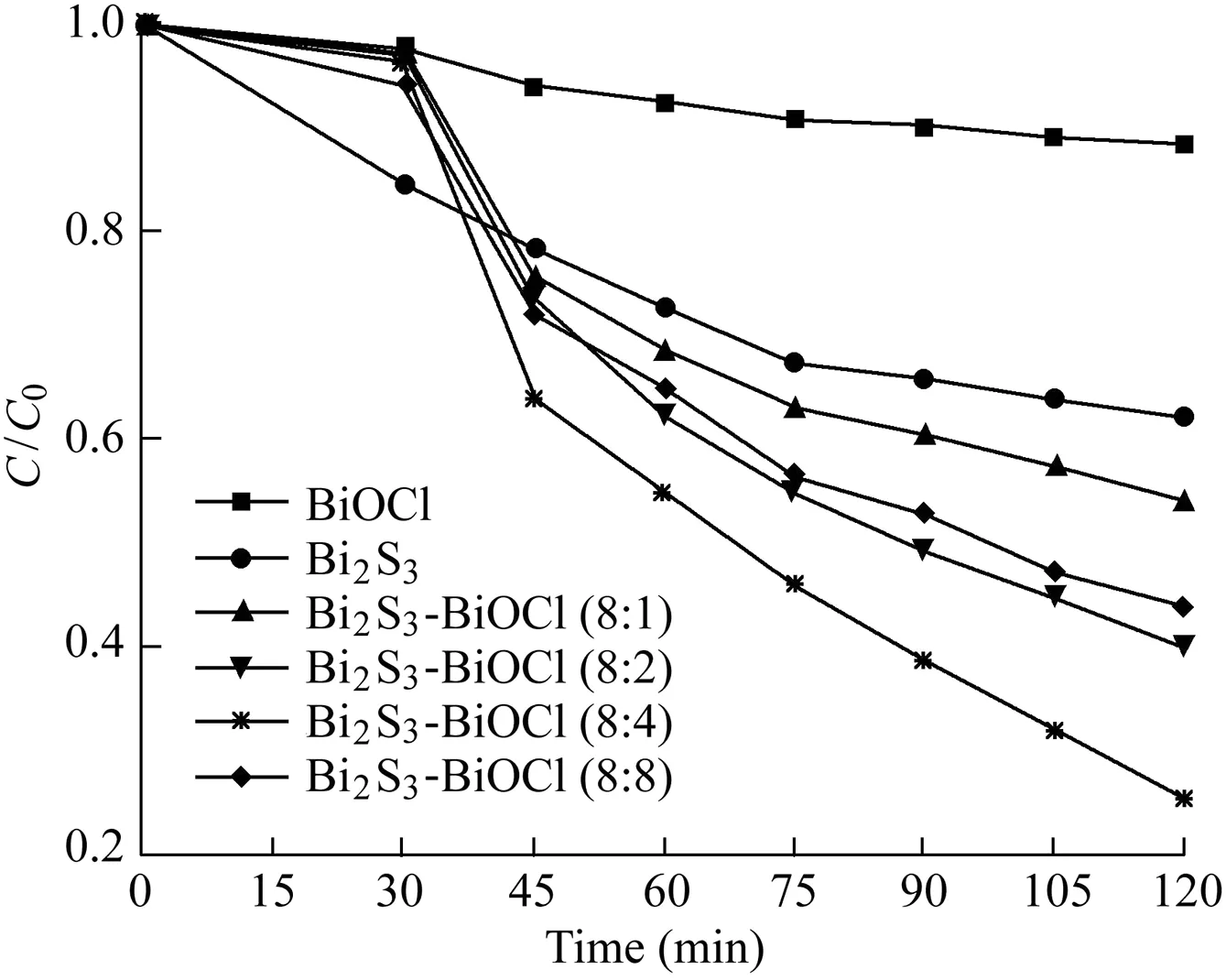
Fig.5.Photocatalytic activities of samples.
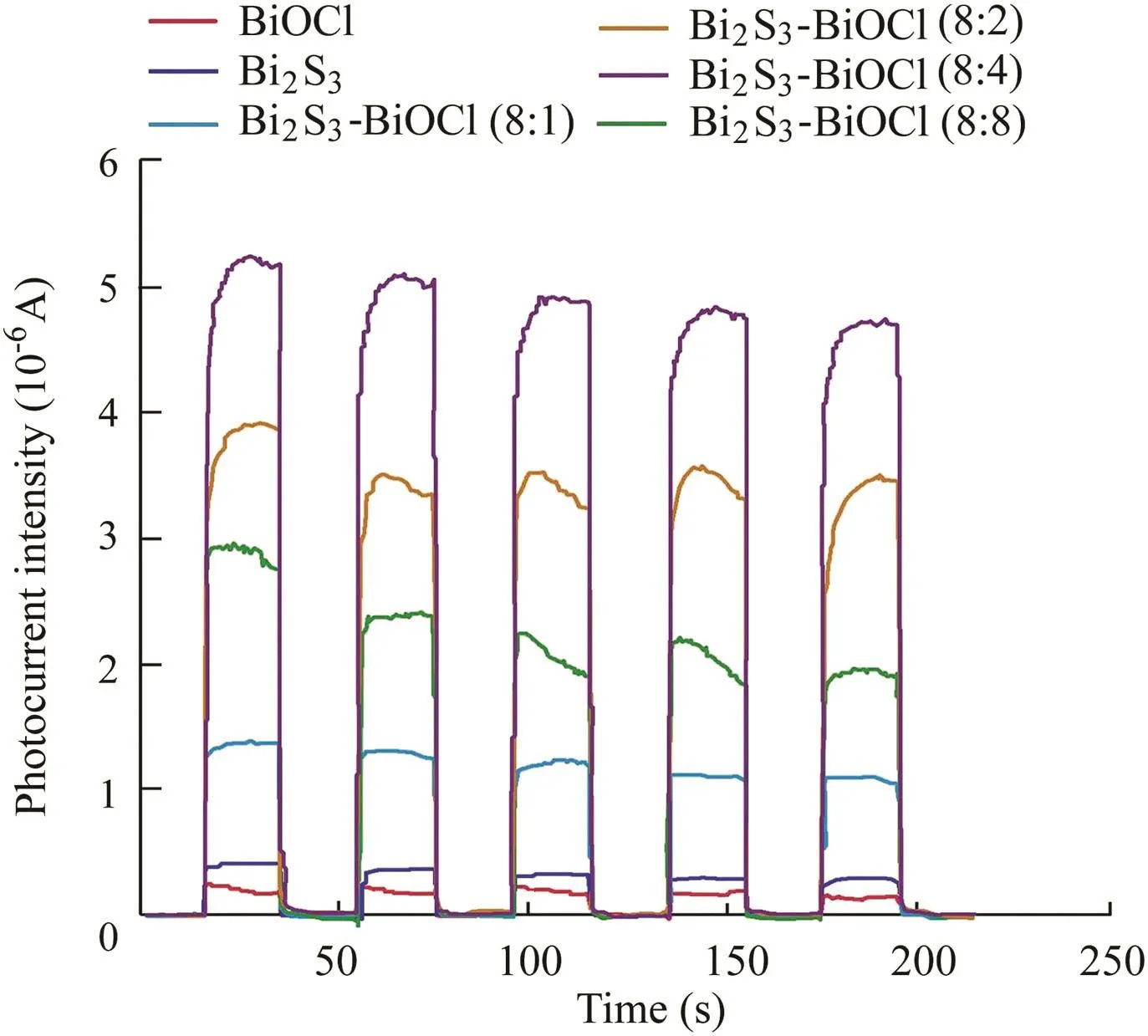
Fig.6.Photocurrent responses of Bi2S3-BiOCl composites under visible light irradiation.
To explore the mechanism of photocatalytic degradation of X-3B by Bi2S3-BiOCl composites is highly significant.As shown in Fig.7,a plausible mechanism for X-3B degradation is proposed.
Under visible light irradiation,Bi2S3is excited enough to generate electron-hole pairs.Some of the photogenerated electrons are then trapped by dissolved O2and produce·O-2,an important active species in oxidation reactions,and the others can be readily transferred to the conduction band(CB)of BiOCl.These electrons on CB of BiOCl cannot react to produce·Ο-2radicals,because the CB potential is 0.11 eV,while the redox potential of O2/·O-2is-0.046 eV(Wang et al.,2013a).The hole(h+)can either react with OH-to produce·OH or directly decompose X-3B dye since it is also a strong oxidant(the valent band(VB)potential of Bi2S3is 1.47 eV,and the redox potential of H2O/·OH is 2.72 eV)(Li et al.,2009).Consequently,the recombination of e-and h+has largely been restrained,and these oxidative species(·O-2,·OH,and h+)can degrade X-3B dye.In addition,BiOCl may degrade X-3B molecules through the photosensitition effect,although it shows little response to visible light because of its wide bandgap.The related equations in this reaction process,including two collaborative processes(photodegradation and photosensitization),are as follows(Nawaz,2017;Cao et al.,2012):
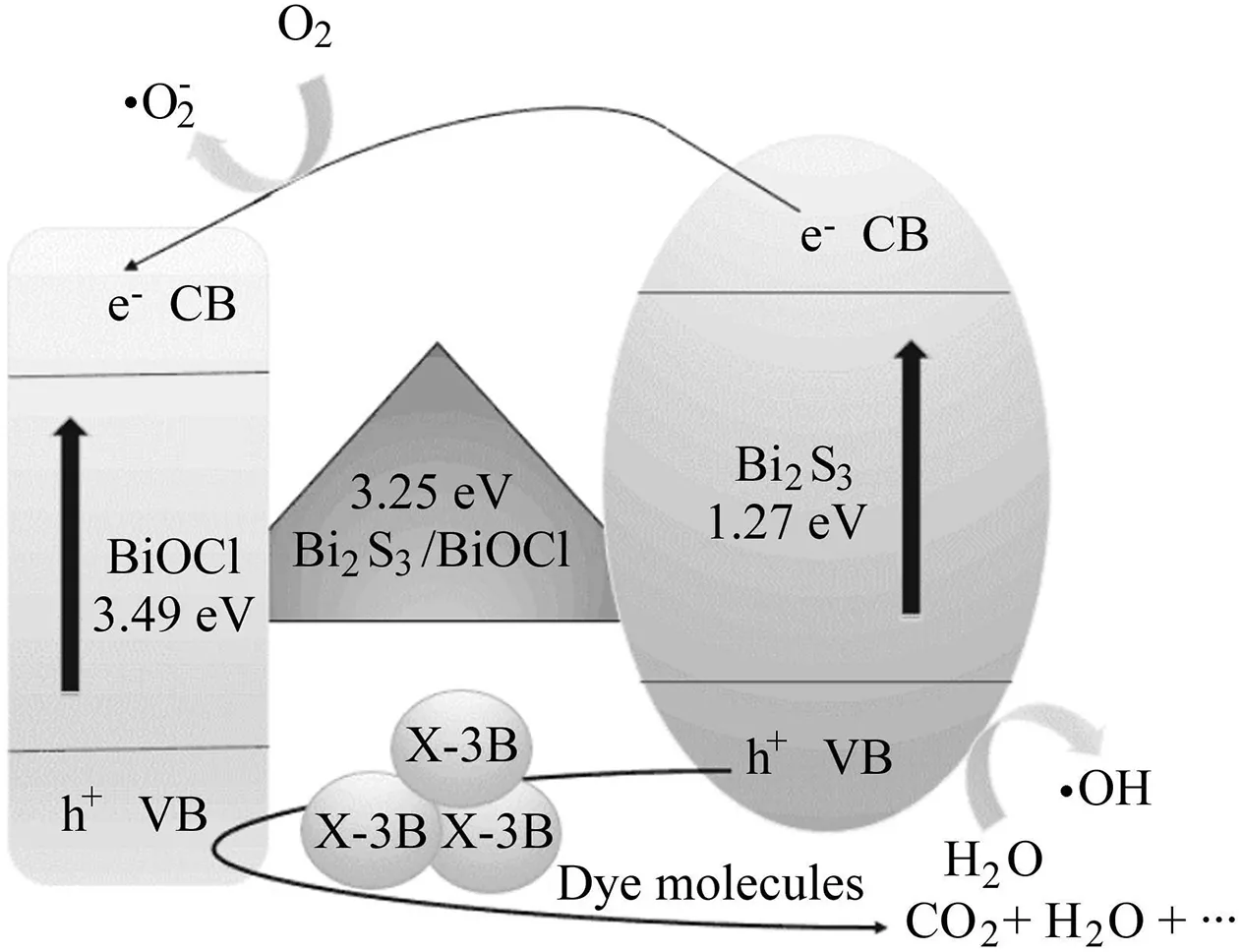
Fig.7.Schematic photocatalytic reaction process of Bi2S3-BiOCl composites with degradation of X-3B under visible light irradiation.
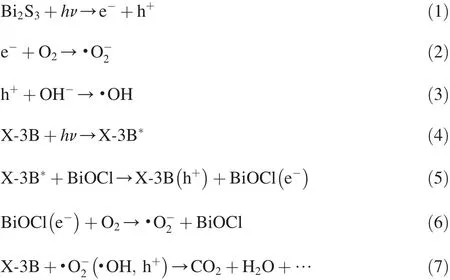
where h is the Planck constant,ν is the frequency,and*means the excited state.
The stability of Bi2S3-BiOCl samples is crucial to their practical applications.Therefore,typical reuse experiments of Bi2S3-BiOCl(8:4)were conducted to evaluate its long-term service properties.The obtained results are shown in Fig.8.The photocatalytic performance of Bi2S3-BiOCl(8:4)has no apparent decreasing trend after three cycles.This indicates that the prepared Bi2S3-BiOCl composite photocatalysts are stable.
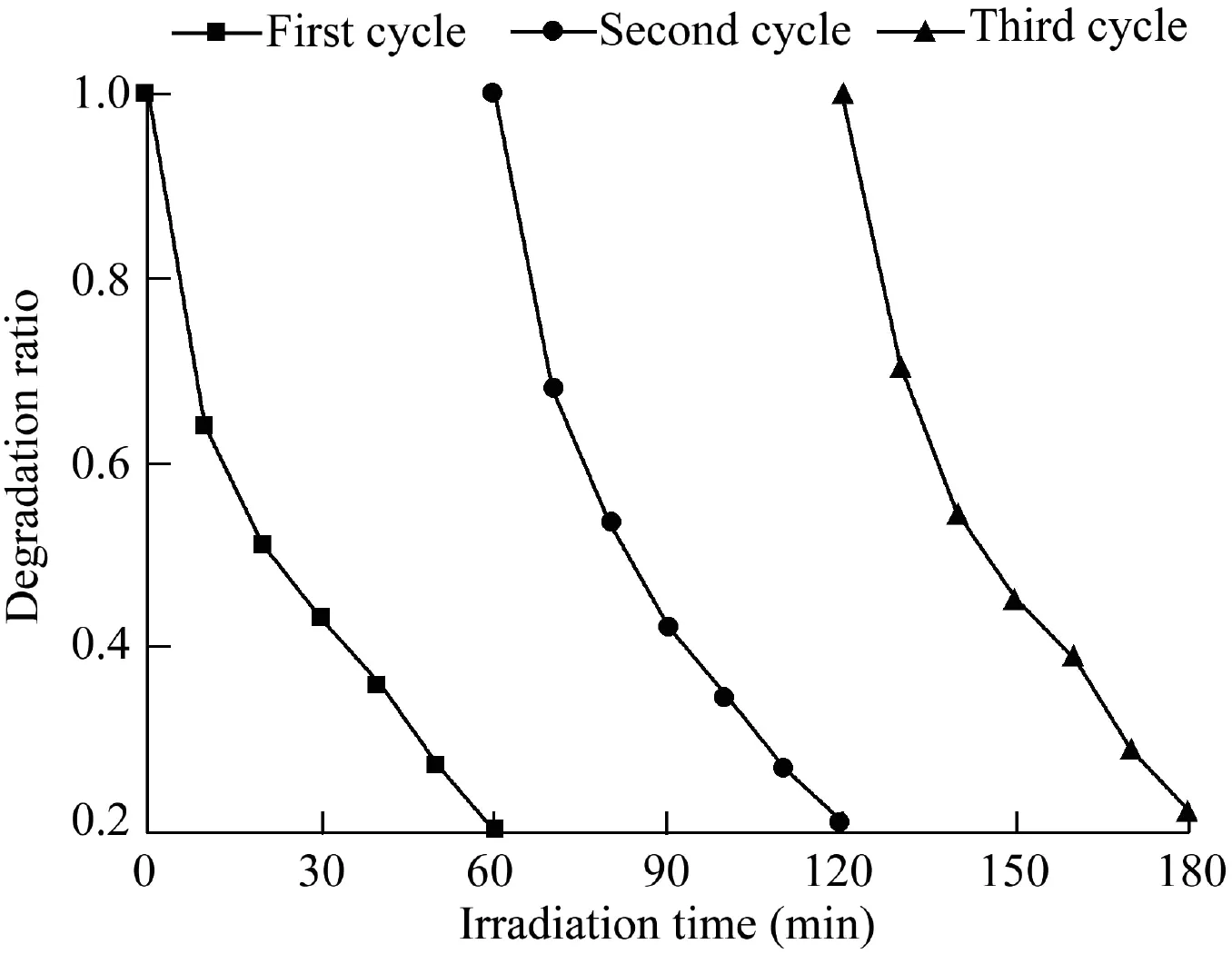
Fig.8.Recyclability of Bi2S3-BiOCl(8:4)under visible light irradiation.
4.Conclusions
Visible light-responsive and square-like Bi2S3-BiOCl composites with different Bi/S ratios were prepared via a twostep anion exchange route.The samples were obtained through a partial anion exchange reaction between the square-like BiOCl and TAA,in which the Bi2S3was produced on the surface of BiOCl.The phase structure,morphology,and optical properties of Bi2S3-BiOCl composites were studied with XRD,SEM,TEM,and DRS.The results show that the coupling of BiOCl with Bi2S3induce enhanced photoabsorption efficiency and a narrower bandgap.The photocatalytic activity under visible light irradiation was investigated through the photocatalytic degradation of X-3B dye.The results show that all Bi2S3-BiOCl composites exhibited much higher activity than pure BiOCl and Bi2S3,and the enhanced activity was ascribed to the formation of Bi2S3on BiOCl nanosheets.Based on investigation of the effect of the Bi/S ratio on the activity of Bi2S3-BiOCl composites,an optimal value(8:4)was obtained.This work can provide ideas concerning the design of more bismuth-based photocatalysts for treatment of pollutants.
Ao,Y.H.,Bao,J.Q.,Wang,P.F.,Wang,C.,Hou,J.,2016a.Bismuth oxychloride modified titanium phosphate nanoplates:A new p-n type heterostructured photocatalyst with high activity for the degradation of different kinds of organic pollutants.J.Colloid Interface Sci.476,71-78.https://doi.org/10.1016/j.jcis.2016.05.021.
Ao,Y.H.,Wang,K.D.,Wang,P.F.,Wang,C.,Hou,J.,2016b.Synthesis of novel 2D-2D p-n heterojunction BiOBr/La2Ti2O7composite photocatalyst with enhanced photocatalytic performance under both UVand visible light irradiation.Appl.Catal.B Environ.194,157-168.https://doi.org/10.1016/j.apcatb.2016.04.050.
Ao,Y.H.,Wang,K.D.,Wang,P.F.,Wang,C.,Hou,J.,2016c.Fabrication of novel p-n heterojunction BiOI/La2Ti2O7composite photocatalysts for enhanced photocatalytic performance under visible light irradiation.Dalton Trans.45(19),7986-7997.https://doi.org/10.1039/c6dt00862c.
Bianchi,C.L.,Gatto,S.,Pirola,C.,Naldoni,A.,Di Michele,A.,Cerrato,G.,Crocella,V.,Capucci,V.,2014.Photocatalytic degradation of acetone,acetaldehyde and toluene in gas-phase:Comparison between nano and micro-sized TiO2.Appl.Catal.B Environ.146,123-130.https://doi.org/10.1016/j.apcatb.2013.02.047.
Cao,J.,Xu,B.Y.,Lin,H.L.,Luo,B.D.,Chen,S.F.,2012.Novel Bi2S3-sensitized BiOCl with highly visible light photocatalytic activity for the removal of rhodamine B.Catal.Commun.26,204-208.https://doi.org/10.1016/j.catcom.2012.05.025.
Cao,J.,Zhou,C.C.,Lin,H.L.,Xu,B.Y.,Chen,S.F.,2013.Surface modifi-cation of m-BiVO4 with wide band-gap semiconductor BiOCl to largely improve the visible light induced photocatalytic activity.Appl.Surf.Sci.284,263-269.https://doi.org/10.1016/j.apsusc.2013.07.092.
Carey,J.H.,Lawrence,J.,Tosine,H.M.,1976.Photodechlorination of PCB's in the presence of titanium dioxide in aqueous suspensions.Bull.Environ.Contam.Toxicol.16(6),697-701.
Chen,L.,Huang,R.,Xiong,M.,Yuan,Q.,He,J.,Jia,J.,Yao,M.Y.,Luo,S.L.,Au,C.T.,Yin,S.F.,2013.Room-temperature synthesis offlower-like BiOX(X=Cl,Br,I)hierarchical structures and their visible-light photocatalytic activity.Inorg.Chem.52(19),11118-11125.https://doi.org/10.1021/ic401349j.
Cheng,H.F.,Huang,B.B.,Qin,X.Y.,Zhang,X.Y.,Dai,Y.,2012.A controlled anion exchange strategy to synthesize Bi2S3nanocrystals/BiOCl hybrid architectures with efficient visible light photoactivity.Chem.Commun.48(1),97-99.https://doi.org/10.1039/c1cc15487g.
Deng,Z.T.,Tang,F.Q.,Muscat,A.J.,2008.Strongbluephotoluminescencefrom single-crystalline bismuth oxychloridenanoplates.Nanotechnology 19(29),295705-295710.https://doi.org/10.1088/0957-4484/19/29/295705.
Ferreira,V.C.,Neves,M.C.,Hillman,A.R.,Monteriro,O.C.,2016.Novel onepot synthesis and sensitisation of new BiOCl-Bi2S3nanostructures from DES medium displaying high photocatalytic activity.RSC Advances 6,77329-77339.https://doi.org/10.1039/C6RA14474H.
Fujishima,A.,Honda,K.,1972.Electrochemical photolysis of water at a semiconductorelectrode.Nature 238(5358),37-38.https://doi.org/10.1038/238037a0.
Jiang,S.H.,Zhou,K.Q.,Shi,Y.Q.,Lo,S.M.,Xu,H.Y.,Hu,Y.,Gui,Z.,2014.In situ synthesis of hierarchicalflower-like Bi2S3/BiOCl composite with enhanced visible light photocatalytic activity.Appl.Surf.Sci.290,313-319.https://doi.org/10.1016/j.apsusc.2013.11.074.
Li,G.T.,Wong,K.H.,Zhang,X.W.,Hu,C.,Yu,J.C.,Chan,R.C.Y.,Wong,P.K.,2009.Degradation of acid orange 7 using magnetic AgBr under visible light:The roles of oxidizing species.Chemosphere 76(9),1185-1191.https://doi.org/10.1016/j.chemosphere.2009.06.027.
Liu,Y.,Shi,Y.D.,Liu,X.,Li,H.X.,2017.A facile solvothermal approach of novel Bi2S3/TiO2/RGO composites with excellent visible light degradation activity for methylene blue.Appl.Surf.Sci.396,58-66.https://doi.org/10.1016/j.apsusc.2016.11.028.
Maile,F.J.,Pfaff,G.,Reynders,P.,2005.Effect pigments:Past,present and future.Prog.Org.Coating 54(3),150-163.https://doi.org/10.1016/j.porgcoat.2005.07.003.
Nawaz,M.,2017.Morphology-controlled preparation ofBi2S3-ZnS chloroplast-like structures,formation mechanism and photocatalytic activityforhydrogenproduction.J.Photochem.Photobiol.Chem.332,326-330.https://doi.org/10.1016/j.jphotochem.2016.09.005.
Qin,X.Y.,Cheng,H.F.,Wang,W.J.,Huang,B.B.,Zhang,X.Y.,Dai,Y.,2013.Three dimensional BiOX(X=Cl,Br and I)hierarchical architectures:Facile ionic liquid-assisted solvothermal synthesis and photocatalysis towards organic dye degradation.Mater.Lett.100,285-288.https://doi.org/10.1016/j.matlet.2013.03.045.
Shenawi-Khalil,S.,Uvarov,V.,Kritsman,Y.,Mennes,E.,Popov,I.,Sasson,Y.,2011.A new family of BiO(ClxBr1-x)visible light sensitive photocatalysts.Catal.Commun.12(12),1136-1141.https://doi.org/10.1016/j.catcom.2011.03.014.
Wang,B.,Li,C.,Cui,H.,Zhang,J.P.,Zhai,J.,Li,Q.,2014.Shifting mechanisms in the initial stage of dye photodegradation by hollow TiO2nanospheres.J.Mater.Sci.49(3),1336-1344.https://doi.org/10.1007/s10853-013-7817-4.
Wang,P.Q.,Bai,Y.,Liu,J.Y.,Fan,Z.,Hu,Y.Q.,2012.Facile synthesis and activity of daylight-driven plasmonic catalyser Ag/AgX(X=Cl,Br).IET Micro Nano Lett.7(8),838-841.https://doi.org/10.1049/mnl.2012.0591.
Wang,Q.Z.,Hui,J.,Li,J.J.,Cai,Y.X.,Yin,S.Q.,Wang,F.P.,Su,B.T.,2013a.Photodegradation of methyl orange with PANI-modified BiOCl photocatalyst under visible light irradiation.Appl.Surf.Sci.283,577-583.https://doi.org/10.1016/j.apsusc.2013.06.149.
Wang,W.J.,Cheng,H.F.,Huang,B.B.,Lin,X.J.,Qin,X.Y.,Zhang,X.Y.,Dai,Y.,2013b.Synthesis of Bi2O2CO3/Bi2S3hierarchical microspheres with heterojunctions and their enhanced visible light-driven photocatalytic degradation of dye pollutants.J.Colloid Interface Sci.402,34-39.https://doi.org/10.1016/j.jcis.2013.03.054.
Wang,Y.Q.,Sun,L.,Fugetsu,B.,2013c.Morphology-controlled synthesis of sunlight-driven plasmonic photocatalysts Ag@AgX(X=Cl,Br)with graphene oxide template.J.Mater.Chem.1(40),12536-12544.https://doi.org/10.1039/c3ta12893h.
Xia,J.X.,Xu,L.,Zhang,J.,Yin,S.,Li,H.M.,Xu,H.,Di,J.,2013.Improved visible light photocatalytic properties of Fe/BiOCl microspheres synthesized via self-doped reactable ionic liquids.CrystEngComm 15(46),10132-10141.https://doi.org/10.1039/C3CE41555D.
Xiong,J.Y.,Cheng,G.,Li,G.F.,Qin,F.,Chen,R.,2011.Well-crystallized square-like 2D BiOCl nanoplates:Mannitol-assisted hydrothermal synthesis and improved visible-light-driven photocatalytic performance.RSC Adv.1(18),1542-1553.https://doi.org/10.1039/C1RA00335F.
Ye,P.,Xie,J.J.,He,Y.M.,Zhang,L.,Wu,T.H.,Wu,Y.,2013.Hydrolytic synthesis offlower-like BiOCl and its photocatalytic performance under visible light.Mater.Lett.108,168-171.
Zhang,J.,Xia,J.X.,Yin,S.,Li,H.M.,Xu,H.,He,M.Q.,Huang,L.Y.,Zhang,Q.,2013a.Improvement of visible light photocatalytic activity overflower-like BiOCl/BiOBr microspheres synthesized by reactable ionic liquids.Colloid.Surface.Physicochem.Eng.Aspect.420,89-95.https://doi.org/10.1016/j.colsurfa.2012.11.054.
Zhang,W.D.,Zhang,Q.,Dong,F.D.,2013b.Visible-light photocatalytic removal of NO in air over BiOX(X=Cl,Br,I)single-crystal nanoplates prepared at room temperature.Ind.Eng.Chem.Res.52(20),6740-6746.https://doi.org/10.1021/ie400615f.
 Water Science and Engineering2017年4期
Water Science and Engineering2017年4期
- Water Science and Engineering的其它文章
- Preface for special section on flood modeling and resilience
- Fenton-like oxidation of azo dye in aqueous solution using magnetic Fe3O4-MnO2nanocomposites as catalysts
- Effects of urban grass coverage on rainfall-induced runoff in Xi'an loess region in China
- Effect of water-sediment regulation and its impact on coastline and suspended sediment concentration in Yellow River Estuary
- Flood management of Dongting Lake after operation of Three Gorges Dam
- A distributed eco-hydrological model and its application
