Molecular docking simulation analysis of the interaction of dietary flavonols with heat shock protein 90
Salam Pradeep Singh,Chitta Ranjan Deb,Sharif Udin Ahmed,Yenisetti Saratchandra, Bolin Kumar Konwar
1Bioinformatics Infrastructure Facility Centre,2Department of Botany,3Department of Zoology,Nagaland University, Lumami 798627,Nagaland,India;
4Department of Molecular Biology and Biotechnology,Tezpur University,Tezpur-784028,Assam,India.
Molecular docking simulation analysis of the interaction of dietary flavonols with heat shock protein 90
Salam Pradeep Singh1,?,Chitta Ranjan Deb2,Sharif Udin Ahmed3,Yenisetti Saratchandra3, Bolin Kumar Konwar4
1Bioinformatics Infrastructure Facility Centre,2Department of Botany,3Department of Zoology,Nagaland University, Lumami 798627,Nagaland,India;
4Department of Molecular Biology and Biotechnology,Tezpur University,Tezpur-784028,Assam,India.
Hsp90 is a major protein involved in the stabilization of various proteins in cancer cells.The present investigation focused on the molecular docking simulation studies of flavanols as inhibitors of Hsp90 at the high affinity adenosine triphosphate(ATP)binding site and analyzed absorption,distribution,metabolism,excretion and toxicity (ADME-toxicity).The molecular docking analysis revealed that the flavanols showed competitive inhibition with ATP molecule at the active site and enhanced pharmacological parameters.
flavonols,cancer,molecular docking,Hsp90,ATP binding site
Introduction
Flavonols,a class of flavonoids that have 3-hydroxyflavone backbone(3-hydroxy-2-phenylchromen-4-one)[1-2],are widely present in a variety of fruits and vegetables[3].Some preliminary reports showed that flavonoids are involved in the modification of allergens, viruses and carcinogens[4-5].Moreover,several in vitro studies showed that flavonoids also have anti-allergic, anti-inflammatory[6],anti-microbial[7-8],anti-cancer[9]and anti-diarrheal activities[10].Additionally,in vitro studies have demonstrated activity of flavonoids against several viruses[11].
Quercetin is usually present in fruits,vegetables, leaves and grains[12].Kaempferol is found in tea,broccoli,Kaempferia galangal,and other fruits,vegetables, leaves and grains[13].while maristin is present in grapes, berries,fruits,vegetables,herbs,as well as other plants and red wine[14].
Hsp90 is an abundant cytosolic chaperone that is involved in the turnover,trafficking and activity of a large number and variety of client proteins such as membrane-associated proteins and soluble protein kinases[15-16].There are several reports of small molecule hsp90 inhibitors that bind to the N-terminal adenosine triphosphate(ATP)binding pocket and inhibit chaperone function of hsp90[17].Hsp90 inhibitors bind to the N-domain ATP-binding pocket and prevent ATP binding,eventually leading to client protein degradation[24].In response to hsp90 inhibition,cancer cells exhibit several types of response,including reversal of transformation,differentiation and apoptosis[18-19]. Hsp90 is also secreted in large quantities and found on the surface of cancer cells[20-21].Hsp90 inhibitorsare currently undergoing clinical evaluation in cancer patients[22-23].
In the present investigation,3 common dietary flavonols viz.quercetin,kaempferol and myricetin were screened against Hsp90 using in silico molecular docking simulation approaches and virtual screening.The molecular docking simulation in this study revealed that quercetin,kaempferol and myricetin exhibited competitive inhibition with ATP molecule at the ATP-binding pocket of Hsp90.
Materials and methods
Protein preparation
The 3 dimensional(3D)crystal structure of Hsp90 N-terminal domain bound to ATP(PDB ID:3T0Z) was retrieved from the Research Collaboratory for Structural Bioinformatics(RCSB)Protein Data Bank (http://www.rcsb.org/).The crystal structure has a resolution of 2.19 A.It also has a structural weight of 26,188.49 Da and amino acid length of 288 and contains only a single chain(Chain A).The enzyme was then imported in the Molegro Virtual Docker (MVD)[25].For molecular docking purpose,all the water molecules were removed because they were considered during the scoring.
Chemical structures
The 2D structures of quercetin(CID5280343),kaempferol(CID5280863),myricetin(CID CID5281672)and ATP(CID5957)were retrieved from the NCBI PubChem database[26].The energy of these compounds were optimized using MM2 force field methods[27]and converted to 3D format and saved as sybyl mol2 file format using Chem Office 2010(Chem Office 2010:CambridgeSoft Corporation)for docking purposes.
Cavity prediction
The cavity or the potential ligand binding site of Hsp90(PDB ID:3T0Z)was predicted using MVD. A cavity which has a volume of 151.04and a surface area of 462.08was predicted.The binding site was set inside a restriction sphere of 15radius with the centre X:12.67,Y:-3.46,Z:14.09.The MolDock grid score was set with a grid resolution of 0.30
Bond flexibility set up
The compounds viz.quercetin,kaempferol,myricetin and ATP molecule was loaded in the MVD.The bond flexibility of these compounds was set. Additionally,the side chain flexibility of the amino acid residues at the potential ligand binding site (Asn51,Ala55,Met98,Gly135,Val136,Gly137 and Thr184)of Hsp90 was set with a tolerance of 1.10 and strength of 0.80 for docking simulations.
Molecular docking simulation
Molecular docking simulation was performed using Molegro Virtual Docker(MVD)6.01.The software is based on a differential evolution algorithm;the solution of the algorithm considers the sum of the intermolecular interaction energy between the ligand and the protein and the intramolecular interaction energy of the ligand.The docking energy scoring function was based on the modified piecewise linear potential (PLP)with new hydrogen bonding and electrostatic terms included[28].The docking algorithm was set with softens potentials during the docking simulation with the side chains of the enzyme made flexibile.The maximum minimization for the residues and the ligand was set at 2,000 steps and the maximum global minimization was set for 2,000 steps.
The MolDock scoring function was also set with a grid resolution of 0.30 A.It was set at a maximum iteration of 1,500 with a simplex evolution size of 50 and a minimum of 10 runs were performed for each compound with threshold energy of 100. Additionally,the simplex evolution was set for 300 steps with a neighbour distance factor of 1.00.The best pose of each compound was selected for subsequent ligand-protein interaction energy analysis.
Absorption,distribution,metabolism,excretion and toxicity(ADME-toxicity)analysis
ADME-toxicity was carried out for quercetin, kaempferol and myricetin using ACD/I-Lab 2.0 (ACD/I-Lab,Version 2.0,Advanced Chemistry Development,Inc,Toronto,ON,Canada).The absorption,solubility,blood brain barrier(BBB)transport,oral bioavailability and distribution of quercetin,kaempferol and myricetin were calculated.Additionally,the LD50and probability of health effects of the 3 compounds were also calculated.Lastly,a comparative analysis was carried out of LD50in mouse(intraperitoneal,oral, intravenous and subcutaneous)for quercetin,kaempferol and myricetin.
Results
Molecular docking simulation was carried out using the MVD.The binding cavity used in the present molecular docking simulation is shown inFig.1.The docking score and scoring results are shown in Table 1.The interaction energy of quercetin,kaempferol and myricetin is-109.87 kJ/mol,-104.73 kJ/mol and-99.48 kJ/mol,respectively,compared to-98.62kJ/mol of ATP molecule.These findings indicated that quercetin,kaempferol and myricetin had more favorable ligand-protein interaction energy than ATP molecule at the binding cavity of Hsp90.In this study,themolecular interaction of quercetin,kaempferol and myricetin lay deep inside the binding pocket of Hsp90,exhibiting both bonded and non-bonded interaction(Fig.2).
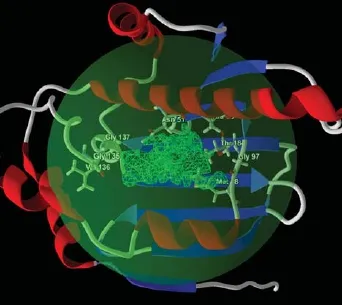
Fig.1The potential ligand binding cavity of Hsp90(PD ID: 3T0Z)with flexible residues predicted using MVD.
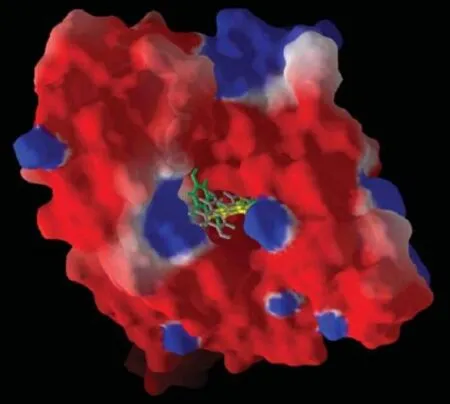
Fig.2Surface map of HSp90 depicting quercetin(green), kaempferol(yellow)and myricetin(grey)lying deep into the binding pocket.
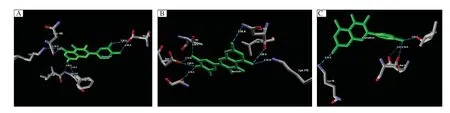
Fig.3Molecular interaction of(A)quercetin(B)myricetin and(C)kaempferol at the active site of Hsp90.Green:steric interaction favorable;turquoise:hydrogen acceptor favorable;yellow:hydrogen donor favorable;red and blue:electrostatic potential of Hsp90.
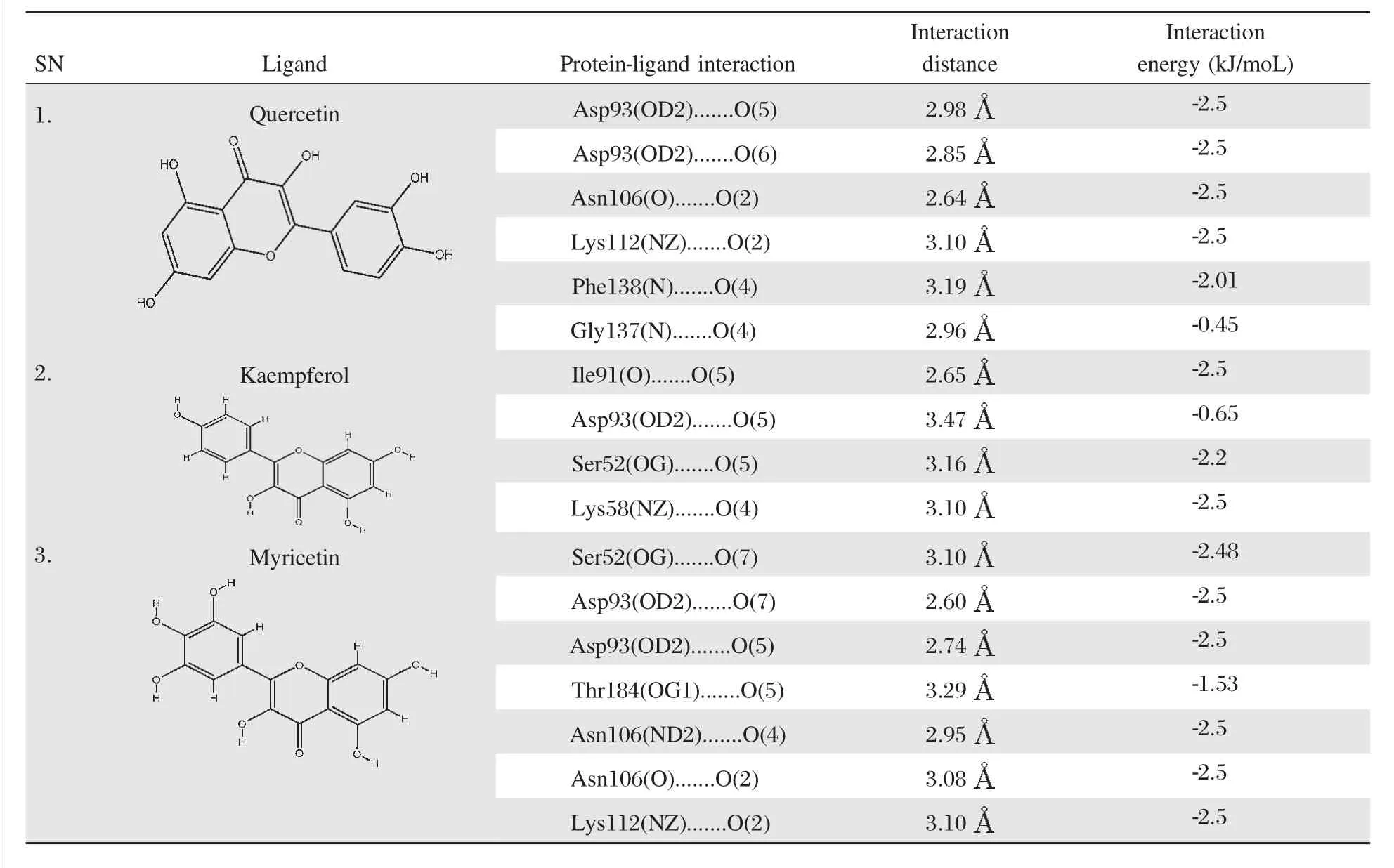
Table 2Molecular interaction analysis of quercetin,kaempferol and myricetin at the active site of Hsp90
Additionally,the molecular interaction analysis for the ligand-protein interaction is shown inTable 2. The average molecular interaction energy of quercetin is-2.07A kJ/mol,while that of kaempferol is-1.96 kJ/ mol and-2.36 kJ/mol for myricetin.The snapshots of ligand-protein interaction depicting the binding mode of quercetin,kaempferol and myricetin are shown in Fig.3A,BandC.Quercetin showed molecular interaction with Asp93,Asn106,Lys112,Gly137 and Phe138 residues of Hsp90 while kaempferol established molecular interaction with Ser52,Lys58,Ile91 and Asp93 residues of Hsp90 and myricetin had molecular interaction with Ser52,Asp93,Asn106,Lys112 and Thr184 residues of Hsp90.The snapshots representing the secondary structures for the molecular interaction of quercetin,kaempferol and myricetin at the active site of Hsp90 are shown inFig.4 A,Band C,respectively.The top three docking hits showed common molecular interaction with Asp93(OD2).
The energy map of Hsp90 that might contribute in steric interaction favourable(green colour),hydrogen acceptor favourable(turquoise colour),hydrogen donor favourable(yellow colour)and electrostatic potential of Hsp90(red and blue colour)with theligand viz.quercetin,kaempferol and myricetin are shown inFig.5 A,BandC,respectively.The electrostatic interaction of quercetin,kaempferol and myricetin as well as the active site of Hsp90 are shown in Fig.6 A,BandC,respectively.
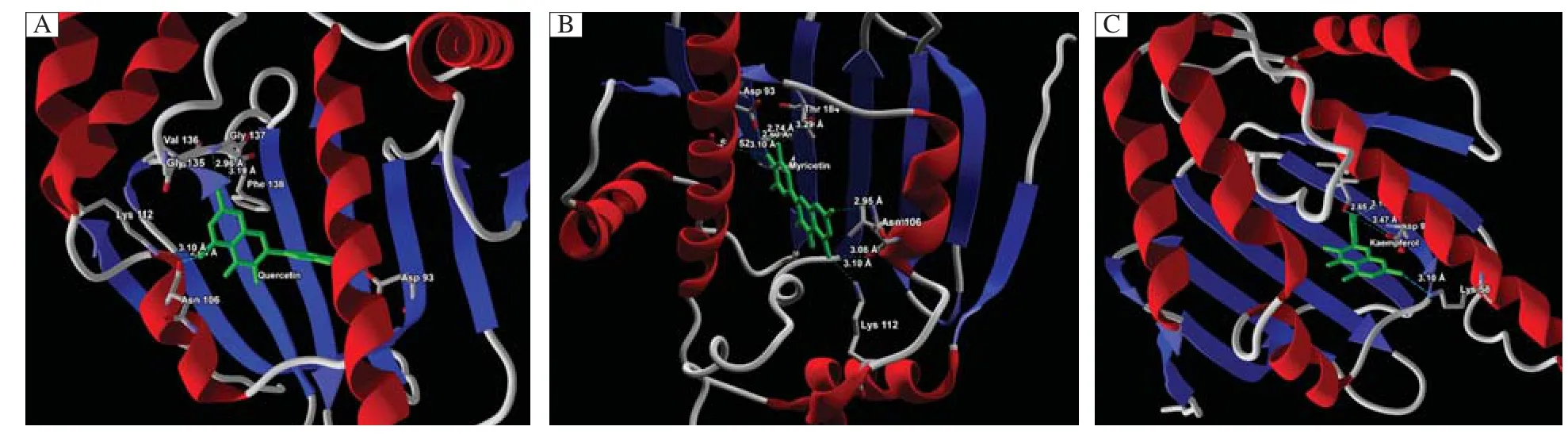
Fig.4Molecular binding mode of(A)quercetin(B)myricetin and(C)kaempferol at the binding site of Hsp90.
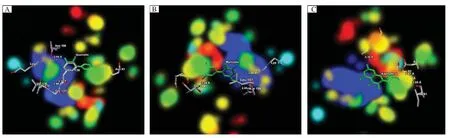
Fig.5Energy map of(A)quercetin(B)myricetin and(C)kaempferol at the binding cavity of Hsp90.
The results of the ADME-toxicity analysis calculated using ACD Ilab 2.0 are shown inTable 4and the comparative graph plot on LD50mousefor quercetin, kaempferol,and myricetin is shown inFig.7.ADME-toxicity analysis showed that kaempferol(0.10 mg/ mL)was readily soluble compared to quercetin(0.14 mg/mL)and myricetin(0.25 mg/mL).For absorption, myricetin showed a bit lower passive absorption of 95%compared quercetin and kaempferol(100%passive absorption).
DISCUSSION
It is revealed from the docking score and scoring result(Table 1)that quercetin,kaempferol and myricetin showed better rerank score than ATP molecule,indicating that the dietary flavonols docked at the active site of the Hsp90(Table 1).The rerank score used in MVD is a weighted combination of the terms used by the MolDock score mixed with a few addition terms which includes the Steric terms which are Lennard-Jones approximations to the steric energy[28].The reranking score function is computationally more expensive than the scoring function used during the docking simulation,but it generally gives better result than the docking score function. The reranking coefficients used the energy parameters such as E-Inter total,E-Inter(protein-ligand),Steric, VdW(Van der Waal’s),HBond(hydrogen bonding energy),E-Intra(tors,ligand atoms),E-Solvation,ETotal etc.In addition,as shown inFig.2,quercetin, kaempferol and myricetin were found to be lying deep inside the binding pocket of Hsp90 which is an indication of a strong molecular interaction exhibiting both bonded and non bonded interaction.
The average molecular interaction energy of the three compounds was also calculated which supports the binding affinity of these compounds at the binding site.Hsp90 is known for its high-affinity for binding the ATP molecule at a region near the N-terminus known as the ATP-binding region and when it is prevented from binding the ATP molecule,Hsp90 eventually is degraded.Hence,these dietary flavonols willserve as a good inhibitor of Hsp90 showing competitive inhibition with ATP molecule as evident from the docking score.Moreover the Lipinski rule of five parameters for quercetin,kaempferol,myricetin and and ATP indicates that these dietary flavonols do not violate the rule of five to be an orally active compound inside the human body.
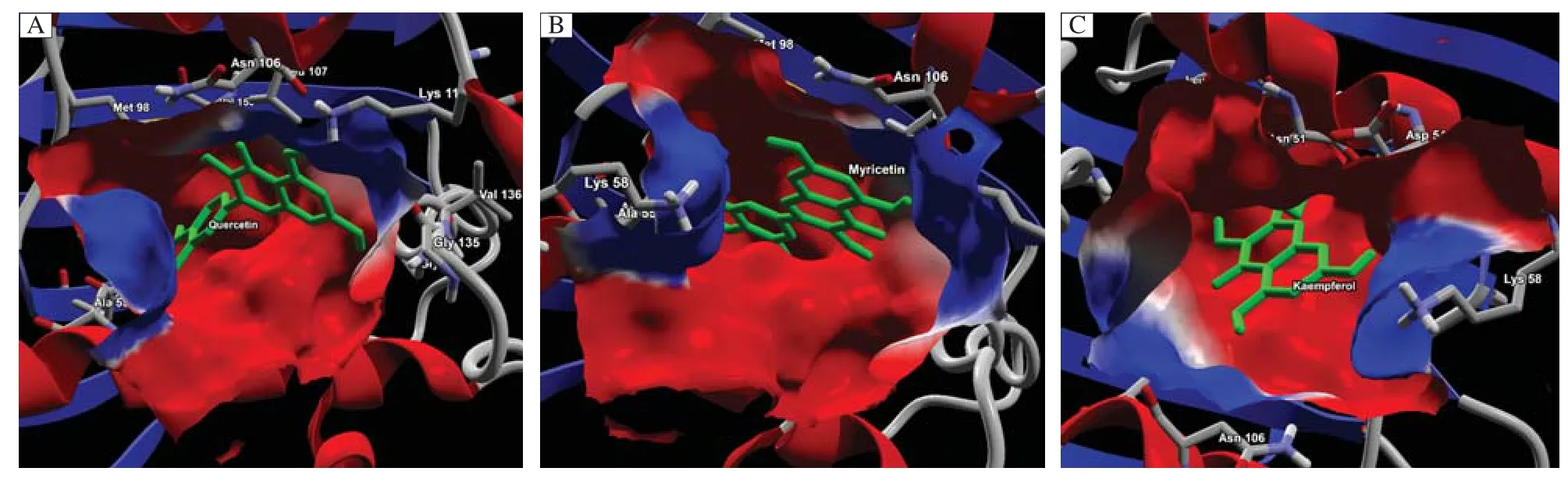
Fig.6Electrostatic interaction(A)quercetin(B)myricetin and(C)kaempferol at the binding cavity of Hsp90.
Table 3Lipinski rule of 5 parameters for quercetin,myricetin,kaempferol and ATP molecule
The comparative graph plot on LD50mousefor quercetin,kaempferol,and myricetin indicated that quercetin,kaempferol,and myricetin had higher LD50mouse(oral).Hence,it can be administered orally instead of intravenously or subcutaneously.In fact,the phytochemicals possessed enhanced pharmacological properties with lesser health effects.Thus,these dietary phytochemicals may serve as lead molecule or a potent inhibitor of Hsp90.
In this study,the authors have carried out molecular docking simulation and molecular interaction analysis of 3 dietary flavonols against Hsp90.The molecular docking score revealed that the dietary phytochemicals viz.quercetin,myricetin and kaempferol showed competitive inhibition with ATP molecule at the high affinity ATP binding site.They inhibited the ATP binding pocket which will lead to the degradation of Hsp90. Furthermore,the inhibition of Hsp90 enzyme will contribute to the decreasing for stabilization of several pro-teins which are involved in tumour growth and cancer. Moreover,there are only preliminary reports of these flavonols reporting for anti-cancer property or inhibiting Hsp90.Additionally,the dietary phytochemicals used in the present study do not violate the Lipinski rule of 5 parameters.
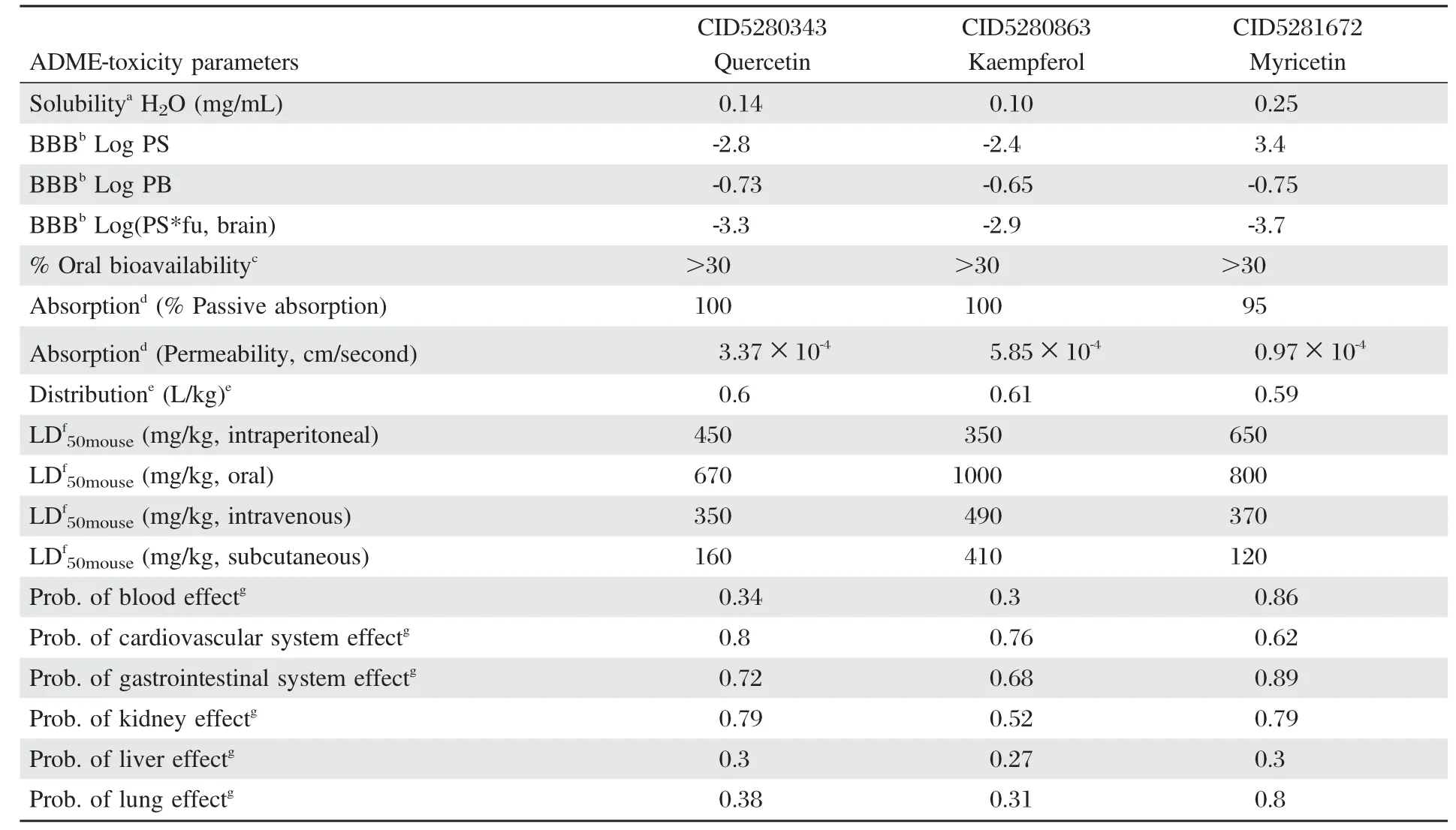
Table 4Predicted ADME-toxicity parameters for quercetin,kaempferol and myricetin
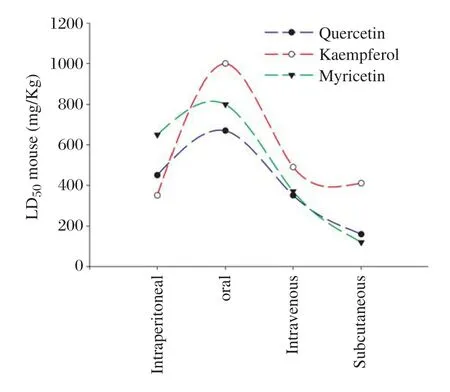
Fig.7LD50mouse(intraperitoneal,oral,intravenous, subcutaneous)graph plot for quercetin,kaempferol and myricetin.
In addition,from the ADME-toxicity analysis,these compounds possessed enhanced pharmacological parameters with lesser LD50 and health effects.Hence,we conclude that,quercetin,myricetin and kaempferol will contribute to fight against cancer thereby inhibit the chaperonic function of Hsp90,which support experimental testing of these compounds.
Acknowledgement
The authors would like to acknowledge Apex Bioinformatics,Department of Biotechnology,and Ministry of Science and Technology,Government of India for providing Bioinformatics Infrastructure Facilityat Nagaland University,Lumami Campus,Nagaland,India.
References
[1]McNaught AD,Wilkinson A,IUPAC.Flavonoids(isoflavonoids and neoflavonoids).IUPAC Compendium of Chemical Terminology,2ndedition.Oxford:Blackwell Scientific,1997.
[2]Galeotti F,Barile E,Curir P,et al.Flavonoids from carnation(Dianthus caryophyllus)and their antifungal activity[J].Phytochem Lett,2008;1(1):44-48.
[3]Spencer JPE.Flavonoids:modulators of brain function?[J]Br J Nutr,2008;99(E-S1):ES60-ES77.
[4]Si D,Wang Y,Zhou YH,et al.Mechanism of CYP2C9 inhibition by flavones and flavonols[J].Drug Metab Dispos,2009;37(3):629-634.
[5]Zhou J,Fang L,Yao WX,et al.Effect of quercetin on heat shock protein expression in HepG2 cells determined by SILAC[J].Zhonghua Zhong Liu Za Zhi.,2011;33(10):737-741.
[6]Yamamoto Y,Gaynor RB.Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer[J].J Clin Invest,2001;107(2):135-142.
[7]Cushnie TPT,Lamb AJ.Antimicrobial activity of flavonoids[J].Int J Antimicrob Agents,2005;26(5):343-356.
[8]Cushnie TPT,Lamb AJ.Recent advances in understanding the antibacterial properties of flavonoids[J].Int J Antimicrob Agents,2011;38(2):99-107.
[9]Margineantu DH,Emerson CB,Diaz D,et al.Hsp90 inhibition decreases mitochondrial protein turnover[J].PLoS One,2007;2(10):e1066.
[10]de Sousa RR,Queiroz KC,Souza AC,et al.Phosphoprotein levels,MAPK activities and NFkappaB expression are affected by fisetin[J].J Enzyme Inhib Med Chem,2007;22(4):439-444.
[11]Gonza′lez ME,Martnez-Abarca F,Carrasco L.Flavonoids:Potent inhibitors of poliovirus RNA synthesis[J].Antivir Chem Chemother,1990;1(3):203-209.
[12]Justesen U,Knuthsen P.Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes[J].Food Chem,2001; 73(2):245-250.
[13]Mustafa RA,Abdul HA,Mohamed S,et al.Total phenolic compounds,flavonoids,and radical scavenging activity of 21 selected tropical plants[J].J Food Sci,75(1):C28-35.
[14]Maggiolini M,Recchia AG,Bonofiglio D,et al.The red wine phenolics piceatannol and myricetin act as agonists for estrogen receptor in human breast cancer cells[J].J Mol Endocrinol,2005;35(2):269-281.
[15]Pratt WB,Toft DO.Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery[J].Exp Biol Med,2003;228(2):111-133.
[16]Zhang H,Burrows F.Targeting multiple signal transduction pathways through inhibition of Hsp90[J].J Mol Med, 2004;82(8):488-499.
[17]Whitesell L,Mimnaugh EG,De Costa B,et al.Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins:essential role for stress proteins in oncogenic transformation[J].Proc Natl Acad Sci USA,1994;91(18):8324-8328.
[18]Lopez-Maderuelo MD,Fernandez-Renart M,Moratilla C, et al.Opposite effects of the Hsp90 inhibitor Geldanamycin:induction of apoptosis in PC12,and differentiation in N2A cells[J].FEBS Lett,2001;490(1-2):23-27.
[19]Chiosis G,Timaul MN,Lucas B,et al.A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells[J].Chem Biol, 2001;8(3):289-299.
[20]Eustace BK.Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness[J].Nat Cell Biol,2004;6(6):507-514.
[21]Sidera L,Patsavoudi E.Extracellular Hsp90:conquering the cell surface[J].Cell Cycle,2008;7(11):1564-1568.
[22]Chiosis G,Tao H.Purine-scaffold Hsp90 inhibitors[J]. I Drugs,2006;9(11):778-782.
[23]Eccles SA,Massey A,Raynaud,et al.NVP-AUY922:a novel heat shock protein 90 inhibitor active against enograft tumor growth,angiogenesis,and metastasis[J]. Cancer Res,2008;68(8):2850-2860.
[24]Pearl LH,Prodromou C.Structure and mechanism of the Hsp90 molecular chaperone machinery[J].Annu Rev Biochem,2006;75:271-294.
[25]Molegro APS(2011)MVD 6.01 Molegro Virtual Docker, DK-8000 Aarhus C,Denmark.
[26]Bolton E,Wang Y,Thiessen PA,et al.PubChem:integrated platform of small molecules and biological activities, 4th edition.Annual reports in computational chemistry. Washington,DC:American Chemical Society,2008, Chapter 12.
[27]Ulrich B,Norman LA.Molecular mechanics(ACS Monograph 177).Washington,DC:American Chemical Society,1982.
[28]Thomsen R,Christensen MH.MolDock:a new technique for high-accuracy molecular docking[J].J Med Chem, 2006;49(11):3315-3321.
[29]ACD/I-Lab,Version 2.0,Advanced Chemistry Development,Inc,Toronto,Canada.
?Salam Pradeep Singh,Bioinformatics Infrastructure Facility Centre,Nagaland University,Lumami-798627, Nagaland,India.Tel/Fax:0369-2268268/0369-2268248, E-mail:salampradeep@gmail.com.
29 November 2013,Revised 02 January 2014,Accepted 16 September 2014,Epub 08 June 2015
R857.3,Document code:A
The authors reported no conflict of interests.
 THE JOURNAL OF BIOMEDICAL RESEARCH2016年1期
THE JOURNAL OF BIOMEDICAL RESEARCH2016年1期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Myocardin-related transcription factor A cooperates with brahmarelated gene 1 to activateP-selectin transcription
- Assessment of malathion and its effects on leukocytes in human blood samples
- Manifestations of type 2 diabetes in corneal endothelial cell density, corneal thickness and intraocular pressure
- Impact of IL28Bgene polymorphisms rs8099917 and rs12980275 on response to pegylated interferon-α/ribavirin therapy in chronic hepatitis C genotype 4 patients
- Circulating thrombospondin-2 in patients with moderate-to-severe chronic heart failure due to coronary artery disease
- Emerging targets for glioblastoma stem cell therapy
