Anticancer Activities of Plant Secondary Metabolites: Rice Callus Suspension Culture as aNew Paradigm
Wusirika Ramakrishna, Anuradha Kumari, Nafeesa Rahman, Pallavi Mandave
Review
Anticancer Activities of Plant Secondary Metabolites: Rice Callus Suspension Culture as aNew Paradigm
Wusirika Ramakrishna1, #, Anuradha Kumari1, #, Nafeesa Rahman2, Pallavi Mandave3
(; )
Plant natural products including alkaloids, polyphenols, terpenoids and flavonoids have been reported to exert anticancer activity by targeting various metabolic pathways. The biological pathways regulated by plant products can serve as novel drug targets. Plant natural compounds or their derivatives used for cancer treatment and some novel plant-based compounds which are used in clinical trials were discussed. Callus suspension culture with secondary metabolites can provide a continuous source of plant pharmaceuticals without time and space limitations. Previous research has shown that rice callus suspension culture can kill >95% cancer cells with no significant effect on the growth of normal cells. The role of candidate genes and metabolites which are likely to be involved in the process and their potential to serve as anticancer and anti-inflammatory agents were discussed. Large scale production of plant callus suspension culture and its constituents can be achieved using elicitors which enhance specific secondary metabolites combined with bioprocess technology.
plant metabolite; cancer metabolism; rice callus suspension culture; cytotoxicity; anticancer agent
Cancer is the leading cause of mortality after cardiovascular diseases. Cancer incidence increases with age with >75% new cases among the age group of 55 years and above (White et al, 2014). Numbers of estimated cancer cases in USA in 2019 are 268600, 228150, 174650, 145600 and 73820 for breast, lung, prostate, colorectal and renal cancers, respectively (American Cancer Society, 2019). Numbers of estimateddeaths due to cancer in USA are 142670, 51020, 41760, 31620 and 14770 for lung, colorectal, breast, prostate and renal cancers, respectively. Cancer is an inherited or sporadic complex genetic disease due to various modifications in gene expression (Wishart, 2015). These modifications can be in the forms of point mutations, insertions, deletions, transpositions and translocation resulting in uncontrolled proliferation, which is the hallmark of cancer.
Proto-oncogenes and tumor suppressor genes are the two categories of genes that conventionally undergo alterations (Lee and Muller, 2010). In cancer, the expression of tumor suppressor genes is inhibited, and proto-oncogenes are upregulated by various mutations. These pro-oncogenic alterations help the mutated cell to bypass every checkpoint of cell division, ultimately invading and spreading to the neighboring tissues. Metastasis is initiated by the formation of new blood vessels from existing vessels called angiogenesis. Various factors responsible for the mutation of normal cell to cancer cell include exposure to asbestos, tobacco, drugs, radiation and oncogenic viruses, ultimately resulting in an alteration in the genetic makeup of the cell. In comparison to normal cells, cancer cells exhibit nuclear pleomorphisms, chromosomal abnormalities, reduction in the cellular gap junction and increase in motility. They possess inherent growth signals, which make them resistant to growth suppression signals. They manifest resistance to apoptosis, a programmed cell death that is necessary for maintaining the homeostasis between cell growth and cell death in the body (Fulda, 2009). These properties of a cancer cell are possible targets for drug development with a focus on halting cell growth, invasion and progression.
Over the years, an enormous number of drugs have been synthesized and used to either prevent or cure cancer (Cragg and Pezzuto, 2016). However, their untoward systemic adverse effects on patients, the rapid development of drug resistance and higher cost of treatment are some of the drawbacks rendering them ineffective in overall cancer management. As a result, despite advances in the development of synthetic oncogenic drugs, cancer patients are increasingly dependent on alternative medicines for treatment due to their safety, availability, affordability, minimal adverseeffects and less chance of developing resistance. Plantsare the only source of medicine in ancient times (Petrovska, 2012; Pan et al, 2014). Surprisingly, they remain a significant source of medicine till now, predominantly in developing countries due to their advantages over commercial synthetic drugs. World Health Organization (WHO) has reported[w1] that about 80% of the world population still relies on the herbal mode of therapy than conventional synthetic drugs (WHO, 2014).
Cancer researchers have therefore focused their attention more on natural products as an alternative source of cancer control and cure. Ancientandof India, Papyrus of Egypt and traditional Chinese medicine provide rich documentations of plant-based preventive and curative methods of ailments and diseases (Lemonnier et al, 2017). Several parts of plants like stems, barks, roots, leaves, seeds and their extracts are used as herbal drugs (Li and Weng, 2017). About 60% of the prescribed anticancer drugs in use are derivatives of plant metabolites. Paclitaxel (), camptothecin (), vincristine and vinblastine () and etoposide () or their water-soluble analogs are some examples of well- known plant-derived anticancer products (Fridlender et al, 2015; Howes, 2018). Several other promising plant natural compounds with anticancer activity and their modes of action are described in Lichota and Gwozdzinski (2018).
Rice serves as a staple food for majority of the world’s population. Although the content of total phenolics and flavonoids of black rice and red rice varieties is higher than the white rice varieties in most cases, some exceptions are observed (Shen et al, 2009; Chutipaijit and Sutjaritvorakul, 2018). Rice and its byproducts such as bran and husk harbor phenolic compounds (ferulic acid and-coumaric acid), phytic acid, tocopherols and tocotrienols, which act as chemopreventive agents (Tan et al, 2017; Yu et al, 2019).
The synthesis of plant secondary metabolites by plant tissue culture is independent of environmental conditions (Smetanska, 2008). Plant cell culture systemoffers a unique opportunity for the large scale production of anticancer compounds due to their industrial level scalability and the advantage over human and bacterial cell cultures due to the lack of human pathogens and bacterial endotoxins (Xu and Zhang, 2014). Several pharmaceutical proteins including serum albumin and taliglucerase alfa are produced using carrot, tobacco and rice cell cultures as well as (Yao et al, 2015). Although callus cultures have been used to produce targeted natural products, there is very little information on the use of plant callus suspension culture as a cytotoxic or anticancer agent. Callus suspension cultureand callus extracts of rice and desert cotton, respectivelyhave been shown to be cytotoxic to cancer cells (Deshpande et al, 2012; Rahman et al, 2016; Kamalanathan and Natarajan, 2018).
The major objectives of this review were to provide an update on plant secondary metabolites with anticancer activity, the use of plant callus suspension cultures forproduction of secondary metabolites and the potential application of rice callus suspension culture with secondary metabolites as an anticancer agent. Further, the biological pathways regulated by secondary metabolites including those found in rice callus suspension culture were discussed.
Secondary metabolites and their anticancer effects
Secondary metabolites are not essential for the growth and development of plants, but they have important accessory activities such as help in defense against herbivory, growth inhibition of competing plants, bacterial and fungal pathogens and aiding in pollination. They possess various anti-inflammatory and anti-oxidant activities (Adebayo et al, 2015). Anti-oxidant capabilities include termination of free radical chains and chelation of redox active metal ions that cause lipid peroxidation. These properties also help with cancer prevention. Secondary metabolites of plants are grouped into alkaloids, terpenoids, polyphenols and flavonoids based on their structures (Singh et al, 2016). Notable anticancer alkaloids include vinblastine,vincristine and camptothecin; terpenoids include lycopeneand gamma-tocopherol; polyphenols include etoposide, resveratrol, curcumin and epigallocatechin gallate (EGCG); and flavonoids include apigenin, genistein and kaempferol. These bioactive compounds exert anticancer effects either independently or synergistically with other compounds through regulation of metabolic and signaling pathways, inhibition of enzymes vital for cancer progression, angiogenesis, microtubule assembly and inducing apoptosis (Kojima-Yuasa et al, 2015). Over the years, several plants and plant extracts are being studied for their antiproliferation properties, and many of them display potential ability to inhibit cancer growth and progression (Grothaus et al, 2010). Table 1 summarizes some investigated plants and their active components showing prominent cancer preventive and inhibitory effects. A comprehensive study on the underlying mechanism of the action of these compounds and other novel compounds and their interactions can explain the biological basis of their anticancer effects. Towards this goal, it is essential to understand the various changes in cancer physiology that differentiate it from a normal proliferating cell so that the side effectsof an anticancer agent on normal cells can be prevented.

Table 1. In vitro examples of plant bioactive compounds and their molecular targets.
ABC transporter, ATP binding cassette transporter; ADAM10, A Disintegrin and metalloproteinase domain-containing protein 10; BCRP, Breast cancer resistance protein; CCNB, Cyclin B; KIF20A, Kinesin family member 20A; CCNB1, Cyclin B1; CDKN1A, Cyclin dependent kinase inhibitor 1A; CDK5, Cyclin-dependent kinase 5; EPHB2, Ephrin type-B receptor 2; ERK, Extracellular signal-regulated kinase; ERα, Estrogen receptor alpha; GADD45α, Growth arrest and DNA damage 45 alpha; GADD45β, Growth arrest and DNA damage 45 beta; GLUT3, Glucose transporter 3; HDAC4, Histone deacetylase 4; IGF-1R, Type-1 insulin-like growth factor receptor; MAPK, Mitogen-activated protein kinase; MDR1, Multidrug resistance protein 1; MMP2, Matrix metalloproteinase 2; MMP9, Matrix metalloproteinase 9; MRP2, Multidrug resistance protein 2; MRP3, Multidrug resistance protein 3; mTOR, Mammalian target of rapamycin; NSCLC, Non-small-cell lung carcinoma; NF-κB, Nuclear factor kappa light chain enhancer of activated B cells; NQO1, NAD(P)H quinone oxidoreductase; PI3K, Phosphatidylinositol-3-kinase; PARP, Poly ADP ribose polymerase; SEMA3E, Semaphorin-3E; SEPP1, Selenoprotein P1; SP-1, Specificity protein 1; STAT3, Signal transducer and activator of transcription 3; TOP2α, Topoisomerase 2-alpha.
Biological pathways serving as targets of plant metabolites
The hallmark of a cancer cell is its uncontrolled rate of proliferation. A cancer cell alters its physiology to meet the nutritional and energy requirements. These alterations are in the form of modifications in metabolicpathways, signaling pathways and enzymatic regulation (Fig. 1). Some of the essentially crucial elements of these altered metabolic pathways are glucose, glutamine,oxygen and adenosine triphosphate (ATP) (Biswal et al, 2017). These metabolic alterations mainly focus on rapid ATP production, synthesis of macromolecules needed for cell progression and regulation of appropriate redox state. In case of scarcity of nutrients and energy, cancer cells are able to modulate their pathways and continue proliferation. Genetic alterations and tumor microenvironment contribute to the abnormal phenotype of cancer. The characteristic changes in metabolism and pathway can serve as important targets for cancer therapy. The challenging task is to find a specific window to differentiate the proliferating cancer cells and proliferating normal cells. The advancement of analytical technology has resulted in the discovery of numerous cancer signaling pathways and processes which are specifically regulated in cancer cells and are important for malignant transformation (Green and Llambi, 2015). Warburg effect is a significant change in cancer metabolism, where energy production is diverted from the normal oxidative phosphorylation to aerobic glycolysis (Warburg, 1956). This shift serves two vital purposes: produce ATP rapidly and produce large quantities of substrate for the biosynthesis of macromolecules needed for cancer progression. This diversion to glycolysis makes glucose essential for cancer cells. It has been reported that glucose transporters (GLUT) are up-regulated in cancer in a cell-specific manner (Thorne and Campbell, 2015). Targeting GLUT presents a viable strategy for cancer inhibition and treatment. Plant extracts have been shown to targetGLUT. For example, naringenin, a flavonoid presents in grapes inhibits glucose uptake in MCF-7 breast cancer cells by inhibiting the phosphoinositide 3-kinase (PI3K) pathway that regulates glucose transporter, GLUT4 (Harmon and Patel, 2004). Another regulator (inhibitor) of glucose transporter is phloretin, a polyphenol presents in apple which reduces tumor progression (Kundu et al, 2014).

Fig. 1. Major players and pathways involved in cancer metabolism and their regulation by plant metabolites.
Curcumin and other plant natural compounds regulating PI3K/Akt/mTOR pathway are shown. Adapted from Porta et al (2014), Eales et al (2016), and Kastenhuber and Lowe (2017). Akt, Protein kinase B; C-Myc, Cellular myelocytomatosis; 4E-BP1, Eukaryotic initiation factor 4E binding protein 1; FKBP-12, Drug FK506 binding protein-12; GLUT, Glucose transporter; HIF-1α, Hypoxia-inducible factor-1 alpha; mTOR, Mammalian or mechanistic target of rapamycin; OCT1, Octamer binding transcription factor-1; PDH, Pyruvate dehydrogenase; PDK, Pyruvate dehydrogenase kinase; PKM2, Pyruvate kinase M2; PTEN, Phosphatase and tensin homolog; PI3K, Phosphatidylinositol-3-kinase; SCO2, Synthesis of cytochrome C oxidase 2; TCA cycle, Tricarboxylic acid cycle; TIGAR, Tumor protein 53 inducible glycolysis and apoptosis regulator.
The PI3K pathway is a common pathway activated by mutations in genes whose protein products are known to modulate processes involved in cancer such as cell proliferation, motility, metabolism and survival (Broecker-Preuss et al, 2017). It is inhibited in normal cells by phosphatase and tensin homolog (PTEN), which is a tumor suppressor. PTEN has been observed to be mutated and suppressed in cancer cells, thereby activating the PI3K pathway.target of the PI3K pathway is AKT1 [w2] (protein kinase B), which mainly regulates glycolytic mechanism of cancer cells through the allocation of glucose transporters and activation of glycolytic enzymes. Flavonoids of various plant extracts and curcumin, a polyphenol from,have been reported to target the PI3K pathway and AKT1 (Mouhid et al, 2017), thereby hindering uncontrolled cell proliferation associated with cancer.
The mechanistic target of rapamycin (mTOR) is an essential pathway for the regulation of cell growth and proliferation. It is responsible for the synthesis of proteins and lipids needed for cancer cells in the presence of sufficient nutrition and energy (Cargnello et al, 2015). This pathway is activated by[w3] (Porta et al, 2014). The mTOR pathway can serve as an effective target for combatting cancer. Curcumin has been reported to inhibit cancer progression by regulating the mTOR pathway (Hamzehzadeh et al, 2018).
Hypoxia-inducible factor 1 (HIF1) is an important transcription factor regulating cancer proliferation, and is generally activated under hypoxic environment. It has been reported to be readily activated in cancer cells even under normoxic environment. In cancer, mTOR pathway is known to upregulate the expression of HIF1, which in turn upregulates enzymes involved in glycolysis as well as glucose transporters (Eales et al, 2016). The hypoxic tumor microenvironment induces HIF1 activating enzymes to produce energy. HIF1also activates the expression of gene encoding vascular endothelial growth factor (VEGF), thereby promoting angiogenesis (Zimna and Kurpisz, 2015). Topotecan, a semisynthetic derivative of pentacyclic alkaloid and camptothecin, which is a metabolite of the Chinese yew, inhibits HIF1 in cervical cancer (Robati et al, 2008). Glyceollin, a phytoalexin from soybean, reduces the expression of HIF1, resulting in the inhibition of HIF1-induced genes (Lee et al, 2014).
AMP-activated protein kinase (AMPK), the energy status sensor of the body, is reported to be suppressed by various signaling pathways and mutations in cancer. Activation of AMPK can, therefore, serve as a good target for inhibiting cancer cell proliferation and growth. AMPK activation leads to cell death in a P53 dependent pathway (He et al, 2014). Oleanolic acid (OA), a triterpenoid presents in a number of medicinal plants,is reported to activate AMPK in cancer cells, which has been shown to suppress the growth of prostate cancer cell line PC-3 and breast cancer cell line MCF-7 (Liu et al, 2014). Curcumin has also been reported to induce apoptosis in ovarian tumor cell via activation of AMPK (Pan et al, 2008).
P53 is known to be an important tumor suppressor, which is down-regulated in cancer. In a normal cell, it detects DNA damage and halts the cell cycle for repair or diverts the cell to apoptosis through caspase activation. P53 has been reported to up-regulate TP53-induced glycolysis and apoptosis regulator (TIGAR) (Kastenhuber and Lowe, 2017) and PTEN, thereby suppressing glycolysis by inhibiting PI3K pathway. Although it is a known tumor suppressor, P53 is also involved in modulating the glycolytic phenotype in cancer by activating the enzyme hexokinase 2, thereby potentiating the macromolecule synthesis in cancer through the pentose phosphate pathway (Simabuco et al, 2018). Several plant metabolites such as curcumin, resveratrol and quercetin induce apoptosis through the p53-dependent pathway (Panda et al, 2017). Most of the cell cycles regulated by p53 are indirect and involve a repressor complex, dimerization partner, RB-like, E2F and MuvB (DREAM)[w4] (Engeland, 2018). Efficient chemotherapeutic drugs can be developed by understanding the mechanisms by which these regulators modulate the cell cycle. Flavonoids like quercetin, genistein and flavopiridol have been reported to inhibit cyclin-dependent kinases (CDKs). Anthocyanins inhibit proliferation of cancer by blocking progression through the cell cycle by targeting P53, P21, cyclin D and cyclin A.
Phytic acids from rice bran up-regulategene in liver cancer cells (Al-Fatlawi et al, 2014). Gamma-oryzanol and tricin from rice bran extracts inhibit G2/M phase whereas gamma-oryzanol, gamma-tocotrienol and phytic acid inhibit G0/G1phase of cell cycle in cancer cells (Yu et al, 2019). Gamma-oryzanol also inhibits nuclear factor kappa light chain enhancer of activated B cells (NF-κB) signaling pathway.
Topoisomerase II is an important enzyme for DNA replication during cell division. It regulates the negative supercoiling of DNA downstream of the replication fork (Ketron and Osheroff, 2014). Topoisomerase covalently binds with the cleaved DNA forming a complex which serves as a target for various conventional anticancer drugs that inhibit cancer cell proliferation. However, this leads to negative consequences for normal cells. Genotoxic agents that target this enzyme-DNA complex are known as ‘topoisomerase poisons’. In addition, topoisomerase catalytic inhibitors reduce catalytic turnover of topoisomerase. Well known examples of eukaryotic topoisomerase II poisons include podophyllotoxin derivatives (etoposide and teniposide), doxorubicin and fluoroquinolones (Pommier, 2013). Phytochemicals such as curcumin, resveratrol, EGCG and isothiocyanates have also been reported to act as topoisomerase poisons and catalytic inhibitors.
Lactate dehydrogenase (LDH) which catalyzes the synthesis of lactate from pyruvate under anaerobic conditions is vital for cancer cells as it shunts oxidative phosphorylation to lactate in the hypoxic tumor microenvironment. LDH-A is one of the two isoforms of LDH, which converts pyruvate to lactate and is overexpressed in cancer cells. LDH-A is a downstream target of HIF1α, promoting lactic acid fermentation to generate nicotinamide adenine dinucleotide (NAD+) in hypoxic conditions that is essential for cancer cell glycolysis. LDH-A knockdown experiments demonstrated activation of the mitochondrial respiratory pathway in cancer cells, which results in repression of tumor progression (Miao et al, 2013). Some plant extracts have been screened to discover their potential in inhibiting LDH, which can serve as an anticancer target (Deiab et al, 2014). For example,was discovered to be a potent LDH inhibitor,which decreases the viability of tumor cells.
Apoptosis pathway is an important target of plant metabolites
Apoptosis is an energy-dependent programmed cell death, which maintains the homeostatic balance between cell proliferation and cell death. Homeostasis is lost in cancer because the apoptotic mechanism is suppressed through various mechanisms (Hanahan and Weinberg, 2011). Many natural anticancer agents have been shown to induce the apoptotic pathway directly or activate apoptosis as a final step in the process. The characteristic features of an apoptotic cell are cell shrinkage, pyknosis and karyorrhexis (nuclear fragmentation). There is another passive process of cell death called necrosis that is energy independent. A thin line of difference exists between these two processes. Researchers on various occasions have found these two processes to be regulated by a common network of pathways (Nikoletopoulou et al, 2013). The determination of cell death by apoptosis or necrosis is dependent on the cell death signal, the developmental stage and the type of cell. Vascular disrupting agents that disrupt established tumor vasculature are found to promote the necrotic death of tumor (Kretzschmann and Furst, 2014).
Apoptosis is grouped into three steps: initiation, execution and engulfment phases. Each step is regulated by caspases. Caspases 2, 8, 9 and 10 perform the initiation phase, and caspases 3, 6 and 7 perform the execution phase (Green and Llambi, 2015). Once a single caspase is activated, it initiates a cascade of activation of other procaspases starting the apoptosis process. Various plant metabolites that directly activate either the initiator caspases or the execution caspases. Quercetin, a well-known flavonoid, induces apoptosis in cancer cells through the activation of caspases 3 and 7 (Khan et al, 2016). Luteolin, a flavonoidfound in broccoli, celery and carrots, induces apoptosis in various cancer cell lines through the activation of caspases3, 8, 9 and 10 (Horinaka et al, 2005). Phytic acids in rice and rice bran have been shown to enhance the expression of caspases 3 and 8 in colon and liver cancer cells (Shafie et al, 2013; Al-Fatlawi et al, 2014). Rice tocotrienols (gamma/delta) also activate caspase 3, leading to apoptosis in cancer cells (Yu et al, 2019).
Two pathways, extrinsic (death receptor pathway) and intrinsic (mitochondrial pathway), are responsible for initiating apoptosis (Fig. 2). Both pathways are dependent on each other for their regulation and activation (Pfeffer and Singh, 2018). The activation of a caspase cascade is a hallmark of apoptosis. The extrinsic pathway begins with the expression of various death receptors on the surface of the cells such as fas ligand (FASL), tumor necrosis factor (TNF) and tumor necrosis factor related apoptosis-inducing ligand (TRAIL). These receptors transmit signals from the surface to the intracellular signaling pathway. Binding of ligand to these receptors activates procaspase 8. Plant extracts have been reported to exert an anticancer effect by increasing the expression of death receptors on cancer cells. For example,extract, rich in phenolic terpenoids, flavonoids, tannins, hydroquinone, phenolic glycosides and triterpenoids, is found to induce apoptosis via upregulation of TNF-α and has been reported to induce apoptosis in ovarian cancer cells by increasingTRAIL (Yi et al, 2014). Luteolin, a flavonoid found in several plants,can enhance expression of death receptors and death receptor downstream factors such as FASL, TRAIL and fas-associated death domain (FADD) proteins in HeLa cells (Ham et al, 2014).
The intrinsic pathway is initiated via various mitochondrial initiated events. These events include an increase in mitochondrial membrane permeability, depletion of transmembrane potential and release of proapoptotic proteins like cytochrome C, second mitochondria-derived activator of caspases (SMAC) and serine protease, a high-temperature requirement protein A2 (HtrA2/Omi) (Pfeffer and Singh, 2018). These proapoptotic proteins activate procaspase 9 and apoptotic protease-activating factor-1 (APAF-1). Severalplant metabolites have been reported to initiate apoptosis in cancer cells by mitochondrial perturbations (Cincin et al, 2015; Green and Llambi, 2015; Zeng et al, 2018). For example, betulinic acid, a pentacyclic triterpenoid found in the bark of white-birched trees, activatesapoptotic cell death by decreasing mitochondrial potential and increasing permeability with no effect on normal proliferating cells. This characteristic makes betulinic acid a promising anticancer agent. Quercetin can also induce apoptosis in DLD-1 colon cancer cell line by decreasing the mitochondrial membrane potential.
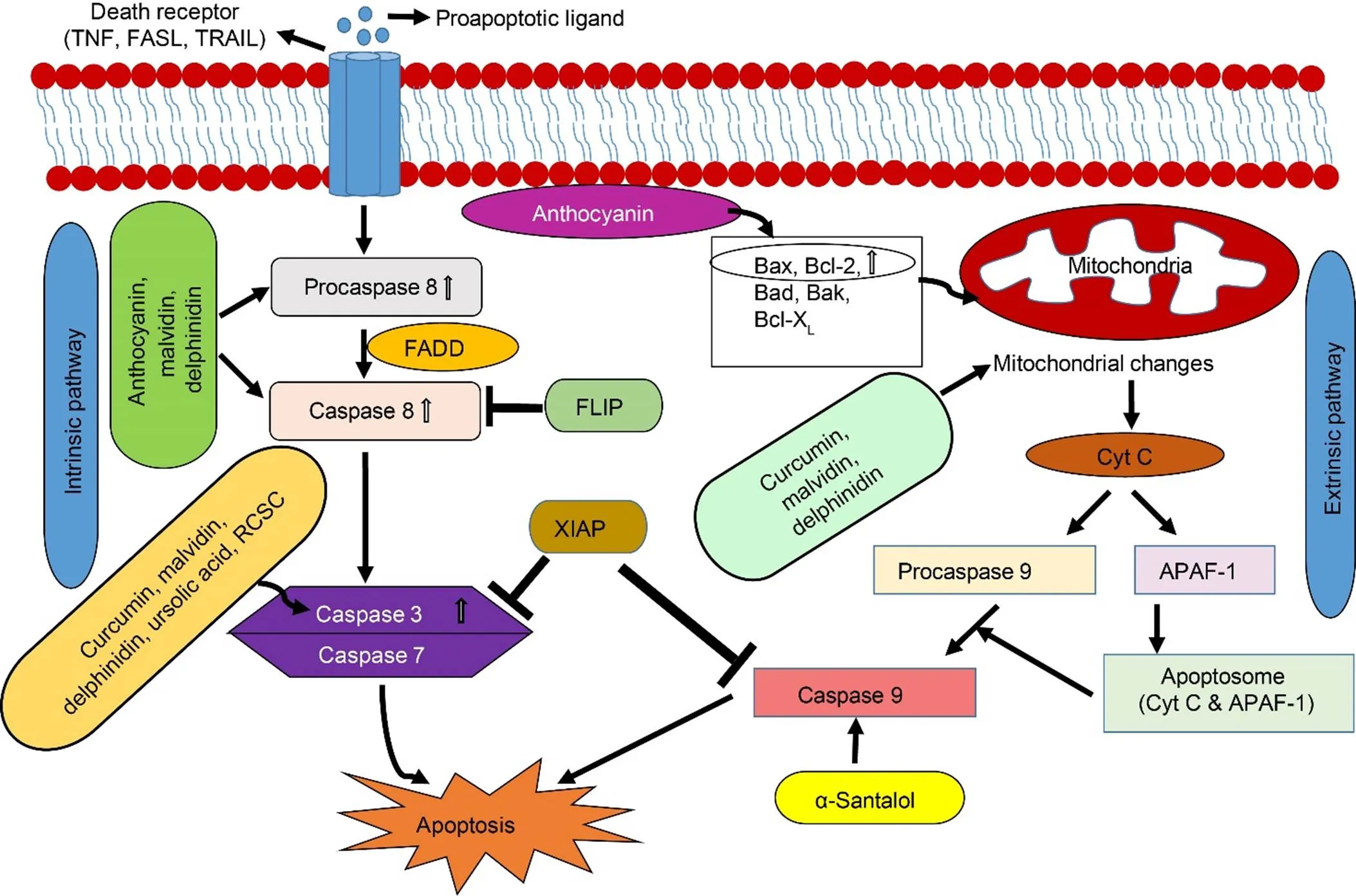
Fig. 2. Initiation phase of apoptosis and their regulation by plant metabolites.
Both pathways (extrinsic and intrinsic) result in the formation of an apoptotic body, which is a diagnostic biomarker of apoptosis. Curcumin, delphinidin and malvidin regulate both intrinsic and extrinsic pathways. Adapted from Kuppusamy et al (2013).APAF-1, Apoptotic protease activating factor-1; Cyt C, Cytochrome C; FADD, FAS-associated death domain; FASL, Fas ligand; FLIP, FADD-like Interleukin-1beta-converting enzyme inhibitory protein; TNF, Tumor necrosis factor; TRAIL, TNF-related apoptosis inducing ligand; XIAP, X-linked inhibitor of apoptosis.
Bcl-2 (B-cell lymphoma 2) family members are rich in pro- and anti-apoptotic proteins, which mainly regulate apoptotic processes associated with mitochondria. Anti-apoptotic proteins include Bcl2, Bcl-x, Bcl-XL (B-cell lymphoma-extra large) and AKT-1 that inhibit apoptosis by preventing caspase activation (Green and Llambi, 2015). Pro-apoptotic proteins include Bcl-10, Bax (BCL2-associated X protein), Bak (Bcl-2 homologous antagonist/killer), and Bid (BH3 interacting-domain death agonist). Phytic acid in rice bran enhances Bax protein and reduces Bcl-XL protein in colorectal and liver cancer cells (Shafie et al, 2013; Al-Fatlawi et al, 2014). Quercetin has been documented to induce apoptosis in cancer cells through the inhibition of activation of PI3K/Akt/mTOR (Rivera et al, 2016). It also increases the expressions of Bax, Bad and Bcl-10. Other pro-apoptotic proteins that initiate apoptosis in a caspase-independent manner include apoptosis-inducingfactor (AIF) and caspase activated DNase (CAD). Once released, these proteins are translocated to the nucleus causing DNA fragmentation, which can initiate the apoptotic pathway (Green and Llambi, 2015). Some bioactive compounds of plants, such as magnolol, a hydroxylated biphenyl,are reported to induce apoptosis in non-small cell lung cancer by DNA fragmentation and release of Bid, Bax and cytochrome C.
The execution pathway is the second phase of apoptosis after the initiation phase. It is activated by caspases 8, 9 and 10, and mediated by caspases 3, 6 and 7. Execution pathway is characterized by the degradationof the nuclear particle, nucleus and cytoskeletal proteins. Caspase 3 on activation inhibits the suppression of endonuclease CAD by ICAD. CAD on activation degrades DNA and condenses chromatin. Even though it is the second important step of apoptosis, no plant metabolites have been reported to activate the execution phase of apoptosis in cancer cells.
The final stage of apoptosis is the engulfment of the apoptotic cell by phagocytes. This is mediated by the attraction of phagocytes through the externalization of phosphatidylserine on the surface of apoptotic cells by a member of tumor necrosis factor receptor superfamily 6 ()[w5] , caspase8 and caspase 3 (Green and Llambi, 2015). Although plant metabolites (berberine, ursolic acid, malvidin, curcumin, etc.) have been shown to exert an anticancer effect by apoptosis on various cancer cell lines by activating the initiation phase, there is a need for more research to determine the effect of these compounds on the execution and engulfment phase of apoptosis. Extensive study to see the effects of these compounds on normal proliferating cells should be performed to verify whether the anticancer effect is efficient for proceeding to clinical trials.
Plant metabolites target angiogenic property associated with cancer
Pathologically initiated angiogenesis is a hallmark of cancer. As cancer progresses, cell proliferation exceeds cell death to a point where tumor cells further away from blood circulation suffering severe nutrition and oxygen insufficiency. Tumor cells can overcome the insufficiency by creating new blood vessels from existing ones by releasing various factors that directly or indirectly induce angiogenesis through proliferation and differentiation of endothelial cells. This phenomenon is called ‘a(chǎn)ngiogenic switch’ (Al-Abd et al, 2017). The angiogenic switch is followed by degradation of extra- cellular matrix and endothelial migrations. Various VEGF and angiopoietin (Ang) family members are involved in the process. Genes encoding VEGF are up-regulated due to hypoxia and signals from several oncogenes such as Ras and Myc. VEGF-A is considered to be the major regulator of angiogenesis which binds specific tyrosine kinase-like receptors that are upregulated in various cancers. Several plant metabolites like flavonoids, tannins, triterpenoids and sulphated carbohydrates inhibit angiogenesis in cancer cells (Wang et al, 2014). Polymethoxylated flavonoids in citrus fruits are shown to have anti-angiogenic activity in cancer cells (Cirmi et al, 2016). Betulinic acid has been reported to inhibit aminopeptidase N enzyme that is overexpressed in various cancers and is involved in the regulation of angiogenesis (Fulda, 2008). Plant extracts are efficient vascular disrupting agents of tumors. They can be subdivided into two classes: ligand directed agents, which include various antibodies and factors targeting endothelial receptors, and small molecule agents, which include various flavonoids and tubulin-binding agents (Kretzschmann and Furst, 2014).
Targeting microtubules for cancer prevention by plant metabolites
Microtubules (MT) are essential structures in the cytoskeleton of the cell, composed of αβ tubulins. They are involved in cell division and possess various motility and signaling functions. They help in segregation of chromosomes and separation into daughter cells during mitosis. Aberration in their assembly and function causes abnormal cell division, cell arrest and apoptosis. These integral functions make them an important target for inhibiting division of cancer cell. Agents targeting microtubules in cancer cells can be divided into two categories: MT-stabilizing and destabilizing agents (Bates and Eastman, 2017). Various microtubule-associated proteins (MAP) help in regulating the dynamics of microtubules which can serve as a potential target for inhibiting microtubules. Although synthetic and semi-synthetic derivatives of plant compounds are currently used to treat cancer, their anti-proliferative effects on normal cells along with cancer cells result in detrimental adverse side effects. These include commercially prescribed cancer drugs, paclitaxel, vincristine and vinblastine. It has been hypothesized that derivatization and purification of plant extract to a single compound make it more toxic because of no synergistic interactions of different compounds present in the plant. Plant extracts have been reported to be more efficient with less toxicity to normal cells (Iqbal et al, 2017). For instance,extract has been reported to bind tubulins of microtubules inhibiting its assembly and ultimately inducing apoptosis (Nam et al, 2002).
Plant natural compounds in clinical trials
Several plant natural compounds or their derivatives that do not belong to the classical anticancer compounds (vinblastine/vincristine, camptothecin, paclitaxel and podophyllotoxin) are in various stages of clinical trials (Pan et al, 2012). Hemoharringtonine (alkaloid ester) is approved for use in the treatment of chronic myeloid leukemia, especially in patients who are resistant to the treatment of tyrosine kinase inhibitors (Seca and Pinto, 2018). Several clinical trials are in progress as single and combined therapies with hemoharringtonine for different cancers. Ingenol mebutate isolated fromsp. has been shown to have cytotoxic as well as immune-modulatory effects due to loss of mitochondrial membrane potential (Ogbourne and Parsons, 2014). It has been approved as a drug for keratosis, a precancerous condition and is under phase I/II clinical trials for skin cancer treatment. Several clinical studies have been conducted with curcumin and are in progress in combination with other anticancer drugs due to its role as a chemosensitizer (Gupta et al, 2013; Seca and Pinto, 2018). Betulinic acid is a triterpene presents in many plants in low quantities. Betulin is present in large quantity in birch bark. It can be converted to betulinic acid which is an effective anticancer agent against a wide range of cancer types (Ali-Seyed et al, 2016). Betulinic acid and combretastatin are in phase I/II clinical trials as anticancer agents. Indigo (indole alkaloid) derivative is in a clinical trial for chronic myeloid leukemia. Lycopene and resveratrol have shown promising results in clinical trials for prostate cancer and colon cancer, respectively (Sahin et al, 2017; Alam et al, 2018).
Plant callus suspension culture as a tool for production of anticancer compounds
Isolation of natural products extracted directly from plants has limitations such as dependence on the availability of space, soil and environmental conditions as well as the effect of extraction and purification processes on the yield and stability of metabolites. Moreover, production and isolation of chemicals or natural compounds from rare or endangered medicinal plants using conventional techniques from plant parts are not possible. The initial overexploitation offor the production of paclitaxel has resulted in the declaration of the plant as near threatened (Howes, 2018). The production of secondary metabolites from plant cells viatechniques under aseptic conditions has the potential of providing an unlimited supply of targeted compounds (Veeresham and Chitti, 2013). Plant cell cultures are being used for the production of not only anticancer compounds but also biopharmaceuticals including therapeutic antibodies for other diseases (Efferth, 2019). Callus is an undifferentiated mass of totipotent cells. Callus extracts have been reported to give superior results compared to extracts from plant parts as seen in the case ofcallus extract which lowered blood sugar level of diabetic rabbits more efficiently than leaf extract (Arumugam et al, 2008). Furthermore, callus extract is shown to be as efficient as leaf extract of stone apple () and miracle fruit () in lowering blood sugar levels of diabetic rabbits (Arumugam et al, 2008; Ahmed et al, 2010). Comparison of callus cultures and plant extracts identi?ed higher antioxidant activity in callus cultures (Hakkim et al, 2007). A callus suspension culture is made by growing callus in liquid plant growth medium for three weeks. During this period, they start releasing their secondary metabolites. The secondary metabolites could also be part of the cells. Callus suspension cultures unlike plant parts offer the advantage of providing an unlimited supply of compounds. Due to rapid growth cycles of cell suspension cultures, they are generally used for the large-scale production of bioactive secondary metabolites such as paclitaxel, vinblastine and vincristine (Vanisree et al, 2004; Georgiev and Maciuk, 2009). Plant cell cultures have been employed for the production of anticancer compounds, resveratrol fromcallus culture (Nandagopal et al, 2017). Aralin, a cytotoxic lectin produced fromcallus culture shows 2-fold higher activity than the compound isolated from plant part due to glycosylation (Tomatsu et al, 2004). The production of an antiproliferative furanoheliangolide was reported incallus culture (dos Santos et al, 2004). Rosmarinic acid and salvianolic acid B produced from callus culture of the Chinese medicinal plant,, show cytotoxic activity against acute lymphoblastic leukemia (Wu et al, 2016). Rice cell suspension cultures have been used to produce human α1-antitrypsin, serum albumin, lysozyme, interleukin-12 (IL-12) and a cytokine, granulocyte-macrophage colony stimulating factor (Xu et al, 2011; Santos et al, 2016). Rice cell cultures give higher expression of human IL-12 and lower proteolytic activity compared to other plant cells (Shin et al, 2010). The high levels of expression in the rice cell culture system are achieved in most cases by the use of an inducible rice α-amylase 3D (RAmy3D) promoter. The production of specific compounds in callus cultures can be enhanced by adding elicitors. This is based on the principle that plants increase the production of specific secondary metabolites when subjected to abiotic (drought, salt, heat, cold and heavy metal, etc.) or biotic (bacteria, fungi, virus, and nematodes, etc.) stress which act as external elicitors for inducing gene expression directly or indirectly. A number of abiotic and biotic elicitors enhance the production of vinca alkaloids incell cultures (Siddiqui et al, 2013).Paclitaxel production increases 2-fold using an endophytic fungus () ofas an elicitor in callus culture of(Wang et al, 2001). In another study, methyl jasmonateis found to enhance paclitaxel levels incallus suspension culture (Tabata, 2004). The addition of methyl jasmonate doubles the production of shikonin derivatives with antitumor activity in callus culture of(Hao et al, 2014). The production of camptothecin incell culture increases 11-fold by UV-B light, and 10-hydroxycamptothecin increases 25-fold by salicylic acid (Pi et al, 2010).
Plant callus suspension culture as a promising anticancer agent
There are only a few studies where plant callus suspension culture or their extracts were evaluated for their cytotoxic activity. The methanol extract of callus suspension culture of green tea () inhibits growth (64%) of the human cervix carcinoma cell line, HEP-2 (Choi et al, 2006). Two flavones, echioidinin and 7--methywogonin, isolated from the callus culture of a medicinal plant, show cytotoxicity on a leukemic cell line (Mohammed et al, 2015). However, this study did not use the callus culture for cytotoxicity studies. In another study, the methanol extract ofcallus treated with ZnO nanoparticles show an increase in flavonoid content and cytotoxic activity on the breast cancer cell line MCF-7 (Karimi et al, 2018). Cytotoxic activity of rice callus suspension culture (RCSC) was reported for the first time in renal and colon cancer cell lines where up to 95% decrease in cell viability was observed with no significant effect on normal lung fibroblast cell line at specific dilutions of RCSC (Fig. 3; Deshpande et al, 2012). Cytotoxic activity of RCSC was found to be as effective as Taxol but without the side effects of killing normal cells. Further extension of this study showed similar effects on lung and breast cancer cell lines where lung cancer cell lines show better response compared to the other cancer cell lines (Rahman et al, 2016). The extent of inhibition in cell proliferation by RCSC in metastatic colon cancer cell line is much greater than non-metastatic colon cancer cell line. A differential response was also observed in breast cancer cell lines where the hormone-dependent cell line shows a better response to RCSC than the hormone-independent cell line. It is likely that specific RCSC compounds may directly or indirectly inhibit the binding of estrogen to receptors on breast cancer cells.
Molecular and cellular mechanisms involved in cytotoxic activity of RCSC
Previous studies showed high levels of LDH in cancer cells, which can be attributed to the Warburg effect where high rates of glycolysis drive pyruvate to lactic acid fermentation. Treatment with RCSC reduces the total cellular LDH levels about 90% in colon and lung cancer cell lines, which is a positive attribute for its anticancer activity (Rahman et al, 2016). RCSC- treated lung cancer cell lines show evidence of apoptotic changes based on analysis of caspases 3 and 7, which are involved in the execution phase of apoptosis. RCSC affects membrane integrity of cancer cells but not normal cells. Real-time PCR analysis identi?ed the upregulation of genes involved in apoptosis and cell cycle inhibition and down-regulation of cancer-promoting genes. A gene encoding FASLG protein, which is a member of the tumor necrosis factor superfamily with a role in apoptosis, is up- regulated. A surprising finding is the up-regulation of a proto-oncogene, c-Jun, which is related to the PI3K-AKT pathway. Some studies have shown the role of c-Jun in apoptosis via alternate signaling pathways involving c-Jun N-terminal protein kinase (JNK) as demonstrated in the cytotoxic activity of tylophorine, an alkaloid from the medicinal plant,(Andrianantoandro, 2012; Yang et al, 2013). The cytotoxic activity of grape seed extract is attributed to apoptosis mediated by JNK (Tyagi et al, 2003). The levels of most integrins increase in cancer cells due to their roles in tyrosine kinase signaling which regulates proliferation and adhesion of cells. However, two integrins, α2β (ITGA2B) and β3 (ITGB3), which are expressed at lower levels in cancer cells, are up-regulated on RCSC treatment. Up-regulation of ITGB3 is shown to suppress the malignant tumors (Kaur et al, 2009). The molecular mechanism proposed for the cytotoxic activity of RCSC is shown in Fig. 4. Scanning electron microscopy analysis shows flatter morphology of the cancer cells treated with RCSC, indicating that they are not at mitosis (Fig. 4; Rahman et al, 2016). Furthermore, sizes of cells are more homogeneous than control, suggesting that these cells are controlled by cell cycle checkpoints. Cells are better attached to each other, suggesting the possible alterations of cell junctions.
Double role of RCSC as a cytotoxic and anti-inflammatory agent
The potential of RCSC to act as an anti-inflammatory agent was shown in three human normal colon cell lines by the treatment with proinflammatory cytokines followed by RCSC (Driscoll et al, 2019). RCSC, its concentrates and its bioactive fractions not only show anti-inflammatory activity in aninflammatory bowel disease model but also display cytotoxic activity on a colon cancer cell line. This is likely due to the regulation of crosstalk and interdependence of cancer and inflammation/ROS mediated by RCSC. The transcription factors NF-κB and signal transducer and activator of transcription 3 (STAT3) are common regulators of cancer and inflammation (Elinav et al, 2013). In addition to NF-κB, C-C motif ligand 5 (CCL5) and C-X-C motif chemokines (CXCL) which are common players between cancer and inflammation are differentially regulated by RCSC based on gene expression studies. The cross talk between cancer and inflammation and the concept that chronic inflammationleads to cancer has resulted in the process of development of cytokine-based therapies including the use of monoclonal antibodies targeted against TNF-α, VEGF and interleukin-6 (IL-6), and treatment with IL-2 (Qu et al, 2018). The above approaches have shown hope significant reduction in inflammation as well as regression of tumors. The cross talk and the underlying molecular mechanisms employed by RCSC can be studied using a 3D cell culture system or organoids developed from cancer patient tissues which serve asmodels (Baker, 2018).

Fig. 3. Effects of rice callus suspension culture (RCSC) and Taxol?on normal and cancer cells.
RCSC reduces the viability of cancer cells with minimal or no effect on the normal cells compared to Taxol?based on Deshpande et al (2012) and Rahman et al (2016).
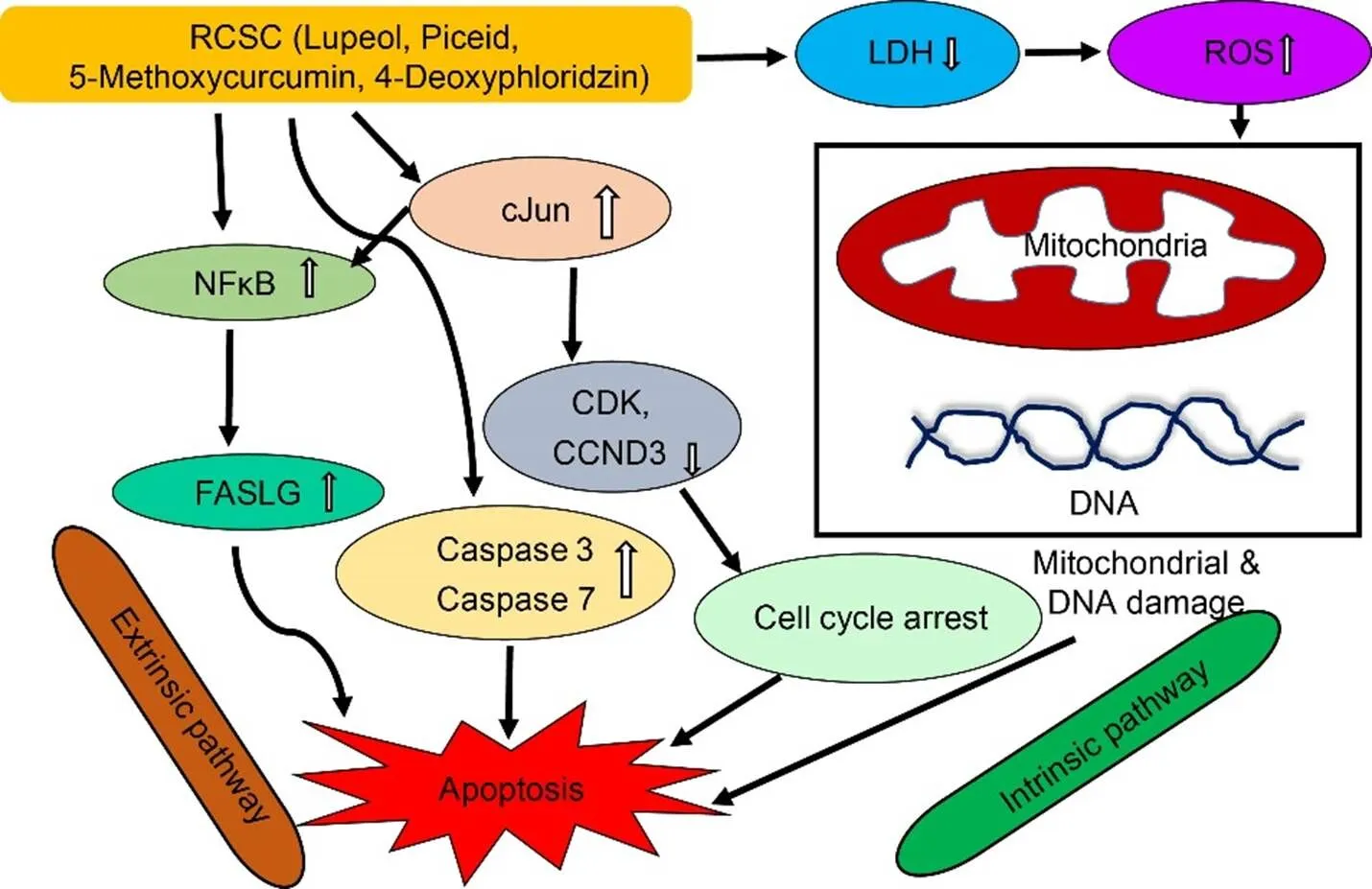
Fig. 4. Molecular mechanisms regulating cytotoxic activity of rice callus suspension culture (RCSC).
RCSC upregulates the transcription factor, NF-κB, which induces the production of the tumor necrosis factor, FASLG, leading to apoptosis via extrinsic pathway. Up-regulation of c-Jun leads to downregulation of cyclins and cyclin-dependent kinases,resulting in apoptosis via intrinsic pathway. c-Jun has also been shown to upregulate NF-κB. RCSC increases ROS levels in cancer cells, leading to DNA damage and loss of membrane integrity. CCND3, Cyclin D3; CDK, Cyclin-dependent kinase; c-Jun, Cellular protooncogene; FASLG, Tumor necrosis factor ligand superfamily member 6; NF-κB, Nuclear factor kappa light chain enhancer of activated B cells; LDH, Lactate dehydrogenase; ROS, Reactive oxygen species.
Unexpected bioactive compounds in RCSC
RCSC isolated fractions employing column chromatography and HPLC are shown to harbor anti-inflammatory and cytotoxic activity (Driscoll et al, 2019). The bioactive fractions of RCSC comprise 4-deoxyphloridzin, 5′-methoxycurcumin, piceid and lupeol (Fig. 3). 4-deoxyphloridzin is a derivative of phloridzin which is abundant in the apple peel. Phloridzin is shown to have anti-aging effects in several organisms through upregulation of antioxidative enzymes and acting against glycation (Xiang et al, 2011). Phloridzin inhibits sodium-glucose transporter 1 (SGLT1) and glucose facilitative transporter 2 (GLUT2) in Caco cells (Manzano and Williamson, 2010). 4-deoxyphloridzin in RCSC might inhibit glucose transporters thereby limiting cancer cells from utilizing glucose as an energy source. 5′-methoxycurcumin is a natural derivative of curcumin, which is a well-known anticancer, antioxidant and anti-inflammatory agent. Piceid is a glycoside form of resveratrol from which resveratrol is synthesized naturally. It is abundant in grapes and red wine among others. Piceid shows tumor cell cytotoxicity as well as higher radical scavenging activity compared to resveratrol (Su et al, 2013). The presence of piceid in RCSC is surprising as rice does not have piceid or resveratrol but transgenic plants which produce piceid or resveratrol have been reported (Baek et al, 2013). Lupeol is a triterpenoid found naturally in some fruits and vegetables. Lupeol is an anticancer and anti-inflammatory agent exerting its effect through NF-κB and PI3K/Akt signaling (Saleem, 2009). Lupeol has been reported in HPLC sub-fraction of black rice bran extract which shows anti-leukemic activity (Suttiarporn et al, 2015). The enzyme, curcuminoid synthase (CUS), a type III polyketide synthase, was identified from rice, which can synthesize bisdemethoxycurcuminfrom coumaroyl-CoA and malonyl-CoA (Katsuyama et al, 2010). The four compounds reported above in RCSC are produced during the three weeks of incubation involved in its production and are not present in rice tissues or callus in significant quantities.
Popular anticancer compounds, often water-insoluble, have low bioavailability and short half-life, which limits their application for cancer treatment (Fridlender et al, 2015). For instance, paclitaxel and curcumin are water insoluble. Some natural compounds have limited absorption in the intestines and liver. Semisynthetic soluble analogs have been developed to overcome the above limitations. Further, nanoparticles and liposomes have been developed as drug carriers for plant natural products (Karimi et al, 2018; Shishir et al, 2019). Liposome-mediated treatment with curcumin enhances cytotoxicity in a skin cancer cell line, breast cancer cell lines, and human glioma cells transplanted in mice (Karewicz et al, 2013; Kangarlou et al, 2017; Zhao et al, 2018). Nanoparticles have been used to enhance the bioavailability of curcumin, epigallocatechin gallate (EGCG)and quercetin (Watkins et al, 2015). For instance, poly(lactic-co-glycolic acid) nanoparticles enhance bioavailability of curcumin to 5.6-fold and lipid nanoparticles to 9.4-fold compared to curcumin alone (Xie et al, 2011; Ji et al, 2016). Similar issues of bioavailability and short half-life as observed with curcumin, EGCG and resveratrol may be encountered with compounds isolated from plant callus suspension culture.
The best way forward for the use of plant callus suspension culture and RCSC for anticancer therapy depends on several factors. If the pharamacological activity of purified bioactive compounds is lower than RCSC, semisynthetic analogs of compounds or a combination of two or more compounds have to be evaluated along with different drug delivery options. If none of the above approaches work, the holistic approach of employing RCSC as an anticancer agent needs to be evaluated, which is similar to the principle of Ayurveda, where a mixture of compounds is used for the treatment of different ailments. An essential criterion to consider in all of the above approaches is the presence of cytotoxic activity on cancer cells with no effect on normal cells.
Large scale production of specific compounds in callus suspension cultures for development of drugs for cancer and other diseases: Drawbacks and challenges
Large scale production of compounds using plant callus suspension cultures can be achieved by cell line selection, optimization of culture conditions and use of elicitors among others (Ochoa-Villarreal et al, 2016). Anticancer compounds which are manufactured by companies using plant suspension cultures include paclitaxel by Phyton Biotech, Germany, berberines by Mitsui chemicals, and podophyllotoxin by Nippon Oil, Japan. The design and operation of bioreactors for commercial production in plant suspension culture have to consider the cell size, number and shearing (Wilson and Roberts, 2012). It is also important to consider the location of the compound(s), cell wall, vacuole or extracellular so that a suitable strategy can be designed for its isolation. Similar approaches can be used for the production of specific compounds from RCSC and medicinal plant callus suspension cultures using different elicitors such as chitosan, ethylene, jasmonate derivatives, sodium chloride and vanadyl sulfate. If diseases other than cancer respond to RCSC or medicinal plant callus suspension cultures, a similar approach described above and bioreactors can be used for large scale production of bioactive phytochemicals and callus cultures. Another alternate method is the upregulation of gene(s) regulating specificmetabolites in callus culture through transgenic approaches.
Mammalian and microbial cells are being used for the production of biopharmaceuticals at least 20 years more than plant cell cultures (Santos et al, 2016). This gave them a distinct advantage with reference to optimization of strains, culture/fermentation conditions and design of bioreactors, which results in higher yield of natural compounds compared to plant cell cultures. Downstream processing includes releasing cellular components by breaking cell membranes which may release biomacromolecules (such as proteases) that destroy biologically active molecules is a drawback in using plant cell cultures (Buyel et al, 2015). Upstream processing includes the design of bioreactors based on the volume of the culture and the fermentation process to be used. Continuous fermentation process of perfusion type is used for plant cell cultures as it is superior to batch and fed-batch due to its dynamic nature where fresh media is added with simultaneous removal of cell free broth. Chemostat method of continuous fermentation process where broth with cells is removed accompanied with the addition of fresh media is also suitable for plant cell cultures. A major challenge in large scale production of secondary metabolites is to prevent microbial contamination in the bioreactor, which can be accomplished by maintaining sterile conditions and the use of antibiotics, if needed. The production of other compounds may inhibit the production of the targeted compound and its isolation. The required metabolite may be produced in low quantities compared to plant parts or it may be produced in large quantities without any biological activity. Further, secondary metabolite production is reduced with the age of the culture. Cells tend to aggregate in plant cell culture which require constant stirring often at high speed and may result in cell damage. There are regulatory issues that need to be addressed. These include quality control of the products, animal testing for toxicity analysis and as a drug followed by clinical trials. Commercial plant cell culture platforms for the production of drugs are not widely established due to the above concerns, which may be alleviated in the future.
Perspectives
Plant compounds show promising prospects in developing effective drugs in treating cancer. It is evident that these components function through different mechanisms such as altering microtubules, inducing apoptotic signals and inhibiting metabolic enzymes. These experimental observations can be used to characterize the inhibitory effects of plant bioactive compounds on cancer cells. It is essential to distinguish the fine line between the normal proliferating cells and cancer cells so that a target can be identified without damaging normal cell physiology. Plant callus suspension cultures have the potential to be new frontiers for the production of compounds with anticancer activity. Success stories exist for the large sacle production of paclitaxel, berberines and podophyllotoxin using plant cell cultures in bioreactors. Plant callus suspension culture as exemplified by RCSC showed a highly desirable trait of selective killing of cancer cells. Although our studies with RCSC on cancer cell lines are promising, further studies are needed to understand genes, proteins, metabolites and interactions between macromolecules responsible for the biological activity of RCSC. These studies have to be expanded to animal model followed by clinical trials. The next step would be to evaluate callus suspension cultures of various medicinal plants for their anticancer activity. If callus suspension cultures and its constituents show the ability to arrest the proliferation of cancer cells belonging to multiple cancer types with no effect on the normal cells, it would be a boon for cancer treatment in the future.
Adebayo S A, Dzoyem J P, Shai L J, Eloff J N. 2015. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African., 15: 159.
Agah S. 2016. Enhanced action of sorghum and cowpea flavonoid mixtures against inflammation. [PhD Thesis].Texas, USA: Texas A & M University.
Ahmed A B A, Rao A S, Rao M V. 2010.callus andleaf extract ofstimulate beta-cells regeneration and anti-diabetic activity in Wistar rats., 17(13): 1033–1039.
Alabsi A M, Ali R, Ali A M, Al-Dubai S A, Harun H, Abu Kasim N H, Alsalahi A. 2012. Apoptosis induction, cell cycle arrest andanticancer activity of gonothalamin in a cancer cell lines., 13(10): 5131–5136.
Alam M N, Almoyad M, Huq F. 2018. Polyphenols in colorectal cancer: Current state of knowledge including clinical trials and molecular mechanism of action., 2018:[w6] .
Al-Abd A M, Alamoudi A J, Abdel-Naim A B, Neamatallah T A, Ashour O M. 2017. Anti-angiogenic agents for the treatment of solid tumors: Potential pathways, therapy and current strategies: A review., 8(6): 591–605.
Al-Fatlawi A A, Al-Fatlawi A A, Irshad M, Zafaryab M, Rizvi M M A, Ahmad A. 2014. Rice bran phytic acid induced apoptosis through regulation ofandgenes in HepG2 human hepatocellular carcinoma cells., 15(8): 3731–3736.
Ali-Seyed M, Jantan I, Vijayaraghavan K, Bukhari S N. 2016. Betulinic acid: Recent advances in chemical modi?cations, effective delivery, and molecular mechanisms of a promising anticancer therapy., 87(4): 517–536.
American Cancer Society. 2019. Cancer Facts & Figures. Atlanta: American Cancer Society.
Andrianantoandro E. 2012. Promoting the apoptotic activity of c-Jun., 5: ec288.
Arumugam S, Kavimani S, Kadalmani B, Ahmed A B A, Akbarsha M A, Rao M V. 2008. Antidiabetic activity of leaf and callus extracts ofin rabbit.,34(3): 317–321.
Baek S H, Shin W C, Ryu H S, Lee D W, Moon E, Seo C S, Hwang E, Lee H S, Ahn M H, Jeon Y, Kang H J, Lee S W, Kim S Y, D’Souza R, Kim H J, Hong S T, Jeon J S. 2013. Creation of resveratrol-enriched rice for the treatment of metabolic syndrome and related diseases., 8(3): e57930.
Baker K. 2018. Organoids provide an important window on inflammation in cancer., 10(5): 151.
Bates D, Eastman A. 2017. Microtubule destabilising agents: Far more than just antimitotic anticancer drugs.,83(2): 255–268.
Berman A Y, Motechin R A, Wiesenfeld M Y, Holz M K. 2017. The therapeutic potential of resveratrol: A review of clinical trials., 1: 35.
Biswal B N, Das S N, Das B K, Rath R. 2017. Alteration of cellular metabolism in cancer cells and its therapeutic prospects., 21(2): 244–251.
Broecker-Preuss M, Becher-Boveleth N, Bockisch A, Dührsen U, Müller S. 2017. Regulation of glucose uptake in lymphoma cell lines by c-MYC- and PI3K-dependent signaling pathways and impact of glycolytic pathways on cell viability., 15: 158.
Buyel J F, Twyman R M, Fischer R. 2015. Extraction and downstream processing of plant-derived recombinant proteins., 33(6): 902–913.
Cargnello M, Tcherkezian J, Roux P P. 2015. The expanding role of mTOR in cancer cell growth and proliferation., 30(2): 169–176.
Chen C C, Sureshbabul M, Chen H W, Lin Y S, Lee J Y, Hong Q S, Yang Y C, Yu S L. 2013. Curcumin suppresses metastasis via Sp-1, FAK inhibition, and E-Cadherin upregulation in colorectal cancer., 2013: e541685.
Choi B Y, Kim B W. 2015. Withaferin: A inhibits colon cancer cell growth by blocking STAT3 transcriptional activity., 20(3): 185–192.
Choi JH, Yoon S K, Lee K H, Seo M S, Kim D H, Hong S B, Kim JY, Paik HD, Kim C H. 2006. Antitumor activity of cell suspensionculture of green tea seed (L.).,11: 396.
Chutipaijit S, Sutjaritvorakul T. 2018. Comparative study of total phenolic compounds, flavonoids and antioxidant capacities in pigmented and non-pigmented rice ofrice varieties., 12: 781–788.
Cincin Z B, Unlu M, Kiran B, Bireller E S, Baran Y, Cakmakoglu B. 2015. Apoptosis effects of quercetin on DLD-1 colon cancer cell.,21(2): 333–338.
Cirmi S, Ferlazzo N, Lombardo G E, Maugeri A, Calapai G, Gangemi S, Navarra M. 2016. Chemopreventive agents and inhibitors of cancer hallmarks: May citrus offer new perspectives?, 8(11): 698.
Cragg G M, Pezzuto J M. 2016. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents., 25: 41–59.
De Angel R E, Smith S M, Glickman R D, Perkins S N, Hursting S D. 2010. Antitumor effects of ursolic acid in a mouse model of postmenopausal breast cancer., 62(8): 1074–1086.
Deiab S, Mazzio E, Zarmouh N, Mack N, Soliman K. 2014. High throughput screening of natural products for LDH inhibition as an anti-mitotic therapeutic target., 28: 585–593.
Deshpande A, Dhadi S R, Hager E J, Ramakrishna W. 2012. Anticancer activity of rice callus suspension culture., 26(7): 1075–1081.
dos Santos P A, Amarante M F C, Pereira A M S, Bertoni B W, Fran?a S C, Pessoa C, de Moraes M O, Costa-Lotufo L V, Pereira M R P, Lopes N P. 2004. Production of an furanoheliangolide bycell culture.(), 52(12): 1433–1435.
Driscoll K, Deshpande A, Chapp A D, Li K F, Datta R, Ramakrishna W. 2019. Anti-inflammatory and immune-modulating effects of rice callus suspension culture (RCSC) and bioactive fractions in aninflammatory bowel disease model.,57: 364–376.
Eales K L, Hollinshead K E R, Tennant D A. 2016. Hypoxia and metabolic adaptation of cancer cells., 5(1): e190.
Ebrahim H Y, Elsayed H E, Mohyeldin M M, Akl M R, Bhattacharjee J, Egbert S, El Sayed KA. 2016. Norstictic acid inhibits breast cancer cell proliferation, migration, invasion, andinvasive growth through targeting C-Met., 30(4): 557–566.
Efferth T. 2019. Biotechnology applications of plant callus cultures., 5(1): 50–59.
Elinav E, Nowarski R, Thaiss C A, Hu B, Jin C C, Flavell R A. 2013. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms.,13(11): 759–771.
Engeland K. 2018. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM., 25(1): 114–132.
Fridlender M, Kapulnik Y, Koltai H. 2015. Plant derived substances with anti-cancer activity: From folklore to practice., 6: 799.
Fulda S. 2008. Betulinic acid for cancer treatment and prevention., 9(6): 1096–1107.
Fulda S. 2009. Tumor resistance to apoptosis., 124(3): 511–515.
Georgiev MI, Weber J, Maciuk A. 2009. Bioprocessing of plant cell cultures for mass production of targeted compounds., 83(5): 809–823.
Green D R, Llambi F. 2015. Cell death signaling., 7: a006080.
Grothaus P G, Cragg G M, Newman D J. 2010. Plant natural productsin anticancer drug discovery., 14(16): 1781–1791.
Gupta S C, Patchva S, Aggarwal B B. 2013. Therapeutic roles of curcumin: Lessons learned from clinical trials., 15: 195–218.
Hakkim F L, Shankar C G, Girija S. 2007. Chemical composition and antioxidant property of holy basil (L.) leaves, stems and in?orescence and theircallus cultures., 55: 9109–9117.
Ham S, Kim K H, Kwon T H, Bak Y, Lee D H, Song Y S, Park S H, Park Y S, Kim M S, Kang J W, Hong J T, Yoon D Y.2014. Luteolin induces intrinsic apoptosis via inhibition of E6/E7 oncogenes and activation of extrinsic and intrinsic signaling pathways in HPV-18-associated cells., 31(6): 2683–2691.
Hamzehzadeh L, Atkin S L, Majeed M, Butler A E, Sahebkar A. 2018. The versatile role of curcumin in cancer prevention and treatment: A focus on PI3K/AKT pathway., 233(10): 6530–6537.
Hanahan D, Weinberg R A. 2011. Hallmarks of cancer: The next generation., 144(5): 646–674.
Harmon A W, Patel Y M. 2004. Naringenin inhibits glucose uptake in MCF-7 breast cancer cells: A mechanism for impaired cellular proliferation., 85(2): 103–110.
Hao H, Lei C Y, Dong Q L, Shen Y L, Chi J T, Ye H C, Wang H. 2014. Effects of exogenous methyl jasmonate on the biosynthesis of shikonin derivatives in callus tissues of.,173(8): 2198–2210.
He G F, Zhang YW, Lee JH, Zeng S H, Wang Y V, Luo Z J, Dong X C, Viollet B, Wahl G M, Lu H. 2014. AMP-activated protein kinase induces p53 by phosphorylating MDMX and inhibiting its activity., 34(2): 148–157.
Honndorf V S, Wiehr S, Rolle A M, Schmitt J, Kreft L, Quintanilla-Martinez L, Kohlhofer U, Reischl G, Maurer A, Boldt K, Schwarz M, Schmidt H, Pichler B J. 2016. Preclinical evaluation of the anti-tumor effects of the natural isoflavone genistein in two xenograft mouse models monitored by [18F]FDG, [18F]FLT, and [64Cu]NODAGA-cetuximab small animal PET., 7(19): 28247–28261.
Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Nakanishi R, Nishino H, Matsui H, Sakai T. 2005. Luteolin induces apoptosis via death receptor 5 upregulation in human malignant tumor cells., 24: 7180–7189.
Howes M J R. 2018. The evolution of anticancer drug discovery from plants., 19(3): 293–294.
Huang C, Lu C K, Tu M C, Chang J H, Chen Y J, Tu Y H, Huang H C. 2016. Polyphenol-richleaf extracts induce apoptosis in human breast and liver cancer cells and in a nude mouse xenograft model., 7: 35874–35893.
Iqbal J, Abbasi BA, Mahmood T, Kanwal S, Ali B, Shah S A, Khalil A T. 2017. Plant-derived anticancer agents: A green anticancer approach., 7(12): 1129–1150.
Ji H Y, Tang J L, Li M T, Ren J M, Zheng N N, Wu L H.2016. Curcumin-loaded solid lipid nanoparticles with Brij78 and TPGS improvedoral bioavailability andintestinal absorption of curcumin., 23(2): 459–470.
Jiang C W, Masood M, Rasul A, Wei W, Wang Y, Ali M, Mustaqeem M, Li J, Li X M. 2017. Altholactone inhibits NF-κB and STAT3 activation and induces reactive oxygen species-mediated apoptosis in prostate cancer DU145 cells., 22(2): 240.
Kamalanathan D, Natarajan D. 2018. Anticancer potential of leaf and leaf-derived callus extracts ofagainst MCF-7 breast cancer cell line., 14(2): 321–327.
Kangarlou S, Ramezanpour S, Balalaie S, Roudbar Mohammadi S, Haririan I. 2017. Curcumin-loaded nanoliposomes linked to homing peptides for integrin targeting and neuropilin-1mediated internalization., 55: 277–285.
Karewicz A, Bielska D, Loboda A, Gzyl-Malcher B, Bednar J, Jozkowicz A, Dulak J, Nowakowska M. 2013. Curcumincontaining liposomes stabilized by thin layers of chitosan derivatives., 109: 307–316.
Karimi N, Behbahani M, Dini G, Razmjou S. 2018. Enhancing the secondary metabolite and anticancer activity ofcallus extracts by treatment with biosynthesized ZnO nanoparticles., 9: 045009.
Kastenhuber E R, Lowe S W. 2017. Putting p53 in context., 170(6): 1062–1078.
Katsuyama Y, Miyazono K I, Tanokura M, Ohnishi Y, Horinouchi S. 2010. Structural and biochemical elucidation of mechanism for decarboxylative condensation of beta-keto acid by curcumin synthase.,286(8): 6659–6668.
Kaur S, Kenny H A, Jagadeeswaran S, Zillhardt M R, Montag A G, Kistner E, Yamada S D, Mitra A K, Lengyel E. 2009. β3-integrin expression on tumor cells inhibits tumor progression, reduces metastasis, and is associated with a favorable prognosis in patients with ovarian cancer., 175: 2184–2196.
Ketron A C, Osheroff N. 2014. Phytochemicals as anticancer and chemopreventive topoisomerase II poisons., 13(1): 19–35.
Khan F, Niaz K, Maqbool F, Hassan F I, Abdollahi M, Nagulapalli Venkata KC, Nabavi SM, Bishayee A.2016. Molecular targets underlying the anticancer effects of quercetin: An update., 8(9): E529.
Kim S, Chen J, Cheng T J, Gindulyte A, He J, He S Q, Li Q L, Shoemaker B A, Thiessen P A, Yu B, Zaslavsky L, Zhang J, Bolton E E. 2019. PubChem 2019 update: Improved access to chemical data., 47: 1102–1109.
Kim S H, Ryu H G, Lee J, Shin J, Harikishore A, Jung HY, Kim YS, Lyu HN, Oh E, Baek NI, Choi KY, Yoon HS, Kim KT. 2015. Ursolic acid exerts anti-cancer activity by suppressing vaccinia-related kinase 1-mediated damage repair in lung cancer cells., 5: 14570.
Kojima-Yuasa A, Huang X D, Matsui-Yuasa I. 2015. Synergistic anticancer activities of natural substances in human hepatocellular carcinoma., 3(4): 260–281.
Kretzschmann V K, Furst T. 2014. Plant-derived vascular disrupting agents: Compounds, actions, and clinical trials., 13(1): 191–206.
Kundu J, Chun K S, Chae I G, Kundu J K. 2014. Phloretin: An apple polyphenol with cancer chemo preventive potential., 2: 17–23.
Kuppusamy P, Yusoff M M, Maniam G P, Govindan N. 2013. A case study: Regulation and functional mechanisms of cancer cells and control its activity using plants and their derivatives., 6(8): 884–892.
Lee E Y H P, Muller W J. 2010. Oncogenes and tumor suppressor genes., 2(10): a003236.
Lee S H, Jee J G, Bae J S, Liu K H, Lee Y M. 2014. A group of novel HIF-1α inhibitors, glyceollins, blocks HIF-1α synthesis and decreases its stability via inhibition of the PI3K/AKT/mTOR pathway and Hsp90 binding., 230(4): 853–862.
Lemonnier N, Zhou GB, Prasher B, Mukerji M, Chen Z, Brahmachari S K, Noble D, Boutron B, Sagner M, Auffray C.[w7] . Traditional knowledge-based medicine: A review of history, principles, and relevance in the present context of P4 systems medicine, 2: pe0011.
Li F S, Weng J K. 2017. Demystifying traditional herbal medicine with modern approach., 3: 17109.
Lichota A, Gwozdzinski K. 2018. Anticancer activity of natural compounds from plant and marine environment., 19(11): 3533.
Liu J, Zheng L H, Wu N, Ma L N, Zhong J T, Liu G, Lin X K. 2014. Oleanolic acid induces metabolic adaptation in cancer cells by activating the AMP-activated protein kinase pathway., 62: 5528–5537.
Liu Y, Yang Z Y, Gong C, Zhang L Y, Yu GL, Gong W.2014. Quercetin enhances apoptotic effect of tumor necrosis factorrelated apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer binding protein homologous protein (CHOP)-death receptor 5 pathway., 105(5): 520–527.
Manzano S G, Williamson G. 2010.Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells., 54(12): 1773–1780.
Miao P, Sheng S L, Sun X G, Liu J J, Huang G. 2013. Lactate dehydrogenase A in cancer: A promising target for diagnosis and therapy., 65(11): 904–910.
Mohammed A, Chiruvella K K, Rao Y K, Geethangili M, Raghavan S C, Ghanta RG. 2015.production of echioidinin, 7--methywogonin from callus cultures ofand their cytotoxicity on cancer cells., 10(10): e0141154.
Mouhid L, Corzo-Martínez M, Torres C, Vázquez L, Reglero G, Fornari T, Ramírez de Molina A. 2017. Improvingefficacy of bioactive molecules: An overview of potentially antitumor phytochemicals and currently available lipid-based delivery systems., 2017: 7351976.
Nam S Y, Yi H K, Lee J C, Kim J C, Song C H, Park J W, Lee D Y, Kim J S, Hwang P H. 2002.extract induces cancer cell apoptosis through inhibition of microtubule assembly., 25(2): 191–196.
Nandagopal K, Halder M, Dash B, Nayak S, Jha S. 2017. Biotechnological approaches for production of anti-cancerous compounds resveratrol, podophyllotoxin and zerumbone., 25: 4693–4717.
Nguyen T T, Yoon S, Yang Y, Lee H B, Oh S, Jeong M H, Kim J J, Yee S T, Cri?an F, Moon C, Lee K Y, Kim K K, Hur J S, Kim H. 2014. Lichen secondary metabolites inexhibit anti-cancer effects on human cancer cells through the induction of apoptosis and suppression of tumorigenic potentials., 9(10): e111575.
Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. 2013. Crosstalk between apoptosis, necrosis and autophagy., 1833(12): 3448–3459.
Ochoa-Villarreal M, Howat S, Hong S, Jang M O, Jin Y W, Lee E K, Loake G J. 2016. Plant cell culture strategies for the production of natural products., 49(3): 149–158.
Ogbourne S M, Parsons P G. 2014. The value of nature’s natural product library for the discovery of new chemical entities: The discovery of ingenol mebutate., 98: 36–44.
Ortiz L M G, Lombardi P, Tillhon M, Scovassi A I. 2014. Berberine, an epiphany against cancer., 19(8): 12349–12367.
Pan L, Chai HB, Kinghorn A D. 2012. Discovery of new anticancer agents from higher plants., 4: 142–156.
Pan SY, Litscher G, Gao SH, Zhou S F, Yu Z L, Chen H Q, Zhang S F, Tang M K, Sun J N, Ko K M. 2014. Historical perspective of traditional indigenous medical practices: The current renaissance and conservation of herbal resources., 2014: 525340.
Pan W, Yang H, Cao C, Song X Z, Wallin B, Kivlin R, Lu S, Hu G, Di W, Wan Y S. 2008. AMPK mediates curcumin-induced cell death in CaOV3 ovarian cancer cells., 20(6): 1553–1559.
Panda A K, Chakraborty D, Sarkar I, Khan T, Sa G. 2017. New insights into therapeutic activity and anticancer properties of curcumin., 9: 31–45.
Petrovska B B. 2012. Historical review of medicinal plants’ usage., 6(11): 1–5.
Pfeffer C M, Singh A T K. 2018. Apoptosis: A target for anticancer therapy., 19(2): 448.
Pi Y, Jiang K J, Hou R, Gong Y F, Lin J, Sun X F, Tang K X. 2010. Examination of camptothecin and 10-hydroxycamptothecin inplant and cell culture, and the affected yields under several cell culture treatments., 34(3): 139–143.
Pommier Y. 2013. Drugging topoisomerases: Lessons and challenges., 8(1): 82–95.
Porta C, Paglino C, Mosca A. 2014. Targeting PI3K/Akt/mTOR signaling in cancer., 4: 64.
Qu X L, Tang Y, Hua S C. 2018. Immunological approaches towards cancer and inflammation: A cross talk., 9: 563.
Rahman N, Dhadi S R, Deshpande A, Ramakrishna W. 2016. Rice callus suspension culture inhibits growth of cell lines of multiple cancer types and induces apoptosis in lung cancer cell line., 16: 427.
Rivera A R, Castillo-Pichardo L, Gerena Y, Dharmawardhane S. 2016. Anti-breast cancer potential of quercetin via the Akt/AMPK/mammalian target of rapamycin (mTOR) signaling cascade., 11(6): e0157251.
Robati M, Holtz D, Dunton C J. 2008. A review of topotecan in combination therapy for advanced cervical cancer.,4(1): 213–218.
Sahin K, Ali S, Sahin N, Orhan C, Kucuk O. 2017. Lycopene: Multitargeted applications in cancer therapy.: Koehn F E. Natural Products and Cancer Drug Discovery. Springer Nature Switzerland AG: Humana Press: 79–108.
Saleem M. 2009. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene.,285(2): 109–115.
Santos R B, Abranches R, Fischer R, Sack M, Holland T. 2016. Putting the spotlight back on plant suspension cultures., 7: 297.
Seca A M L, Pinto D C G A. 2018. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application., 19(1): 263.
Seyed MA, Jantan I, Bukhari S N A. 2014. Emerging anticancer potentials of goniothalamin and its molecular mechanisms., 2014: 536508.
Shafie N H, Esa N M, Ithnin H, Saad N, Pandurangan A K. 2013. Pro-apoptotic effect of rice bran inositol hexaphosphate (IP6) on HT-29 colorectal cancer cells., 14(12): 23545–23558.
Shen Y, Jin L, Xiao P, Lu Y, Bao J S. 2009. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight., 49(1): 106–111.
Shin Y J, Lee N J, Kim J, An X H, Yang M S, Kwon T H. 2010. High-level production of bioactive heterodimeric protein human interleukin-12 in rice., 46(5): 347–351.
Shishir M R I, Karim N, Gowd V, Zheng X D, Chen W. 2019. Liposomal delivery of natural product: A promising approach in health research., 85: 177–200.
Siddiqui Z H, Mujib A, Mahmooduzzafar, Aslam J, Hakeem K R, ParweenT. 2013.production of secondary metabolites using elicitor in: A case study.: Hakeem K, Ahmad P, Ozturk M. Crop Improvement. Springer, Boston, MA: 401–419.
Simabuco F M, Morale M G, Pavan I C B, Morelli A P, Silva F R, Tamura R E. 2018. p53 and metabolism: From mechanism to therapeutics., 9: 23780–23823.
Singh S, Sharma B, Kanwar S S, Kumar A. 2016. Lead phytochemicals for anticancer drug development.,7: 1667.
Smetanska I. 2008. Production of secondary metabolites using plant cell cultures., 111: 187–228.
Su D, Cheng Y, Liu M, Liu D Z, Cui H, Zhang B L, Zhou S Y, Yang T H, Mei Q B. 2013. Comparision of piceid and resveratrol in antioxidation and antiproliferation activities., 8(1): e54505.
Suttiarporn P, Chumpolsri W, Mahatheeranont S, Luangkamin S, Teepsawang S, Leardkamolkarn V. 2015. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice., 7(3): 1672–1687.
Tabata H. 2004. Paclitaxel production by plant-cell-culture technology., 87: 1–23.
Tan B L, Norhaizan M E. 2017. Scientific evidence of rice by-products for cancer prevention: Chemopreventive properties of waste products from rice milling on carcinogenesisand., 2017: 9017902.
Thorne J L, Campbell M J. 2015. Nuclear receptors and the Warburg effect in cancer., 137(7): 1519–1527.
Tomatsu M, Mujin T, Shibamoto N, Tashiro F, Ikuta A. 2004. Production of aralin, a selective cytotoxic lectin against human transformed cells, in callus culture of., 70(5): 469–471.
Tsai J R, Liu P L, Chen Y H, Chou S H, Cheng Y J, Hwang J J, Chong I W. 2015. Curcumin inhibits non-small cell lung cancer cells metastasis through the adiponectin/NF-κb/MMPs signaling pathway., 10(12): e0144462.
Tyagi A, Agarwal R, Agarwal C. 2003. Grape seed extract inhibits EGF-induced and constitutively active mitogenic signaling but activates JNK in human prostate carcinoma DU145 cells: Possiblerole in antiproliferation and apoptosis., 22: 1302–1316.
Vanisree M, Lee C Y, Lo S F, Nalawade S M, Lin C Y, Tsay HS. 2004. Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures.,45: 1–22.
Veeresham C, Chitti P. 2013. Therapeutic agents from tissue cultures of medicinal plants., 1: 118.
Wang C G, Wu J Y, Mei X G. 2001. Enhancement of taxol production and excretion incell culture by fungal elicitation and medium renewal., 55: 404–410.
Wang L W, Wang J H, Fang L Y, Zheng Z L, Zhi D X, Wang S Y, Li S M, Ho CT, Zhao H. 2014. Anticancer activities of citrus peel polymethoxyflavones related to angiogenesis and others., 2014: 453972.
Warburg O. 1956. On the origin of cancer cells., 123: 309–314.
Watkins R, Wu L, Zhang C M, Davis R M, Xu B. 2015. Natural product-based nanomedicine: Recent advances and issues., 10(1): 6055–6074.
Watson G W, Beaver L M, Williams D E, Dashwood R H, Ho E. 2013. Phytochemicals from cruciferous vegetables, epigenetics, and prostate cancer prevention., 15(4): 951–961.
White M C, Holman D M, Boehm J E, Peipins L A, Grossman M, Henley S J. 2014. Age and cancer risk: A potentially modifiable relationship., 46(3): 7–15.
Wilson S A, Roberts S C. 2012. Recent advances towards development and commercialization of plant cell culture processes for synthesis of biomolecules., 10(3): 249–268.
Wishart D S. 2015. Is cancer a genetic disease or a metabolic disease?, 2(6): 478–479.
World Health Organization (WHO). 2014–2023. Traditional, complementary and integrative medicine. http://www.who.int/medicines/areas/traditional/en/.
Wu C F, Karioti A, Rohr D, Bilia A R, Efferth T. 2016. Production of rosmarinic acid and salvianolic acid B from callus culture ofwith cytotoxicity towards acute lymphoblastic leukemia cells., 201: 292–297.
Xiang L, Sun K Y, Lu J, Weng Y F, Taoka A, Sakagami Y, Qi J H. 2011. Anti-aging effects of phloridzin, an apple polyphenol, on yeast via the SOD and Sir2 genes., 75(5): 854–858.
Xie X X, Tao Q, Zou Y, Zhang F Y, Guo M, Wang Y, Wang H, Zhou Q, Yu S Q.2011. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: Characterizations and mechanisms., 59: 9280–9289.
Xu J F, Ge X M, Dolan M C. 2011. Towards high-yield production of pharmaceutical proteins with plant cell suspension cultures., 29(3): 278–299.
Xu J F, Zhang N N. 2014. On the way to commercializing plant cell culture platform for biopharmaceuticals: Present status and prospect., 2(6): 499–518.
Yang C W, Lee Y Z, Hsu H Y, Wu C M, Chang H Y, Chao Y S, Lee S J. 2013. c-Jun-mediated anticancer mechanisms of tylophorine., 34(6): 1304–1314.
Yao J, Weng Y Q, Dickey A, Wang K Y. 2015. Plants as factories for human pharmaceuticals: Applications and challenges., 16(12): 28549–28565.
Youns M, Hegazy W A H. 2017. The natural flavonoid fisetin inhibits cellular proliferation of hepatic, colorectal, and pancreatic cancer cells through modulation of multiple signaling pathways., 12(1): e0169335.
Yu Y H, Zhang J J, Wang J, Sun B G. 2019. The anti-cancer activity and potential clinical application of rice bran extracts and fermentation products., 9: 18060–18069.
Zeng A Q, Yu Y, Yao Y Q, Yang F F, Liao M Y, Song L J, Li Y L, Yu Y, Li Y J, Deng Y L, Yang S P, Zeng C J, Liu P, Xie Y M, Yang J L, Zhang Y W, Ye T H, Wei Y Q. 2018. Betulinic acid impairs metastasis and reduces immunosuppressive cells in breast cancer models., 9(3): 3794–3804.
Zhao M, Zhao M N, Fu C, Yu Y, Fu A L. 2018. Targeted therapy of intracranial glioma model mice with curcumin nanoliposomes., 13: 1601–1610.
Zimna A, Kurpisz M. 2015. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: Applications and therapies., 2015: 549412.
Copyright ? 2021, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2020.11.004
14 March 2020;
9 May 2020
Wusirika Ramakrishna (rk.wusirika@cup.edu.in; wusirika@gmail.com)
(Managing Editor: Wang Caihong)
- Rice Science的其它文章
- Different Hypotheses for Resistance Loss of Rice Varieties to Magnaporthe oryzae
- Development of Heat Tolerant Two-Line Hybrid Rice Restorer Line Carrying Dominant Locus of OsHTAS
- Identification of QTLs for Cadmium Tolerance During Seedling Stage and Validation of qCDSL1 in Rice
- Genetic and Geographic Patterns of Duplicate DPL Genes Causing Genetic Incompatibility Within Rice: Implications for Multiple Domestication Events in Rice
- Physiochemical Properties of Resistant Starch and Its Enhancement Approaches in Rice
- Visualizing Meiotic Chromosome Pairing and Segregation in Interspecific Hybrids of Rice by Genomic in situHybridization

