Synthesis and characterization ofcopolymers ofpoly(m-xylylene adipamide)and poly(ethylene terephthalate)oligomers by melt copolycondensation
FengleiBi,Jianqiang Shao ,Zhenhao Xi,Ling Zhao ,*,DiLiu
1 ShanghaiKey Laboratory of Multiphase Materials ChemicalEngineering,East China University ofScience and Technology,Shanghai200237,China
2 Beijing Sanlian Hope Shin-Gosen TechnicalService Co.,Ltd.,Beijing 100102,China
1.Introduction
Poly(m-xylylene adipamide)(MXD6)isa kind ofsemi-aromatic crystalline nylon resin with distinguished properties such as high rigidity and strength,high modulus,good dimensionalstability,and prepared by the polycondensation of adipic acid with m-xylylenediamine(MXDA)[1].The most remarkable peculiarity of MXD6 is concerned by its excellent gas barrier properties against O2and CO2,which is much lower than that of other conventionalpolyamide resins,ethylene-vinylalcoholcopolymers(EVOH)and poly(vinylidene chloride)copolymers(PVDC)etc.[2].Owing to this excellent gas barrier properties,especially in high humidity conditions,MXD6 can offer important processing bene fits by blending with other polymers,such as polyethylene,polypropylene,and poly(ethylene terephthalate)(PET).It can also be appropriately used as engineering structuralmaterials,in particular,when reinforced with glass fiber[3–7].Therefore,MXD6 is attracted in the technical,scienti fic and commercialinterest,especially in the field ofmultilayer food packaging material application such as multilayer and stretch-blown bottles[3],molding compounds and nono filaments[2].However MXD6 is easy to be fractured in the processing process,especially in molding process,so it is important to improve the toughness of MXD6.
Polymer blends and copolymerization are the most conventional methods which can improve many properties including elasticity,toughness,melting temperature,glass transition temperature etc.There have been remarkable numbers ofresearch studies ofpolymer blending that occur between polyamide and polyester,such as poly(butylene terephthalate)/nylon-6(PBT/PA6)[8–12],poly(butylene terephthalate)/nylon-66(PBT/PA66)[13–16],poly(ethylene terephthalate)/nylon-6(PET/PA6)[17–19],PET/nylon-66(PET/PA66)[18,20],and PET/Poly(m-xylylene adipamide)(PET/MXD6)[4,21–25].But it is found that polyamide/polyester blends are incompatible and proper compatibilizers are needed,or the copolymers can be got with the help ofan appropriate catalyst[4,9–12,22–24,26–31],for example sodium p-toluene sulfonic acid.Chemicalinterchange reactions may be occurred when polycondensates such as polyamides,polyesters and polymers with reactive functionalgroups at the chain terminals or in the backbone are mixed in the molten state,leading to the formation of random,block,segmented,or grafted copolymers,and then affecting the properties of the originalpolycondensates[27].But most polymer blending or copolymerization are occurred between higher molecular weightpolymers in melting state,thermaldegradation usually cannot be neglected,which would result in breaking polymer chain linkage as wellas some side reactions to reduce the quality ofpolymer products.Since polymer oligomers,bearing more reactive function groups atthe chain ends or in the backbone,such as polyester oligomer and polyamide oligomer,could directly react with each other[32,33],that might achieve polymer product with quite good quality.
In fact,some features ofPET are very close to thatofMXD6,including almost the same reaction temperature and vacuum condition in polycondensation process,similar rheologicaland crystallization behavior and temperature processing window of PET and MXD6 overlapping[2].Furthermore,PET is easy to form fiber and has good toughness,the MXD6/PET copolymers by copolycondensation with a smallamount of PET oligomers should have improved toughness compared to neat MXD6.In this work,PET oligomers are added during MXD6 oligomer polycondensation process to synthesize MXD6/PET copolymers,and then the thermaland mechanicalproperties ofprepared MXD6/PET copolymers are evaluated.
2.Experimental
2.1.Materials
Pure terephthalic acid(PTA;polymer grade),ethylene glycol(EG;polymer grade),adipic acid(≥99.5%,analytical reagent),sulphuric acid(H2SO4;95%–98%,analytical reagent),methanoic acid(CH2O2;≥98%,analyticalreagent),phenol(crystallization point≥40 °C,analyticalreagent)and 1,1,2,2-tetrachloroethane(Cl2CHCHCl2;≥99.0%,analyticalreagent)were supplied by Lingfeng Chemical Reagent(Shanghai)Co.,Ltd.m-xylylenediamine(MXDA;99.5%,polymer grade)was purchased from CAC ShanghaiChemicalCo.,Ltd.Antimony trioxide(Sb2O3;analyticalreagent)used as the catalyst was purchased from Aladdin.
2.2.Synthesis of PET oligomers
PET oligomers were synthesized through two stages,namely,direct esteri fication and prepolycondensation process.In the firststage,a mixture of83.06 g PTA,62.07 g EG,and the catalyst Sb2O3(200μg·g-1PTA)was vigorously stirred at temperature of 230°C in a 500 ml flask equipped with Vigreux column at the atmospheric pressure under the nitrogen atmosphere for nearly 9 h,and small molecule by-product(H2O)was removed out of the system.Then the esters being exposed to a vacuum of4.3 kPa were polymerized for another 2 h at 260°C in the second stage to obtain PET oligomers with a certain molecular weight.
2.3.Synthesis of MXD6 oligomers
MXD6 oligomers were prepared by directly melting polycondensation and adding 68.1 g MXDAto 73.1 g molten adipic acid atatmospheric pressure.The reaction was also carried out in a 500 ml flask equipped with Vigreux column at the atmospheric pressure under the nitrogen atmosphere.When the adipic acid was completely melted at 165°C,MXDAwas slowly dropped into and the temperature ofreaction system gradually rose with the continued removalofby-product H2Oforabout 1.5 h.Then the reaction was further carried out for another 0.5 h at 260°C to get the MXD6 oligomers.
2.4.Copolycondensation of MXD6/PET
A small amount of the prepared PET oligomers(1–5 g·(100 g MXDA)-1)were added into MXD6 oligomers melt.The oligomer mixture was stirred for another 0.5 h at 260°C under the nitrogen atmosphere,then gradually exposed to vacuum condition of1.4 kPa for 1 h and about400 Pa for another 1.5 h respectively to obtain MXD6/PET copolymers finally.MXD6 oligomers also experienced the same process to produce pure MXD6 with high molecular weightas a reference sample.
3.Characterization
Before the characterization,the MXD6/PET copolymer samples was puri fied to eliminate the unreacted PET oligomers,thatwas,the obtained copolymer was dissolved in formic acid,re-precipitated in methanol,filtered,adequately washed by deionized water and dried for 24 h at 90°C in vacuum oven.
3.1.FTIR spectroscopy
FTIR spectra ofallthe samples were acquired with Nicolet5700 Fourier Transform Infrared Spectrometer(U.S.Thermo Nicolet Company Electron Corporation)and collected in the region of 4000–400 cm-1.The measurements were carried outby applying 32 scans with a resolution of4 cm-1.
3.2.NMR spectroscopy
The structures of the resulting copolycondensation polymers were performed with a BRUKERAVANCE 400 Superconducting Fourier Transform Nuclear Magnetic Resonance Spectrometer(Germany)at 400 MHz,using deuterated tri fluoroacetic acid(CF3COOD)as a solvent.
3.3.Intrinsic viscosity
The intrinsic viscosity of PET oligomers was carried out at a concentration of 0.5 g·dl-1solution in a phenol-1,1,2,2-tetrachloroethane(1:1,w/w)using an Ubbelohde viscometer placed in a water bath at 25°C.
The relation ofintrinsic viscosity([η])and number-average molecular weightfor PET was expressed by the empirical equation,as follows[34]

The relative viscosity of the MXD6 oligomers and MXD6/PET copolymers was performed at a concentration of 1 g·dl-1[35]solution in concentrated H2SO4also with an Ubbelohde viscometer placed in a water bath at 25°C.
An empirical linear correlation between relative viscosity(ηr)and number-average molecular weightfor MXD6 was reported as follows[35]:
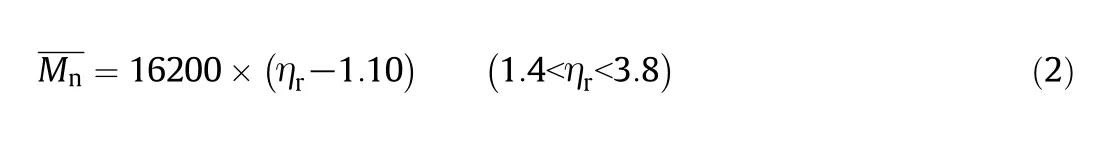
3.4.Differentialscanning calorimetry(DSC)
The thermalbehaviorsofpolymerand copolymerswere analyzed on a NETZSCH DSC 204 HP with the Thermal Analysis Data Station which provided the thermalanalysis data under nitrogen atmosphere.Dried samples of about 10 mg placed in aluminum crucible were heated from room temperature to 270 °C at 10 °C·min-1and held for 5 min to erase any prior thermal history before cooling to 30°C at 5 °C·min-1.The samples were then reheated up to 300 °C and cooled down to 30 °C at10 °C·min-1and 5 °C·min-1,respectively.
Isothermalcrystallization behaviors were performed that the samples were firstly heated from 30 °C to 270 °C at10 °C·min-1and maintained for 5 min to erase any prior thermal history.And then,these melted samples were rapidly cooled from 270°C to the speci fied isothermal crystallization temperatures at 160,180,185,190,195 and 200°C,respectively.These crystallization behaviors were recorded as function of time.The samples were held at each selected temperature for adequate time to allow complete crystallization to occur.After that,the samples were continuously cooled down to 30°C,and then were re-heated from 30 °C to 300 °C at10 °C·min-1to record the melting behaviors of the previously formed crystallinity.
The melt crystallization temperature Tmc,melting temperature Tmwere determined from the DSC curves of the second heating cycle.
3.5.Mechanicalproperties analysis
The specimens for mechanicalproperties testing were prepared by injection molding using Haake Minilab,Minijet(Thermo Fisher).The tensile properties of polymer and copolymer samples were tested following ASTMD638–2003 test method.And the testing was carried on an American Instron Corporation 3300 Series Dual Column desktop electronic universaltesting machine.The Izod notched impact strength ofpolycondensates and copolymers was tested following ASTMD-256 test method.Eight specimens were tested for each sample and the average results were reported.
3.6.Scanning electron micrographs
Scanning electron micrographs of impact fracture surfaces were observed on a NOVA Nano SEM 450 scanning electron microscope(SEM)operating atacceleration voltage of3 kV.Allthe sample surfaces were made conductive before testing by the deposition ofa layerofgold for 1 min on a United Kingdom Quorum Technologies Ltd.,Q150RS.
4.Results and Discussion
4.1.Interchange reaction between MXD6 oligomers and PET oligomers
The number-average molecular weight of synthesized PET oligomers was about 3100 g·mol-1,and the one MXD6 oligomer was about 12300 g·mol-1.
It is wellknown that,exchange reactions taking place between two bifunctional condensation polymers with different chemical nature could generate a four-component copolycondensate[36–38].PET oligomers and MXD6 oligomers are mixed under reaction conditions and could yield ester interchange reactions to form copolymers.Two possible exchange reaction routes[26]may be followed,namely,outer amide-inner carbonyl interchange reaction and inner amide-inner carbonylinterchange reaction.For a particular case of the MXD6/PET system,when ester-amide exchange reactions occur between one bifunctional polyamide A1B1 and another bifunctionalpolyester A2B2 can lead to the following structuralchanges presented in Fig.1.
This interchange reaction between MXD6 oligomers and PET oligomers and the structure of MXD6/PET copolymers were characterized using the FTIR technique.The FTIR spectra for PET oligomers,MXD6 and MXD6/PET copolymers are shown in Fig.2,respectively.Itis clear that the neat PET oligomers have a strong absorption band at 1719 cm-1corresponding to the stretching vibration ofcarbonylgroups(-C=O)from the repeated ester units.And the infrared spectrum of neat MXD6 reveals the characteristic absorption bands of–CONH–group[39].The intense band at 1646 cm-1is assigned to the amide I vibration which mainly involves the C=O stretching vibration peak,and the bands at 1546 cm-1and 1251 cm-1are assigned to the amide IIvibrations which correspond to the coupled N–H in-plane deformation and C–N stretching modes.Compared with the neat polymers,it should be pointed out that another new peak with MXD6/PET copolymers at 1723 cm-1(indicated by the arrows)near to the –CONH– stretching vibration peak(1646 cm-1and 1546 cm-1)of MXD6 has been observed which has been proved to be the C=O stretching vibration peak of PET oligomers in Fig.2.This indicates that the interchange reaction do happen between these two oligomers to form copolymer(A1B2),as shown in Fig.1.Moreover,the intensity of the new peak gradually enhances with the adding content of PET oligomers.
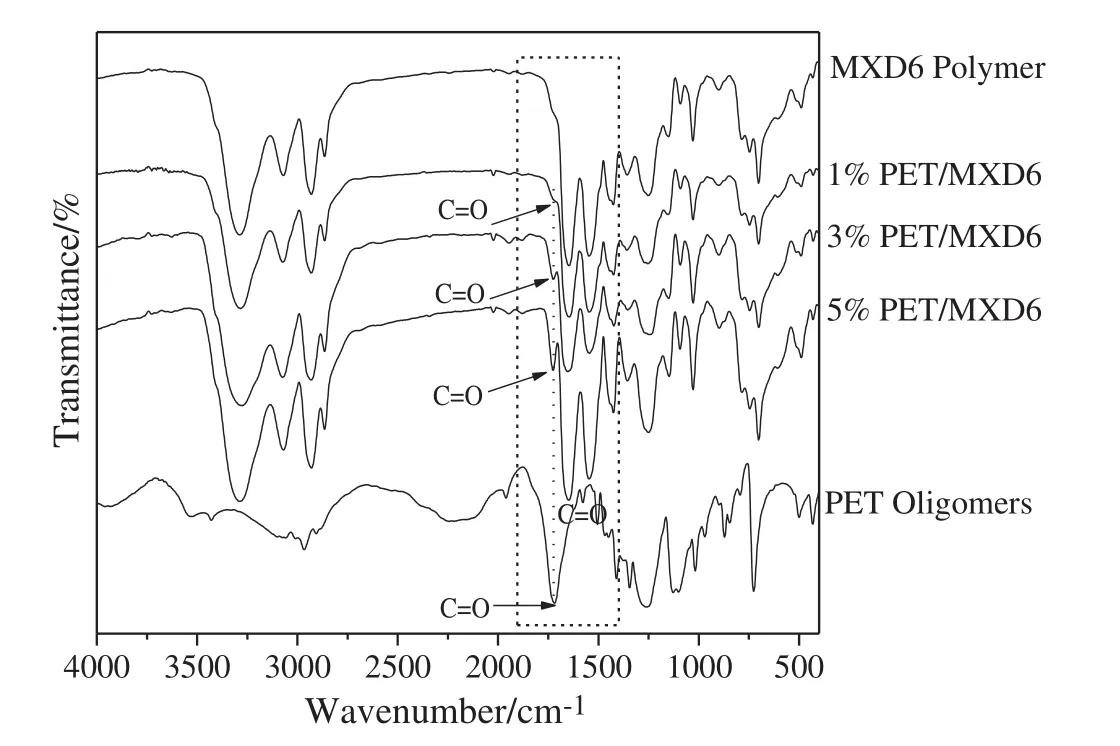
Fig.2.Infrared spectra of synthesized PET oligomers,MXD6/PET copolymers and neat MXD6 polymer.Note the peaks in the 1720 cm-1(indicated by the arrows)of copolymers.
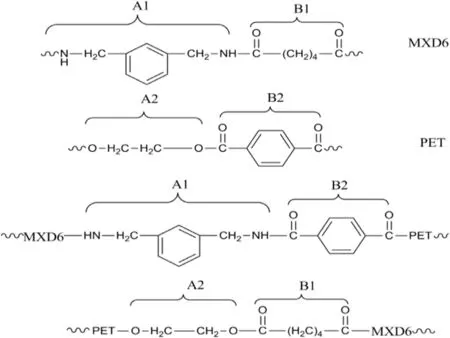
Fig.1.Scheme ofstructuralchanges expected from the ester-amide interchange reaction.
Polymersamples ofneatMXD6,PET oligomers and the various crude and puri fied MXD6/PET copolymers were further con firmed using1H NMR spectroscopy.Spectra of a and b in Fig.3 show the typical 400 MHz1H NMR spectra of the neat PET oligomers and MXD6 in the deuterated tri fluoroacetic acid(TFA-d)respectively,while spectra ofa and b in Fig.4 displayed the1H NMR spectra of crude and puri fied MXD6/PET copolymers respectively.
It can be seen that allpeaks originate from the polymer MXD6 in Fig.3(b)atthe following position:δ=8.52(singlet),δ=7.31(singlet),δ=7.20(singlet),δ=4.53(singlet),δ=2.62(singlet),δ=1.80(singlet)in Fig.4(a).There are two new peaks for MXD6/PET copolymers which appear atδ=8.09 andδ=7.87 in Fig.4(a),while at δ=8.17 andδ=7.95 in Fig.4(b).Allof these signals belong to benzene ring protons of the neat PET oligomers in the copolymer.Furthermore,the distance between the two new peaks is all0.22,as shown in Fig.4.That means the interchange reactions had surely occurred between MXD6 oligomers and PET oligomers.It is con firmed that the amide groups along the MXD6 oligomers chains are able to attack the carboxylic ends or ester groups of PET oligomers[27].It can also be seen that there is a peak appeared atδ=8.52 both the neat MXD6 and raw copolymers,but it disappears in the puri fied copolymers,that is because of the reaction between amino group-terminated(δ=8.52)and formic acid when the crude copolymers are puri fied.
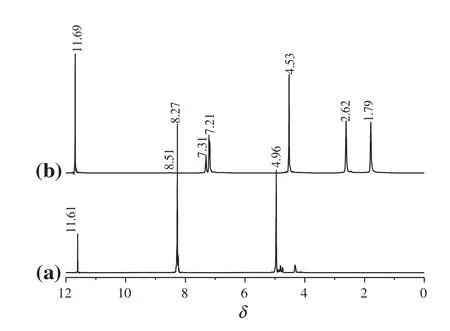
Fig.3.1H NMR spectra of PET oligomers(a)and MXD6(b).

Fig.4.1H NMR spectra for crude(a)and puri fied(b)5 wt%MXD6/PET copolymers.
1H NMR spectra of the synthesized copolymers with differently adding content of PET oligomers are also shown in Fig.5.It can be seen that the peak intensity ofbenzene ring of PET(δ=8.09,δ=7.87)increases with the increasing contents of PET oligomers while the peak intensity ofamino group-terminated(δ=8.52)accordingly decreases.Itcan be attributed that more amino group-terminated should be reacted with PET oligomers and more PET chain segments willbe contained in the copolymers with the increasing contents of PET oligomers.
Based on the previous discussion,itcan be considered the formation of A1B2(m-xylylene units/terephthalate units)sequences by linking MXD6 and PET blocks in the formed block copolymer,which has been described four-component copolycondensate(shown in Fig.1).
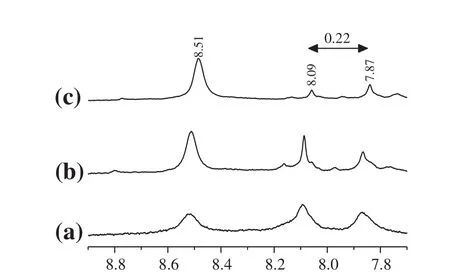
Fig.5.Sections of the 1H NMR spectra of synthesized copolymers with different mass ratios of PET oligomers to MXDA:(a)5%;(b)3%;(c)1%.
4.2.Thermalproperties of MXD6/PET copolymer
The effectofPET oligomers on the melting temperature Tmand crystallization temperature Tmcof the MXD6/PET copolymerswasexamined by DSC thermograms.The second heating scans of the neat MXD6 polymers and the MXD6/PET copolymers are presented in Figs.6 and 7,respectively.All the samples of the MXD6/PET copolymers have the similar intrinsic viscosity values,so there is a smallin fluence of the molecular weightdifferences on the thermalbehaviors.The corresponding glass transition temperature,peak crystallization temperature,peak melting temperature and their enthalpies are listed in Table 1.
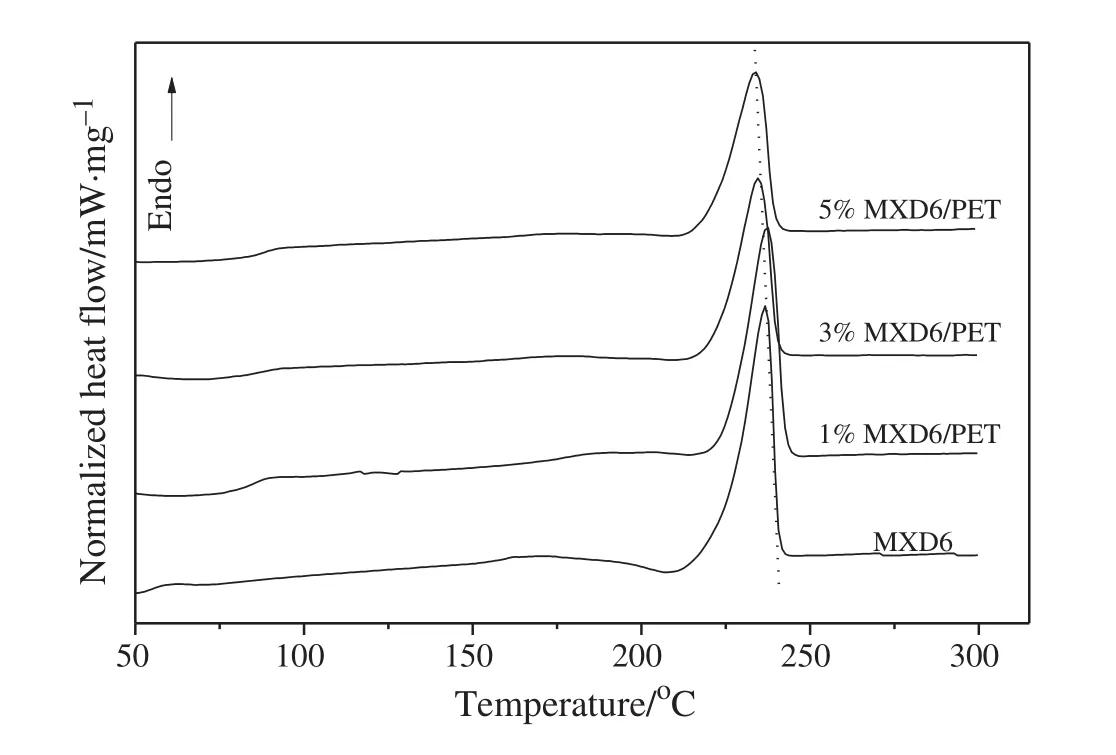
Fig.6.The melt temperature curves of MXD6 polymer and copolymers.
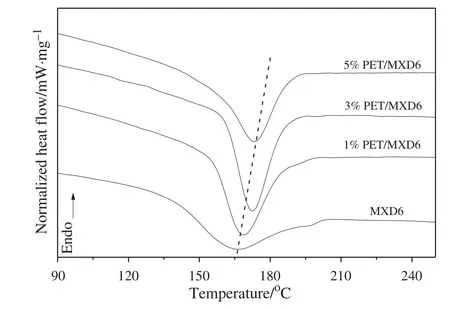
Fig.7.The crystallization temperature curves of MXD6 polymer and copolymers.
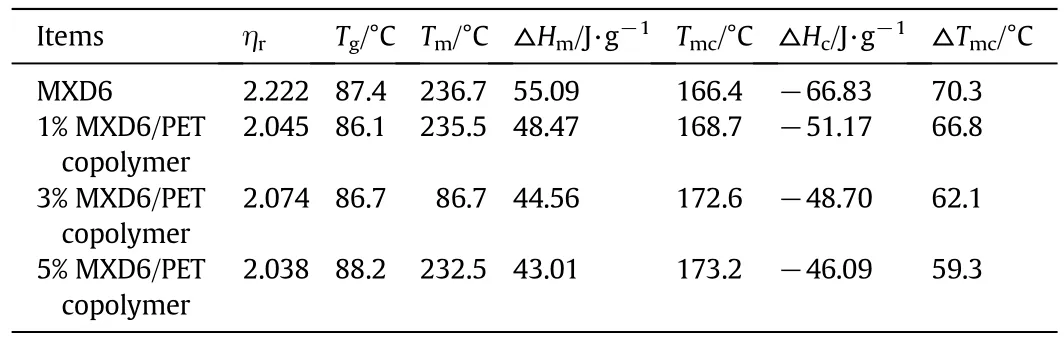
Table 1 The thermalproperties ofcopolymer and oligomers
As seen in Table 1,the relative viscosity ofMXD6/PET copolymers is decreased compared with the neat MXD6,that is because the melting MXD6 blocks are in fluenced by the presence of the PET blocks and/or above all the formed heterosequence of the aromatic amide mxylylene terephthalamide units(A1B2)by exchange reactions[27].There isa slightdifference among the relative viscosity ofMXD6/PET copolymers,it also indicates that the average molar masses of the formed copolymers does not have much variation.
Due to the low contentof PET segments in copolymers as wellas the relatively small difference between the Tgof neat MXD6(89°C)and neat PET(80°C)[27],there is only a single Tgthat appeared in the heating scans of the copolymers,and almost identicalto the values of neat MXD6.Only one melting endothermpeak existed in MXD6/PET copolymers in Fig.6,indicating that the PET segments have been introduced the structure of MXD6 and there is a single phase in MXD6/PET copolymers.The PET segments are introduced into MXD6 matrix which will break the regularity of neat MXD6,and then reduces the crystallinity of copolymers.Decreased△Hcis associated to the lower crystallinity of the copolymers.The reduction of△Hmis for the same reason as the decreased△Hc,both of which can be attributed to the lower crystallinity of the copolymers.
Itis wellknown thatthe velocity ofcrystallization process for MXD6 is inferior to thatof PA6 and PA66 because of the weak symmetry and steric hindrance of meta-oriented aromatic ring in its main chain.A broader crystallization peak could show the crystallization feature of MXD6 in Fig.7.Cooling thermograms clearly show the effectofPET oligomer blocks on the crystallization process ofmelting MXD6.The degree ofsupercooling(△Tmc)is decreased and melting crystallization temperature(Tmc)of copolymers is gradually increased with increasing PET oligomer content,as seen from Table 1,itis conducive to crystallization and enhances the crystallization rate for MXD6/PET copolymer.When the meltcopolymer is cooling from high temperature,partof PET firstly forms solid particles in the melt and acts as the heterogeneous crystal nucleus for MXD6 continuous phases.As heterogeneous nucleation is dominated,the nucleation and crystallization are quickly completed in the high temperature range,and then sharpened peak shape and sped the crystallization rate.While there are homogeneous and heterogeneousnucleation in the system with increasing PET componentcontent,and due to the heterogeneous nucleation is prior to the homogeneous nucleation in the melt crystallization process,it leads to the increased MXD6 crystallization temperature.Compared with that of the neat MXD6,the range ofcrystallization is narrower and the peak is sharper for MXD6/PET copolymers in Fig.7.Atthe same time the more PET symmetricalstructures are introduced into the MXD6 matrix,the crystallization of copolymers becomes much easier,the crystallization performance is much better and the crystallization velocity is faster than that of the neat MXD6.
According to the reports[40,41],the equilibriummelting temperature Tm0of a crystallizable polymer is important in the analysis of crystal growth rate data in order to establish the degree of supercooling,because it is used as a reference temperature from which the driving force for crystallization is observed.Therefore,the equilibrium melting temperature is also determined for MXD6/PET copolymers.
Isothermally crystallized MXD6 has been found to existtwo melting peaks[42],when crystallized in the lower temperature range from 170 to 180°C.In the higher crystallization temperature range,from 185 to 195°C,itdisplays three melting peaks.PET also generally exhibits three melting peaks[43],when it is isothermally crystallized at temperatures from 180 to 205°C.These peaks have been designated as Tm1,Tm2and Tm3.However,there are many different interpretations of the observed multiple melting peaks.According to the common interpretation[40],the first melting peak Tm1may represent smallportions ofmetastable crystalline material,the second melting peak Tm2stands for the quality of crystalline structures formed during primary crystallization and the third peak Tm3represents the reorganization and recrystallization that occurs during heating at the programmed rate in the DSC.The second peak is used to provide data used for extrapolation to the intercept(Tm0)with a theoreticalline based on equivalent melting and crystallization temperatures[40].
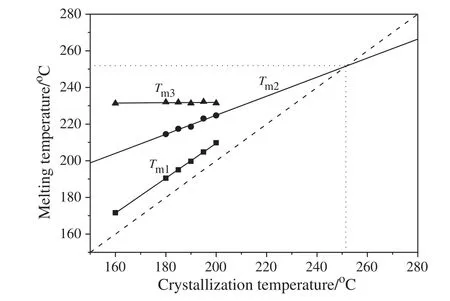
Fig.9.Variation with crystallization temperature ofmultiple melting peaks:T m1,T m2,and T m3 for the 5 wt%MXD6/PET copolymer after complete isothermal crystallization.Extrapolation of the T m2 line is used for determination of T m 0.
Fig.8 displays the DSC thermalphenomena for MXD6/PET copolymer samples recorded after complete crystallization from the melt state at different crystallization temperatures ranging from 160°C to 200°C.These endothermic peaks are assigned as Tm1,Tm2and Tm3for the lowest,medium and highesttemperature melting endotherms,respectively.The results show clearly that the melting behaviors strongly depend on the crystallization temperature Tc.Isothermally crystallized copolymers,processing at the lower crystallization temperature 160°C,have also been found to exhibittwo melting peaks.But,itclearly shows three melting peaks for isothermally crystallized copolymers at the higher crystallization temperature range from 180 to 200°C.This thermal phenomenon is similar to the isothermally crystallized PET[43]and MXD6[42].
Fig.9 shows the plot of the melting temperatures as the function of crystallization temperature for MXD6/PET copolymers.It can be seen that both peaks Tm1and Tm2moved towards higher temperatures with the increasing crystallization temperatures,while peak Tm3almost does notchange with the crystallization temperature and is constant.It is also noticed that the difference between Tm1and Tcis fairly constant and equals to(10±0.5)°C.Then the equilibrium melting temperature Tm0can be determined by extrapolating the Tm2line to higher temperature which willintercept with the Tm=Tcline.On basic of the calculation method of Hoffman and Weeks[41],the intersection of the least-squared fit with the line Tm=Tcprovides the value of equilibrium melting temperature.Then,the equilibrium melting temperaturecan be evaluated to be equal to 251.8°C,which is relatively close to the values ofneat MXD6(250 ±2)°C[42].

4.3.Mechanicalproperties of MXD6/PET copolymer
The values of mechanicalproperties of MXD6/PET copolymers are listed in Table 2.As seen from Table 2,it is clear that some mechanical properties of the copolymers increased obviously after adding a small amount of the PET oligomers.Since PET has a better tensile property than that of MXD6,so that PET chain segments being introduced intoMXD6 blocks can improve the tensile property of copolymers.Meanwhile,PET oligomers destroy the homopolymer regularity of MXD6 chain and lead to the lower crystallinity,the arrangement of molecular chain is looser,and the impact strengths ofcopolymers are also improved.
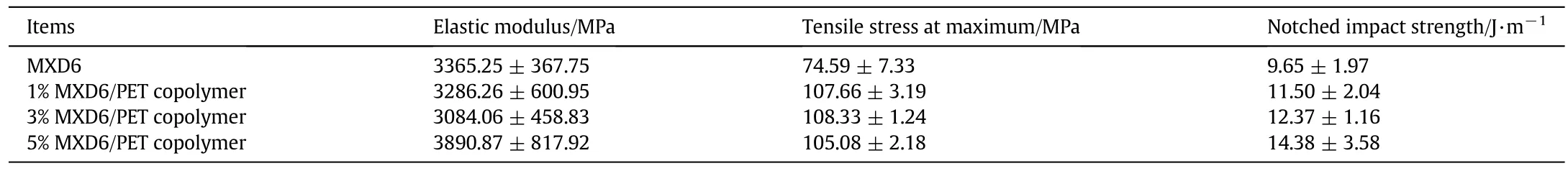
Table 2 The mechanicalproperties of MXD6 and MXD6/PET copolymers
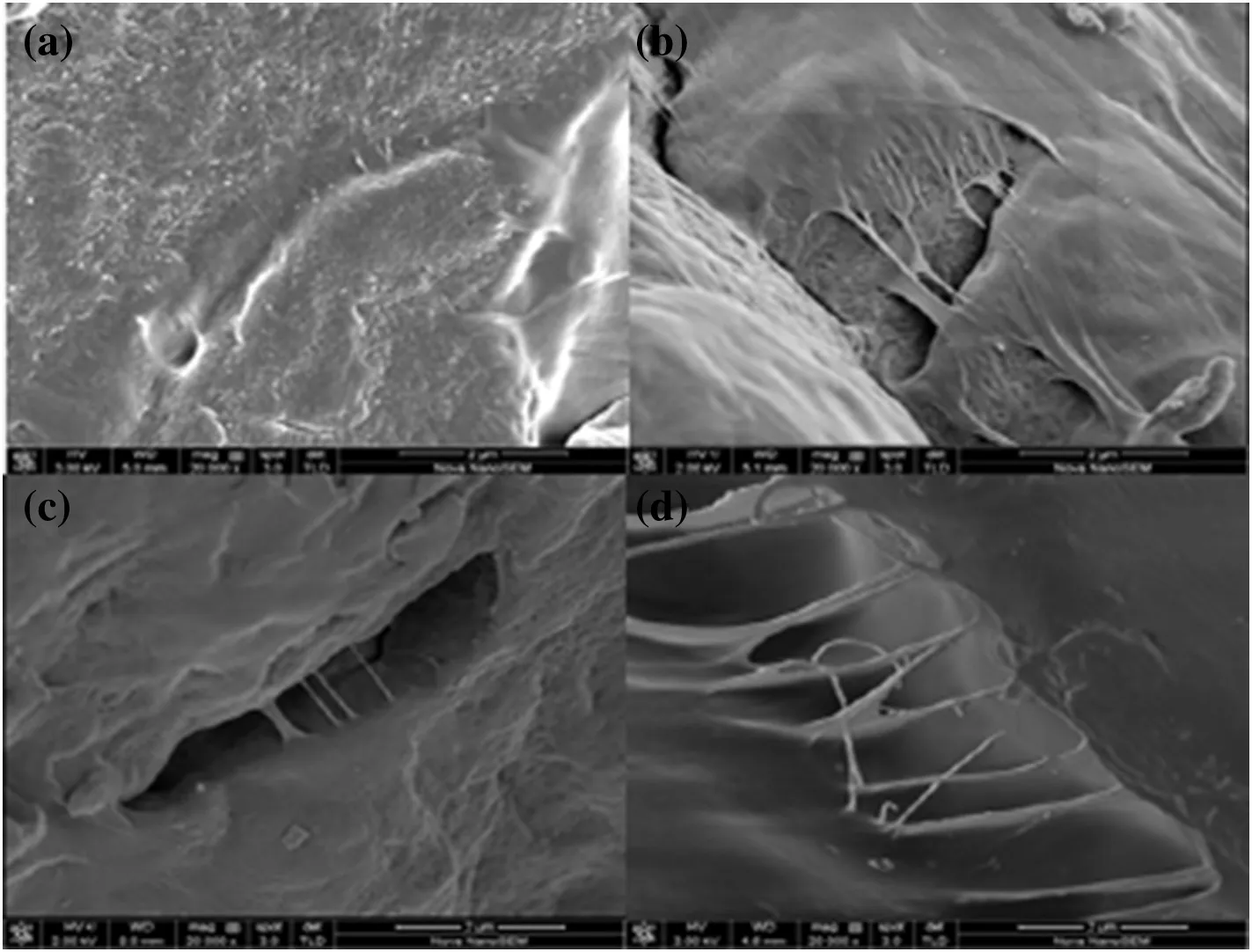
Fig.10.Scanning electron micrographs of MXD6(a)1 wt%(b)3 wt%(c)and 5 wt%(d)MXD6/PET copolymer.
Scanning electron micrographs chosen at same magni fication under identicalconditions of the impact fracture surfaces of MXD6,1 wt%,3 wt%and 5 wt%MXD6/PET copolymers are given in Fig.10 respectively.
It can be seen that the neat MXD6 has a homogeneous structure,while the MXD6/PET copolymers are obviously somewhat different.The micro fibrilstructures in the impact fracture surface are generated for MXD6/PET copolymers as shown in Fig.10(b)to(d),and more micro fibril structure can be formed with the increasing contents of PET oligomers.When the samples suffer the externalforce,the micro fibril can absorb lots of energy and enhance the material toughness,which reveals the features ofductile fracture for MXD6/PET copolymers.Meanwhile,the formed copolymers can contribute to improve the compatibility ofMXD6 and PET,which makesa good interface adhesion.That should be a proof why the mechanical properties of MXD6/PET copolymers are improved than those ofneat MXD6.
5.Conclusions
MXD6/PET copolymers containing 1wt%–5 wt%PET are synthesized by the interchange reactions between MXD6 oligomers and PET oligomers in molten state.The chemicalstructure of the synthesized copolymers has been characterized by FT-IR and1H NMR.It proves that the formation of A1B2(m-xylylene units/terephthalate units)sequences by link MXD6 and PET blocks in the formed MXD6/PET copolymers.
The measurement of thermal properties of copolymers indicates that the melting temperature of MXD6/PET copolymers decreases,while the crystallization temperature of copolymers increases with the increasing ofadding amount of PET oligomers.Isothermalcrystallization behavioralresults indicate that the equilibrium temperature Tm0of 5 wt%MXD6/PET copolymers is evaluated to be equalto 251.8°C,which is close to that ofneat MXD6.
The measurement of the mechanical properties of MXD6/PET copolymers compared with that of neat MXD6 indicates that both of the tensile stress at maximum and the notched impact strength of MXD6/PET copolymers are improved almost 50%than that of neat MXD6.Morphological examination reveals that the micro fibril structures are generated in the impact fracture surface of MXD6/PET copolymers,which leads to the ductile fracture for copolymers.
[1]E.F.Carlston,F.G.Lum,m-Xylylenediamine polyamide resins,Ind.Eng.Chem.49(8)(1957)1239–1240.
[2]Mitsubishi Gas Chemical Catalog,MX-Nylon,Vol.52000.
[3]Z.H.Xi,L.K.Chen,Y.Zhao,L.Zhao,Experimental and modeling study of melt polycondensation process of PA-MXD6,Macromol.Symp.333(1)(2013)172–179.
[4]Y.S.Hu,V.Prattipati,A.Hiltner,E.Baer,S.Mehta,Improving gas barrier of PET by blending with aromatic polyamides,Polymer 46(8)(2005)2685–2698.
[5]P.J.Cahill,J.A.Richardson,R.V.Wass,Copolyamide active-passive oxygen barrier resins,US Pat.,6506463(2003).
[6]R.Germonprez,Y.J.Kim,Barrier compositions and film made therefrom having improved opticaland oxygen barrier properties,US Pat.,5314987(1994).
[7]Y.J.Kim,R.Germonprez,R.L.Kaas,A.Mehta,Barrier compositions and articles made therefrom,US Pat.,6239210(1999).
[8]F.Samperi,M.Montaudo,C.Puglisi,R.Alicata,G.Montaudo,Essential role of chain ends in the Ny6/PBT exchange.A combined NMR and MALDIapproach,Macromolecules 36(19)(2003)7143–7154.
[9]N.Wakita,Melt elasticity of incompatible blends of poly(butylene terephthalate)(PBT)and polyamide 6(PA6),Polym.Eng.Sci.33(13)(1993)781–788.
[10]R.Scaffaro,L.Botta,F.P.La,Mantia,P.Magagnini,D.Acierno,M.Gleria,R.Bertani,Effect ofadding new phosphazene compounds to poly(butylene terephthalate)/polyamide blends.I:preliminary study in a batch mixer,Polym.Degrad.Stab.90(2)(2005)234–243.
[11]R.Scaffaro,L.Botta,F.P.La,Mantia,M.Gleria,R.Bertani,F.Samperi,G.Scaltro,Effect of adding new phosphazene compounds to poly(butylene terephthalate)/polyamide blends.II:Effect of different polyamides on the properties of extruded samples,Polym.Degrad.Stab.91(10)(2006)2265–2274.
[12]R.Jeziórska,Studies on reactive compatibilisation of polyamide 6/poly(butylene terephthalate)blends by low molecular weight bis-oxazoline,Polym.Degrad.Stab.90(2)(2005)224–233.
[13]C.C.Huang,F.C.Chang,Reactive compatibilization of polymer blends of poly(butylene terephthalate)(PBT)and polyamide-6,6(PA66):1.Rheological and thermalproperties,Polymer 38(9)(1997)2135–2141.
[14]C.C.Huang,F.C.Chang,Reactive compatibilization of polymer blends of poly(butylene terephthalate)and polyamide 6,6:2.Morphologicaland mechanical properties,Polymer 38(17)(1997)4287–4293.
[15]X.L.Xie,S.C.Tjong,R.K.Y.Li,Study on in situ reinforcing and toughening of a semiflexible thermotropic copolyesteramide in PBT/PA66 blends,J.Appl.Polym.Sci.77(9)(2000)1975–1988.
[16]J.John,M.Bhattacharya,Synthesis and properties of reactively compatibilized polyester and polyamide blends,Polym.Int.49(8)(2000)860–866.
[17]S.Fakirov,M.Evstatiev,S.Petrovich,Micro fibrillar reinforced composites from binary and ternary blends of polyesters and nylon 6,Macromolecules 26(19)(1993)5219–5226.
[18]Z.Denchev,H.R.Kricheldorf,S.Fakirov,Sequentialreordering in condensation copolymers,6.Average block lengths in poly(ethylene terephthalate)–polyamide 6 copolymers as revealed by NMR spectroscopy,Macromol.Chem.Phys.202(4)(2001)574–586.
[19]F.Samperi,C.Puglisi,R.Alicata,G.Montando,Essential role of chain ends in the nylon-6/poly(ethylene terephthalate)exchange,J.Polym.Sci.Polym.Chem.41(18)(2003)2778–2793.
[20]X.G.Xie,D.C.Wu,X.L.Xie,DSC analysis of PET/PA66 blends and study on their compatibility,J.Wuhan Inst.Chem.Technol.2(1)(2003)7–10.
[21]Y.S.Hu,V.Prattipati,A.Hiltner,E.Baer,S.Mehta,Improving transparency of stretched PET/MXD6 blends by modifying PET with isophthalate,Polymer 46(14)(2005)5202–5210.
[22]V.Prattipati,Y.S.Hu,S.Bandi,D.A.Schiraldi,A.Hiltner,E.Baer,S.Mehta,Effect of compatibilization on the oxygen-barrier properties of poly(ethylene terephthalate)/poly(m-xylylene adipamide)blends,J.Appl.Polym.Sci.97(3)(2005)1361–1370.
[23]V.Prattipati,Y.S.Hu,S.Bandi,S.Mehta,D.A.Schiraldi,A.Hiltner,E.Baer,Improving the transparency of stretched poly(ethylene terephthalate)/polyamide blends,J.Appl.Polym.Sci.99(1)(2006)225–235.
[24]M.Fereydoon,S.H.Tabatabaei,A.Ajji,Rheological,crystalstructure,barrier,and mechanicalproperties ofPA6 and MXD6 nanocomposite films,J.Polym.Eng.Sci.54(11)(2014)2617–2631.
[25]S.R.Turner,G.W.Connell,S.L.Stafford,J.D.Hewa,Polyester-polyamide blends with reduced gas permeability and low haze,US Pat.,6444283(2002).
[26]F.Xie,Y.W.Kim,S.A.Jabarin,The interchange reaction between poly(ethylene terephthalate)and poly(m-xylylene adipamide),J.Appl.Polym.Sci.112(6)(2009)3449–3461.
[27]F.Samperi,M.S.Montaudo,S.Battiato,D.Carbone,C.Puglisi,Characterization of copolyesteramides from reactive blending of PET and MXD6 in the molten state,J.Polym.Sci.Polym.Chem.48(22)(2010)5135–5155.
[28] ?.?zen,G.Bozoklu,C.Dalg??dir,O.Yücel,E.ünsal,M.?akmak,Y.Z.Mencelo?lu,Improvement in gas permeability of biaxially stretched PET films blended with high barrier polymers:The role of chemistry and processing conditions,Eur.Polym.J.46(2)(2010)226–237.
[29]X.D.Wang,Study on the Preparation and Properties of PET/MXD6 Blends(M.S.Thesis)Tongji Univ.,China,2003.
[30]Y.Wang,S.A.Jabarin,Novel preparation method for enhancing nanoparticle dispersion and barrier properties of poly(ethylene terephthalate)and poly(mxylylene adipamide),J.Appl.Polym.Sci.129(3)(2013)1455–1465.
[31]S.Bandi,S.Mehta,D.A.Schiraldi,The mechanism ofcolor generation in poly(ethylene terephthalate)/polyamide blends,Polym.Degrad.Stab.88(2)(2005)341–348.
[32]R.R.Wu,W.T.Shi,Study on PET-PA66 copolymer,Chin.J.Polym.Sci.10(4)(1992)356–360.
[33]S.Joachim,B.P.Michael,G.Freddy,Block copolymers made of polyethylene terephthalate and a polyamide made of meta-xylylenediamine and adipinic acid,CN Pat.,101072834(2005).
[34]J.Zhang,Synthesis of High Molecular Weight of PET and the SSP Process Optimization Research(Ph.D.Thesis)Zhejiang Univ.,China,2011.
[35]B.DeRoover,G.Coppens,J.Devaux,R.Legras,A.Momtaz,Contribution to poly(mxylylene adipamide)characterization:Hydrolysis,condensation,and oxidation in the melt,J.Polym.Sci.Polym.Chem.34(6)(1996)1039–1047.
[36]A.M.Aerdts,K.L.L.Eersels,G.Groeninckx,Transamidation in melt-mixed aliphatic and aromatic polyamides.1.Determination of the degree ofrandomness and number-average block length by means of13C NMR,Macromolecules 29(3)(1996)1041–1045.
[37]J.Devaux,P.Godard,J.P.Mercier,Bisphenol-A polycarbonate–poly(butylene terephthalate)transesteri fication.I.Theoreticalstudy of the structure and of the degree of randomness in four-component copolycondensates,J.Polym.Sci.Polym.Phys.Ed.20(10)(1982)1875–1880.
[38]J.Devaux,P.Godard,J.P.Mercier,R.Touillaux,J.M.Dereppe,Bisphenol-A polycarbonate–poly(butylene terephthalate)transesteri fication.II.Structure analysis of the reaction products by IR and1H and13C NMR,J.Polym.Sci.Polym.Phys.Ed.20(10)(1982)1881–1894.
[39]N.Yoda,I.Matsubara,Studies on structure and properties ofaromatic polyamides.II.Structuralelucidation of poly(m-xylylene adipamide),J.Polym.Sci.A 2(1)(1964)253–265.
[40]F.Xie,E.A.Lofgren,S.A.Jabarin,Melting and crystallization behavior of poly(ethylene terephthalate)and poly(m-xylylene adipamide)blends,J.Appl.Polym.Sci.118(4)(2010)2153–2164.
[41]J.D.Hoffman,J.J.Weeks,Melting process and the equilibrium melting temperature of polychlorotri fluoroethylene,J.Res.Natl.Bur.Stand.A 66(1)(1962)13–28.
[42]B.B.Doudou,E.Dargent,J.Grenet,Crystallization and melting behaviour ofpoly(mxylene adipamide),J.Therm.Anal.Calorim.85(2)(2006)409–415.
[43]C.Zhou,S.B.Clough,Multiple melting endotherms ofpoly(ethylene terephthalate),Polym.Eng.Sci.28(2)(1988)65–68.
 Chinese Journal of Chemical Engineering2016年9期
Chinese Journal of Chemical Engineering2016年9期
- Chinese Journal of Chemical Engineering的其它文章
- In situ synthesis ofhydrophobic magnesium hydroxide nanoparticles in a novelimpinging stream-rotating packed bed reactor☆
- Enhancing the hydration reactivity ofhemi-hydrate phosphogypsum through a morphology-controlled preparation technology☆
- Improvement of CO2 capture performance ofcalcium-based absorbent modi fied with palygorskite☆
- Adsorption behavior ofcarbon dioxide and methane in bituminous coal:A molecular simulation study☆
- Characterization of the adsorption behavior ofaqueous cadmium on nanozero-valent iron based on orthogonalexperiment and surface complexation modeling☆
- Enhanced cold active lipase production by metagenomic library recombinant clone CALIP3 with a step-wise temperature and dissolved oxygen levelcontrolstrategy☆
