An Extraction Process for Optimal Utilization of Naphtha Based on Molecule Management
(State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
An Extraction Process for Optimal Utilization of Naphtha Based on Molecule Management
Wang Tianxiao; Shen Benxian; Sun Hui
(State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
In this paper, the separation of aromatics from light naphtha by using extraction process was investigated for improving the utilization effciency of naphtha. It is indicated that, using a mixture of propylene carbonate-diethylene glycol as the solvent, the optimal extraction conditions cover: a volume fraction of propylene carbonate in the mixed solvent of 0.3, a solvent to feed ratio of 8, and an extraction temperature of 308 K. Through the extraction process, the aromatics mass fraction increases from 10.05% in naphtha to 27.74% in extract oil. It is found that the aromatics yield of extract oil,RA, reaches 92.11%. As a result, in comparison with naphtha, the potential aromatics content of extract oil increases impressively by 18.03%. Meanwhile, the aromatics content of raffnate oil decreases to 1.33%, and the normal paraffn yield of raffnate oil,Rp, is 76.61%. Accordingly, higher total olefns yields can be obtained when using raffnate oil as the raw material for steam cracking. The present results show that the utilization effciency of naphtha is improved through extraction process.
optimal utilization; naphtha; extraction; aromatics separation
1 Introduction
Naphtha is the main feed for the steam cracking process and the catalytic reforming process to produce basic chemicals for the industry. As the demand for naphtha in both processes increases, the contradiction emerges. Hence, the optimal utilization of naphtha attracts extensive research interests in the petrochemical industry.
The ineffcient utilization of naphtha is being referred to frequently because naphthenes which comprise the potential aromatic content in naphtha are preferred in the catalytic reforming process, while aromatic hydrocarbons can lead to coking during the steam cracking process[1-2]. Therefore, separating aromatic components from naphtha, which is used as a feed for catalytic reforming process, is necessary for the optimal utilization of naphtha fraction. However, aromatics can hardly be separated from naphtha by traditional distillation because of the presence of azeotropes and components with close boiling points[3-4]. The molecule management strategy, which aims at separating the components from a mixture according to different usage requirements of products in molecule, exhibits the superiority in improving the utilization effciency of naphtha[2,5]. As normal paraffins are the preferred components in steam cracking process, the adsorption technology using 5 A zeolite for separating normal paraffins from naphtha is an effcient way for optimal utilization of naphtha[1-2,6-8]. However, the presence of olefns in naphtha leads to coke deposition on 5 A zeolite, which could largely reduce the performance of separation process[9]. Extraction could be considered to be a simple and practical way for separation of naphtha containing olefns. Upon applying an extraction process by using a solvent with high capacity in the separation of aromatics from naphtha, the extract oil with high potential aromatic content can be separated from naphtha as a good feed for catalytic reforming process, meanwhile, the remaining raffnate oil with low aromatics content and relatively highn-paraffin content is a good feed for steam cracking process.
The aromatics extraction process for naphtha has been widely studied[4,10-12], followed by distillation for solvent recovery. A number of polar solvents, such as sulfolane (SUL)[13], diethylene glycol (DEG)[14], triethylene glycol[15], tetraethylene glycol[16-17], propylene carbonate(PC)[18-20], ionic liquids[21], and other solvents[22], have been studied for separating aromatics from naphtha. The conventional extraction processes, such as the liquid-liquid extraction, the extractive distillation and the azeotropic distillation, have been used for the separation of aromatic hydrocarbons from the mixture with aromatics content exceeding 20%[12]. However, it is a great challenge to separate aromatics from the naphtha which contains less than 20% of aromatics by using the extraction process.
The present work is aimed at the optimal utilization of light naphtha via liquid-liquid extraction process. Assisted by the Hansen solubility parameter, propylene carbonate (PC) was selected as a suitable solvent for extraction. Then the extraction conditions for PC were optimized. Thereafter, diethylene glycol (DEG) was selected as the cosolvent, and the optimal conditions for the mixed solvent (PC+DEG) were studied through the orthogonal test.
2 Experimental
2.1 Materials
The light naphtha was obtained from the Shandong Jingbo Petrochemical Co., Ltd., with itsn-paraffins mainly consisting ofn-propane ton-decane and its boiling points ranging from 313 K to 433 K. The contents ofn-paraffins, iso-paraffins, olefins, cycloparaffins and aromatics analyzed by gas-chromatograph were 25.90%, 33.73%, 0.54%, 29.78% and 10.05%, respectively, which could change slightly due to the environment and storage conditions.N,N-dimethyl formamide (DMF),N-methylpyrrolidone (NMP), diethylene glycol (DEG), triethylene glycol (TEG) and dimethyl sulfoxide (DMSO) were supplied by the Lingfeng Chemical Reagent Co., Ltd. in Shanghai, with the quality of all materials meeting the chemical reagent purity. Sulfolane (SUL) and propylene carbonate (PC) were supplied by the Sinopharm Chemical Reagent Co., Ltd. in Shanghai, with the quality of all materials meeting the chemical reagent purity. Di-(2-ethylhexyl) sebacate (dioctyl sebacate, DOC) was supplied by the Huakai Resin Co., Ltd. in Jining, with its quality reaching the technically pure grade. Naphtha and solvents were used without further purifcation.
2.2 Extraction experiments
The flow diagram of extraction process is shown in Figure 1 (b). The extraction process includes an extraction column, a washing tower and a distillation column. The washing tower and distillation column were used to remove the solvent from the raffinate phase and the extract phase, respectively.
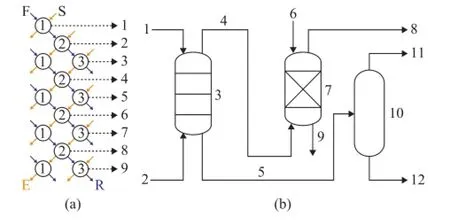
Figure 1 Flow diagrams of three-stage counter-current extraction experiment (a) and the extraction process (b)
The flow diagram of the three-stage counter-current extraction experiment[23]is shown in Figure 1 (a), which is used to simulate a three-stage counter-current extraction column. The single-stage extraction experiment and the three-stage counter-current extraction experiment were conducted by using liquid separating funnels and thermostatic bath. Each extraction was followed by shaking for at least 5 min and then settling for at least 10 min to form two clear phases under specifed conditions. The recovery of solvent was carried out for handling both the extract phase and the raffnate phase, respectively, to obtain the extract oil (E) and the raffnate oil (R), respectively. Due to the strong polarity which solvents possess, a small quantity of solvent existing in the raffnate phase can be removed via washing with water. Washing was conducted at a water/oil ratio of 1.0 in order to obtain raffnate oil from the raffnate phase. In consideration of the great difference in boiling point between oil and solvent, with the exception of DMF, the extract oil could be separated from the extract phase through distillation. DMF was removed from the extract phase by washing at a water/oil ratio of 1.0 owing to its similar boiling point to naphtha.
The water to oil ratio and solvent to feed ratio (S/F) are expressed in volume ratio. The PC content of mixed solvent is expressed in volume fraction.
2.3 Composition analysis
The composition of extract oil and raffnate oil was analyzed by a GC-920 gas chromatograph (Shanghai Haixin Chromatograph Instrument Co., Ltd.) equipped with a flame ionization detector (FID) and a PONA column (0.3 mm i.d.×20 m). The hydrocarbon group compositions of naphtha and products were determined by employing the RIPP-Gasoline Composition Analyzer Software V2.01 developed by the Research Institute of Petroleum Processing.
2.4 Hansen solubility parameter (HSP)
HSP was used as a convenient way to predict the miscibility of solvents by solubility parameter “distance”(Ra). Hansen[24-27]divides the solubility parameter[28-29]into several individual parts. The relationship between total solubility parameter and HSP is:
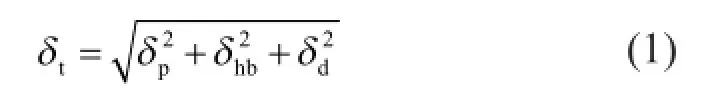
where the subscript d represents dispersion, p represents polar, and hb represents hydrogen bonding.
Based on HSP, the solubility parameter “distance”between solvent and solute,Ra, is calculated by equation (2)[26]. To use HSP, a detailed database can be found in the literature[24,30-31].
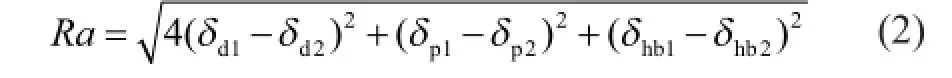
2.5 Orthogonal analysis
The optimal solvent composition and extraction conditions for mixed solvent were determined by orthogonal test. Three indexes were selected for analysis of experimental results. Both direct analysis and variance analysis were used to evaluate the results. To make general score for each scheme of conditions, weights for evaluation indexes were computed subjectively by the Analytic Hierarchy Process[32](AHP) and objectively by the entropy method[33]through MATLAB. To make the full use of information, the optimal weight was calculated by considering both subjective weight and objective weight based on the sum of square of deviations[34].
Variance analysis was carried out for evaluating the indexes to distinguish whether the results of orthogonal test were caused by factors or error. The sum of square of deviations,SS, for factor A is calculated by equation (3).
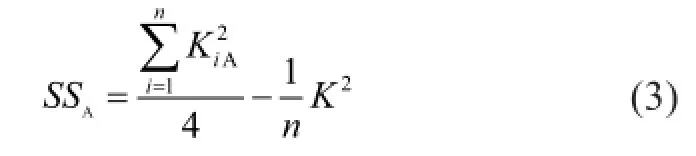
whereKis the sum of indexes,n=16 is the experimental time.
2.6 Product evaluation
To investigate the performance of extraction process for optimal utilization of naphtha, the performance of naphtha and extract oil for catalytic reforming process were evaluated by the potential aromatics content,P, which is defned as the aromatics content of product when all of the cycloparaffin components are converted into aromatics. In this study, the potential aromatics contents of C6, C7and C8hydrocarbons were calculated for performance evaluation.

whereMiis the molecular weight of cycloparaffn componenti,Niis the mass fraction of cycloparaffn componenti, andAiis the mass fraction of aromatic componenti.
3 Results and Discussion
3.1 Solvent selection
The selection of solvent was performed by means of the Hansen solubility parameter. Suitable solvents were selected atT=313 K andS/F=3 through single-stage extraction.
Asn-paraffins are the preferred components for steam cracking process, they possess low reactivity in catalytic reforming process. To preventn-paraffns from being extracted into the extract oil, the HSP ofn-paraffns is calculated to fnd the solvents having overwhelming aromatics solubility overn-paraffns solubility. In case of more than two components in a mixture, the HSP for the mixture can be calculated by equation (5)[24]. The HSP for the mixture ofn-paraffns is listed in Table 1.
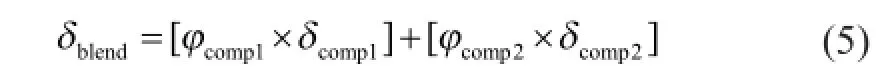
whereφiis the volume fraction of componentiin the mixture.
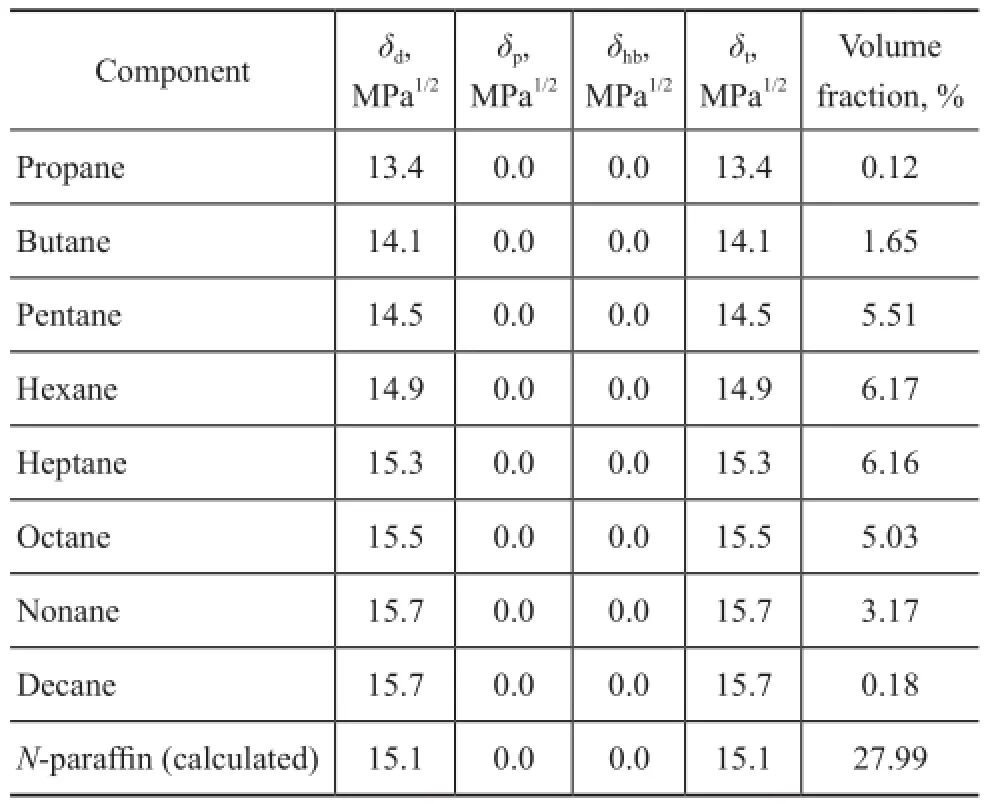
Table 1 HSP of n-paraffins[24]
To determine the appropriate solubility parameter “distance”Ra, DMF, NMP, SUL, DEG and DOC were used in the preliminary study. The result shows that NMP and DOC are good solvents for naphtha, which can even be miscible with naphtha atS/F=1; DMF becomes miscible with naphtha at the stated conditions, and can extract about 30% of naphtha atS/F=1; and DEG and SUL have certain solubility in naphtha.
A suitable solvent for extractive separation of naphtha needs a considerable solubility for aromatics and a poor solubility forn-paraffins, and a certain solubility (not too much) for naphtha to balance the products. In this context, we have to find a solvent with HSP similar to the aromatic ones (with a small amount of polar force and hydrogen-bonding force), but unlike then-paraffnic ones (without polar force or hydrogen-bonding force). As regards the naphtha in this study, DMF is considered as a good solvent at the boundary surface of HSP solubility sphere with its radius ton-paraffnRaDMF=18.3 MPa1/2calculated by equation (2). The less theRais, the closer the position of solvent to the position of naphtha would be. In other words, solvents inside the sphere are considered as good solvents, and the bad ones are outside or close to it. To exclude good solvents for naphtha, theRaof appropriate solvents is determined to be larger thanRaDMF.
In order to display the solubility of solvent clearly, the Hansen three-dimensional solubility parameter sphere for the naphtha-solvent system is established for 16 kinds of conventional solvents. The coordinates of the solubility volume center are the HSP ofn-paraffin (δd=15.1,δp=0.0,δhb=0.0) withRa=18.3 MPa1/2. As shown in Figure 2, NMP, DOC and DMF are inside the sphere indicating their high solubility to naphtha, while SUL, DEG are outside the sphere, which agrees well with our preliminary results.
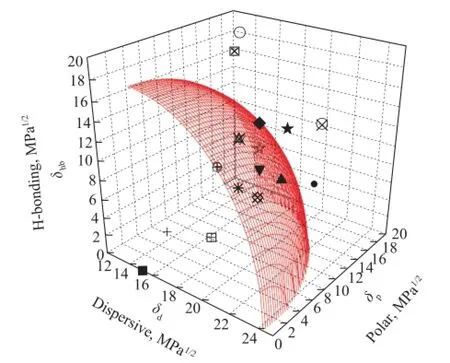
Figure 2 Hansen three-dimensional solubility sphere for then-paraf fi n-solvent system
To prevent the solvent from getting miscible with naphtha, DMF, DEG, SUL, PC, TEG and DMSO were obtained as solvents for extraction. Then the single-stage extraction experiments were performed by using 6 solvents mentioned above.
The yield of aromatics in the extract oil,RA, is defned as the percentage of aromatic components extracted from naphtha into extract oil.

At the same time, then-paraffn yield of raffnate oil,Rp, is defned by equation (7). The aromatics recovery refers toRA,and then-paraffn recovery refers toRp.

The yields of aromatics and extract oil are shown in Figure 3. The results indicate that PC, DMF and DMSO have better capacity for extractive separation of naphtha, which means a lowerS/Fratio for aromatics recovery. The yield of aromatics decreases in the following order: DMF>DMSO>PC>SUL>DEG>TEG.
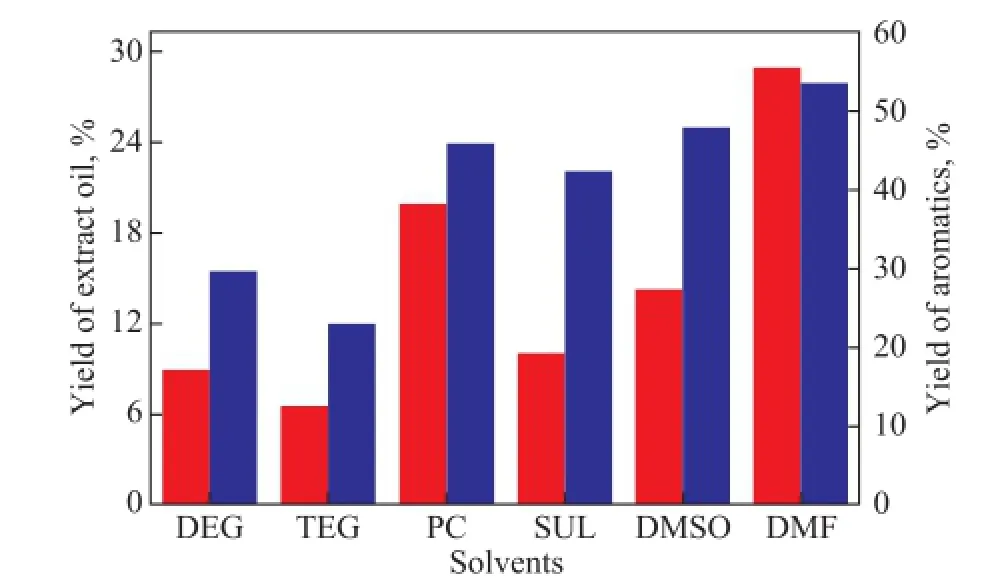
Figure 3 Yields of aromatics and extract oil. Extraction runs were operated atT=313 K andS/F=3 for DEG, TEG, PC, SUL, DMSO and atT=313 K andS/F=1 for DMF
Asn-paraffin components are good feedstocks for steam cracking process, solvents should have the selectivity for the separation of aromatics andn-paraffns. The selectivity of the solvent,S, is defned by equation (8). The selectivity of solvents is shown in Figure 4, which decreases in the following order: SUL>DMSO>TEG>PC>DEG>DMF.

By taking solubility and selectivity into consideration, it is indicated that DMF, DMSO, PC and SUL show advantages in extractive separation of naphtha. DMF, PC and DMSO are found to have high solubility, while DMF possesses low selectivity. SUL and DMSO have high selectivity, but low thermal stability. As a result, PC is selected as a suitable solvent for extractive separation of light naphtha.
3.2 Extraction conditions optimization for PC
3.2.1 Effect of temperature
Once the solvent was selected, the effect of temperature (T) and solvent to feed ratio (S/F) on extraction were studied through the three-stage counter-current extraction experiment. Via the three-stage counter-current extraction, the performance of PC in the multi-stage countercurrent extraction was also tested.
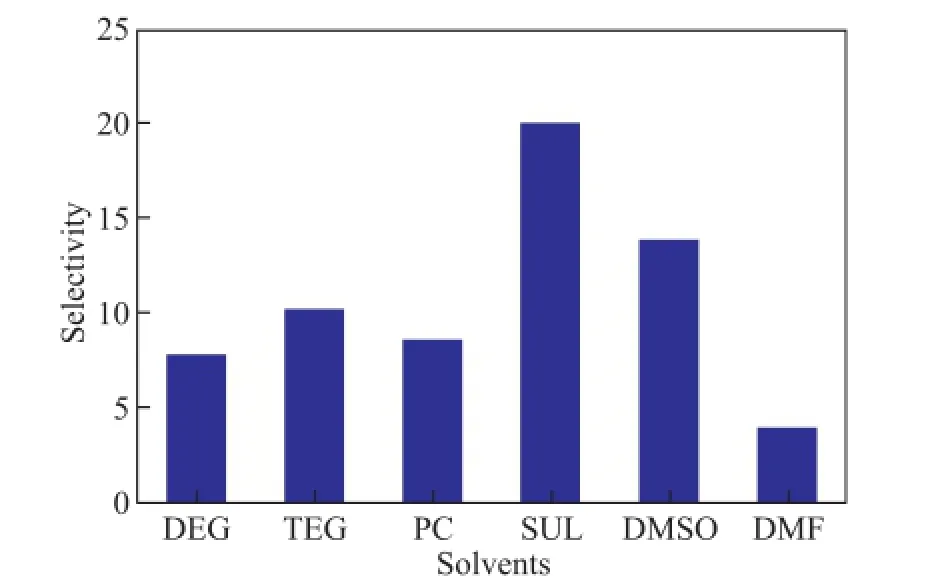
Figure 4 Selectivity of solvents. Extraction runs took place atT=313 K andS/F=3 for DEG, TEG, PC, SUL, DMSO and atT=313 K andS/F=1 for DMF
Firstly, the effect of temperature on the composition of products was studied at a temperature of 303, 308, 313, 318 and 323 K, respectively, and aS/Fratio of 6. In Figure 5, it can be seen that the aromatics content of raffinate oil is reduced to about 1.0%, which indicates that PC has a satisfactory capacity for separating aromatics from naphtha in the multi-stage counter-current extraction. With the temperature increasing from 303 K to 323 K, the aromatics content of extract oil decreases significantly because of the increasing solubility for other components; then-paraffns content of raffnate oil increases and reaches a plateau at 318 K, then decreases due to the increasing solubility ofn-paraffns in solvent at a higher temperature. Upon considering the composition of extraction products, the optimal extraction temperature is 313 K.
3.2.2 Effect ofS/Fratio
The effect of solvent to feed ratio (S/F)on the composition of extraction products was studied at aS/Fratio of 2, 4, 6, 8, and 10, respectively, and an extraction temperature of 313 K. The results are shown in Figure 6. The aromatics content of raffnate oil is reduced to about 1.0% when theS/Fratio is higher than 6. With the increase ofS/Fratio, the aromatics content of extract oil decreases signifcantly while then-paraffins content of extract oil increases. Meanwhile, then-paraffins content of raffinate oil increases, and iso-paraffns content of raffnate oil increases when theS/Fratio increases from 2 to 6, then decreases with a further increase in the S/F ratio. To separate aro-matics fromn-paraffns, the optimal solvent to feed ratio is 6.
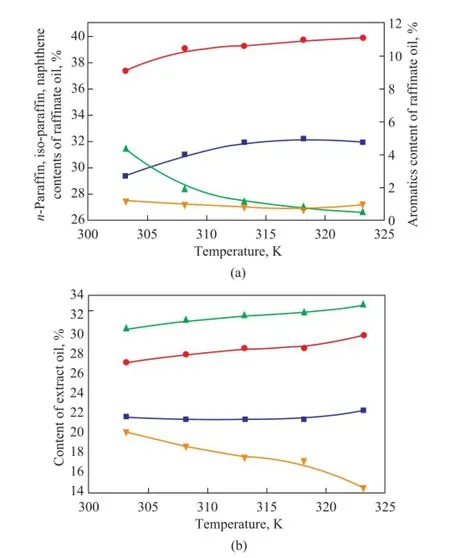
Figure 5 Effect of temperature on the composition of raf fi nate oil (a) and extract oil (b) at aS/Fratio of 6
By using an optimal conditions ofS/F=6 andT=313 K throughout the three-stage counter-current extraction process, pure PC can increase the aromatics content from 10.05% in naphtha to 17.58% in extract oil and reduce the aromatics content of raffnate oil to 0.86%. The yield of extract oil is 45.39%. Hence, PC shows high aromatics solubility under optimal conditions. However, then-paraffns content of extract oil reaches 21.38%, which indicates that the solubility of PC ton-paraffins is also relatively high.
3.3 Analysis of orthogonal test
Sincen-paraffn components can lead to high total olefns yield in steam cracking process but low aromatics yield in catalytic reforming process, they should remain in raffnate oil based on the idea of molecule management. To improve the recovery ofn-paraffins in raffinate oil, diethylene glycol (DEG) has been selected as a cosolvent, which has lower solubility ton-paraffins and a close boiling point (518 K) to PC (515 K). Orthogonal test was designed to determine the optimal conditions suitable to the mixed solvent.
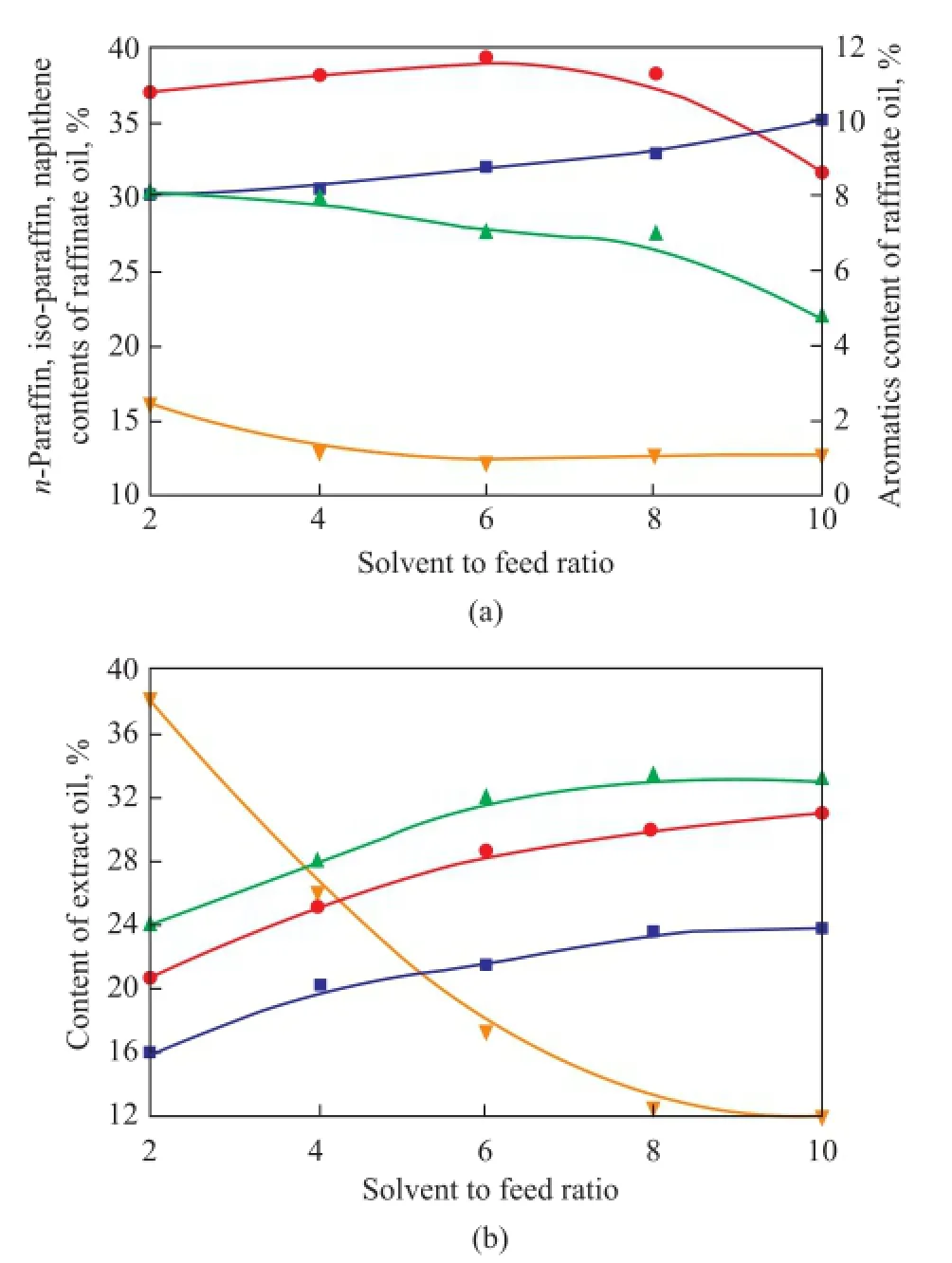
Figure 6 Effect ofS/Fratio on the composition of raf fi nate oil (a) and extract oil (b) atT=313 K
The major factors considered in the orthogonal test covered: the volume fraction of PC in the mixed solvent (x), the solvent to feed volume ratio (S/F), and the extraction temperature (T). Other conditions, such as the pressure, were limited in orthogonal test to reduce error. The [L16(45)] orthogonal table was chosen in this study. As shown in Table 2, each factor has four levels in equal interval, respectively. The aromatics yield of extract oil (RA) and the distribution coefficient ofn-paraffins (Kp) were used to evaluate the extraction performance. Meanwhile, the solubility (defined as the yield of extract oil, %) was also considered in order to obtain the comparative yields of extract oil and raffnate oil.

According to the properties of indexes, the evaluation matrix was normalized by equation (10).RAandKpare beneft types, which means that they are positively related to the evaluation. And the solubility is a quantitative type with the desired value of 0.5.
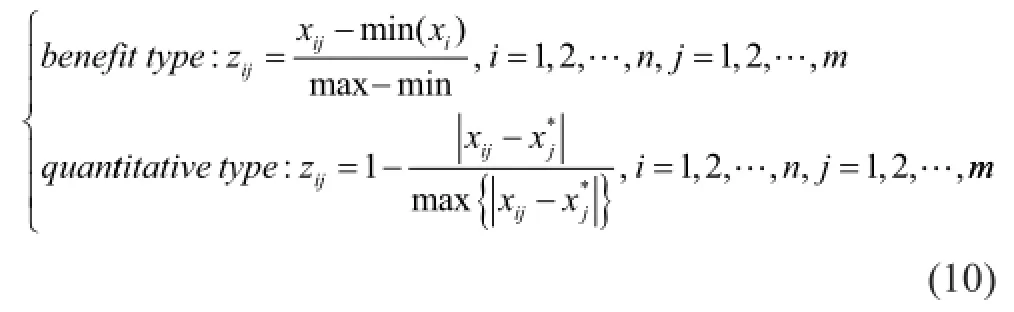
To calculate the subjective weightwAof evaluation indexes, the judgment matrixAof AHP is defined according to the importance of indexes. To improve the recovery ofn-paraffins, the distribution coefficient ofn-paraffins (Kp) is considered as the most important evaluation index for orthogonal test. Then the aromatics yield of extract oil (RA) is in the second place. To keep the balance of products, solubility is also considered. The consistency ratio (CR) of matrixAis 0.015 8. Hence, the AHP weightwAcalculated by matrixAis available.

By using MATLAB, the subjective weightwAand the objective weightwSof evaluation indexes were calculated. As a result, the optimized combination weight vectorWcwas obtained to calculate the general scores of orthogonal test.
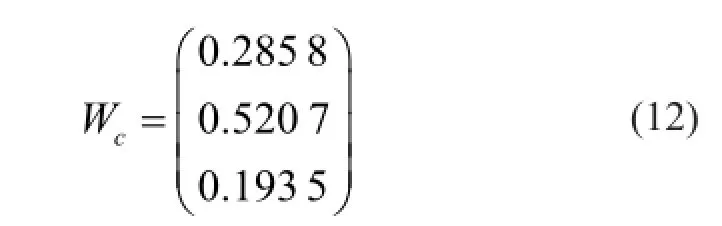
3.3.1 Direct analysis
By using the weight vectorWcobtained above, scores were calculated for the normalized matrixZ. The result of direct analysis is shown in Table 2. The range of scores for each factor,R=(knm)max-(knm)min, was calculated to investigate the impact of factors on the results. Larger impact of the factor on scores is made with largerR.
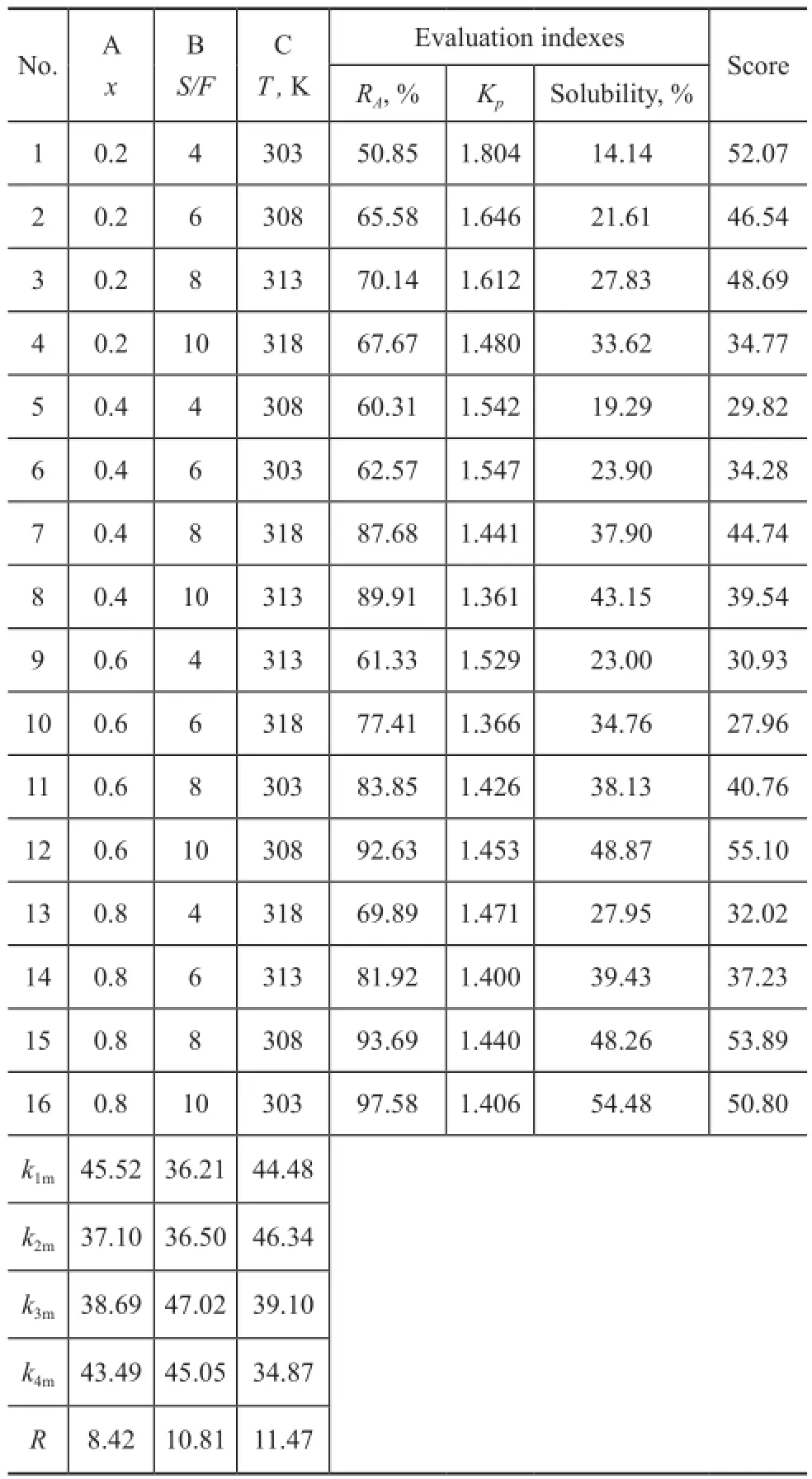
Table 2 Results of orthogonal test
Through the direct analysis, the effect of factors on score follows the order: C>B>A. The result indicates that the best scheme of conditions for orthogonal test is A1B3C2, which impliesx=0.2,S/F=8, andT=308 K.
3.3.2 Variance analysis
The result of variance analysis is shown in Table 3. The degree of freedomfandFvalue were calculated by the statistical method. By comparingFvalue with the critical value, the factor withFvalue being larger than critical value (α=0.01) is marked as (* *); the factor withFvalue being larger than critical value (α=0.05) is marked as (*).
If the factor’s sum of squares of deviations is smaller than the errorQ, then the factor will be considered as erroneous, in other words, the new errorQ*, the degree of freedomf, and theFvalues of factors should be recalculated. The 4thand 5throws of [L16(45)] in the orthogonal table were used to calculate the original errorQ. In Table 3, the results show that factors A and B have great significance toRAwithFvalues being larger than the critical value (α=0.01), while the factor C has no significance to the index. Factors A and B have a 99% signifcance toKp, and the factor C has a 95% signifcance to it.Kpis the most important index, which weighs 52.07% in the score. Hence, these factors are all major factors of extraction and evaluation. The variance analysis result of solubility is similar to theRAone. Factors A and B have a 99% significance to the solubility. However, the temperature shows no significance to the solubility. This result might be ascribed to the limited variation of temperature investigated in this orthogonal test.
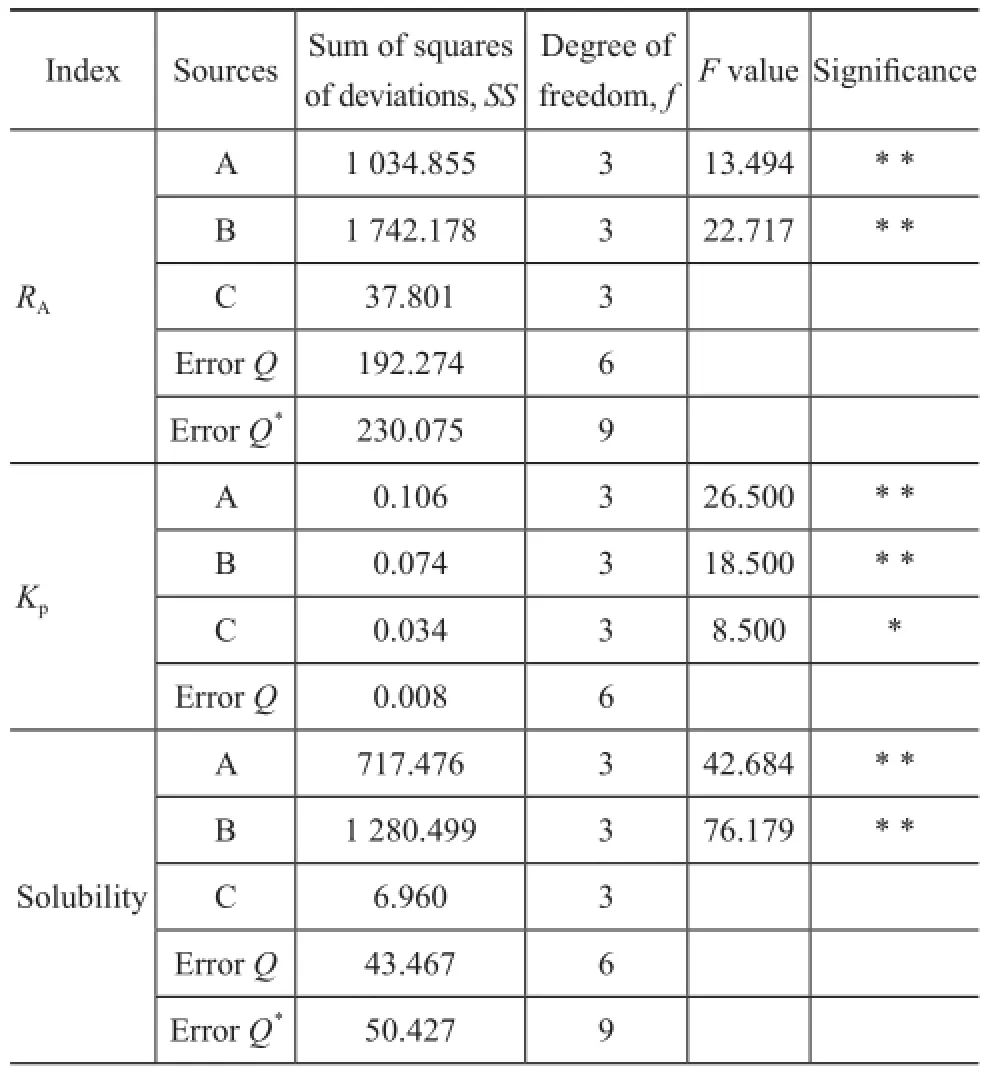
Table 3 Results of variance analysis on RA, Kp, and solubility
It can be concluded that all the factors have great signifcance to the extraction operation, therefore, the test error can be ignored, and the result of orthogonal test is reliable.
3.3.3 Three-stage counter-current extraction for mixed solvent
To investigate the performance of multi-stage extraction by using the mixed solvent, the three-stage countercurrent extraction experiment was operated at the best scheme of conditions for orthogonal test. The result is shown in Table 4.
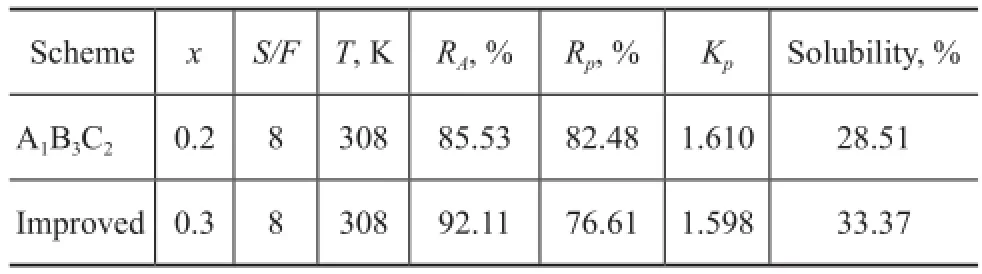
Table 4 Result of three-stage counter-current extraction by using mixed solvent
The three-stage counter-current extraction by using mixed solvent achieves a relatively highKp, andRpreaches 82.48%, whileRAis below 90%. To realize the separation of aromatics from naphtha, the satisfedRAshould be over 90%. As a result of variance analysis,RAincreases significantly with the increase ofxandS/Fratio. The load of extraction process will increase if theS/Fratio increases. Hence, it is an efficient way to increase the volume fraction of PC in the mixed solvent to improveRA. The influence ofxon extraction is shown in Figure 7. It is indicated that the solubility of mixed solvent to aromatics increases with the increase ofx, while the solubility ton-paraffins also increases in some degree, which leads to the decrease ofKpandRp. It can be noticed that the general score andKpdecrease signifcantly whenxincreases from 0.2 to 0.4. To ensure the recovery ofn-paraffns in the raffnate oil,xshould not be larger than 0.4. Therefore, further experiment was carried out under conditions leading to increasingxslightly from 0.2 to 0.3, viz.:x=0.3,S/F=8, andT=308 K, which is between the two investigated schemes of conditions in orthogonal test. The result is shown in Table 4. With the increase ofxfrom 0.2 to 0.3,RAincreases to a satisfactory value of 92.11% as expected, andRpdecreases slightly from 82.48% to 76.61%. The yield of extract oil is 33.37%. To achieve high aromatics yield of extract oil and relatively highn-paraffins yield of raffnate oil, the optimal conditions suitable to the mixed solvent throughout the three-stage countercurrent extraction are:x=0.3,S/F=8, andT=308 K.
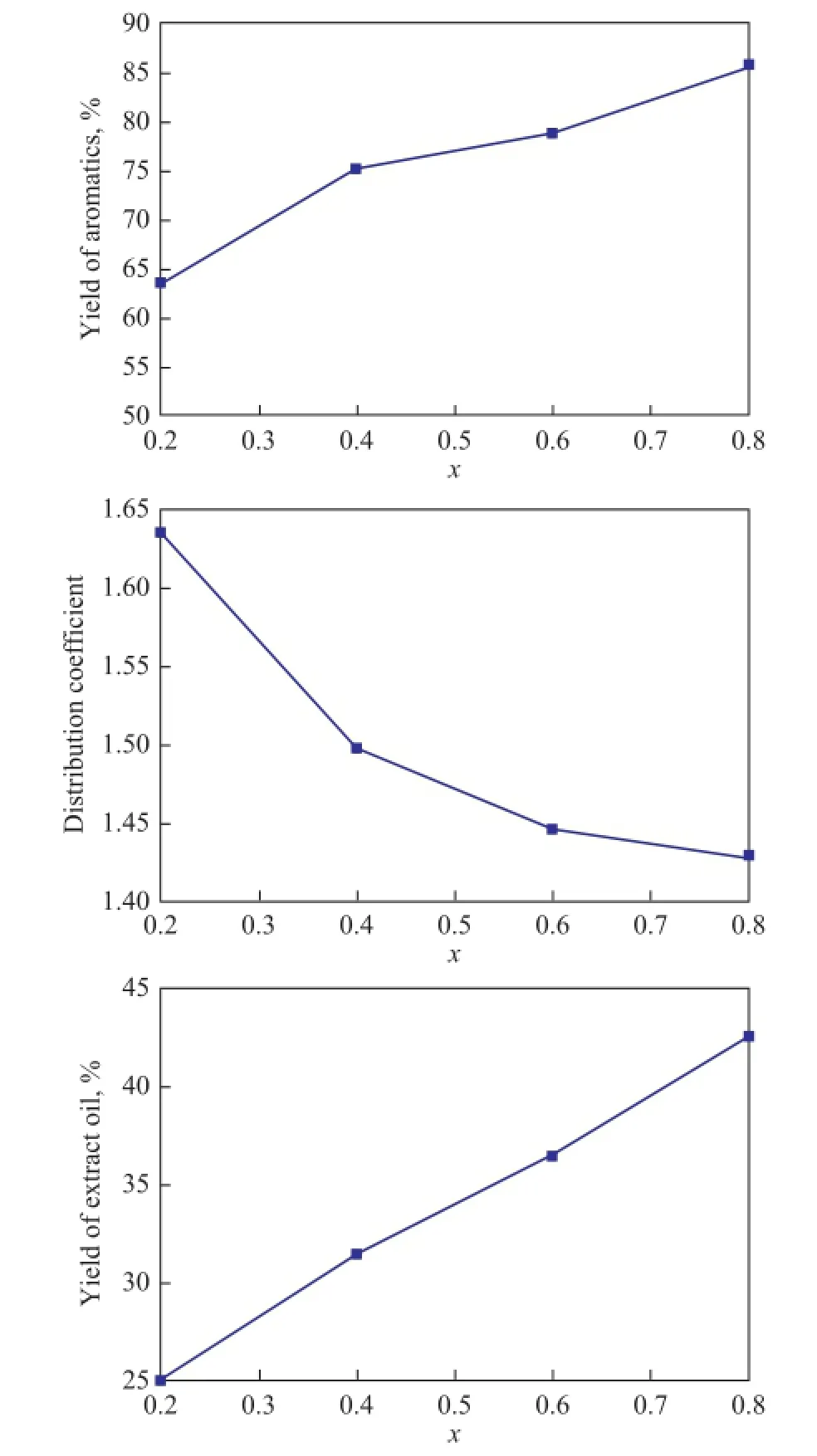
Figure 7 The in fl uence of factorxon extraction
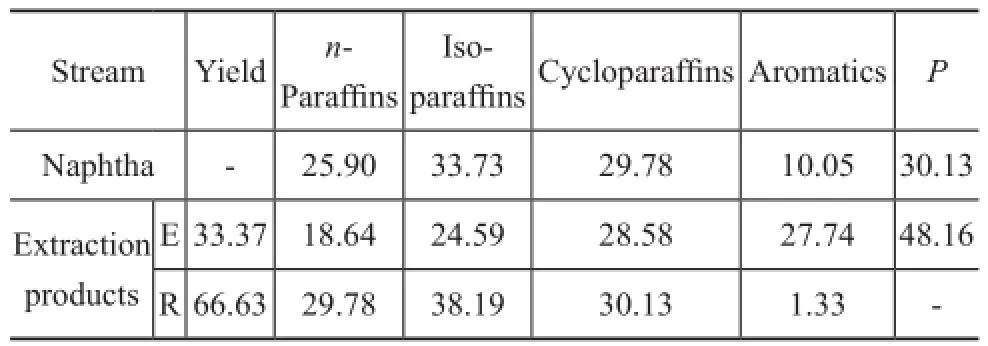
Table 5 Composition of naphtha and extraction products %
The composition of naphtha and extraction products obtained under optimal conditions is shown in Table 5. It is indicated that the aromatic content of extract oil increases to 27.74%. Meanwhile, the aromatics content of raffnate oil decreases significantly to 1.33%, and then-paraffin content of raffinate oil increases to 29.78%. It is found that the extraction process by using the mixed solvent can extract most of aromatics into extract oil, and an acceptablen-paraffn yield of raffnate oil is also obtained.
The performance of naphtha and extraction products was evaluated according to the demand of processes. For catalytic reforming process, the potential aromatics content of raw material can be calculated by equation (4); for steam cracking process, the total olefins (ethylene, propylene and butadiene) yields (yt) of raw material can be estimated by equation (13)[35], which is an empirical equation regressed by raw materials with similar composition. As compared with naphtha, the potential aromatic content of extract oil increases by 18.03%. Meanwhile, the total olefins yields of raffinate oil may increase by 2% due to the increase inn-paraffn content. As a result, the application of PC-DEG mixed solvent in the extraction process boosts a greater capacity in improving the utilization effciency of naphtha.

whereXnis the correctedn-paraffins content excluding the aromatics content (except for ethyl benzene).
4 Conclusions
Based on the molecule management, the extract oil with high potential aromatic content has been separated from light naphtha by using extraction process. A mixture of propylene carbonate and diethylene glycol has been found as a suitable solvent. In addition, the optimal extraction conditions include: a volume fraction of PC in mixed solvent of 0.3, a solvent to feed ratio of 8, and an extraction temperature of 308 K. By employing the extraction process, the aromatics content increases from 10.05% in naphtha to 27.74% in the extract oil. Furthermore, the aromatics yield of extract oil reaches 92.11%. As a result, the potential aromatics content increases impressively from 30.13% in naphtha to 48.16% in the extract oil. Meanwhile, the aromatics content of raffinate oil decreases to 1.33% while then-paraffin content increases from 25.90% in naphtha to 29.78% in the raffnate oil. Then-paraffn yield of raffnate oil can reach 76.61%. Consequently, as compared with naphtha, a higher total olefins yield can be obtained when using raffinate oil as the raw material for steam cracking. Hence, the current extraction process achieves an effcient separation of aromatics from naphtha, and the utilizationeffciency of naphtha is improved largely.
Acknowledgements: This work was financially supported by the Natural Science Foundation of Shanghai, China (16ZR1408100) and the Fundamental Research Funds for the Central Universities of China (22A201514010).
[1] Shen B, Liu J, Chen H, et al. Method for optimizing naphtha utilization by molecular sieve adsorption: China, CN1710030A[P]
[2] Liu J, Shen B, Sun H. Adsorption behaviour of normal paraffns in a fxed bed adsorber containing 5 ? molecular sieves[J]. Adsorption Science and Technology, 2006, 24(4): 311-320
[3] Meindersma G W, Podt A, Klaren M B, et al. Separation of aromatic and aliphatic hydrocarbons with ionic liquids[J]. Chemical Engineering Communications, 2006, 193(11): 1384-1396
[4] Rawat B S, Gulati I B. Liquid-liquid equilibrium studies for separation of aromatics[J]. Journal of Applied Chemistry and Biotechnology, 1976, 26(8): 425-35
[5] Sun H, Shen B, Liu J.n-Paraffns adsorption with 5A zeolites: The effect of binder on adsorption equilibria[J]. Separation and Purification Technology, 2008, 64(1): 135-139
[6] Miano F. Adsorption of hydrocarbon vapour mixtures onto zeolite 5A[J]. Colloids and Surfaces A—Physicochemical and Engineering Aspects, 1996, 110(1): 95-104
[7] Barcia P S, Silva J A C, Rodrigues A E. Octane upgrading of C5/C6light naphtha by layered pressure swing adsorption[J]. Energy Fuels, 2010, 24(9): 5116-5130
[8] Cao J, Shen B X, Liu J C. Optimal operation of simulated moving bed technology on utilization of naphtha resource[J]. Separation Science and Technology, 2012, 48(2): 246-253
[9] Silva J A C, Mata V G, Dias M M, et al. Effect of coke in the equilibrium and kinetics of sorption on 5 A molecular sieve zeolites[J]. Industrial and Engineering Chemistry Research Fundamentals, 2000, 39(4): 1030-1034
[10] Hamid S H, Ali M A. Comparative study of solvents for the extraction of aromatics from naphtha[J]. Energy Sources, Part A: Recvoery, Utilization and Environmental Effects, 1996, 18(1): 65-84
[11] Weissermel K, Arpe H-J. Aromatics-Production and Conversion[M]//Industrial Organic Chemistry, Forth Edition. Weinheim: Wiley-VCH Verlag GmbH, 2008
[12] Meindersma G W, Hansmeier A R, De Haan A B. Ionic liquids for aromatics extraction. Present status and future outlook[J]. Industrial and Engineering Chemistry Research, 2010, 49(16): 7530-7540
[13] Gaile A A, Zalishchevskii G D, Gafur N N, et al. Removal of aromatic hydrocarbons from reforming naphtha. Combined extraction-extractive-azeotropic distillation process[J]. Chemistry and Technology of Fuels and Oils, 2004, 40(4): 215-221
[14] Rodrigues M F M, Pinheiro R S, De Sant’ana H B, et al. Removal of aromatic hydrocarbons from hydrocarbon mixture using glycols at 303.15 K and 333.15 K and atmospheric pressure: Experimental and calculated data by NRTL and UNIQUAC models[J]. Fluid Phase Equilibria, 2015, 387: 135-142
[15] Banerjee T, Sahoo R K, Rath S S, et al. Multicomponent liquid-liquid equilibria prediction for aromatic extraction systems using COSMO-RS[J]. Industrial and Engineering Chemistry Research, 2007, 46(4): 1292-1304
[16] Saha M, Rawat B S, Khanna M K, et al. Liquid-liquid equilibrium studies on toluene+heptane+solvent[J]. Journal of Chemical and Engineering Data, 1998, 43(3): 422-426
[17] Wang W, Gou Z, Zhu S. Liquid-liquid equilibria for aromatics extraction systems with tetraethylene glycol[J]. Journal of Chemical and Engineering Data, 1998, 43(1): 81-83
[18] Ali S H, Lababidi H M S, Merchant S Q, et al. Extraction of aromatics from naphtha reformate using propylene carbonate[J]. Fluid Phase Equilibria, 2003, 214(1): 25-38
[19] Fahim M A, Merchant S Q. Liquid-liquid equilibria of systems containing propylene carbonate and some hydrocarbons[J]. Journal of Chemical and Engineering Data, 1998, 43(5): 884-888
[20] Lababidi H M S, Ali S H, Fahim M A. Optimization of aromatics extraction of naphtha reformate by propylene carbonate/diethylene glycol mixed solvent[J]. Industrial and Engineering Chemistry Research, 2006, 45(14): 5086-5097.
[21] Meindersma G W, De Haan A B. Conceptual process design for aromatic/aliphatic separation with ionic liquids[J]. Chemical Engineering Research and Design,2008, 86(7): 745-752.
[22] Rodriguez N R, Requejo P F, Kroon M C. Aliphatic-aromatic separation using deep eutectic solvents as extracting agents[J]. Industrial and Engineering Chemistry Research, 2015, 54(45): 11404-11412.
[23] Deng Xiu, Wu Junsheng. Chemical Separation Engineering[M]. Beijing: Science Press, 2013. (in Chinese)
[24] Hansen C M. Hansen Solubility Parameters: A User’s Handbook[M]. Boca Raton, FL: CRC Press LLC, 2007.
[25] Hansen C M. Three-dimensional solubility parameterkey to paint-component affnities: I. Solvents, plasticizers, polymers, and resins[J]. Journal of Paint Technology, 1967, 39(505): 104-117.
[26] Hansen C M. Three-dimensional solubility parameter-key to paint component affnities. II. Dyes, emulsifers, mutual solubility and compatibility, and pigments[J]. Journal of Paint Technology, 1967, 39(511): 505-510.
[27] Hansen C M, Skaarup K. Three-dimensional solubility parameter-key to paint component affnities. III. Independent calculation of the parameter components[J]. Journal of Paint Technology, 1967, 39(511): 511-514.
[28] Hildebrand J H. The Solubility of Non-Electrolytes[M]. Reinhold, NY: Reinhold Pub. Corp., 1950.
[29] Hildebrand J H, Scott R L. Regular Solutions[M]. Upper Saddle River, NJ: Prentice-Hall, 1962.
[30] Barton A F M. CRC Handbook of Solubility Parameters and Other Cohesion Parameters[M]. Boca Raton, FL: CRC Press, Inc., 1983.
[31] Heremisinoff N P. Industrial Solvents Handbook, Second Edition[M]. New York: Marcel Dekker, 2003.
[32] Saaty T L. How to make a decision: The analytic hierarchy process[J]. European Journal of Operational Research, 1990, 48(1): 9-26
[33] Shannon C E. A mathematical theory of communication[J]. The Bell System Technical Journal, 1948, 27(3): 379-423
[34] Chen Wei, Xia Jianhua. An optimal weights combination method considering both subjective and objective weight information[J]. Mathematics in Practice and Theory, 2007, 37(1): 17-22 (in Chinese)
[35] Wang D Q. Recombination for making good use of naphtha by solvent extraction technology[D]. East China University of Science and Technology, Shanghai, 2008 (in Chinese)
Received date: 2016-07-07; Accepted date: 2016-07-24.
Dr. Sun Hui, Telephone: +86-21-64252916; E-mail: sunhui@ecust.edu.cn.
- 中國(guó)煉油與石油化工的其它文章
- Effect of Magnetic Field on Tribological Properties of Lubricating Oils with and without Tricresyl Phosphate
- Preparation and Lubricating Properties of A New Antibacterial Emulsion Containing Nano-TiO2for Cold Rolling Strips
- Prediction of Coke Yield of FCC Unit Using Different Arti fi cial Neural Network Models
- Hydrodynamic Characteristics in an External Loop Airlift Slurry Reactor
- Microwave-Assisted Synthesis of Poly(Aspartic Acid-Itaconic Acid) Copolymer and Its Characterization
- Effects of Solution Chemistry Conditions and Adsorbent Surface Properties on Adsorption of Ni(II) on Laiyang Bentonite

