Microwave-Assisted Synthesis of Poly(Aspartic Acid-Itaconic Acid) Copolymer and Its Characterization
Zhang Yuling; Jiang Yijian; Hu Zhiguang; Wei Hanxiao; Guo Jun; Wang Jilong
(1. Department of Environmental Science & Engineering, North China Electric Power University, Baoding 071003; 2. Department of Computer, North China Electric Power University, Baoding 071003)
Microwave-Assisted Synthesis of Poly(Aspartic Acid-Itaconic Acid) Copolymer and Its Characterization
Zhang Yuling1; Jiang Yijian1; Hu Zhiguang1; Wei Hanxiao1; Guo Jun2; Wang Jilong1
(1. Department of Environmental Science & Engineering, North China Electric Power University, Baoding 071003; 2. Department of Computer, North China Electric Power University, Baoding 071003)
Poly(aspartic acid-itaconic acid) copolymer was synthesized from aspartic acid (Asp) and itaconic acid (Ita) under microwave irradiation. The effects of microwave power, microwave irradiation time, molar ratio of itaconic acid and aspartic acid, catalyst type, catalyst and organic solvent content on copolymer yield, and the performance for inhibition of CaCO3fouling were investigated. It was found that the product yield achieved a highest record of 95% when the amount of catalyst NaH2PO4was 0.012 mol, the amount of organic solvent propylene carbonate was 16 mL, the molar ratio of Asp/Ita was 3:1, the microwave output power was 1200 W and the irradiation time was 5.5 min. And the product performance for inhibition of calcium carbonate also reached a highest value of 94.38%. Structural characterization of the product showed that the product was the aspartic acid-itaconic acid copolymer.
aspartic acid; itaconic acid; copolymerization; scale inhibition
1 Introduction
In recent years, with the aggravation of water pollution, the fresh water resource is diminishing[1]. In order to improve this condition, water treatment agents are used in industries throughout the world[2]and are being improved all the time. Polyaspartic acid (PASP), a new type of polymer material[3], is a green water treatment agent and is widely used in the field of circulating cooling water, boiler water, oily water, etc., thanks to its nontoxicity, biodegradability[4-6], excellent scale and corrosion inhibition performance[7], and good inhibitory performance to CaSO4, CaCO3[8], Ca3(PO4)2
[3], etc. However, there is a slight difference between the polyaspartic acid and the currently marketed water treatment agents in terms of scale inhibition properties. In order to improve the scale inhibition performance, numerous efforts have been made to introduce hydrophobic group, carboxylic group, sulfonic group and phosphonyl radical to the side chain of PASP[9-11]. For example, Chen, et al. introduced amino group to the side chain of PASP to obtain Ser-PASP[12]. Xu, et al. obtained polyaspartic acid-melamine grafted copolymer by the reaction of polysuccinimide (PSI) with melamine[2]. However, the preparation of these copolymers is complex and the yield is not high[12].
In this paper, carboxylic group has been introduced to the side chain of PASP to improve its scale inhibition performance via the addition of itaconic acid (Ita). The introduction of carboxyl group makes the modifed aspartic acid chelate Ca2+stronger and the chelating molecules mixed into dirt layer to disturb the growth of microcrystals to improve the scale inhibition performance of modified aspartic acid[13]. During the synthesis of modified polyaspartic acid, there are several ways to synthesize the intermediate. Compared with the traditional heating methods, microwave radiation has gained extensive attention due to its high efficiency, energy saving and environmentally-friendly nature[14]. Thus the poly(aspartic acid-itaconic acid) copolymer (PAI) was synthesized from aspartic acid and itaconic acid under microwave irradiation in this paper. Its structure was characterized by the Fourier transform infrared spectrometer (FTIR) and its scale performance was also investigated.
2 Experimental
2.1 Materials and Equipment
The L-aspartic acid (99%) was obtained from the Shanghai Yuanye Biological Technology Co., Ltd. The itaconic acid (99%) was purchased from the Changsha Jingkang New Material Technology Co., Ltd. Other chemicals were commercially available and were used without further purifcation.
Instruments used in the present research included a microwave oven made by the Nanjing Sanle Microwave Technology Development Co., Ltd., which was available on several power settings (200 W, 400 W, 600 W, 800 W, 1 000 W, and 1 200 W), a Spectrum One Fourier transform infrared spectrometer (FTIR) made by the American PerkinElmer Co., Ltd., an electric vacuum drying oven made by the Shanghai Shuli Instrument Co., Ltd, an electric heated water bath pot made by the Beijing Medical Instrument Factory and a digital thermostat water bath pot made by the Jiangsu Jintan Universal Scientific Instrument Factory.
2.2 Synthesis of PAI
Aspartic acid, itaconic acid and catalyst were accurately weighed, stirred well after adding organic solvent and then reacted for 5.5 min under microwave irradiation to obtain a yellow fuffy product — polysuccinimide derivative (PSID). PSID was fully hydrolyzed by adding an appropriate amount of 6 mol/L NaOH solution. The color of the solution after hydroxylation turned into orange. The pH value was adjusted to 3.84 by using hydrochloric acid, and then excess anhydrous ethanol was added into the solution. The target product (PAI) was obtained after fltering, drying and grinding.
2.3 Calculation of PAI yield
Certain amount of PSID was weighed accurately and was fully dissolved by adding excess DMF. It was fltered and dried until a steady state was obtained. The resulting precipitate, including the unreacted aspartic acid or itaconic acid, was weighed accurately. The yield was calculated as:
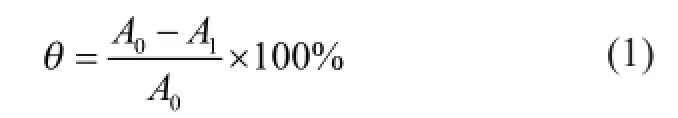
whereA0is the mass of reactants (g);A1is the mass of precipitate (g).
2.4 Characterization of PAI
Two mg of PAI were weighed and then mixed with 100—200 mg of dried KBr powder. The mixture was milled for 1—2 min by a ball mill, transferred to a suitable mold and then pressed into a transparent sheet followed by evacuation under vacuum. The KBr blank tablet was used as the reference for IR spectrometric analysis.
2.5 Measurement of scale inhibition performance of PAI
The scale inhibition rate of a scale inhibitor suited for calcium carbonate was measured by the static method according to the national standard GB/T 16632—2008“Determination of scale inhibition performance of water treatment agents——calcium carbonate deposition”. Its scale inhibition performance was calculated as:
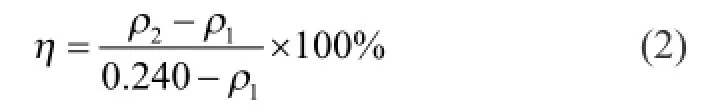
whereρ1is the concentration of calcium ions (Ca2+, mg/ mL) in the blank test solution after measurement;ρ2is the concentration of calcium ions (Ca2+, mg/mL) in the test solution with treatment agent after measurement; 0.240 is the concentration of calcium ions (Ca2+, mg/mL) in the test solution before measurement.
3 Result and Discussion
3.1 Copolymerization process of PAI
The copolymerization process of aspartic acid and itaconic acid is shown in Figure 1. The intermediate product PSID was obtained by copolymerization of aspartic acid and itaconic acid, and then PAI was hydrolyzed from PSID. The PAI yield was determined directly from PSID yield. Therefore, the yield of PAI should be studied by examining PSID yield.
3.2 Choice of reaction system
Since the total reaction materials constituted 0.02 mol, and the amount of organic solvent was 16 mL, the molar ratio of catalyst/reactant was 0.05. The test results under different conditions are shown in Table 1.
The following phenomena can be observed from Table 1: (1) Polymerization did not occur between aspartic acid monomers, and aspartic acid did not enter into polymerization reactions with itaconic acid in the absenceof catalysts and organic solvent.
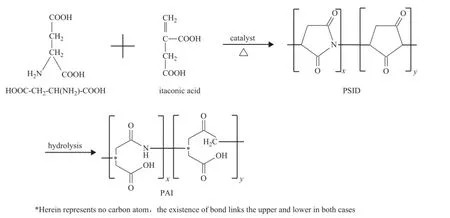
Figure 1 Condensation of L-aspartic acid and itaconic acid
(2) Without catalysts, aspartic acid and itaconic acid in an organic solvent did not react completely.
(3) Even in the presence of catalysts, the desired product could not be obtained from aspartic acid and itaconic acid with dimethylformamide serving as the organic solvent.
(4) Only in the presence of catalyst and the organic solvent propylene carbonate, the product with good performance and satisfactory yields could be formed.
The results showed that using the appropriate catalyst and solvent, the copolymerization of aspartic acid and itaconic acid could occur under microwave irradiation. This fact showed that the ideal product could be obtained by adjusting the technical parameters.
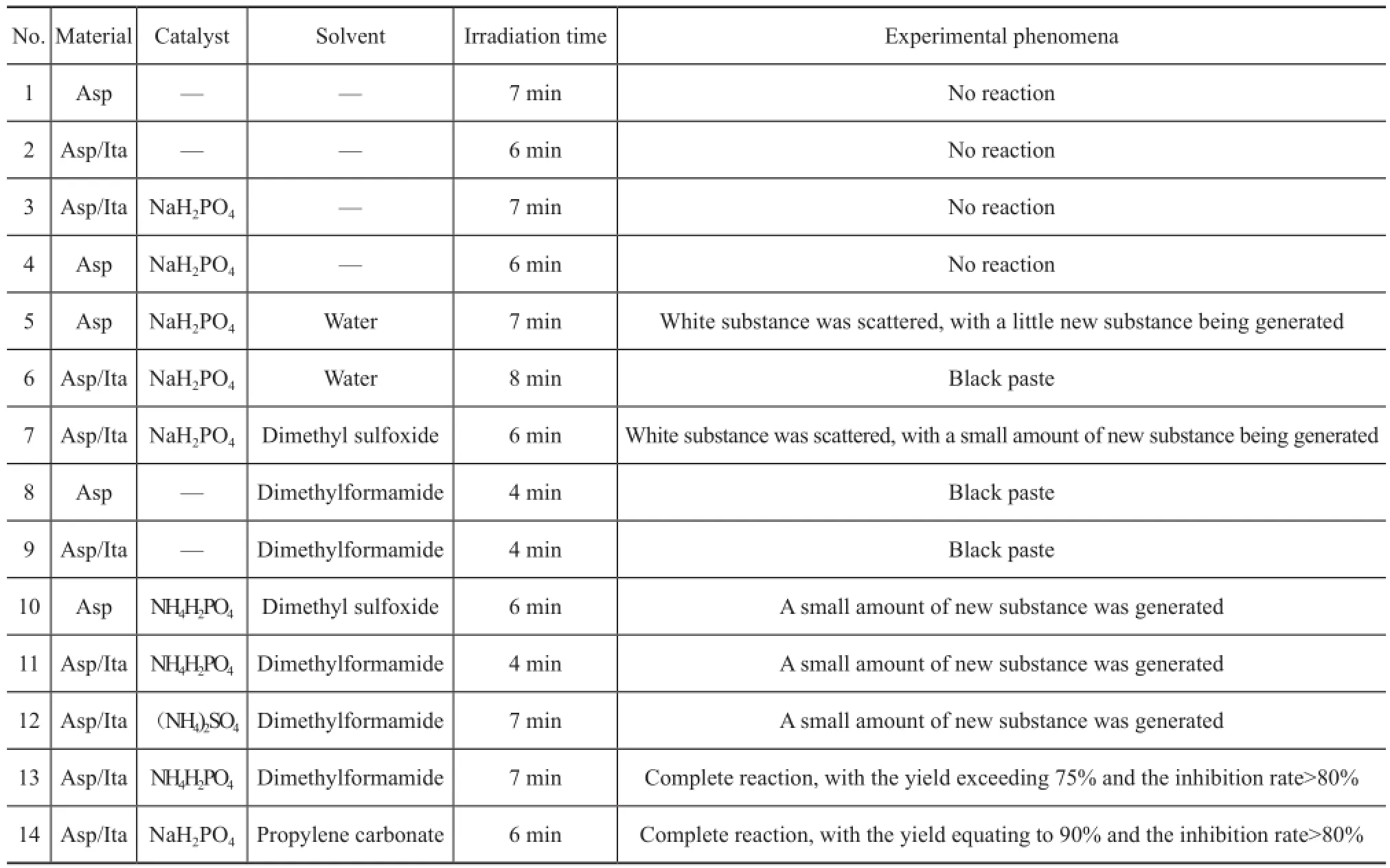
Table 1 Experimental phenomenon of different reaction systems
3.3 Characterization of products
The synthesized product PAI was purifed twice via ethanol precipitation. A small amount of sample was milled with KBr and pelleted for testing. The result is shown in Figure 2.
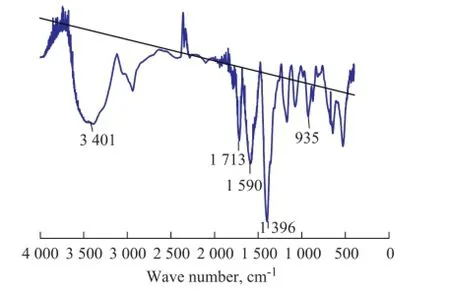
Figure 2 IR spectra of PAI
As shown in Figure 2, there was a remarkable band at 3 000—3 700 cm-1, indicating to the characteristic absorption peak of NH species, which verifed clearly the polymerization reaction during the synthesis; the band at 1 713 cm-1was assigned to the C=O vibration of the carboxyl group (—COOH); a strong band at 1 590 cm-1was assigned to the NH vibration of the amido group (—CONH); the absorption peak near 1 396 cm-1could be the bending vibration of —CH2— and —CH— radicals or the absorption peak of symmetric stretching vibration and asymmetric stretching vibration of —COO— in carboxylic acid salts; the fnger print band at 935 cm-1explained that —COOH groups existed in the polymer albeit not in the monomer. Thus it could be inferred that PAI had been successfully synthesized.
3.4 Effect of reaction conditions on the yield and scale inhibition performance
3.4.1 Effect of catalysts on the yield and scale inhibition performance
The experimental conditions were as follows: 0.2 mol of Asp and Ita were weighed to adjust the molar ratio of Asp/Ita at 2:1; 18 mL of propylene carbonate was used as the solvent; the amount of catalysts of various types was equal to 0.010 mol; and the microwave power was 1 200 W, with the microwave irradiation time equating to 6 min. Under these conditions, the effect of different catalysts on PSID yield and scale inhibition performance of PAI was investigated. The results are shown in Figure 3.
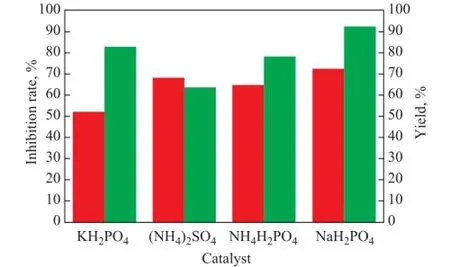
Figure 3 Effect of different catalysts on the yield of PSID and scale inhibition performance of PAI
As shown in Figure 3, the PSID yield was the highest and the scale inhibition performance of PAI to CaCO3was the best when sodium dihydrogen phosphate was used as the catalyst. Furthermore, the PSID yield and the inhibition rate of the product obtained in the presence of ammonium dihydrogen phosphate serving as the catalyst were close to those of the product obtained when sodium dihydrogen phosphate was used as the catalyst. However, sodium dihydrogen phosphate was selected as the catalyst because the ammonium salt was easily decomposed to produce ammonia at a high temperature resulting in environmental pollution.
3.4.2 Effect of the molar ratio of Asp/Ita on the PSID yield and scale inhibition performance of PAI
During the polymerization reaction 0.010 mol of sodium dihydrogen phosphate was used as the catalyst and 18 mL of propylene carbonate were used as the solvent, while the microwave power was 1 200 W, with the microwave irradiation time equating to 6 min. Under these conditions, 0.2 mol of Asp and Ita were weighed accurately and the ratio of Asp/Ita was varied. The effect of the molar ratio of Asp/Ita on the PSID yield and the scale inhibition performance of PAI was investigated. The results are shown in Figure 4.
As shown in Figure 4, the PSID yield was increased signifcantly with the increase in the proportion of itaconic acid whenn(Asp):n(Ita) was in the range of 9:1 to 7:3; the yield started to drop when then(Asp):n(Ita) exceeded 7:3. The inhibition rate of PAI to CaCO3achieved a best value of 88.56% when the molar ratio of Asp/Ita was 3:1. Upon comprehensively taking into account the yield and scaleinhibition performance, the best molar ratio of ASP/ITA was chosen as 3:1.
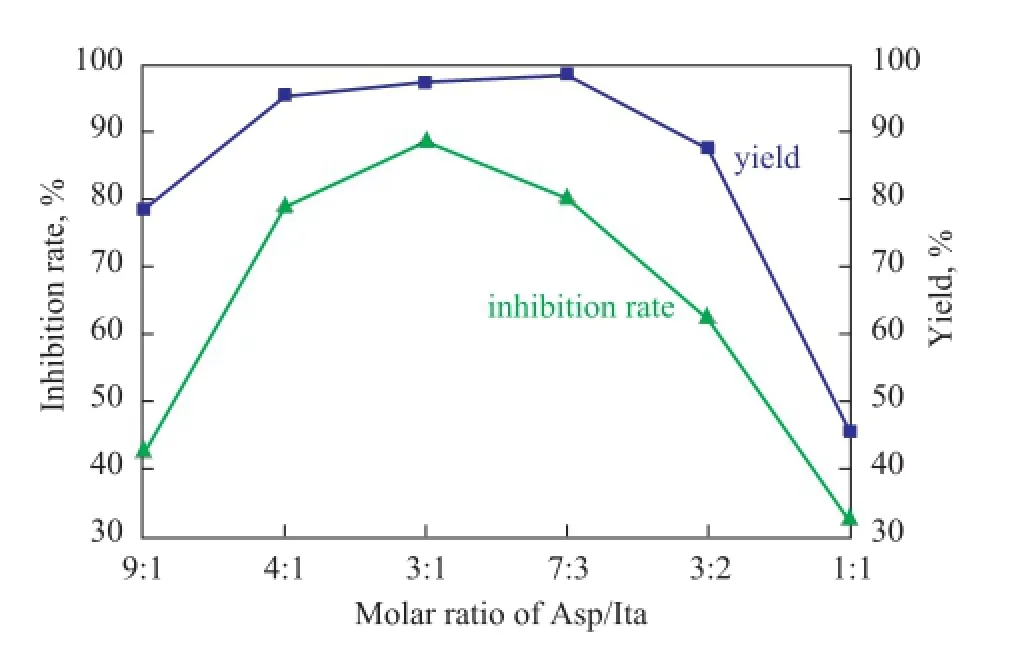
Figure 4 Effect of the molar ratio of Asp/Ita on the yield ofPSID and scale inhibition performance of PAI
3.4.3 Effect of microwave irradiation time on the yield and scale inhibition performance
During the polymerization reaction 0.2 mol of Asp and Ita were weighed with the molar ratio of Asp/Ita set at 3:1, and 0.010 mol of sodium dihydrogen phosphate was used as the catalyst, while 18 mL of propylene carbonate were used as the solvent, with the microwave power being specified at 1 200. Under these conditions, the effect of microwave irradiation time on PSID yield and scale inhibition performance of PAI was investigated. The test results are shown in Figure 5.
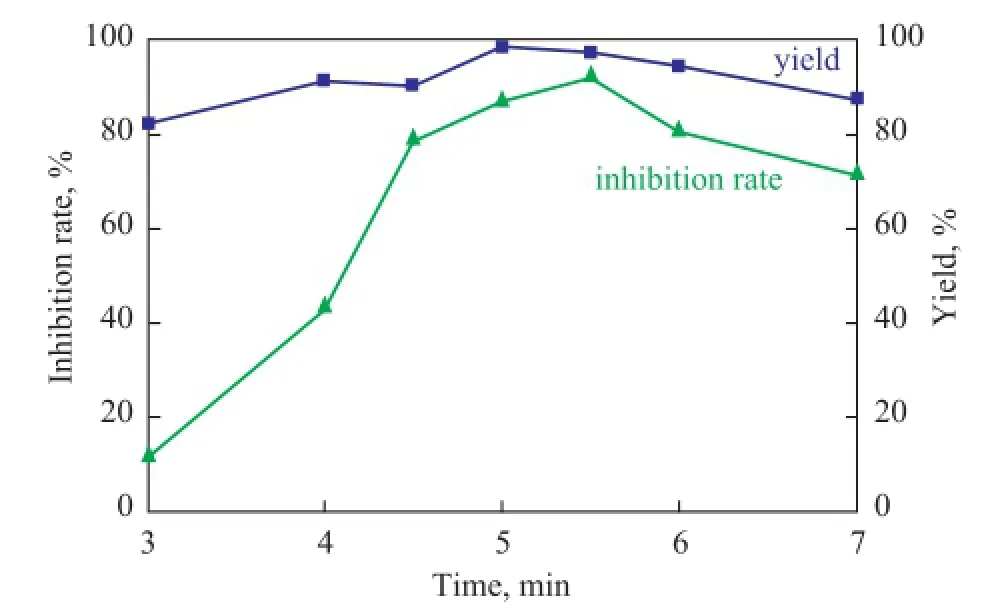
Figure 5 Effect of microwave irradiation time on the yield of PSID and scale inhibition performance of PAI
As shown in Figure 5, the PSID yield and inhibition rate of PAI were on the rise with the increase of microwave irradiation time in the range of 3 min to 5.5 min and declined when the microwave irradiation time exceeded 5.5 min. Perhaps the polymerization reaction could take place easily in the initial stage while the material concentrations were still high. When the microwave irradiation time was longer than 5.5 min, it would result in carbonized deterioration of PSID partially. So the yields and inhibition rate declined. Therefore, the best microwave irradiation time was set as 5.5 min.
3.4.4 Effect of microwave power on the yield and scale inhibition performance
During the polymerization reaction 0.2 mol of Asp and Ita were weighed with the molar ratio of Asp/Ita being specified at 3:1, and 0.010 mol of sodium dihydrogen phosphate were used as the catalyst, while 18 mL of propylene carbonate was used as the solvent with the microwave irradiation time set at 5.5 min. Under these conditions, the effect of microwave power on the PSID yield and scale inhibition performance of PAI was investigated. The results are shown in Figure 6.
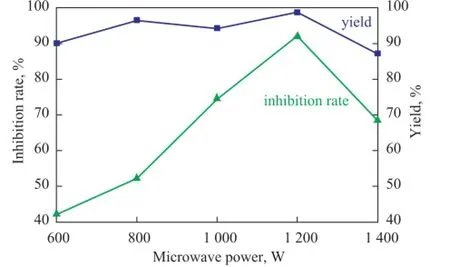
Figure 6 Effect of microwave power on the yield of PSID and scale inhibition performance of PAI
As shown in Figure 6, if the microwave irradiation energy was provided with a power of less than 600 W, which was lower than the activation energy required for conducting the reaction, the reaction would not occur, and the PSID yield and the inhibition rate of PAI were on the rise with the increase of microwave power in the range of 600 W—1 200 W, but they would decline if the microwave irradiation energy was higher than 1 200 W. The PSID yield was increased when the majority of the amino acid monomer was transformed to polymer with the increase of the microwave power. But the PSID yield and the inhibition rate of PAI declined as the microwave irradiation energy exceeding 1 200 W might cause overheating, which could result in the carbonized deterioration of PSID partially. Therefore, the best microwave power was specifed at 1 200 W.
3.4.5 Effect of catalyst amount on the yield and scale inhibition performance
During the polymerization reaction 0.2 mol of Asp and Ita were weighed, with the molar ratio of Asp/Ita specified at 3:1, and sodium dihydrogen phosphate was used as the catalyst, while 18 mL of propylene carbonate were used as the solvent, with the microwave power set at 1 200 W. Under these conditions, the effect of catalyst amount on the PSID yield and the scale inhibition performance of PAI was investigated. The results are shown in Figure 7.
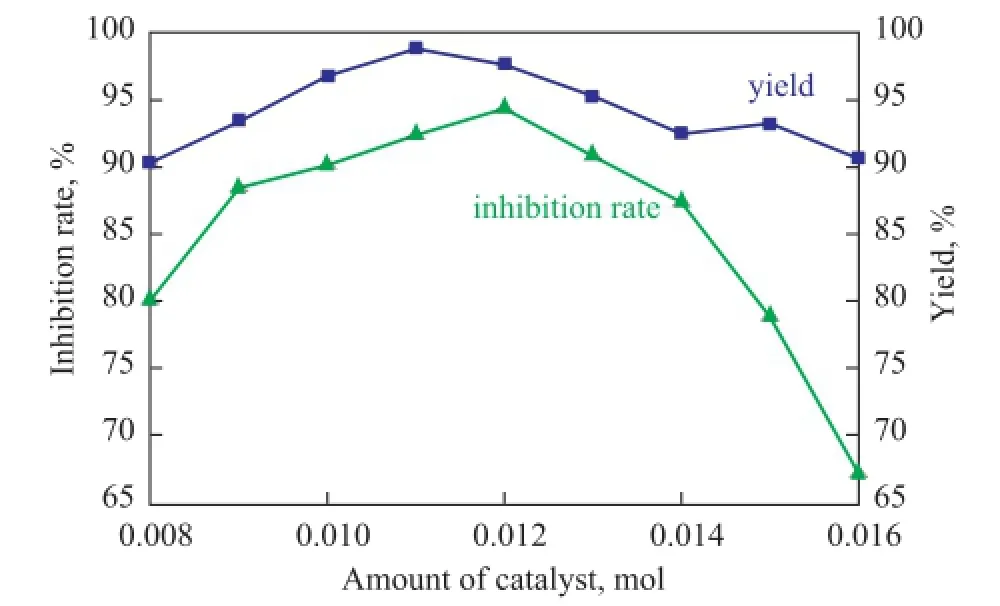
Figure 7 Effect of amount of catalyst on the yield of PSID and scale inhibition performance of PAI
As shown in Figure 7, with the increase of the amount of catalyst, the PSID production frst increased and then leveled off, while the inhibition rate of PAI at frst increased and then decreased. The required activation energy was lowered by the added catalyst and the PSID yield was enhanced. When the catalyst amount reached 0.012 mol, the PSID yield started to level off. The reason why the inhibition rate at frst increased and then decreased was thought to be associated with the copolymer molecular weight, which increased with an increasing catalyst concentration. When the catalyst amount exceeded 0.012 mol, the molecular weight of copolymer could become too large which might lead to a decline in the scale inhibition ability of the product. Therefore, the optimum amount of catalyst was specifed at 0.012 mol.
3.4.6 Effect of amount of organic solvent on the yield and scale inhibition performance
During the polymerization reaction 0.2 mol of Asp and Ita were weighed, with the molar ratio of Asp/Ita set at 3:1, and 0.010 mol of sodium dihydrogen phosphate was used as the catalyst, while propylene carbonate was used as the solvent, with the microwave irradiation time set at 5.5 min and the microwave power specified at 1 200 W. Under these conditions, the effect of organic solvent amount on the yield of PSID and the scale inhibition performance of PAI was investigated. The results are shown in Figure 8.
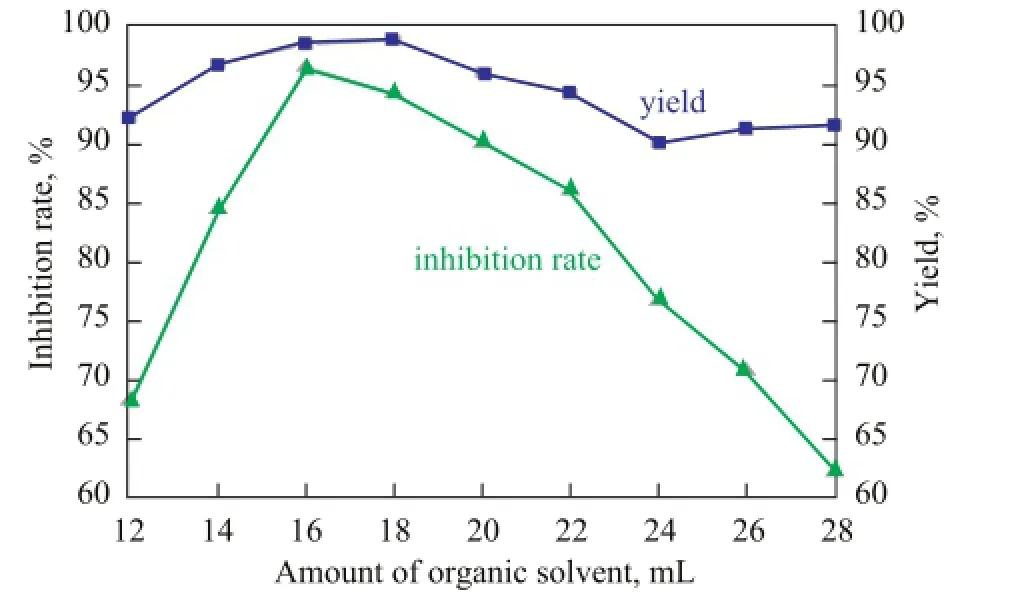
Figure 8 Effect of organic solvent amount on the yield of PSID and THE scale inhibition performance of PAI
As shown in Figure 8, the PSID yield and the inhibition rate of PAI exhibited a growing trend with the increase of the amount of organic solvent and the inhibition rate of PAI reached a peak value of 96.62% when the amount of organic solvent was 16 mL. When the amount of the solvent was over 16 mL, the PSID yield and the inhibition rate of PAI began to decline. It was thought that too much organic solvent could reduce the concentration of the reactants which would result in the decrease of PSID yield and also the absorbed microwave energy, resulting in the decrease of copolymer molecular weight, which would lead to a decline of the inhibition rate of PAI. Therefore, the optimum amount of organic solvent was 16 mL.
4 Conclusions
The poly(aspartic acid-itaconic acid) copolymer (PAI) was synthesized by the reaction of aspartic acid and itaconic acid. The optimum conditions for synthesis of PAI covered: an Asp/Ita molar ratio of 3:1 using 16 mL of propylene carbonate as the solvent in the presence of 0.012 mol of sodium dihydrogen phosphate functioning as the catalyst under microwave irradiation with a power of 1 200 W for 5.5 min. The product yield was more than 95% and the best scale inhibition rate to CaCO3was 94.38%. Compared with other modified PASP, a better scale inhibition performance was attributed to the carbox-ylic group in PAI, which could enhance the chelating ability on Ca2+ions to increase the solubility of calcium deposits.
Acknowledgments: This work was supported fnancially from the National Natural Science Foundation of China (Grant No. 51308211), the State Key Laboratory of Urban Water Resource and Environment (HIT) (Grant No. ES200903) and the Fundamental Research Funds for the Central Universities (Grant No. 2015MS63).
[1] Antony A, Low J H, Gray S, et al. Scale formation and control in high pressure membrane water treatment systems: A review[J]. Membr Sci, 2012, 383(1): 1-16
[2] Xu Y, Zhao L L, Wang L N, et al. Synthesis of polyaspartic acid-melamine grafted copolymer and evaluation of its scale inhibition performance and dispersion capacity for ferric oxide[J]. Desalination, 2012, 286: 285-289
[3] Cheng J F, Yang W L, Liu H M, et al. Research of Fenton reagents on treating azithromycin wastewater[J]. Industrial Water Treatment, 2009, 29(8): 66-68 (in Chinese)
[4] Le T X, Munekage Y, Kato S I. Antibiotic resistance in bacteria from shrimp farming in mangrove areas[J]. Science of the Total Environment, 2005, 349(1):95-105
[5] Guo J H, Severtson S J. Application of classical nucleation theory to characterize the infuence of carboxylate-containing additives on CaCO3nucleation at high temperature, pH, and ionic strength[J]. Ind Eng Chem Res, 2003, 42(14): 3480-3486
[6] Annibaldi V, Rooney A D, Breslin C B. Corrosion protection of copper using polypyrrole electrosynthesised from a salicylate solution[J]. Corros Sci, 2012, 59(3): 179-185
[7] Zhao Y S, Li Y Q, Peng L. Synthesis of modifed polyaspartic acid copolymer and its scale inhibiting performance to calcium sulfate[J]. Chemistry and Bioengineering, 2011, 28 (3): 81-84
[8] Shang X M, Liu Q, Li G L. Synthesis of polyaspartic acid copolymer and evaluation its scale properties[J]. Modern Chemical Industry, 2011, 31(S2): 50-52 (in Chinese)
[9] Zhang Y X, Wu J H, Hao S C, et al. Synthesis and inhibition effciency of a novel quadripolymer inhibitor[J]. Chin J Chem Eng, 2007, 15(4): 600-605
[10] Gu X X, Qiu F X, Zhou X, et al. Preparation and application of polymers as inhibitors for calcium carbonate and calcium phosphate scales[J]. Int J Polym Mater Polym Biomater, 2012, 62: 323-329
[11] Kumar N M; Kumar G S, Varaprasad K. Development of anti-scale poly(aspartic acid-citric acid) dual polymer systems for water treatment[J]. Environmental Technology, 2014, 35(23): 2903-2909
[12] Chen J X, Xu L H, Han J. Synthesis of modifed polyaspartic acid and evaluation of its scale inhibition and dispersion capacity[J]. Desalination, 2015, 358: 42-48
[13] Guo X H, Shi M C, Du S Z. Advance in research of polyepoxysuccinic acid[J]. Corrosion Science and Protection Technology, 2014, 26(4): 82-84 (in Chinese)
[14] Zheng B Z, Zheng J Y, Yu T T, et al. Fast microwaveassisted synthesis of Au-Ag bimetallic nanoclusters with strong yellow emission and their response to mercury (II) ions[J]. Sensors and Actuators B Chemical, 2015, 221: 386-392
Received date: 2016-04-19; Accepted date: 2016-07-14.
Dr. Zhang Yuling, Telephone: +86-312-7525514; E-mail: zhangyuling_hit@163.com.
- 中國煉油與石油化工的其它文章
- Effect of Magnetic Field on Tribological Properties of Lubricating Oils with and without Tricresyl Phosphate
- Preparation and Lubricating Properties of A New Antibacterial Emulsion Containing Nano-TiO2for Cold Rolling Strips
- Prediction of Coke Yield of FCC Unit Using Different Arti fi cial Neural Network Models
- An Extraction Process for Optimal Utilization of Naphtha Based on Molecule Management
- Hydrodynamic Characteristics in an External Loop Airlift Slurry Reactor
- Effects of Solution Chemistry Conditions and Adsorbent Surface Properties on Adsorption of Ni(II) on Laiyang Bentonite

