Effects of Solution Chemistry Conditions and Adsorbent Surface Properties on Adsorption of Ni(II) on Laiyang Bentonite
(College of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590)
Effects of Solution Chemistry Conditions and Adsorbent Surface Properties on Adsorption of Ni(II) on Laiyang Bentonite
Cao Xiaoqiang; Yan Bingqi; Xue Jianliang; Wang Qian; Wang Yaping; Huang Yongqing; Zhang Yan; Lyu Xianjun
(College of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590)
The effects of solution chemistry conditions and adsorbent surface properties on the adsorption of Ni(II) on Laiyang bentonite were investigated via the batch technique. Potentiometric and mass titration techniques were employed in the batch experimental methods, and the results showed that the point of zero net proton charge (PZNPC) of bentonite at different ionic strength denoted pHPZNPCto be 8.2±0.1. The removal of Ni(II) from the solution increased with an increasing bentonite dosage, with the maximum removal effciency equating up to 99%. The adsorption of Ni(II) on bentonite increased with an increasing pH value at a pH value of <8.5, and reached a Ni(II) removal effciency of >99% at a pH value of >10.2. The Ni(II) adsorption performance exhibited different responses to cations (K+, Na+) but was not infuenced by the background anions (NO3-, Cl-, and ClO4
nickel, surface properties, adsorption, waste catalyst, bentonite
1 Introduction
With the development in the petrochemical industry, the amount of catalysts used has been increasing annually. The presence of heavy metals in spent catalyst is a potentially adverse factor that can affect the environment. Heavy metals contamination in human environment is a major concern because of the potential health threats to plants, animals, and humans by such elements. Nickel is a toxic heavy metal widely used in the petrochemical, electroplating, stainless steel manufacture, and storage battery industries[1]. Nickel can accumulate in soil and water reservoirs, leading to harmful effects on humans. Therefore, decreasing the nickel concentration before discharge to the environment is important to protect environmental and human health.
Heavy metal ions have been removed from wastewater through various methods, such as adsorption, chemical precipitation, ion exchange, membrane separation, and electrolysis froth fotation[2-3]. However, the application of these conventional techniques is limited by their complicated operation and relatively high cost. Adsorption is an economical, convenient, and effective alternative method to remove heavy metals[4]. However, adsorption is limited by its tedious regeneration process and by the high cost of conventional adsorbents (e.g., activated carbon); hence, the development of cheaper but equally effective substitutes is needed. Numerous adsorbents, such as solid humic acid, hematite, red mud, and clay materials, have been used to remove heavy metal ions. Clay materials such as vermiculite, kaolinite, palygorskite, and bentonite have been widely studied for heavy metal adsorption from wastewater[5-6].
For decades, bentonite has been extensively investigatedin the field of heavy metal removal because of its high cation exchange capacity, excellent mechanical and chemical stability, and low cost. Bentonite is mainly composed of montmorillonite, which is a 2:1-type aluminosilicate clay mineral. The montmorillonite is composed of two tetrahedrally coordinated sheets of silicon ions surrounding a sandwiched octahedrally coordinated sheet of aluminum ions. Substitutions within the lattice structure of trivalent aluminum for quadrivalent silicon in the tetrahedral sheet and of low-valence ions, particularly magnesium, for trivalent aluminum in the octahedral sheet produce a net negative charge on the clay surface. The charge imbalance is offset by exchangeable cations, such as H+, Na+, or Ca2+, on the layer surfaces[7]. In consideration of the crystal chemical features of montmorillonite, the removal of heavy metal ions from aqueous solutions by using bentonite involves two distinct mechanisms: (1) an ion exchange reaction at permanent-charge sites and (2) formation of complexes with surface hydroxyl groups[8]. The adsorption of heavy metal ions on bentonite is influenced by many factors, including the initial concentration of heavy metal ions, the adsorbent dosage, the contact time, the pH value, the ionic strength, and the foreign ions. Among these infuential factors, the pH value and the ionic strength (i.e., background electrolyte concentration) are most important because both can affect the species of heavy metal ions, the surface potential and the surface chemical properties of bentonite, and the affnity between heavy metal ions and bentonite, thereby infuencing also the ion exchange and surface complexation reaction[9].
The adsorption ability of bentonite is generally determined by the surface chemical nature and key parameters of solutions[10-11]. Hence, a local bentonite from Laiyang County (Shandong, China) was used in this study as an adsorbent to remove Ni(II) from aqueous solutions. The Laiyang bentonite (LYB) was characterized through the zeta potential and the acid-base potentiometric titration analyses. The influence of several parameters, such as the bentonite dosage, the pH value and the ionic strength of solution, the foreign ions, the reaction time, and the temperature, were investigated via the batch technique. The Langmuir, Freundlich, and Dubinin-Radushkevich (D-R) models were used to analyze the adsorption equilibrium. Thermodynamic parameters, such as the Gibbs free energy (ΔG), the enthalpy change (ΔH), and the entropy change (ΔS) of adsorption, were evaluated, and the adsorption mechanism was discussed in detail following the experimental results. This study aimed to evaluate the bentonite surface properties, such as the point of zero net proton charge (PZNPC), the surface charge density and the zeta potentials, in relation to its potentialities as an adsorbent for heavy metal removal in aqueous solutions. It also aimed to investigate the adsorption characteristics of LYB for removal of Ni(II) from aqueous solutions.
2 Experimental
2.1 Materials
The bentonite sample was obtained from the Laiyang county (Shandong, China). The cation exchange capacity was 0.56 meq/g, which was determined using the ammonium acetate saturation method. A stock solution of Ni(II) species was prepared by dissolving a required amount of Ni(NO3)2·6H2O (Sinopharm GR) in the double-distilled water at room temperature. Other reagents, such as HNO3, NaOH, and CH3COONH4, were purchased in compliance with the analytical reagent purity and used directly without any further purifcation.
2.2 Methods
Adsorption studies were performed via the batch technique. Known amounts of bentonite in polytetrafuoroethene conical fasks containing 40 mL of known concentration of Ni(II) solution were shaken at 100 rpm using a thermostatic shaker for each corresponding time interval atT= 293 K, 313 K, and 333 K, respectively. The solutions were then centrifuged (Thermo Fisher Ltd.) at 5 000 r/min for 10 min. The supernatant was fltered, and the fltrate was analyzed for Ni(II) concentration by fame atomic absorption spectrophotometry (FAAS, Shimadzu Ltd.). The adsorption effciency (η) and adsorption capacity (qe) of bentonite were calculated using Eqs. (1) and (2), respectively:
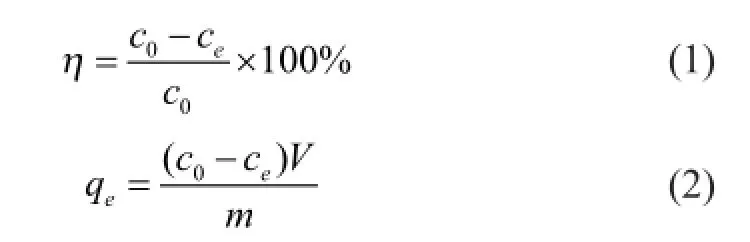
whereqeis the adsorbed mass of Ni(II) per gram of ben-tonite (mg/g),c0is the initial Ni(II) concentration in solution (mg/L),ceis the equilibrium Ni(II) concentration in solution (mg/L),mis the mass of the bentonite used (g), andVis the volume of solution (L).
The PZNPC of bentonite was determined using potentiometric and mass titration techniques as described by Guo, et al.[12]and Avena, et al.[13], respectively. The acid-base potentiometric titration was performed to measure the surface proton adsorption. Experiments for charge determination were carried out under ambient temperature using 0.5 g of bentonite in 40 mL of NaNO3electrolyte solution, and the resulting dispersion was allowed to equilibrate for 12 h in a thermostatic shaker operating at a revolution rate of 100 r/min and a temperature of 293±0.1 K. The suspension was then titrated with a standard NaOH solution, and the pH value was measured and recorded when the potential variation was lower than 0.5 mV/min. Titration was stopped approximately at a pH value of 11. To check the reversibility, the same suspension was titrated with a standard HNO3solution. A blank titration experiment was carried out following the same method. In all cases, the pH value was measured using a digital pH meter (Mettler-Toledo International Inc.). As regards the potentiometric titration results, the net surface charge densityσpwas calculated using Eq. (3):
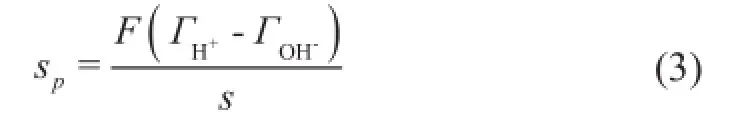
whereσpis the surface charge (C/m2),Fis the Faraday constant, andsis the specific surface area of bentonite sample (m2/g).andare the adsorbed amounts of H+and OH-ions (mmol/g) during titration, respectively. In this manner, the dependence of the surface charge density on pH value and electrolyte concentration was obtained.
The mass titration experiments were performed to determine the PZNPC of bentonite using the same conditions of acid-base potentiometric titration. Portions of 0.05 g of dry bentonite sample were added to 40 mL of NaNO3solution at a given ionic strength and a pH value in the range of between 2 and 11. After each solid addition, the suspension was shaken for 30 minutes in a thermostatic shaker at a revolution rate of 100 rpm and a temperature of 293 ± 0.1 K; the pH value was measured and recorded when the potential variation was lower than 0.5 mV/min. After equilibration, a new 0.05 g portion of the sample was added, and this procedure was repeated until the suspension pH value did no further change following addition of the sample.
The electrophoretic mobility of the bentonite sample at different pH values and ionic strength was obtained using a ZetaPlus (Brookhaven Instruments Corporation) and then converted to the zeta potential using the Helmholtz-Smoluchowski equation[14].
3 Results and Discussion
3.1 Potentiometric titration
pHPZNPCis defned as the pH where the net proton surface charge is equal to zero. As shown in Figure 1, the surface charge of bentonite was positive below pH 8.2 because of proton uptake along the surface but became negative when the net proton release occurred above pH 8.2. Thus, the pHPZNPCfor the bentonite was determined to be approximately 8.2, which was similar to previous fndings[13,15-16]. In Figure 1, the ionic strength effect along the entire pH range studied was not signifcant, which agreed well with the previous studies[14,17].
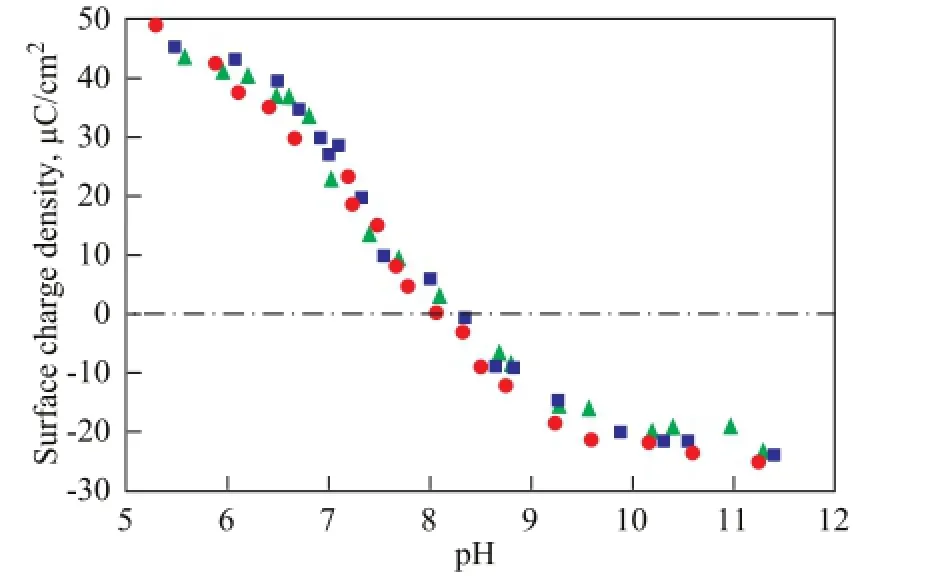
Figure 1 Surface charge density of bentonite as a function of pH at different ionic strength
3.2 Mass titration
Mass titration data are presented in Figure 2. The pH gradually changed with the addition of solids and asymptotically approached a limiting value. The direction of pH variation depended on the initial pH of the solution. The pHPZNPCestimated in this way was approximately 8.3; this pH value was a good approximation of the results of potentiometric titration.
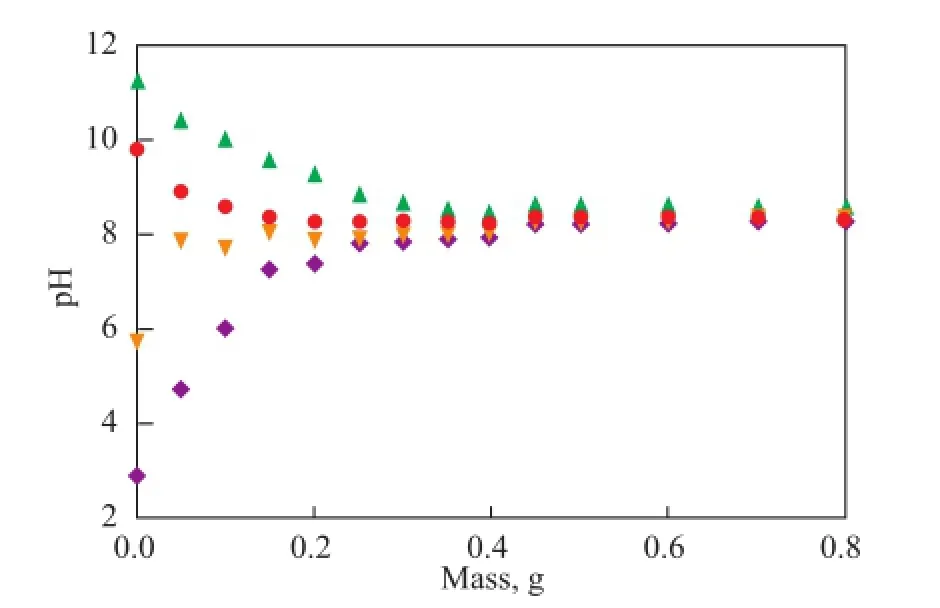
Figure 2 Mass titration curves for the bentonite obtained at ionic strength of 0.01 mol/L
3.3 Zeta potential measurements
The zeta potentials of bentonite measured as a function of pH at different ionic strength are shown in Figure 3. Bentonite showed negative zeta potentials throughout the pH range of 3—11 at ionic strength of 0.001 mol/L, 0.01 mol/L, and 0.1 mol/L, respectively. Among the many electrokinetic measurements for bentonite and montmorillonite, the zeta potentials were generally negative (-30 mV to -50 mV) at pH between 2—12 without an isoelectric point (IEP)[18-19]. The particle zeta potential increased with the pH value. This result can be ascribed to the previous fnding[14]that the generation of positive charges on the bentonite surface could largely compensate for the permanent negative charges of the basal plane faces as the pH value decreases at lower ionic strength. However, the contribution of the positive charges at the edge sites to the total surface charge might not be enough to reach a positive value; hence, an IEP was not observed in the pH range that was studied thereupon. An increase in the ionic strength of the bentonite suspension might cause a contraction of the electric double layer, a decrease of the surface potential
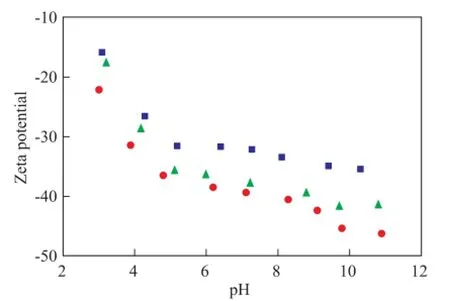
Figure 3 Zeta potentials of the bentonite suspension as a function of pH at different ionic strength
(and zeta potential), and fnally a coagulation of the bentonite suspension, which could agree well with the double layer theory.
3.4 Effect of adsorbent dosage on Ni(II) adsorption on bentonite
The adsorption of Ni(II) on bentonite as a function of adsorbent dosage is shown in Figure 4. The removal of Ni(II) from solution increased with an increasing adsorbent dosage; the maximum removal of 99% (approx.) could be achieved with an adsorbent dosage of 0.025 g/mL.
The increase in removal effciency was negligible at higher adsorbent dosages. The functional groups on the bentonite surfaces increased with an increasing adsorbent dosage, rendering more surface sites available to form complexes with Ni(II) on the bentonite surfaces. The results indicated that the adsorption capacity (qe) of Ni(II) on bentonite decreased with an increasing adsorbent dosage. The solid surface was composed of sites with a spectrum of binding energies. At a low adsorbent dosage, all types of surface sites were entirely exposed for adsorption and the surface became rapidly saturated, which increased the adsorption capacity. However, at a high adsorbent dosage, the availability of high-energy sites decreased and a large fraction of low-energy sites became occupied, which would decrease the adsorption capacity[20]. Moreover, a high adsorbent dosage increased the probability of collision between bentonite particles and could lead to particle aggregation, causing a decrease in the total surface area andan increase in the diffusional path length, both of which could contribute to the decrease in adsorption capacity.
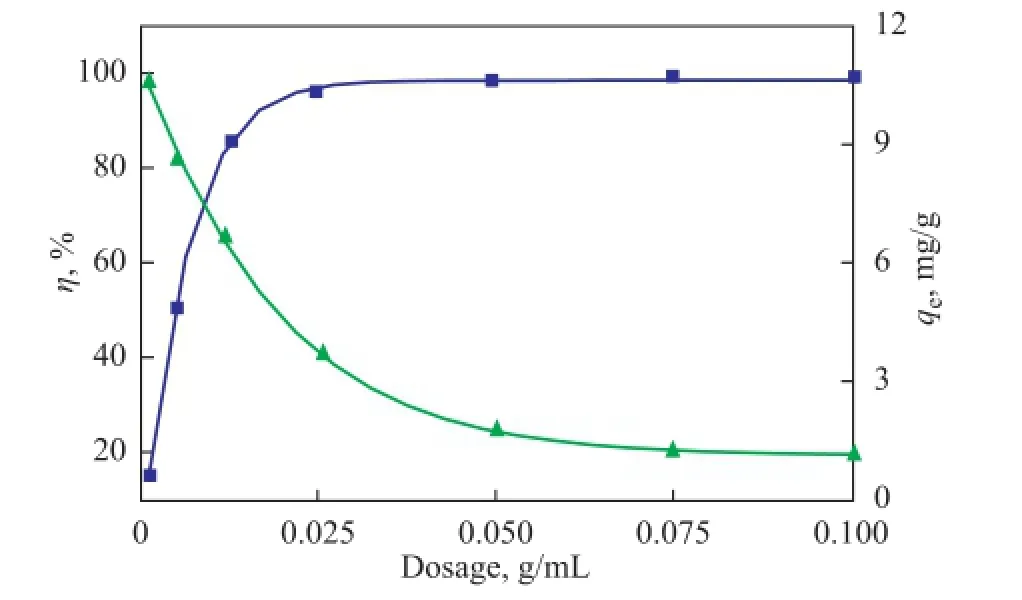
Figure 4 Effect of adsorbent dosage on Ni(II) adsorption on bentonite (V=40 mL, pH=7.5±0.1,T=293 K,c0=100 mg/L)
3.5 Effects of pH and ionic strength on Ni(II) adsorption on bentonite
The adsorption of Ni(II) on bentonite as a function of pH value and ionic strength is shown in Figure 5. The adsorption of Ni(II) on bentonite was obviously affected by the pH value. The adsorption curves can be divided into three regions: (1) in region I, the effciency for removal of Ni(II) increased sharply as the solution pH value was increased from 1.6 to 8.5; (2) in region II, the effciency for removal of Ni(II) on bentonite gradually increased at pH values between 8.5—10.2; (3) in region III, the removal efficiency remained high (approximately at 99%) at a pH of >10.2. This phenomenon implies the formation of complicated surface complexes and represents different adsorption mechanisms[21]. The exact speciation of Ni(II) signifcantly infuenced the removal effciency; therefore, the removal selectivity of Ni(II) by bentonite was infuenced by the character of Ni(II) species that predominated at a particular solution pH value. Nickel species exist in the forms of Ni2+, Ni(OH)+, Ni(OH)2, Ni(OH)3-, and Ni(OH)42-at different pH values. The precipitation curve of Ni(II) is shown in Figure 6. Ni(II) began to precipitate at pH of 8.7. The predominant nickel species was Ni2+at pH<8.5. Therefore, the low Ni(II) adsorption rate that occurred at pH<8.5 (region I) can be attributed partly to the competition between H+/Na+and Ni2+on the surface sites. At pH values between 8.5—10.2, Ni2+coexisted with Ni(OH)2in the solution, and the removal of Ni(II) was attributed to the surface precipitation and ion-exchange process (region II). Depending on the pH value and metal ion concentration, the hydrolytic action of Ni(II) might generate various complexes, such as Ni(OH)2, Ni(OH)3-, and Ni(OH)4
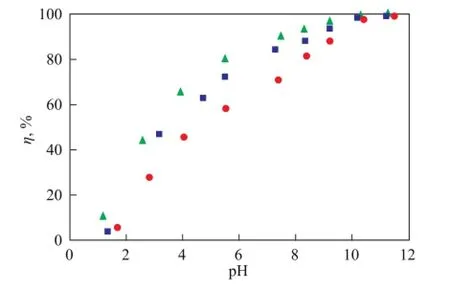
Figure 5 Effect of ionic strength on Ni (II) adsorption on bentonite as a function of solution pH value (V=40 mL, pH=7.5 ± 0.1,T=293 K,c0=100 mg/L)
2-, at high pH values. These complexes participated in the adsorption and precipitation onto the bentonite surfaces. Consequently, the formation of Ni(II) species with OH-could contribute to the promotion of Ni(II) removal at a pH of above 10.2 (region III)[22].

Figure 6 Relative proportion of Ni(II) species as a function of pH value
Figure 5 also shows the effect of ionic strength on Ni(II) adsorption on the bentonite. As shown in Figure 5, the adsorption of Ni(II) on bentonite increased with a decreasing ionic strength at pH<10.2. This phenomenon could be attributed to three reasons: (1) The presence of electrolytes could significantly expand the thickness of diffused electric double layer surrounding the bentonite and Ni(II) species in solution, which inhibited the bentonite particles and Ni(II) ions from approaching each other and decreased the uptake of Ni(II) ions because of the decreased electrostatic attraction[23]; (2) The ionic strength of solution could influence the activity coefficient of Ni(II) ions, which would limit their transfer onto the bentonite surface[24]; (3) The presence of electrolytes could affect the particle aggregation by infuencing electrostatic interactions. Increased ionic strength reduces electrostatic repulsion and increases particle aggregation of bentonite, thereby reducing the amount of available binding sites and decreasing the adsorption of Ni(II) on the bentonite[24-25]. In general, the outer-sphere complexes or the cation exchange with -Na or -H on the bentonite surface are infuenced by ionic strength, whereas the inner-sphere surface complexation is affected by pH value[26]. Basedon the theory mentioned above, we can deduce that Ni(II) adsorption on the bentonite at pH<8.5 was mainly attributed to the ion exchange or outer-sphere surface complexation, whereas the surface precipitation and inner-sphere surface complexation were main mechanisms of Ni(II) adsorption on bentonite at pH>8.5.
3.6 Effects of foreign ions on Ni(II) adsorption on bentonite
The effects of foreign ions on Ni(II) adsorption on bentonite as a function of solution pH value are shown in Figures 7 and 8. In Figure 7, the adsorption of Ni(II) on bentonite was not influenced by the background electrolyte anions because the anions might form complexes with the oxygen-containing functional groups on the bentonite surface[16]. As shown in Figure 8, the adsorption of Ni(II) on bentonite was obviously dependent on electrolyte cations at a pH of <8, and the effect of cations on Ni(II) adsorption on bentonite was in the sequence of K+>Na+; however, a slightly drastic difference was observed at a higher pH value. Before the addition of Ni(II) ions, bentonite had not been pre-equilibrated with the background electrolyte ions. Therefore, this phenomenon suggests that the affinity between K+and bentonite was greater than that between Na+and Ni(II), and K+could easily enter the bentonite interlayer via ionic exchange reaction, which might be attributed to the smaller ionic radius of K+(0.232 nm) than Na+(0.276 nm)[27]. However, at a pH of >8, a slight difference was observed between Ni(II) adsorption in K+and Na+solutions, which might be attributed to the inner-sphere surface complexation and surface hydrolysis precipitation at high pH values[28].
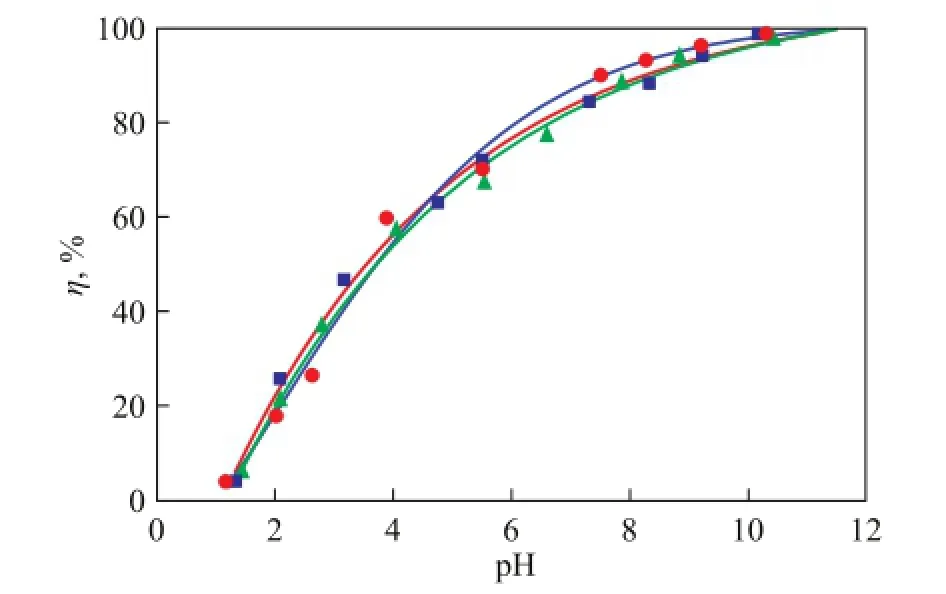
Figure 7 Effect of foreign anions on Ni(II) adsorption on bentonite as a function of solution pH value (V=40 mL,T=293 K,c0=100 mg/L)

Figure 8 Effect of foreign cations on Ni(II) adsorption on bentonite as a function of solution pH value (V=40 mL,T=293 K,c0=100 mg/L)
3.7 Adsorption isotherms
For adsorption isotherm modeling, the Langmuir, Freundlich, and D-R models were conducted to establish the relationship between the amount of Ni(II) adsorbed on bentonite and the concentration of Ni(II) remaining in the solution. The Langmuir isotherm model describes the monolayer adsorption process that takes place on a homogeneous surface. It can be expressed as[29]
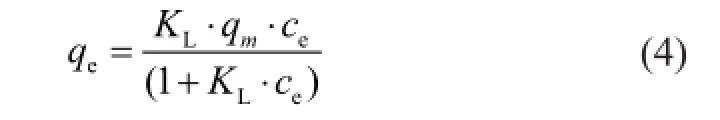
whereceandqeare already defned above,qmis the maximum adsorption capacity, which is the total adsorbed Ni(II) ions at complete monolayer coverage (mg/g), andKLis a constant that is related to the adsorption energy (L/ mg). This equation can be linearized as follows:
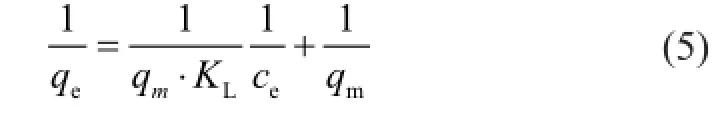
From the data of 1/qevs. 1/ce,KLandqmcan be determined from the slope and intercept, respectively.
The Freundlich expression is an exponential equation that represents the adsorption data at low and intermediate concentrations on heterogeneous surfaces. It can be expressed as[30]:

whereceandqeare already defned above,KF(mg1-n·Ln/g) is correlated with the adsorption capacity when the equilibrium concentration of metal ions is 1, andnrepresents the degree of dependence of adsorption with equilibrium concentration. This equation can be linearized as follows:
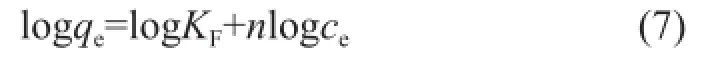
From the data of logqevs. logce,nandKFcan be determined from the slope and intercept, respectively.
The D-R isotherm model can be used to describe the adsorption on both homogeneous and heterogeneous surfaces. It can be expressed as[31]:

whereqeis the adsorbed molar mass of Ni(II) per gram of bentonite (mol/g),qmis the maximum adsorption capacity (mol/g),βis a constant that is related to the adsorption energy (mol2/kJ2), andεis the Polanyi potential. The value ofεcan be calculated by the following equation:
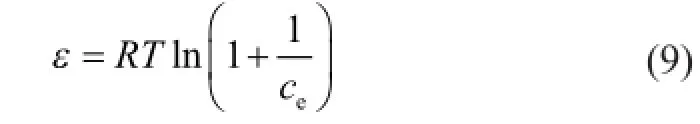
whereTis the absolute temperature in Kelvin (K) andRis the ideal gas constant (8.314 J/mol/K).
Equation (8) can be linearized as follows:

Based on the data of logqevs.ε2,βandqmcan be determined from the slope and intercept, respectively.
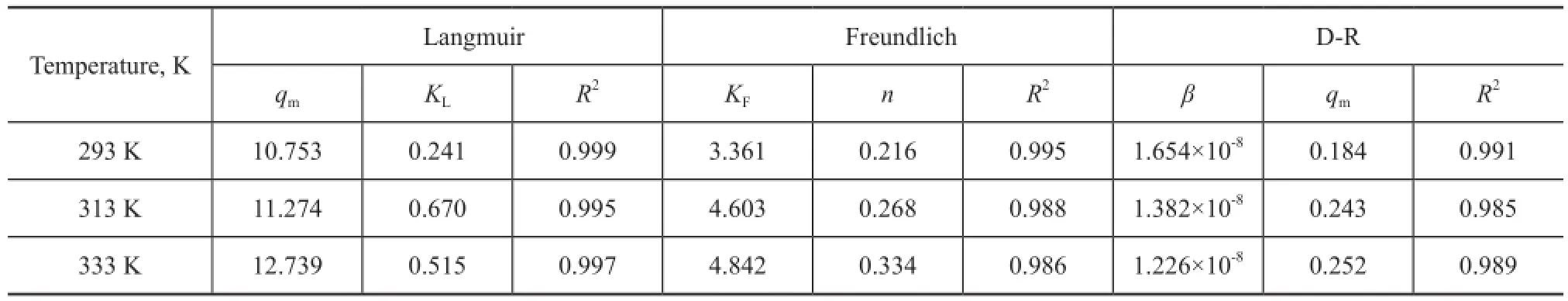
Table 1 Adsorption isotherm parameters for Ni(II) adsorption on LYB
The parameters calculated from the three models are listed in Table 1. The correlation coeffcients for the three models are close to 1.R2values indicated that the Langmuir model simulated the experimental data better than the other two models, which indicated that the binding energy on the entire surface of bentonite was uniform. In other words, the entire surface had identical adsorption activity, and therefore the adsorbed Ni(II) ions did not interact or compete with each other and were adsorbed through forming an almost complete monolayer that covered the bentonite particles. The value ofnfor the Freundlich model was < 1, indicating that Ni(II) ions were favorably adsorbed by bentonite at all temperatures studied and that a nonlinear adsorption of Ni(II) occurred on the bentonite surfaces[32]. The values of adsorption capacity (qm) calculated by the D-R model were higher than those calculated by the Langmuir model, which might be attributed to the different assumptions used in the two models; theqmcalculated by the D-R model was the total adsorbed Ni(II) ions on the total specifc micropores of bentonite, but theqmcalculated by the Langmuir model was related to the total adsorbed Ni(II) ions in the case of complete monolayer coverage.
3.8 Effect of temperature and thermodynamic analysis
Figure 9 shows the dynamic curves of Ni(II) adsorption on bentonite at different temperatures. The adsorption dynamic curve at high temperatures was higher than that at low temperatures, which indicated that the adsorption on bentonite was enhanced with an increasing temperature. The thermodynamic parameters for Ni(II) adsorption on bentonite can be calculated by the Van’t Hoff equation[33]:
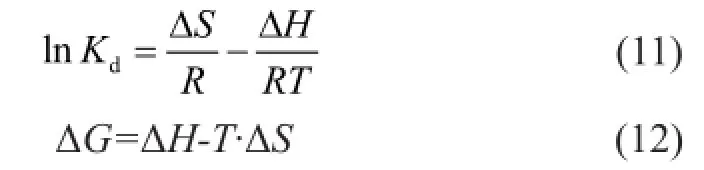
where ΔSand ΔHare the entropy change (kJ/mol K) and enthalpy change (kJ/mol) of adsorption, respectively, ΔGis the Gibbs free energy (kJ/mol),RandTare defned above, andKdis the distribution coeffcient for the adsorption[34-35].

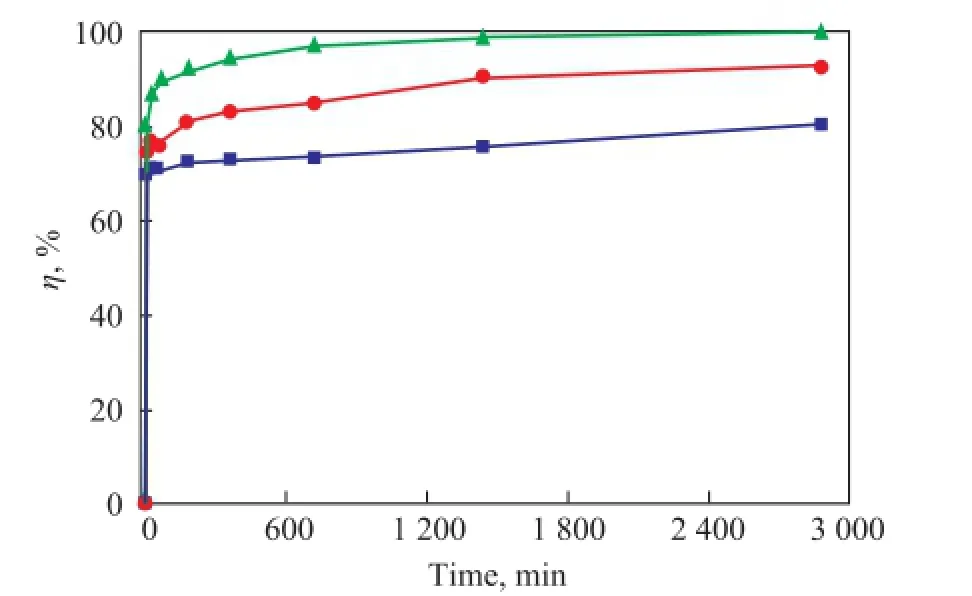
Figure 9 Dynamic curves of Ni(II) adsorption on bentonite at different temperatures (m/V=0.025 g/mL, pH=7.5±0.1,c0=100 mg/L)
wherec0,ce,V, andmare already defned above, and the values of ΔSand ΔHare calculated from the slope and intercept of linear regression of lnKdvs. (1/T), respectively. The calculated thermodynamic parameters are listed in Table 2. The values of ΔHwere positive, suggesting that the adsorption was endothermic. The observed phenomenon may be attributed to four reasons: (1) The exchange of Ni(II) ions with H+/Na+needs to frst break the O-H/ O-Na bonds, which is an endothermic process[16]; (2) The increase in temperature may lead to the increase in the proportion and activity of Ni(II) ions in the solution, the affnity of Ni(II) ions to the surface, or the potential charge of bentonite surface[36]; (3) The diffusion rate of Ni(II) into bentonite pores may increase with temperature[37]; (4) The Ni(II) ions in the solution are all hydrated, and the hydration sheath of Ni(II) must be destroyed before Ni(II) is adsorbed on bentonite. The dehydration process needs energy, which is favored at high temperatures[38]. ΔGand ΔSwere negative and positive, respectively, which indicated that the adsorption reaction was spontaneous. ΔGbecame more negative with an increasing temperature, implying more effcient adsorption at higher temperatures. The positive value of ΔSimplied some structural changes in the sorbate and adsorbent during adsorption, which induced the disorder of the solid solution system during the uptake of Ni(II) on bentonite.
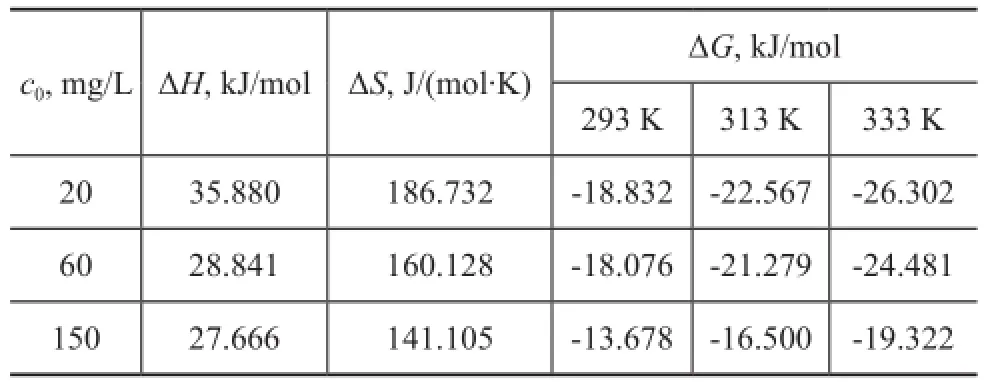
Table 2 Thermodynamic parameters for Ni(II) adsorption on LYB
4 Conclusions
The surface properties and adsorption characteristics of local LYB for Ni(II) adsorption were studied. The PZNPC of bentonite at different ionic strength presented the pHPZNPCto be 8.2±0.1. The bentonite showed negative zeta potentials in the pH range of 3—11 at three ionic strength values of 0.001 M, 0.01 M, and 0.1 M. The removal of Ni(II) from solution increased with an increasing bentonite dosage; the maximum removal effciency of approximately 99% could be achieved with an adsorbent dosage of 0.025 g/mL. The removal of Ni (II) increased with an increasing pH value from 1.6 to 10.2. The adsorption of Ni(II) on bentonite increased with a decreasing ionic strength at pH<10.2. The adsorption of Ni(II) on bentonite at low pH values was influenced by foreign cations but not by foreign anions. Based on the above-mentioned analysis results, we can conclude that the adsorption of Ni(II) on bentonite was dominated by ion exchange or outer-sphere surface complexation at a pH value of <8.5 and by inner-sphere surface complexation and surface precipitation at a pH value of >8.5. The adsorption isotherms of Ni(II) on bentonite can be described well by the Langmuir isotherm model. The thermodynamic analysis results showed that the adsorption of Ni(II) ions on bentonite was endothermic and spontaneous. The positive value of ΔHconfrmed the endothermic nature of adsorption.
Acknowledgements: Financial supports from the National Natural Science Foundation of China (51204104, 21201111,50774050, and 51474140), the Sponsored Research Foundation for Young Scientist of Shandong Province (BS2012CL026, BS2013CL001, and BS2013NJ019), the Shandong Postdoctoral Innovation Project (201202028), the China Postdoctoral Science Foundation (2012M521367), and the Support Plan for Innovative Research Team of Shandong University of Science and Technology (2012KYTD102) are acknowledged.
[1] Ajmal M, Rao R A K, Ahmad R, et al. Adsorption studies on Citrus reticulata (fruit peel of orange): removal and recovery of Ni (II) from electroplating wastewater [J]. Journal of Hazardous Materials, 2000, 79(1/2): 117-131
[2] Roundhill D M. Novel strategies for the removal of toxic metals from soils and waters [J]. Journal of Chemical Education, 2004, 81(2): 275-282
[3] Han R, Zhang J, Zou W, et al. Biosorption of copper (II) and lead (II) from aqueous solution by chaff in a fxed-bed column [J]. Journal of Hazardous Materials, 2006, 133(1/3): 262-268
[4] Bhattacharya A K, Mandal S N, Das S K. Adsorption of Zn (II) from aqueous solution by using different adsorbents[J].Chemical Engineering Journal, 2006, 123(1/2): 43-51
[5] Novakovi? T, Ro?i? L, Petrovi? S, et al. Synthesis and characterization of acid-activated Serbian smectite clays obtained by statistically designed experiments [J]. Chemical Engineering Journal, 2008, 137(2): 436-442
[6] Christianah O I, Mi-Hwa B, Dong-Su K. Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions [J]. Journal of Hazardous Materials, 2009, 166(1): 538-546
[7] Abollino O, Aceto M, Malandrino M, et al. Adsorption of heavy metals on Na-montmorillonite: Effect of pH and organic substances [J]. Water Research, 2003, 37(7):1619-1627
[8] Eren E, Afsin B, Onal Y. Removal of lead ions by acid activated and manganese oxide-coated bentonite[J]. Journal of Hazardous Materials, 2009, 161(2/3): 677-685
[9] Benedicto A, Degueldre C, Missana T. Gallium sorption on montmorillonite and illite colloids: Experimental study and modeling by ionic exchange and surface complexation [J]. Applied Geochemistry, 2014, 40(1): 43-50
[10] Juang R S, Lin S H, Tsao K H. Mechanism of sorption of phenols from aqueous solutions onto surfactant-modifed montmorillonite[J]. Journal of Colloid & Interface Science, 2002, 254(2): 234-241
[11] Luckham P F, Rossi S. The colloidal and rheological properties of bentonite suspensions [J]. Advances in Colloid & Interface Science, 1999, 82(1/3): 43-92
[12] Guo Z, Xu J, Shi K, et al. Eu (III) adsorption/desorption on Na-bentonite: Experimental and modeling studies [J]. Colloids & Surfaces A: Physicochemical & Engineering Aspects, 2009, 339(1/3): 126-133
[13] Avena M J, Pauli C P D. Proton adsorption and electrokinetics of an Argentinean montmorillonite [J]. Journal of Colloid & Interface Science, 1998, 202(1): 195-204
[14] Min H B, Lee S Y. Colloidal stability of bentonite clay considering surface charge properties as a function of pH and ionic strength [J]. Journal of Industrial & Engineering Chemistry, 2010, 16(5): 837-841
[15] Xu D, Zhou X, Wang X. Adsorption and desorption of Ni2+on Na-montmorillonite: Effect of pH, ionic strength, fulvic acid, humic acid and addition sequences [J]. Applied Clay Science, 2008, 39(3/4): 133-141
[16] Wu X L, Zhao D, Yang S T. Impact of solution chemistry conditions on the sorption behavior of Cu(II) on Lin’an montmorillonite[J]. Desalination, 2011, 269(1): 84-91
[17] Kraepiel A M L, Keller K, Morel F M M. On the acid?base chemistry of permanently charged minerals [J]. Environmental Science & Technology, 1998, 32(19): 2829-2838
[18] Durán J D G, Ramos-Tejada M M, Arroyo F J, et al. Rheological and electrokinetic properties of sodium montmorillonite suspensions: I. rheological properties and interparticle energy of interaction[J]. Journal of Colloid & Interface Science, 2000, 229(1): 107-117
[19] Niriella D, Carnahan R P. Comparison study of zeta potential values of bentonite in salt solutions [J]. Journal of Dispersion Science and Technology, 2006, 27(1): 123-131
[20] Huang J, Liu Y, Wang X. Selective adsorption of tannin from flavonoids by organically modified attapulgite clay [J]. Journal of Hazardous Materials, 2008, 160(2): 382-387.
[21] Kowal-Fouchard A, Drot R, Simoni E, et al. Use of spectroscopic techniques for uranium (VI)/montmorillonite interaction modeling[J]. Environmental Science & Technology, 2004, 38(5): 1399-1407
[22] Cabrera C, Gabaldón C, Marzal P. Sorption characteristics of heavy metal ions by a natural zeolite[J]. Journal of Chemical Technology and Biotechnology, 2005, 80(4): 477-481
[23] Wang X S, Huang J, Hu H Q, et al. Determination of kinetic and equilibrium parameters of the batch adsorption of Ni (II) from aqueous solutions by Na-montmorillonite [J]. Journal of Hazardous Materials, 2007, 142(1): 468-476
[24] And C C, Wang X. Adsorption of Ni (II) from aqueous solution using oxidized multiwall carbon nanotubes[J]. Industrial & Engineering Chemistry Research, 2006, 45(26): 9144-9149
[25] Yang S, Li J, Lu Y, et al. Sorption of Ni (II) on GMZ bentonite: effects of pH, ionic strength, foreign ions, humic acid and temperature [J]. Applied Radiation & Isotopes, 2009, 67(9): 1600-1608
[26] Changlun C, Xiangke W. Sorption of Th (IV) to silica as a function of pH, humic/fulvic acid, ionic strength, electrolyte type[J]. Applied Radiation & Isotopes, 2007, 65(2): 155-163
[27] Esmadi F, Simm J. Sorption of cobalt (II) by amorphous ferric hydroxide [J]. Colloids & Surfaces A: Physicochem-ical & Engineering Aspects, 1995, 104(2/3): 265-270
[28] Chen C L, Wang X K. Infuence of pH, soil humic/fulvic acid, ionic strength and foreign ions on sorption of thorium (IV) onto γ-Al2O3[J]. Applied Geochemistry, 2007, 22(2): 436-445
[29] Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum [J]. Journal of the American Chemical Society, 1918, 40(9): 1361-1403
[30] Jiang M Q, Jin X Y, Lu X Q, et al. Adsorption of Pb (II), Cd (II), Ni (II) and Cu (II) onto natural kaolinite clay[J]. Desalination, 2010, 252(1/3): 33-39
[31] Karapinar N, Donat R. Adsorption behaviour of Cu2+and Cd2+onto natural bentonite [J]. Desalination, 2009, 249(1): 123-129
[32] Malik P K. Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: a case study of acid yellow [J]. Dyes & Pigments, 2003, 56(3): 239-249
[33] Sheng G, Wang S, Hu J, et al. Adsorption of Pb (II) on diatomite as affected via aqueous solution chemistry and temperature[J]. Colloids & Surfaces A: Physicochemical & Engineering Aspects, 2009, 339(1/3): 159-166
[34] Chen D, Chen J, Luan X, et al. Characterization of anioncationic surfactants modifed montmorillonite and its application for the removal of methyl orange[J]. Chemical Engineering Journal, 2011, 171(3): 1150-1158
[35] Vieira M G A, Neto A F A, Gimenes M L, et al. Sorption kinetics and equilibrium for the removal of nickel ions from aqueous phase on calcined Bofe bentonite clay[J]. Journal of Hazardous Materials, 2010, 177(1/3): 362-371
[36] Partey F, Norman D, Ndur S, et al. Arsenic sorption onto laterite iron concretions: Temperature effect[J]. Journal of Colloid & Interface Science, 2008, 321(2): 493-500
[37] Behera S K, Kim J H, Guo X, et al. Adsorption equilibrium and kinetics of polyvinyl alcohol from aqueous solution on powdered activated carbon [J]. Journal of Hazardous Materials, 2008, 153(3): 1207-1214
[38] Li J, Hu J, Sheng G, et al. Effect of pH, ionic strength, foreign ions and temperature on the adsorption of Cu (II) from aqueous solution to GMZ bentonite[J]. Colloids & Surfaces A: Physicochemical & Engineering Aspects, 2009, 349(1/3): 195-201
Received date: 2016-05-09; Accepted date: 2016-07-25.
Dr. Lyu Xianjun, Telephone: +86-532-86057105; E-mail: lu_ xianjun@163.com.
-). The adsorption of Ni(II) was dominated by the outer-sphere surface complexation and ion exchange with Na+/H+on bentonite surface at low pH value, whereas the inner-sphere surface complexation and surface precipitation were the main adsorption mechanisms at high pH value. The adsorption isotherms of Ni(II) on bentonite can be described well by the Langmuir model. The thermodynamic parameters of adsorption, including the Gibbs free energy, the enthalpy change, and the entropy change, at different temperatures indicated that the adsorption of Ni(II) on bentonite was endothermic and spontaneous.
- 中國(guó)煉油與石油化工的其它文章
- Effect of Magnetic Field on Tribological Properties of Lubricating Oils with and without Tricresyl Phosphate
- Preparation and Lubricating Properties of A New Antibacterial Emulsion Containing Nano-TiO2for Cold Rolling Strips
- Prediction of Coke Yield of FCC Unit Using Different Arti fi cial Neural Network Models
- An Extraction Process for Optimal Utilization of Naphtha Based on Molecule Management
- Hydrodynamic Characteristics in an External Loop Airlift Slurry Reactor
- Microwave-Assisted Synthesis of Poly(Aspartic Acid-Itaconic Acid) Copolymer and Its Characterization

