Gradient high performance liquid chromatography method for simultaneous determination of ilaprazole and its related impurities in commercial tablets
Short Communication
Gradient high performance liquid chromatography method for simultaneous determination of ilaprazole and its related impurities in commercial tablets
Shang Wang,Dong Zhang,Yingli Wang,Xiaohong Liu*,Yan Liu,Lu Xu*
Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
ARTICLEINFO
Article history:
Received 28 March 2014
Received in revised form
31 August 2014
Accepted 3 September 2014
Available online 22 September 2014
Ilaprazole
A
A methodology(HPLC)proposed in this paper for simultaneously quantitative determination of ilaprazole and its related impurities in commercial tablets was developed and validated.The chromatographic separation was carried out by gradient elution using an Agilent C8column(4.6 mm×250 mm,5 μm)which was maintained at 25°C.The mobile phase composed of solvent A(methanol)and solvent B(solution consisting 0.02 mmol/l monopotassium phosphate and 0.025 mmol/l sodium hydroxide)was at a fl ow rate of 1.0 ml/min.The samples were detected and quanti fi ed at 237 nm using an ultraviolet absorbance detector.Calibration curves of all analytes from 0.5 to 3.5 μg/ml were good linearity(r≥0.9990)and recovery was greater than 99.5%for each analyte.The lower limit of detection(LLOD)and quanti fi cation(LOQ)of this analytical method were 10 ng/ml and 25 ng/ml for all impurities,respectively.The stress studies indicated that the degradation products could not interfere with the detection of ilaprazole and its related impurities and the assay can thus be considered stability-indicating.The method precisions were in the range of 0.41-1.21 while the instrument precisions were in the range of 0.38-0.95 in terms of peak area RSD%for all impurities,respectively.This method is considered stabilityindicating and is applicable for accurate and simultaneous measuring of the ilaprazole and its related impurities in commercial enteric-coated tablets.
?2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1. Introduction
Ilaprazole,[IY-81149,2(((4-methoxy-3-methyl)-2-pyridinyl)-methylsul fi nyl)-5-(1H-pyrrol-1-lyl)1H-benzimidazole](Fig.1A),is a potent inhibitor of the enzyme gastric proton pump H+/K+-ATPase in inhibiting gastric acid secretion,which includes gastric and duodenal ulcer,re fl ux oesophagitis and Zollinger-Ellison syndrome[1-3].Unlike other proton pump inhibitors(PPIs),ilaprazole with a longer plasma half-life (about 3.6 h)[4]produces a greater and prolongs acidsuppressing effect[5],avoiding the occurrence of nocturnal acid break(NAB)during the PPIs therapy[6].Besides,a greater safety could be assured by at least two factors.One is the signi fi cant reduction of dosage and lower toxicity for ilaprazole comparing to omeprazole[7].And the other one is the low variability of the pharmacokinetics and pharmacodynamics of ilaprazoleattributed to itsmetabolism independentof CYP2C19[8],which played the predominant role in metabolism of other PPIs[9,10].
Recently,therewerealotofeffortscommittedto researching metabolism in vivo[11-14];however,there was only one paper reporting a UPLC method for the simultaneous determination of ilaprazole and its impurities in tablets[15].A ternary liquid system(acetonitrile,methanol and ammonium acetate buffer)was used to separated fi ve known impurities. In light of the requirements of a more widelyavailable method and better quality control for ilaprazole involved solid preparations,the development of a novel analytical method was demanded and necessary.Therefore,we developed a sensitive,selective and accurate gradient HPLC method,using a simple methanol-water binary system,for the quanti fi cation of ilaprazole and seven known impurities including ilaprazole sulfur ether and ilaprazole sulphone(Fig.1B and C)and impurity A,B,C,D,E(structures not for publication)in commercial tablets.
2. Materials and methods
2.1. Materials
Ilaprazole(5 mg enteric-coated tablet)were purchased from Liyzon Pharmaceutical Group Inc.(Zhuhai,China).Ilaprazole and related impurities(ilaprazole sulphone,ilaprazole sulfur ether and impurity A,B,C,D,and E)(purity>99.0%,purities were detected by HPLC using area normalization method and structures were validated by nuclear magnetism resonance (NMR)and mass spectrum(MS))were supplied by Medicinal Chemistry Lab of Shenyang Pharmaceutical University(Shenyang,China).HPLC-grade methanol was from Fisher Scienti fi cWorldwide(Shanghai)Co.,Ltd.(Shanghai,China). Deionized-distilled water was used throughout this study. All other reagents were of analytical grade.
2.2. HPLC operating conditions
HPLC separations were performed by an Agilent C8column (4.6 mm ×250 mm,5 μm)(Agilent,USA).Analytical HPLC apparatus was consisted of an L-2130 pump(Hitachi,Japan) and an L-2400 ultraviolet absorbance detector(Hitachi,Japan).
Gradient HPLC method was used for the analysis of ilaprazole and its related impurities at 25°C by using an ultraviolet absorbance detector at a wavelength of 237 nm.The mobile phase consisted of solvent A(methanol)and solvent B (0.02 mmol/l monopotassium phosphate and 0.025 mmol/l sodium hydroxide)with a fl ow rate of 1.0 ml/min.The initial mobile phase composition was maintained at 42%solvent A for 30 min,changed linearly to 55%(30-35 min)and held 30 min(35-65 min),then followed by a return to the initial conditions within 5 min(65-70 min)and kept 5 min (70-75 min)for the chromatograph column equilibrium.The mobile phase was fi ltered through a 0.22 μm PTFE fi lter(Millipore,USA)and ultrasounded for degasi fi cation before use. The injection volume was of 20 μl.
2.3. Preparation of stock and standard solutions
On account of the instability of ilaprazole,its freshly solution was prepared immediately before use and protected from light.Stock solutions(100 μg/ml)of ilaprazole impurities were separately prepared with methanol.The stock solutions of different impurity were kept from light and stored at 4°C for four weeks with no evidence of decomposition.
Aliquots of the stock solutions of different impurity were transferred into10 ml volumetric fl asks with bulb pipettesand the solutions were made up to volume with methanol to yield fi nal concentrations of 0.5,1,1.5,2,2.5,3 and 3.5 μg/ml for each impurity.
Twenty commercial tablets were weighed,crushed and mixed with a mortar and pestlefor 15 min.A portion ofpowder equivalent to 10 mg ilaprazole was precisely weighed to 10 ml volumetric fl ask and 5 mlmethanolwas added.The volumetric fl askwassonicatedfor10mintocompletelydissolveilaprazole andthenthesolutionsweremadeuptovolumewithmethanol. After centrifugation for 5 min at 13,000 rpm,aliquots of the solutions were fi ltered through 0.22 μm nylon fi lters.
2.4. System suitability test
System suitability test was conducted by injections of the system suitability solution(n=6).The acceptance criteria were as follows:relative standard deviation(RSD)for peak areas within 2%,column plates greater than 5000,the USP tailing factor within 1.5,and the resolution greater than 2.0.
2.5. Forced degradation studies of ilaprazole
The conditions for forced degradation studies were screened and fi nally fi xed as follows to obtain 10-20%degradation of ilaprazole[16].
2.5.1. Acid degradation
Solution containing 1 mg/ml of ilaprazole was treated with 1 mol/l HCl for 30 s.The resultant solution was neutralized immediately as needed and analyzed.
2.5.2. Alkali degradation
Solution containing 1 mg/ml of ilaprazole was disposed with 1 mol/l NaOH for 2 h.The resultant solutions were neutralized and analyzed promptly.
2.5.3. Oxidative condition
Solution containing 1 mg/ml ilaprazole was obtained with 30% w/v H2O2,and the resultant solutions were analyzed 1 h later.
2.5.4. Thermal degradation study
Tabletpowdersweresubjectedto 100°C inanovenfor1 h,and then 1 mg/ml ilaprazole solution was achieved with methanol for analysis.
2.5.5. Photostability study
Tablet powders were exposed to 4500 lx±500 lx light for 20 days to determine the effect of irradiation on the stability of the drug.The sample solution(equivalent to approximately 1 mg/ml of ilaprazole)was prepared for further HPLC analysis.
3. Results and discussion
3.1. HPLC method development and optimization
As we all know,more impurities controlled were bene fi cial to guarantee quality of commercial drugs and to ensure safety of drug therapy.From this prespective,the development of method with a capability of more known impurities detected than the reported method in the literature[15]was necessary and salutary.At the begin of method development,an isocratic elution(42%solvent A as the initial mobile phase composition described in section“2.2”)was used to separate ilaprazole and seven kown impurities,leading to a total runtime over 120 min.Considering the polarity difference of ilaprazole and its related impurities,a gradient HPLC method was developed to achieve a shorter runtime and desired sensitivity.An Agilent C8column(4.6 mm×250 mm,5 μm) (Agilent,USA)maintained at 25°C was used for the separation and this method was validated to assure the reliability of determination of ilaprazole and its impurities.The composition,pH and the fl ow rate of the mobile phase were changed to optimize the separation conditions.A gradient HPLC method which was described in section“2.2”was selected for further studies after several preliminary investigatory chromatographic runs.Under the experimental conditions,all the peaks were well de fi ned and free from tailing.The deliberate changes in the mobile phase composition and fl ow rate were evaluated to test the method robustness.
3.2. Validation of the method
The developed method was characterized by evaluation of speci fi city,limit of detection(LLOD),limit of quantization (LOQ), linearity, precision, accuracy, and robustness/ ruggedness.
3.2.1. System suitability test
The results of system suitability test indicated that all the parameters obtained were within the acceptable limits.Fig.2 shows the chromatogram of the system suitability test solution.
3.2.2. Speci fi city
The speci fi city of the method was guaranteed by observing potential interferences caused by degradation products under stress conditions.The stress testing studies indicated a preferable speci fi city of this method for both ilaprazole and its related impurities.The results of stress testing are shown in Fig.3.
3.2.3. LLOD and LOQ
The LOQs for ilaprazole impurity A,B,C,D,E,ilaprazole sulphone and ilaprazole sulfur ether corresponding to a signalto-noise ratio of 10 were 25 ng/ml,200 ng/ml,75 ng/ml, 125 ng/ml,750 ng/ml,200 ng/ml and 200 ng/ml,respectively.And the LLODs for ilaprazole related impurities corresponding to a signal-to-noise ratio of 3 were 10 ng/ml,80 ng/ml, 30 ng/ml,50 ng/ml,300 ng/ml,80 ng/ml and 80 ng/ml.The resultant RSD values(%)for these studies were≤2.0%.
3.2.4. Linearity
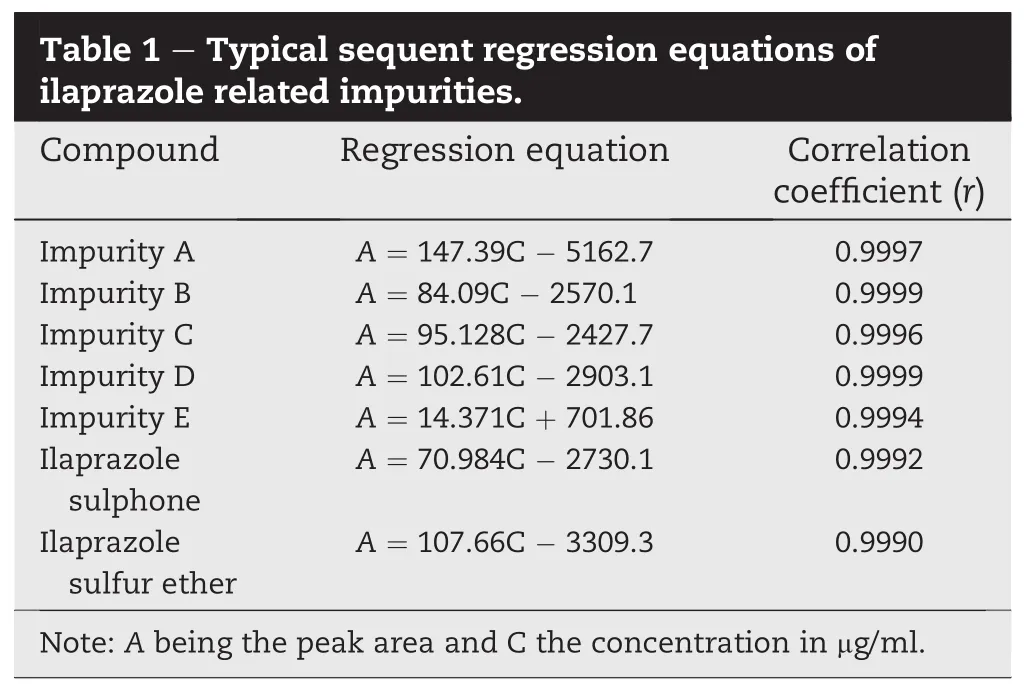
The linearity was performed with the standard solutions of ilaprazole impurities(n=7)over the concentration range of 0.5-3.5 μg/ml,respectively.Least squares linear regression analysis of the calibration curve was employed to establish the linearity.Typical sequent regression equations for ilaprazole impurity A,B,C,D,E,ilaprazole sulphone and ilaprazole sulfur ether are listed in Table 1.
3.2.5. Precision
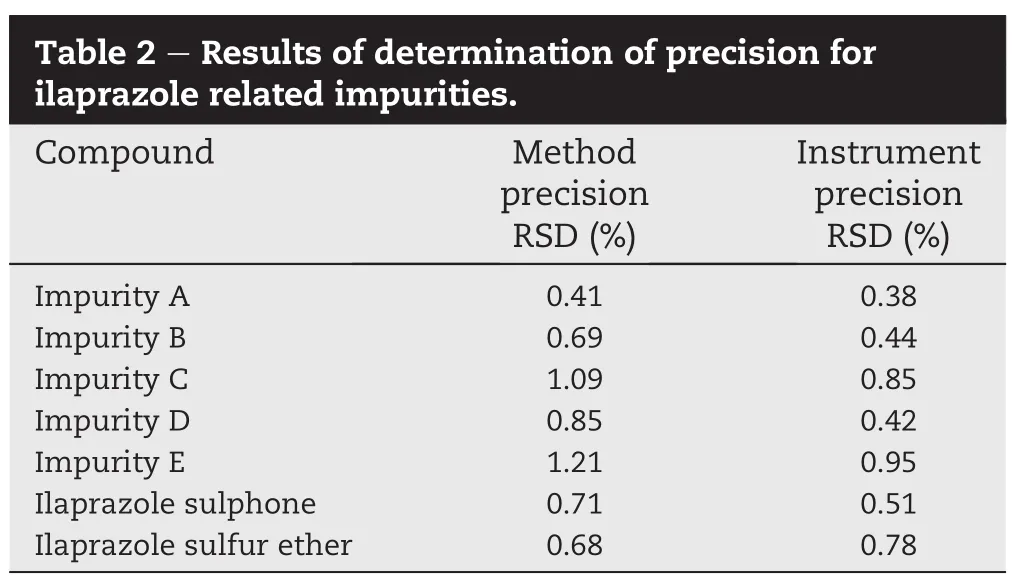
The precision of the HPLC method were assessed by analyzing 6 replicate sample solutions in three different concentrations of mixed impurities standard solutions(i.e.0.8,1.0,1.2 μg/ml). The same sample solution(1.0 μg/ml)was injected 6 times to investigate the instrument precision.The RSD(%)of theproposed method precision and instrument precision were all less than 1.21 and 0.95%for impurities A,B,C,D,E,ilaprazole sulphone and ilaprazole sulfur ether(Table 2).
3.2.6. Accuracy
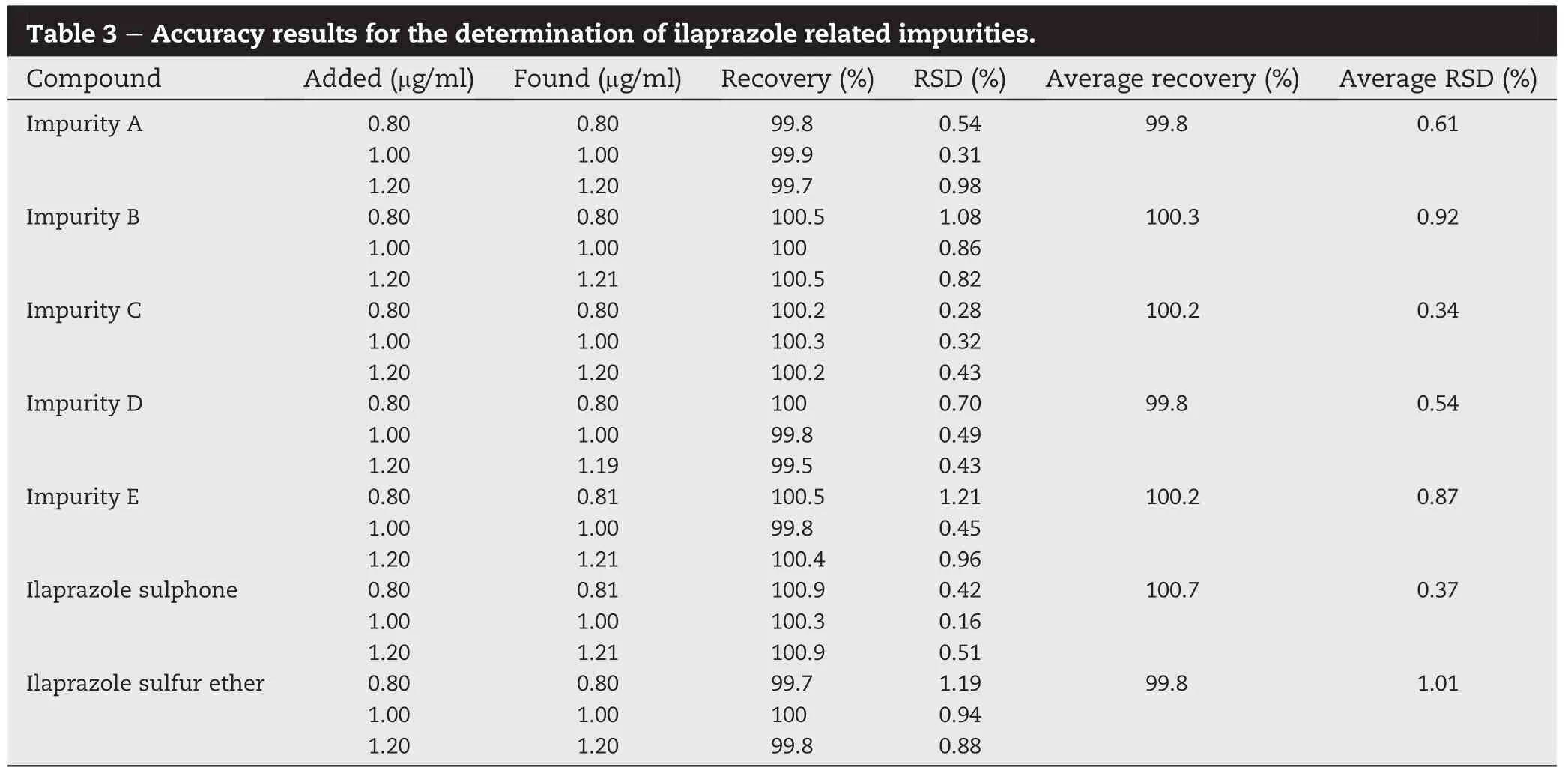
Results of method accuracy are summarized in Table 3.The accuracy for the determination of ilaprazole and its impurities was determined by preparing drug substance sample at 80, 100 and 120%of the target(1 μg/ml).The apparent recovery of ilaprazole and its impurities were found to be from 99.5 to 100.9%.The average percent recovery was 99.8(RSD%0.61), 100.3(RSD%0.92),100.2%(RSD%0.34),99.8%(RSD%0.54), 100.2%(RSD%0.87),100.7%(RSD%0.37)and 99.8%(RSD%1.01) for ilaprazole and its related impurities.
3.2.7. Stability
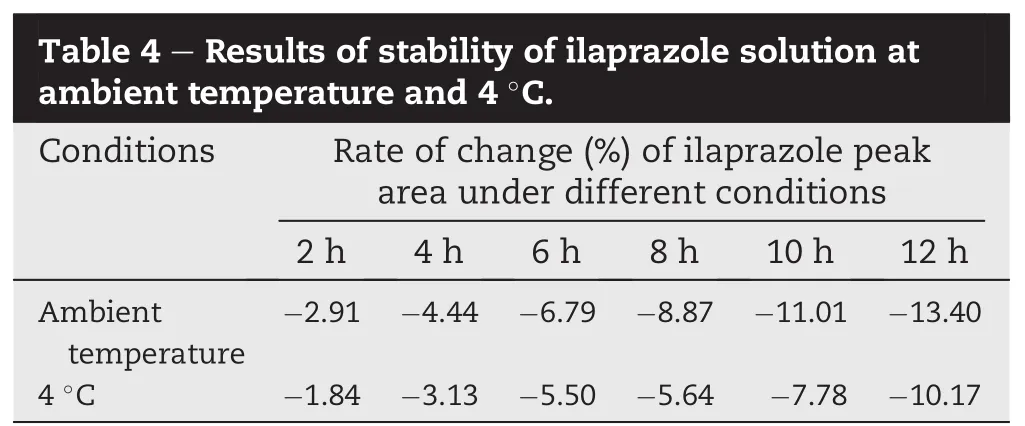
The stability of ilaprazole in methanol was evaluated at ambient temperature and 4°C in refrigeration for 12 h(Table 4).Compared with ambient temperature,the stability of sample was improved to some extent,however,the change of ilaprazole peak area was approximated 2%in 2 h,so the fresh sample needed to be prepared prior to use.The mixed impurities solution was stable at ambient temperature for 12 h(Table 5).No signi fi cant changes(<2%)of the chromatographic responses were observed forthe stock solutions of different impurity,relative to freshly prepared standards(Table 6).
3.2.8. Robustness
The robustness of the HPLC method was studied under a variety of conditions including changes of fl ow rate and the proportion of buffer and organic phase.The small deliberate variations of these parameters were not signi fi cantly affected the reproducibility of the proposed method,which proved good robustness of the HPLC method.
4. Conclusions
A sensitive,selective and accurate gradient HPLC method has been developed and validated for the quantization of ilaprazole and its related impurities in commercial tablets.The stress testing results revealed that the method was speci fi c and selective.Validation experiments demonstrated that linear,precise and accurate of method met the acceptancecriteria.The method proposed here was found to be suf fi cient for quantitation of ilaprazole and its related impurities in commercial enteric-coated tablets.

?

REFERENCES
[1]Welage LS,Berardi RR.Evaluation of omeprazole, lansoprazole,pantoprazole,and rabeprazole in the treatment of acid-related diseases.J Am Pharm Assoc(Wash) 2000;40:52-62.
[2]Welage LS.Pharmacologic properties of proton pump inhibitors.Pharmacotherapy 2003;23:74S-80S.
[3]Dekel R,Morse C,Fass R.The role of proton pump inhibitors in gastro-oesophageal re fl ux disease.Drugs 2004;64:277-295.
[4]Du YQ,Guo WY,Zou DW,et al.Acid inhibition effect of ilaprazole on Helicobacter pylori-negative healthy volunteers:an open randomized cross-over study.J Dig Dis 2012;13:113-119.
[5]Periclou AP,Goldwater R,Lee SM,et al.A comparative pharmacodynamic study of IY-81149 versus omeprazole in patients with gastroesophageal re fl ux disease.Clin Pharmacol Ther 2000;68:304-311.
[6]Nzeako UC,Murray JA.An evaluation of the clinical implications of acid breakthrough in patients on proton pump inhibitor therapy.Aliment Pharmacol Ther 2002;16:1309-1316.
[7]Kwon D,Chae JB,Park CW,et al.Effects of IY-81149,a newly developed proton pump inhibitor,on gastric acid secretion in vitro and in vivo.Arzneimittelforschung 2001;51:204-213.
[8]Li Y,Zhang W,Guo D,et al.Pharmacokinetics of the new proton pump inhibitor ilaprazole in Chinese healthy subjects in relation to CYP3A5 and CYP2C19 genotypes.Clin Chim Acta 2008;391:60-67.
[9]Yamazaki H,Inoue K,Shaw PM,et al.Different contributions of cytochrome P450 2C19 and 3A4 in the oxidation of omeprazole by human liver microsomes:effects of contents of these two forms in individual human samples. J Pharmacol Exp Ther 1997;283:434-442.
[10]Pearce RE,Rodrigues AD,Goldstein JA,et al.Identi fi cation of the human P450 enzymes involved in lansoprazole metabolism.J Pharmacol Exp Ther 1996;277:805-816.
[11]Zhou G,Shi S,Zhang W,et al.Identi fi cation of ilaprazole metabolites in human urine by HPLC-ESI-MS/MS and HPLC-NMR experiments.Biomed Chromatogr 2010;24:1130-1135.
[12]Shin JS,Lee JY,Cho KH,et al.The pharmacokinetics, pharmacodynamics and safety of oral doses of ilaprazole 10, 20 and 40 mg and esomeprazole 40 mg in healthy subjects:a randomised,open-label crossover study.Aliment Pharmacol Ther 2014;40:548-561.
[13]Cho H,Choi MK,Cho DY,et al.Effect of CYP2C19 genetic polymorphism on pharmacokinetics and pharmacodynamics of a new proton pump inhibitor, ilaprazole.J Clin Pharmacol 2012;52:976-984.
[14]Cao S,Zhou G,Ou-Yang DS,et al.Pharmacokinetic interactions between ilaprazole and clarithromycin following ilaprazole,clarithromycin and amoxicillin triple therapy.Acta Pharmacol Sin 2012;33:1095-1100.
[15]Satheesh B,Sree Ganesh KK,Saravanan D,et al. Simultaneous determination of ilaprazole and its related compounds in pharmaceutical dosage forms by UPLC.J Liq Chrom Rel Technol 2013;36:2968-2981.
[16]Canavesi R,Aprile S,Varese E,et al.Development and validation of a stability-indicating LC-UV method for the determination of pantethine and its degradation product based on a forced degradation study.J Pharm Biomed Anal 2014;97:141-150.
*Corresponding authors.Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China.Tel.:+86 24 23986293/ 6325;fax:+86 24 23986293/6325.
E-mail addresses:xulu1974@hotmail.com,LVJ221@163.com(L.Xu).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.09.001
1818-0876/?2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Enteric-coated tablets Related impurities
HPLC
Validation
 Asian Journal of Pharmacentical Sciences2015年2期
Asian Journal of Pharmacentical Sciences2015年2期
- Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- A developed HPLC method for the determination of Alogliptin Benzoate and its potential impurities in bulk drug and tablets
- Mycosynthesis,characterization and antibacterial properties of AgNPs against multidrug resistant (MDR)bacterial pathogens of female infertility cases
- Can semipermeable membranes coating materials in fl uence in vivo performance for paliperidone tri-layer ascending release osmotic pump tablet: In vitro evaluation and in vivo pharmacokinetics study
- A new self-emulsifying formulation of mefenamic acid with enhanced drug dissolution
- Design and evaluation of nicorandil extended-release tablet
