Can semipermeable membranes coating materials in fl uence in vivo performance for paliperidone tri-layer ascending release osmotic pump tablet: In vitro evaluation and in vivo pharmacokinetics study
Can semipermeable membranes coating materials in fl uence in vivo performance for paliperidone tri-layer ascending release osmotic pump tablet: In vitro evaluation and in vivo pharmacokinetics study
ARTICLEINFO
Article history:
Received 18 April 2014
Received in revised form
2 November 2014
Accepted 3 December 2014
Available online 23 December 2014
Ascending release
Tri-layer osmotic pump
Paliperidone
Cellulose acetate
Pharmacokinetics
In vitro-in vivo correlation
A
One purpose of this study was to develop a paliperidone(PAL)tri-layer ascending release push-pull osmotic pump(TA-PPOP)tablet which could meet the needs of clinical applications.And another purpose was to investigate whether different coating materials in fl uenced in vivo performance of TA-PPOP.The ascending release mechanism of this trilayer delivery system on theory was elaborated.TA-PPOP was prepared by means of coating with cellulose acetate(CA)or ethyl cellulose(EC).Several important in fl uence factors such as different core tablet compositions and different coating solution ingredients involved in the formulation procedure were investigated.The optimization of formulation and process was conducted by comparing different in vitro release behaviors of PAL.In vitro dissolution studies indicated that both the two formulations of different coating materials were able to deliver PAL at an ascending release rate during the whole 24 h test.The in vivo pharmacokinetics study showed that both self-made PPOP tablets with different coating had a good in vitro-in vivo correlation(IVIVC)and were bioequivalent with the brand product,which demonstrated no signi fi cant in fl uence of the coating materials on the in vivo release acceleration of TA-PPOP.
?2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/3.0/).
1. Introduction
As a benzisoxazole derivative,an active metabolite of risperidone and mediating through a combination of central dopamine Type 2(D2)and serotonin Type 2(5HT2A)receptor antagonism,paliperidone(PAL)is approved by FDA for the treatment of central nervous system(CNS)disease schizophrenia[1].Comparing to risperidone,it's an advanced second-generation atypical antipsychotic[2,3].Also,it's reported that PAL can provide positive affective symptoms in the treatment of adults with schizoaffective disorder[4].
Because of the multiple dosing,long administration cycle and complicating of the treatment of CNS disease[5,6],a lot of sustained release and controlled-release dosages are used to ease suffering of the patients and cure the formidable disease. One satisfying example is the osmotic pump tablet(OPT) which drivers drug ingredients by osmotic pressure and exhibits zero-order release behavior.Besides,the release kinetic is only rest with the osmotic pressure of the system and free from the release medium pH,stirring speed and gastrointestinal peristalsis[7].Rose and Nelson designed a simple longterm injector which was capable of delivering fl uid at a constant rate for a long time in 1955.This was the rudiment of the osmotic pump[8].ALZA pharmaceutical company developed the elementary osmotic pump(EOP)[9]to deliver watersoluble drugs via osmotic pressure and then Theeuwes and Higuchi[10]elaborated the basic principle of the osmotic pump in the 1970s.According to the Biopharmaceutical Classi fi cation System(BCS),Class II drugs possess the characteristics of high permeability and poor solubility.In order to achieve a high pressure and procure a smooth release for the poor solubility drugs such as the former model drug PAL, Malaterre carried out the push-pull osmotic pump(PPOP)[11] which consisted of a bi-layer core surrounded by a semipermeable membrane with a laser-drilled ori fi ce.The boost layerpolymerswelledandthedrugsuspension was drivenout through the release ori fi ce.
However,this kind of simple zero-order release dosage form still cannot settle the root curing dilemma upon the CNS drugs.Firstly,thetherapeuticprocessrequiresdosagetitration considering different patients with the same kind of CNS disease have changed dosage need.It's clear that dosage titration is often utilized in the individualized treatment of reliving cancer-related breakthrough pain which is a challenging clinical phenomenon[12].As to CNS drugs,on one hand, dosage titration can avoid the dosage is too low,which resultinginthecuringprocessinvain;ontheotherhand,itcan decrease the adverse effect[13].Secondly,acute tolerance often shows up after the CNS drugs are administered to patients[14].Thirdly,a series of adverse effects will take place duringthetreatmentofCNSdisease[15].Moreover,sideeffects will often decrease a patient's willingness to adhere to treatment while physical and psychiatric comorbidities add to the complexity of treatment[16].So,it's urgent to develop some kinds of non-zero order release dosages of PPOP to work around this issue.In recent years,ascending release[17], delayedsustained-release[18]andsandwich-shaped[19]PPOP have been exhibited by the researchers.Among these, ascending release PPOP showed great advantages which could not only provide a steady and slow drug release rate avoiding thehighinitialplasmaconcentrationtokeepeffective,tolerant andsafe,butalsomaintainasmooth,controlledandprolonged therapeutic window decreasing the adverse effects and drug excitationandimprovingthecompliance[20].Thecommercial product Invega?(3 mg)[21]which was chosen as the reference preparation in this study is a tri-layer ascendingreleasetablet.
Heming Xu[22]had found a proper kind of polyethylene oxide and developed a novel bi-layer ascending release osmotic pump tablet.It differed a lot with the commercial product on the tablet structure and the manufacture process was easier.The study explained the key point to achieve nonzero order drug release rate of bi-layer osmotic pumptablet on theoreticalanalysis.Inordertoobservethehydrationstatusof the drug layers the author cut the tablet into halves and photographedthem.Also,theauthordesignedasmallexperiment to further estimate the in fl uence of the ori fi ce size on drug release.These were the characteristics of this study.However, the paper had some drawbacks.Firstly,in fl uences of coating solution ingredients involved with important factors were not taken into concern.Secondly,the determination method of plasma paliperidone concentration was performed by HPLC, which was not accurate.Thirdly,concentration-time pro fi les of paliperidone in plasma showed the peak plasma concentration was about 80 μg/ml,which was unauthentic and likely to the unit of ng/ml.So,to solve these issues and make further investigationto the osmotic pumptablet,this studydeveloped an extremely similar tri-layer ascending release tablet to the commercial one as a me-too drug.Comparing to the investigation of Xu,different core tablet,different manufacture process,different release theory and diverse in vivo experimental design and evaluation were performed.Particularly,we investigated the in fl uences ofcoatingsolutioningredients and appliedUPLC-MS/MS methodto preciselymeasurethe plasma concentration and made comparison to different coating material.These were the advantages and progress in the study.
When it comes to the coating of OPT,different semipermeable membranes coating materials are alternative,such as cellulose acetate and ethyl cellulose.The semipermeable membrane isanimportantaspectoftheosmoticpumpthanks to its role upon controlling the permeation rate of the water. Assumption that the same core tablets are prepared and coated with cellulose acetate and ethyl cellulose,respectively. Also,theinvitrodissolutionbehaviorsofthetwotabletsarethe same.Whether in vivo pharmacokinetics of them will still be the same?In this paper,we attempt to answer this question.
This study exploited two distinct kinds of coating tablets: formulation 1 was tri-layer core tablets coating by cellulose acetate(CA);formulation 2 was tri-layer core tablets coating by ethyl cellulose(EC).The theory of tri-layer ascending release PPOP,in vitro evaluation,in vivo pharmacokinetics study and IVIVC of the two formulations were investigated.
2. Materials and methods
2.1. Materials
Paliperidone (PAL)wassynthesized by pharmaceutical chemistry laboratory of Shenyang pharmaceutical university.(Shenyang,China).Commercially availablepaliperidone extended-release tablets Invega?(3 mg)which acted as the reference preparation in this study were purchased from Janssen Cilag Manufacturing L.L.C.(Xian-janssen,China). PovidoneK30,Hydroxy-propylmethylcellulose(HPMC), magnesium stearate were bought from Anhui Shanhe Pharm. S.P.A.(Anhui,China).Polyethylene oxide(PEO)was a gift from Dow Chemical Co.,Ltd.(New Jersey,USA).Cellulose acetate (CA)wasfromSinopharmChemicalReagentCo.,Ltd. (Shanghai,China).Ethyl cellulose(EC)was from Tianjin Bodi Chemical Co.,Ltd.(Tianjin,China).Acetone,ethanol and polyethylene glycol 1500(PEG 1500)were purchased from Yuwang Chemical Reagent Co.,Ltd.(Shandong,China). Lactose monohydrate was from MEGGLE Co.,Ltd.(Wasserburg am Inn,Germany).Sodium chloride(NaCl)and ammonium formate were analytical grade.UPLC-grade methanol was purchased from Fisher Scienti fi c(Pittsburgh,PA).
2.2. Design and theory of tri-layer ascending release oral osmotic tablet
The TA-PPOP delivery system was designed to be a capsuleshaped tablet[23].It consisted of an osmotically active trilayercorecontainingtwodifferentdrugconcentration layers(the fi rst drug layer and the second drug layer)and an osmotic push layer,surrounded by an ethyl cellulose or cellulose acetate semipermeable membrane.Drug was released through a laser-drilled hole which stands on the fi rst drug layer dome of the tablet.In the aqueous environment of the gastrointestinal tract(GIT),water is imbibed by osmosis activity gradient which controlled by osmotic excipients across the semipermeable membrane into the system core.The composition and thickness of the membrane also determined the rate of water absorbed to the core.Then the drug layers were hydrated becoming a gel-like suspension and the push layer started to swell at the same time.As a result,the drugs were driven out through the ori fi ce by the expanding bottom layer.
A theory can be used to describe the drug releasing process [24-26].Generally,the drug release rate(dm/dt)of a single drug core and the volume imbibition rate of water(dv/dt)of oral osmotic tablet can be described like this:
where Csis the mass concentration of the drug in suspension, A is the membrane area,k is the osmotic membrane permeability,h is the membrane thickness,π is the osmotic pressure.
As to a multicompartment core oral osmotic tablet, different drug layers have various drug concentrations.So the mass release rate should be calculated by this equation: where n represents the number of the drug layer.In this multicompartment system,the bottom layer acts as the push layer which contains no drug(C1=0).Subsequent layers maybe have different drug concentrations(C2≠C3≠…≠Cn). Different release pro fi les will be achieved if the drug suspensions from these layers are drove out continuously.For instance,when C1=0 and C2=C3=Cn,zero order release kinetics will be got.When C1=0 and C2>C3>Cn,ascending release kinetics will be performed.When C1=0 and C2<C3<Cn,descending release kinetics will be generated.
As to the tri-layer ascending release oral osmotic tablet (TA-PPOP)in this study,n equals three.And we design the fi rst drug layer containing a lower drug concentration than the second layer,which provides the necessary concentration gradient to achieve a gradual but ascending release of the drug.Here C2>C3.The time goes by.The fi rst drug layer is pushed out fi rstly.After most of the drug is released from the fi rst compartment,drug release begins from the second compartment at an ascending releasing rate.
Take each single drug layer into consideration,the drug release rate of the two layers are calculated as follows:
where(dm/dt)3and(dm/dt)2stands for the drug release rate of the fi rst drug layer and the second drug layer,respectively.
Ignoring other factors,it can infer that(dv/dt)3≈(dv/dt)2. Adding C2>C3,throughout the whole 24 h releasing period, releasing rate of the fi rst layer and the second layer can be displayed as follows:
So,as a result,tri-layer oral osmotic tablet designed by this shape and this composition can achieve an ascending drug release rate to become TA-PPOP.
2.3. Preparation of the formulations
The formulation of core tablets was consisted of paliperidone, Povidone K30,Hydroxy-propyl methyl cellulose,magnesium stearate,polyethylene oxide,lactose monohydrate,sodium chloride,butylated hydroxytoluene,stearic acid and iron oxides.Details see Table 1.
Active pharmaceutical ingredient(API)PAL and all the excipients were passed through 80-mesh sieve before use, respectively.The fi rst drug layer blend was prepared by mixing PAL and other excipients in a sealed polyethylene bag.The second drug layer blend was prepared by mixing a different percentage of PAL and certain excipients in the same kind of bag. Thethirddruglayer(pushlayer)blendwasestablishedbymixingthe rest of excipients in the same way.Three bags mentioned formerly were operated by hand for 15 min to obtain a homogeneous physical mixture.Then,by preparing a damp mass, screening the damp mass into granules,drying the granulation, sizing the granulation through dry screening,adding lubricant and blending,the wet granulation process was accomplished.

Core tablets were compressed by a single-punch tablet machine equipped with a particular shallow recessed punch (5 mmdiameter)(TDPsingle-punch,ShanghaiPharmaceutical Factory,China).The compress process was as follows:Firstly, all the three layers were accurately weighted,respectively; secondly,the fi rst drug layer powder was loaded and precompressed manually;thirdly,the second layer drug powder was loaded and pre-compressed as the same way; fi nally,the push layer powder was added into the punch groove and compressed into tri-layer tablets.
Weprepared two separate kindsofcoating tablets: formulation 1 was tri-layer core tablets coating by cellulose acetate(CA);formulation 2 was tri-layer core tablets coating by ethyl cellulose(EC).The coating solution of formulation 1 was developed by dissolving cellulose acetate and/or PEG 1500 into a precise percentage of acetone-water.The coating solution of formulation 2 was developed by dissolving ethyl cellulose and/or PVP K30 in some kind percentage of alcoholwater.The two kinds of formulations were coated both by a traditional coating pan(BY300A,Shanghai Huanghai Machinery Factory,Shanghai,China).Drying temperature was set at about 32~42°C,rotating rate of the pan was 40~45 rpm, spraying rate was 5 ml/min.Under this circumstance,the core tablets were sprayed and covered a homogeneous coating membrane of dissimilar material,respectively.To clear away the residual solvent and aging the membrane,the coating tablets were dried for 10 h at 40°C in the oven.
Drug release ori fi ce was drilled by hand by using a kind of twist-drill.We purchased a series of different sizes of the twist-drills(Dongguan Shunjia hardware Machinery Factory, Dongguan,China)and we can obtain the diameter from the speci fi cation so that we could drill some needed ori fi ces.
2.4. In vitro dissolution test
Invitrodissolution testwasaccomplishedat 37±0.5°C,50rpm according to the USP paddle method[27].To preventing the tablets fl oating on the surface of the medium after releasing all thedrugs,sinkerswas usedin theexperiment.The testwas operated by using a dissolution apparatus(ZRS-8G Test Dissolution Tester,China).A volume of 500 ml NaCl 2 gm/L (0.2%w/w)in 0.0825 mol/L HCl(pH 1.0±0.5)was picked as the dissolution medium.In consideration of decreasing the relative error and keeping the precision of the experiments,prepared tablets(formulation 1 and formulation 2)were weighted to be equivalent to 3 mg of PAL as well as the marketed Invega?.Then they were added into the dissolution medium respectively at thesametime.A volumeof10mlsampleswere withdrawn after 2,4,8,12,14 and 24 h and reinstated by equal volume of fresh medium synchronously to sustain the specifi edvolumeof the medium.The sampleswere fi lteredthrough a 0.45 μm millipore fi lter and analyzed by HPLC.
TheanalysiswascarriedoutbyanHPLCsystemconsistingof a CM-5110 pump,a CM-5210 auto-sampler,a CM-5310 column oven maintained at 35°C,a CM-5410/5420 UV-VIS detector set at 275 nm and Chromaster workstation(Hitachi,Japan).Before the test,a methodological study was carried out.The results indicated there was no interference in UV absorption between the excipients and the drug and the analytic method satis fi ed the requirements of the concentrations determination of samples ranging from 2 to 24 h.The separations were performed at roomtemperaturebyusingaC18column(4.6×150mm,particle size 5 μm,Huapu Co.,Ltd.,China).The mobile phase was 0.05 mol/L ammonium formate buffer solution(pH 3.3±0.1) acetonitrile-methanol(75:8:17,v/v),pumped at a fl ow rate of 1.5 ml/min.They were fi ltered through a 0.45 μm membrane fi lter and degassed by ultrasonication before use.
Both of marketed Invega?and prepared formulations (formulation 1 and formulation 2)showed an ascending drug release rate.To evaluate this release behavior,we chose the similarity factor(f2).The calculation formula was characterized as follows[28]: wherein n stands for the numbers of the samples,Rtand Ttrepresents the drug release percentages of the marketed Invega?and prepared formulation 1 and formulation 2 at time t,respectively.We admitted that the two drug release dates were the same as each other if the similarity factor(f2)was between 50 and 100.
2.5. In vivo evaluation
2.5.1. Experimental design
Sixhealthy beagledogs(GeneralHospitalofShenyangMilitary Region,China)were chosen.This study was performed in accordance with the“Guiding Principles in the Use of Animals in Toxicology”[29]adopted by the Society of Toxicology of the US in July 1989 and revised in March 1999.A 3-period crossover single-dose with a washout period in between experiment was designed.All the dogs were fasted overnight and free of water prior to the experiments.Six dogs were separated into three groups randomly and preparations(reference marketed Invega?,formulation 1 and formulation 2)containing 6 mg(each dog gives 2 tablets)of PLA were administered to the three groups,respectively.During the whole in vivo experiments,5 ml of blood sample were withdrawn inheparinized tubes at 0 h before dosing and at 2,4,8,12,16,20, 22,24,26,30,32,36,48,60,72,84,96 h after dosing.All the samples were centrifuged immediately at 3000 rpm for 15 min to get the separated plasma.Then the plasma was kept frozen at-20°C until analysis.
2.5.2. Determination of paliperidone in dog plasma
The determination was performed by using ultra performance liquidchromatography-tandemmassspectrometry(UPLC-MS/ MS)withaWatersTandemQuadrupole(TQ)Detector(Waters). According to the investigation of Hongming Chen,diazepam (DIA)was chosen as the internal standard.The separation was conducted on a C18column(50×2.1 mm,2.6 μm;Phenomenex Co.,USA)at40°C.Themobilephasewaswater(containing0.1% formicacid)andmethanolata fl owrateof0.3ml/min.Injection volume was 5 μl.UV detector was set at 275 nm[30].
Plasma sample preparation method was as follows:200 μl plasma was fi rst added.Then 50 μl internal standard solution DIA(500 ng/ml)and 50 μl methanol were added.They were mixed by vortex for 1 min to be homogeneous.To extract the drugs,2 ml redistilled diethyl ether was then added to the mixture.After vortex-mixing for 3 min,the samples were centrifuged at 3500 rpm for 10 min.The supernatant was collected and dried at 37°C under a stable stream of nitrogen. Theextractionsampleswerereconstitutedby100μlmethanolwater when they were to be determined[30].The concentration of PAL was determined by a standard linear calibration curveintheconcentrationrangeof1-1000ng/ml(r>0.99).The lower limit of quanti fi cation(LLOQ)for PAL was 1 ng/ml.
2.5.3. Pharmacokinetic data analysis
With the calculation of DAS2.1.1 software(Mathematical Pharmacology Professional Communities of China,Shanghai, China),a series of pharmacokinetics parameters were obtained,which including the peak plasma concentration(Cmax), the time to reach peak plasma concentration(Tmax),the area under the curve from 0 to 96 h(AUC0-96),the area under the plasmaconcentration-timecurvefromzerotoin fi nity (AUC0-∞).The value of each formulation was the mean values of the six dogs.The relative bioavailability(F)[31]of formulation 1 and formulation 2 to the reference tablets(Invega?) was calculated using the following equation:F(%)=(AUC0-96test/AUC0-96reference)× 100%.Verge for differences to be considered signi fi cant was de fi ned at p value<0.05.
2.5.4. In vitro-in vivo correlations(IVIVC)evaluation
An IVIVC for paliperidone was evaluated by plotting the fraction dissolved(Ft)in vitro versus the fraction of drug absorbed(Fa)in vivo.Fawas calculated according to the Wagner-Nelson model[32]:
whereCtisthedrugplasmaconcentration attimet;Ctdt are areas under the curve from time zero to t;Ctdt are areas underthecurvefromtimezerotoin fi nity;keistheelimination rate.Linear regression analysis was conducted and the coef ficient correlation(r2)was used to evaluate the IVIVC.
3. Results and discussion
3.1. In fl uence of different core tablet compositions of tri-layer tablet(coating by CA)
In order to obtain the optimal formulation,distinctive compositions of the core tablet and relevant in vitro release behavior were investigated.We concluded that the molecular weight(Mw)of PEO,the amount of PEO in drug containing layer and the amount of NaCl in the push layer signi fi cantly affected the in vitro PAL release performances.It was showed in the Fig.1A that the drug release rate was greatly in fl uenced by the Mw of PEO.Because of the poor solubility of PAL,it was dif fi cult to form the drug suspension in the osmotic pump without adaptive polymer materials which could avoid the precipitation and separation of the drug after the hydration of thedruglayer.MwofPEOfrom200,000,300,000to400,000were chosen in this study.The result showed the highest release rate was achieved and the dissolution within 24 h was complete when using PEO(Mw 200,000).Along with the Mw of PEO in drug containing layer increased,the releasing rate was decreased and the dissolution was not outright.This was because the higher the Mw of PEO in drug containing layer was,the larger amount of water was needed to reach the hydration of the drug layer.When the volume imbibition rate of water was constant,the PEO(Mw 400,000)required longer time to suspend the drug layer.What's more,the viscosity of PAL suspension increased,resulting in an extremely slow releasing rate.Taking these reasons into consideration,we picked the Mw 200,000 as the polymer suspension.The in fl uence of the amount of PEO in drug containing layer was demonstrated in Fig.1B.The drug releasing rate rose and the dissolutionat 24h wascompletealongwiththeincreaseofthe amount of PEO.The drug could not be suspended entirely with a small quantity of PEO.Under that circumstance,the drug was tackled in the coating membrane and the membrane was deformed even though the push force of the bottom layer was constant.It was clear that osmotic pump release drugs by the osmoticpressure.DifferentamountofNaClwhichactedasthe osmotic agent in the push layer showed different in vitro PAL releasebehaviors(Fig.1C).Appropriatequantityoftheosmotic agent could achieve an ascending osmotic pressure gradient across the membrane.PAL was pushed out too fast through the ori fi ces if the amount of NaClwas too high while therewas not enough power to push itself out of the ori fi ce at an ascending rate totally if the quantity of NaCl was too low.The hydration and expanding rate of the push layer was affected upon the osmotic agent.In this approach,an ideal ascending releasing rate could obtain by regulating the osmotic agent.
3.2. In fl uence of the coating of tri-layer tablet
3.2.1. In fl uence of the water amount in the coating solution (coating by CA)
It was observed in Fig.1D that water amount in the coating solution in fl uenced the ascending release considerably.The result showed that the drug release acceleration reduced gradually when the water amount in the coating solution went down.The solution was prepared by dissolving cellulose acetate and PEG 1500 which used as the porogen into the acetone-water.PEG 1500 and the water would help forming the porous fi lm structure during the coating process.If larger amount of water was added to the coating solution,the fi lm structure would become more porous.Thus,the volume imbibition rate of water increased,which resulting in faster hydration rates of push layer and drug layer and thereby faster ascending drug releasing rate.However,the maximal amount of water of the coating solution was limited because the cellulose acetate would not dissolve in the acetone-water solution completely when there were too many hydrophilic solvents.
3.2.2. In fl uence of the Mw of PEG in the coating solution(coating by CA)
It was showed in Fig.2A that the ascending releasing rate was signi fi cantly in fl uenced by the Mw of PEG.The releasing rate was also decreased along with the Mw of PEG in the coating solution reduced.Generally,proper additive ingredient was needed to be added into the coating solution.On one hand,it could aggrandize toughness and strength of the coating membrane;on the other hand,it couldadjust the permeability of the fi lm to achieve the requested drug releasing rate.The study chose PEG because it was water-soluble and could reach these requires.The PEG was homogeneously dispersed in the coating solution and coated surrounding the core tables with cellulose acetate after being sprayed.It was well known that HOCH2(CH2OCH2)mCH2OH represented the molecular formula of PEG,where m represented the average number of oxyethylene groups.Mw of PEG was related to the m value.A higher Mw was accompanied with a bigger m value.Due to the water-solubility of PEG which was caused by oxyethylene groups,higher Mw of PEG could present more water-soluble and obtain more porous fi lm structure.Lots of pores were formed when the tablets were dried.As a result,the tablet with PEG 6000 achieved the highest ascending releasing rate. But we did not hope the drug been driven out too fast.So PEG 1500 was chosen to add into the coating solution in the end.
3.2.3. In fl uence of the amount of PEG of in the coating solution(coating by CA)
Different amount of PEG in the coating solution would cause a variety drug releasing rate(Fig.2B).It could conclude that the drug was driven out faster at an ascending releasing rate if a larger quantity of PEG was added into the coating solution. The drug would be released slowly and even could not be pushed out completely when the PEG component was not enough.It was clear that the water amount in the coating solution in fl uenced the ascending release considerably.PEG was used to form a porous fi lm structure and the water was used to dissolve this kind of porogen.So there was a similarity between the two factors.Both of them could enhance the permeability of the membrane.In this study,very little water could in fi ltrate through the membrane into the core tablet when 0.02 g PEG was used.Thereby,neither the drug layer nor the push layer could receive an abundant impetus to drive out the drugs.So,an appropriate ascending releasing rate could obtain by adjusting the amount of PEG in the coating solution.
3.2.4. In fl uence of coating weight gain of tri-layer tablet (coating by CA)
Fig.2C described ascendingreleasing behavior of PAL from the tri-layer PPOP tablets with different coating weight gain.The result indicated that the ascending releasing rate was affected by the coating weight gain.It was evident to fi nd that the drug ascending releasing characteristic decreased when the coating weight gain went up.The coating membrane was utilized to control the penetration rate of the water between the core tablet and the gastrointestinal tract environment. Water penetration decreased if the coating weight gain increased and the hydration of the drug layer and the push layer became slower,resulting in the decline of the ascending releasing rate.Therefore,the coating weight gain was a particularly important factor to the osmotic pump tablets.On one hand,it would be hard to drive out the drugs from the ori fi ce and release completely when the coating membrane was too thick.The whole releasing process would be affected owing to the lack of enough push forces under a low penetration of water,especially the end stage of the drug releasing. On the other hand,the coating membrane was inclined to weaker with a smaller thickness,resulting in a much more rapid ascending drug release rate and even an easier break of the fi lm against the enormous push impetus caused by the osmotic pressure.
3.2.5. In fl uence of different sizes of the ori fi ces of tri-layer tablet(coating by CA)
Fig.2D indicated that no signi fi cant in fl uence of different sizes of the ori fi ces on the ascending releasing behavior.Three different kinds of tablets were prepared and the sizes of the ori fi ces varied from 0.5 mm,0.8 mm-1.0 mm.The dissolution of the tablets with different sizes of ori fi ces displayed similar pro fi les(f2>50).It could conclude from the fi gure that the root drug driven force of the osmotic pump came from the osmotic pressure across the membrane.Although the sizes of the ori fi ces were distinctive,the drug releasing rate was constant. The ori fi ce was just used to let the drug been expelled. Comparing to the huge in fl uence of osmotic pressure,the size of the ori fi ce could not be taken into account.
3.3. Ascending drug release behavior of the formulation 1,formulation 2 and the reference preparation
The dissolution of three different formulations was studied in the Fig.3.Tri-layer core tablets were prepared fi rst.Formulation 1 was tri-layer core tablets coating by cellulose acetate (CA)while formulation 2 was ethyl cellulose(EC)coating.The coating solution of formulation 1 was developed by dissolving cellulose acetate and PEG 1500 into acetone-water.The coating solution of formulation 2 was developed by dissolving ethyl cellulose and PVP K30 into alcohol-water.The extensional coating solution composed of cellulose acetate and ethyl cellulose were made by formula as showed in Table 2. Fig.3 described the release behavior of PAL from the three different formulations.It was obvious that the CA coating and EC coating tri-layer ascending releasing osmotic pump tablets had similar in vitro dissolution behavior.Also,they displayed similar pro fi les with the reference preparation.

3.4. In vivo evaluation
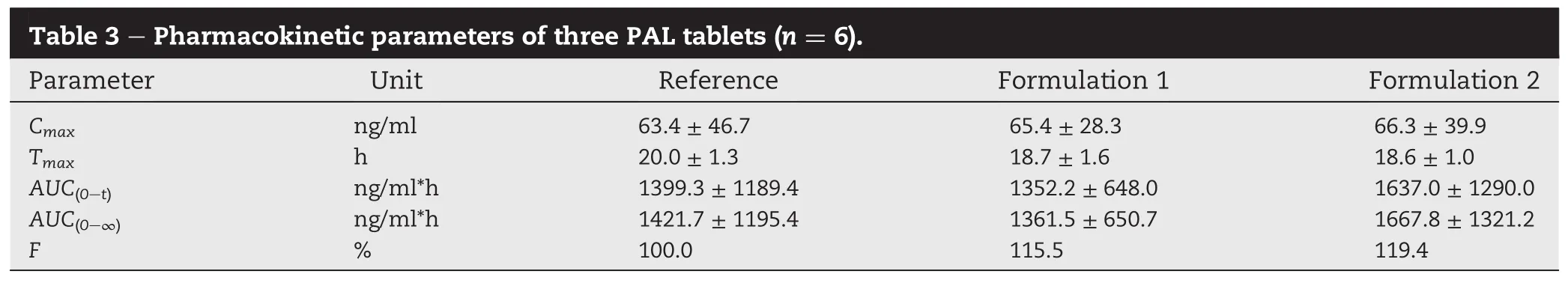
The PAL plasma concentration-time pro fi les of two different coating POPP tablets and commercial reference tablets in beagle dogs were listed in Fig.4.The main pharmacokinetic parameters of PAL were shown in Table 3.It was showed that the release pro fi les of both the CA coating and EC coating PPOP reached the peak plasma concentration level at about 18.6 h after dosing while the reference tablet was at about 20 h.The PALplasmaconcentrationwasincreasedsteadilyuntil reaching to the highest level,and then the plasma concentration decreased gradually in a prolong period which showed aperfectascendingreleasingbehaviorand sustainedreleasing function.The relative bioavailability of the two self-made formulations was 115.5%(CA coating)and 119.4% (EC coating),respectively.It was concluded from the result that the in vivo pharmacokinetics of the two different coating materials of TA-PPOP were still similar.No signi fi cant in fl uence of the coating materials on the release acceleration was found.
So,the hypothesis in the introduction was correct and could be proved.In this study,drug release motive power was mainly came from the osmotic pressureof the pumpand push force of the push layer.The content of drug in each tablet was very small and was released into the outer system for extremely long time to continue to 24 h.It could infer that the drug would be absorbed and metabolism immediately as long as the drug was released into the gastrointestinal tract.Both the two coating materials acted as the semipermeable membranes.They would still keep their characteristics and functions as before when the residual tablets were excreted from the body because they were neither dissolved nor absorbed by the bio-system.CA or EC coating materials were only acted as carrier,as a result they had no signi fi cant in fl uence upon in vivo performance for paliperidone tri-layer ascending release osmotic pump tablet.
A level A of IVIVC,point-to-point relationship[11]was performed.The results of linear regression between in vitro dissolution and in vivo absorption for the two self-made formulations were displayed in Figs.5 and 6.It was showed that both of the CA coating and EC coating tablets had an excellent linearity with r2being 0.9914 and 0.9963,respectively.So,it was concluded that there was a close correlation between the in vitro dissolution and in vivo absorption.
4. Conclusion
A TA-PPOP which could drive out drugs at an ascending releasing rate and deal with complicated therapy issues of the CNS drugs was developed in this study.The mechanism of the delivery on theory was elaborated and it could infer that TAPPOP with different releasing behavior could be worked out by adjusting drug loading in different layers.A series of in fl uencing factors were investigated.It was observed that the molecular weight of PEO,the amount of PEO in drug containing layer,the amount of NaCl in the push layer,water amount in the coating solution,the molecular weight of PEG, the amount of PEG in the coating solution and coating weight gain signi fi cantly affected the in vitro PAL release performances.CA coating and EC coating tablets were prepared and the in vitro dissolution test showed both similar to the selfmade tablets and the reference preparation.The in vivo pharmacokinetics study demonstrated that both self-made PPOP tablets with different coating could obviously control therelease rateof PALwith a good IVIVC,whichdemonstrated no signi fi cant in fl uence of the coating materials on the release accelerationofTA-PPOP.Moreover,thetwo kindsofself-made PPOPtabletswerebioequivalentwiththebrandproduct.Thus, this TA-PPOP model has great potential in the CNS drugs' development.
REFERENCES
[1]Yang LPH.Oral paliperidone.CNS Drugs 2011;25:523-538.
[2]Citrome L.Oral paliperidone extended-release:chemistry, pharmacodynamics,pharmacokinetics and metabolism, clinical ef fi cacy,safety and tolerability.Expert Opin Drug Metab Toxicol 2012;8:873-888.
[3]Nussbaum AM,Stroup TS.Oral paliperidone for schizophrenia.Cochrane Database Syst Rev 2012;3:12-18.
[4]Canuso CM,Schooler N,Carothers J,et al.Paliperidone extended-release in schizoaffective disorder:a randomized, controlled study comparing a fl exible dose with placebo in patients treated with and without antidepressants and/or mood stabilizers.J Clin Psychopharmacol 2010;30:487-495.
[5]Sibley JT,Olszynski WP,Decoteau WE,et al.The incidence and prognosis of central nervous system disease in systemic lupus erythematosus.J Rheumatol 1992;19:47-52.
[6]Ming G,Song H.Adult neurogenesis in the mammalian central nervous system.Annu Rev Neuro Sci 2005;28:223-250.
[7]Verma RK,Krishna DM,Garg S.Formulation aspects in the development of osmotically controlled oral drug delivery systems.J Control Release 2002;79:7-27.
[8]Rose S,Nelson JF.A continuous long-term injector.Aust J Exp Biol Med Sci 1955;33:415-416.
[9]Higuchi T,Leeper HM.Improved osmotic dispenser employing magnesium sulphate and magnesium chloride. U.S.Patent 3,760,804;1973,9.
[10]Theeuwes F,Sheet M.Osmotic dispensing device for releasing bene fi cial agent.U.S.Patent 3,845,770;1974.
[11]Malaterre V,Ogorka J,Loggia N,et al.Approach to design push-pull osmotic pumps.Int J Pharm 2009;376:56-62.
[12]Portenoy RK,Payne R,Coluzzi P,et al.Oral transmucosal fentanyl citrate(OTFC)for the treatment of breakthrough pain in cancer patients:a controlled dose titration study. Pain 1999;79:303-312.
[13]Pajouhesh H,Lenz GR.Medicinal chemical properties of successful central nervous system drugs.NeuroRx 2005;2:541-553.
[14]Manning C,Scandale L,Manning EJ,et al.Central nervous system effects of meclizine and dimenhydrinate:evidence of acute tolerance to antihistamines.J Clin Pharm 1992;32:996-1002.
[15]Puzantian T.Central nervous system adverse effects with efavirenz:case report and review.Pharmacotherapy J Hum Pharm Drug Ther 2002;22:930-933.
[16]Chue PS,MacKenzie EM,Chue JA,et al.The pharmacology and formulation of paliperidone extended release.Expert Rev Neurother 2012;12:1399-1410.
[17]Thyssen A,Cleton A,Talluri K,et al.No pharmacokinetic interaction between paliperidone extended-release tablets and trimethoprim in healthy subjects.Hum Psychopharmacol 2009;24:532-539.
[18]Verma S,VanRyzin C,Sinaii N,et al.A pharmacokinetic and pharmacodynamic study of delayed-and extended-release hydrocortisone(ChronocortTM)vs.conventional hydrocortisone(CortefTM)in the treatment of congenital adrenal hyperplasia.Clin Endocrinol 2010;72:441-447.
[19]Zhang Y,Zhang Z,Wu F.A novel pulsed-release system based on swelling and osmotic pumping mechanism. J Control Release 2003;89:47-55.
[20]Owen RT.Extended-release paliperidone:ef fi cacy,safety and tolerability pro fi le of a new atypical antipsychotic.Drugs Today(Barc)2007;43:249-258.
[21]Janicak PG,Winans EA,Paliperidone ER.a review of the clinical trial data.Neuropsychiatr Dis Treat 2007;3:869-872. [22]Xu Heming,Li Zhao,Pan Hao,et al.A novel bi-layer ascending release osmotic pump tablet:in vitro investigation and in vivo investigation in pharmacokinetic study and IVIVC evaluation.Int J Pharm 2013;458:181-187.
[23]Leslie C.Oral paliperidone extended-release:chemistry, pharmacodynamics,pharmacokinetics and metabolism, clinical ef fi cacy,safety and tolerability.Drug Eval 2012;8:873-888.
[24]Michael JR,Jonathan H,Michel SR.Modi fi ed-release drug delivery technology,vol.1.CRC Press;2003.p.110-130.
[25]Charman SA,Charman WN.Oral modi fi ed-release delivery systems.Modi fi ed-release drug delivery Tech,vol.1;2002. p.1-19.
[26]Ikegami K,Tagawa K,Osawa T.Bioavailability and in vivo release behavior of controlled-release multiple-unit theophylline dosage forms in beagle dogs,cynomolgus monkeys,and gottingen minipigs.J Pharm Sci 2006;95:1888-1895.
[27]Nagata S,Jin-nai A,Hirai K,et al.Evaluation of dissolution of osmotic-controlled release paliperidone tablets using the reciprocating cylinder method.Yakugaku Zasshi 2012;133:405-410.
[28]Moore JW,Flanner HH.Mathematical comparison of curves with an emphasis on in vitro dissolution pro fi les.Pharm Technol 1996;20:64-74.
[29]Society of Toxicology(SOT).Guiding principles in the use of animals in toxicology.1999.
[30]Chen Hongming,Zhao Longshan,Leng Donglei,et al. Development and validation of a rapid and sensitive UHPLCeMS/MS method for determination of paliperidone in the beagle dog plasma.A J Pharm Sci 2014;9:286-292.
[31]Hollman P,Van T,Buysman M,et al.Relative bioavailability of the antioxidant fl avonoid quercetin from various foods in man.FEBS Lett 1997;418:152-156.
[32]Wagner JG,Pernarowski M.Biopharmaceutics and relevant pharmacokinetics.Pharm Res 1971;20:340-350.
Guangjing Lia,Yongjun Wanga,Hongming Chena,Donglei Lenga, Panqin Mab,Yanjie Donga,Lifang Gaoa,Zhonggui Hea,*
aSchool of Pharmacy,Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China bKangya of Ningxia Pharmaceuticals Co.Ltd,China
*Corresponding author.Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China.Tel./fax:+86 24 23986321. E-mail address:hezhonggui@vip.163.com(Z.He).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.12.002
1818-0876/?2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/3.0/).
 Asian Journal of Pharmacentical Sciences2015年2期
Asian Journal of Pharmacentical Sciences2015年2期
- Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- A developed HPLC method for the determination of Alogliptin Benzoate and its potential impurities in bulk drug and tablets
- Gradient high performance liquid chromatography method for simultaneous determination of ilaprazole and its related impurities in commercial tablets
- Mycosynthesis,characterization and antibacterial properties of AgNPs against multidrug resistant (MDR)bacterial pathogens of female infertility cases
- A new self-emulsifying formulation of mefenamic acid with enhanced drug dissolution
- Design and evaluation of nicorandil extended-release tablet
