A new self-emulsifying formulation of mefenamic acid with enhanced drug dissolution
A new self-emulsifying formulation of mefenamic acid with enhanced drug dissolution
ARTICLEINFO
Article history:
Received 16 August 2014
Received in revised form
29 September 2014
Accepted 11 October 2014
Available online 20 December 2014
Self-emulsifying formulation Poorly water-soluble drug
A
To enhance the dissolution of poorly soluble mefenamic acid,self-emulsifying formulation (SEF),composing of oil,surfactant and co-surfactant,was formulated.Among the oils and surfactants studied,Imwitor?742,Tween?60,Cremophore?EL and Transcutol?HP were selected as they showed maximal solubility to mefenamic acid.The ternary phase diagram was constructed to fi nd optimal concentration that provided the highest drug loading.The droplet size after dispersion and drug dissolution of selected formulations were investigated.The results showed that the formulation containing Imwitor?742,Tween?60 and Transcutol?HP(10:30:60)can encapsulate high amount of mefenamic acid.The dissolution study demonstrated that,in the medium containing surfactant,nearly 100%of mefenamic acid were dissolved from SEF within 5 min while 80%of drugs were dissolved from the commercial product in 45 min.In phosphate buffer(without surfactant),80%of drug were dissolved from the developed SEF within 5 min while only about 13%of drug were dissolved in 45 min,from the commercial product.The results suggested that the SEF can enhance the dissolution of poorly soluble drug and has a potential to enhance drug absorption and improve bioavailability of drug.
?2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1. Introduction
Mefenamic acid,a non-steroidal anti-in fl ammatory drug (NSAID)of the enolic acid class,is widely used in mild to moderate pain including headache,dental pain,dysmenorrheal,rheumatoid arthritis,osteoarthritis and other joint disorders.The solubility of mefenamic acid in water is 0.04 mg/ml[1].Mefenamic acid is rapidly absorbed after oraladministration.Following a single 1-g oral dose,mean peak plasma levels ranging from 10 to 20 mg/ml have been reported.Peak plasma levels are attained in 2-4 h and the elimination half-life approximates 2 h[2,3].Mefenamic acid belongs to class II category under the biopharmaceutical classi fi cation system (BCS),i.e.,itis inherently highly permeable through biological membranes,but exhibits low aqueous solubility.Rate of absorption and/or extent of bioavailabilityforsuch insolublehydrophobicdrugare controlled by rate of dissolution in gastro-intestinal fl uids. However,its oral bioavailability is very low,probably due to poor solubility in water and insuf fi cient dissolution rate[1-3].
Various approaches for improving drug dissolution rate have been reported in literature,including reducing the particle size,solid dispersion,inclusion complex formation,solubilization in surfactant system,using prodrugs and drug derivatization,lipid-based formulation,and self-emulsifying drug delivery system[4].Among these approaches,selfemulsifying drug delivery system(SEDDS)is found to be a prominent approach to improve solubility and drug dissolution.SEDDS is an isotropic mixture of oil,surfactant,cosurfactant and drug,which spontaneously forms thermodynamically stable oil-in-water emulsions when introduced into aqueous phase under gentle agitation conditions,similar to those which would be encountered in the GI tract[5-7].
Poorly water-soluble drugs can be dissolved in SEDDS, allowing them to be encapsulated as dosage form for oral administration.Upon contact with aqueous phase of GI tract, the SEDDS formulations are self-emulsifying and very fi ne dispersions are then formed spontaneously with the aid of GI motility[8]because the free energy required to form the emulsion is either low and positive or negative.Therefore,the drug remains in solution in the GI lumen,avoiding the dissolution step which often limits the rate of absorption of poorly water-soluble drugs from the crystalline state[9]. SEDDS appears to be an attractive choice of formulation as it requires simple manufacturing equipment.This is because it is physically stable lipid-based solution and omits the need of high energy emulsi fi cation process,and thus reduces the manufacturing cost.Moreover,greater dissolution rate of SEDDS reveals the reduction in drug dose and possibly reduce the dose-related side effects.
Inthisstudy,we developed anewself-emulsifying formulation(SEF)of mefenamic acid to enhance the dissolution rate of mefenamic acid.Physical properties and dissolution pro fi lesofSEFwereevaluated in comparison to mefenamic acid powders and commercial capsules.
2. Materials and methods
2.1. Materials
Mefenamic acid,clove oil,olive oil and rice bran oil were purchased from P.C.Drug Center(Thailand).Caprylic/capric triglycerides(Miglyol?812)and caprylic/capricglyceride (Imwitor?742)were purchased form Sasol(Germany).Oleic acid was purchased from Sigma-Aldrich(USA).Polyoxyethylene 20 sorbitan monolaurate(Tween?20),Polyoxyethylene (20) sorbitan monostearate (Tween?60), polyoxyethylene 20 sorbitan monooleate(Tween?80),sorbitan monolaurate(Span?20)and sorbitan monostearate (Span?60)were purchased from Fluka(USA).Polyoxyl 40 hydrogenated castor oil(Cremophor?RH40)and polyoxyl 35 castor oil(Cremophor?EL)were a gift form BASF(Thai)Co., Ltd.(Thailand).Diethylene glycolmonoethyl ether(Transcutol?HP)was supported by Gattefosse(Saint-Priest Cedex, France).Other chemicals were of reagent or analytical grade and used without further puri fi cation.Distilled water was used in all preparations.
2.2. Determination of drug solubility in various vehicles
Solubility of mefenamic acid was determined in various vehicles by adding excess amount of mefenamic acid(500 mg)in 10 mL of a pure vehicle in glass tubes.The drug suspension was equilibrated at 25°C in a thermostatically controlled bath for 72 h.After equilibration,the tubes were centrifuged at 12,000 rpm for 20 min and the clear supernatants were analyzed for mefenamic acid with a high performance liquid chromatography(HPLC,model JASCO PU-2089 plus quaternary gradient inert pump,and a JASCO UV-2070plus multiwavelength UV-vis detector,Jasco,Japan)using Luna 5u C18 column(5 μm,4.6 nm ×25 cm)(Phenomenex,USA).The mobile phase composing of acetonitrile,pH 5.0 phosphate buffer and tetrahydrofuran(23:20:7)was fi ltered through a 0.22-μm membrane fi lter,and degassed in a sonicator bath before use.The fl ow rate of mobile phase was 1 mL/min,and the UV detection wavelength was 254 nm.
2.3. Construction of ternary phase diagram and preparation of SEF
Based on the solubility studies,the oil(Imwitor?742),surfactant(Tween?60 or Cremophor?EL)and co-surfactant (Transcutol?HP)were chosen for the construction of ternary phase diagram.A series of SEF were prepared using various concentrations(10-80% v/v)ofoil,surfactantandcosurfactant.The oil,surfactant and co-surfactant were mixed at ambient temperature(25°C),until clear solution was obtained.Then,excess amount of mefenamic acid(500 mg)was added to the mixtures and mixed thoroughly.The resultant formulations were shaken at ambient temperature(25°C)for 72 h,in the same manner as mentioned above,before further analysis of mefenamic acid content,as described above.
2.4. Physical characterization
Visual observation of self-emulsi fi cation:Evaluation of the self-emulsifying properties of SEF was visually observed(i.e., until a clear homogenous system was obtained).
Droplet size analysis:The droplet size of emulsion formed after reconstitution of SEF in water(200 times)was determined by static laser light scattering(model LA-950,Horiba, Japan).Reconstituted samples were withdrawn and diluted to a fi nal concentration of approximately 0.05%(w/w)with distilled water.A relative refractive index of 1.2(ratio of the indices between the oil and water phase)was used.All measurements were repeated 3 times and the values of mean diameter were reported.The span,which is the width of thedistribution based on the 10%,50%and 90%quartile,was also calculated from the following equation:
where D(v,0.5)is the volume median diameter where 50%of the volume distribution is above and 50%is below,D(v,0.9)is the volume median diameter where 90%of the distribution is below this value,and D(v,0.1)is the volume median diameter where 10%of the volume distribution is below this value.
2.5. In vitro dissolution study
The in vitro dissolution of mefenamic acid from SEF formulations was compared to that of mefenamic acid powder and commercial product(mefenamic acid capsules)purchased from the retail pharmacy in Thailand.The dissolution test was carried out using USP dissolution apparatus I(PharmaT-est,Germany)with 900 mL of 0.05 M Tris buffer(pH 9.0)with 1%(w/v)sodium lauryl sulfate(SLS)or phosphate buffer(pH 7.4)as dissolution medium at 37±0.5°C.The basket rotation speed was adjusted to 100 rpm.Selected drug-loaded SEF formulations(equivalent to 250 mg of mefenamic acid) fi lled in hard capsules were put into a basket before placing in a dissolution vessel.During the study,5 mL aliquots were removed at predetermined time intervals from the dissolution medium and 5 mL of fresh medium were replaced.Samples were withdrawn from the dissolution vessels at 2,5,10,20,30, 45 and 60 min,and passed through 0.45-μm nylon membrane fi lters before analysis.The amount of mefenamic acid dissolved in the dissolution medium was determined by HPLC,as mentioned above.The dissolution experiments were carried out in triplicate.
2.6. Statistical analysis
Analysis of variance(ANOVA)and Levene's test for homogeneity of variance were carried out using SPSS version 10.0 for Windows(SPSS Inc.,USA).Post hoc testing(P<0.05)of the multiple comparisons was performed by either the Scheff′e or Games-Howell test depending on whether Levene's test was insigni fi cant or signi fi cant,respectively.
3. Results and discussion
3.1. Solubility of mefenamic acid in various vehicles
As oil,surfactant and co-surfactant play a vital role to increase the solubility of drug and drug loading in SEF,these components should be carefully chosen to have maximal drug solubility along with good miscibility with each other to produce a stable formulation[10].The solubility of mefenamic acid in various vehicles is presented in Table 1.Higher solubility of mefenamic acid in the oil phase was important criterion,as it could help the emulsion to maintain drug in solubilized form. Mefenamicacid exhibited high solubility in clove oil (9.95±0.68 mg/ml)but this oil can irritate mucus membranes and cause skin irritation.Thus,it is not suitable for using as oil phase in SEF.It is shown that the solubility of mefenamic acid in Imwitor?742 was high(7.88±0.62 mg/ml),as Imwitor?742 (caprylic/capric glyceride)is amphiphilic compound with surface active property.In addition,some hydroxyl groups within the glycerol ester of Imwitor?742 are free,contributing to its polarity and excellent solvent properties for many drugs. Therefore,Imwitor?742 was then selected as an oil component for further study.
Various non-ionic surfactants have been widely used due to their relatively low toxicity.The results shown in Table 1 suggest that mefenamic acid was highly soluble in hydrophilic surfactant,e.g.,Tween?and Cremophor?.Tween?60 wasfoundtohavethemaximalsolubilizingcapacity (44.60±0.52 mg/ml)while Span?20 demonstrated the minimal solubilizing capacity(6.07±0.52 mg/ml)for mefenamic acid.Cremophor?ELalsoshowedhighdrugsolubility (37.62±2.76 mg/ml).It is observed that mefenamic acid was highly soluble in co-surfactant;the highest solubility was found in Transcutol?HP(38.76±2.42 mg/ml).Based on the solubility of mefenamic acid in different oils,surfactants and co-surfactants,we have selected Imwitor?742 as oil,Cremophor?EL and Tween?60 as surfactant and Transcutol?HP as co-surfactant for the preparation of SEF.
3.2. Preparation of SEF
Ternary phase diagrams were constructed to obtain the appropriate components of oil,surfactant and co-surfactant that can result in spontaneously formed emulsion.Surfactant and co-surfactant are preferentially adsorbed at the interface,reducing the interfacial energy as well as providing a mechanical barrier to coalescence.Fig.1 shows the ternary phase diagram of Imwitor?742,Cremophor?EL or Tween?60,and Transcutol?HP.Fourteen different combinations for each surfactant(Cremophor?EL or Tween?60)were prepared.It was observed that the incorporation of highconcentration of co-surfactant(Transcutol?HP)increased the spontaneity of the spontaneous emulsi fi cation process, as shown by the highlighted region in the ternary phase diagram(Fig.1).The ef fi ciency of emulsi fi cation was good when the surfactant/co-surfactant concentration was more than 50%(v/v)of the SEF.
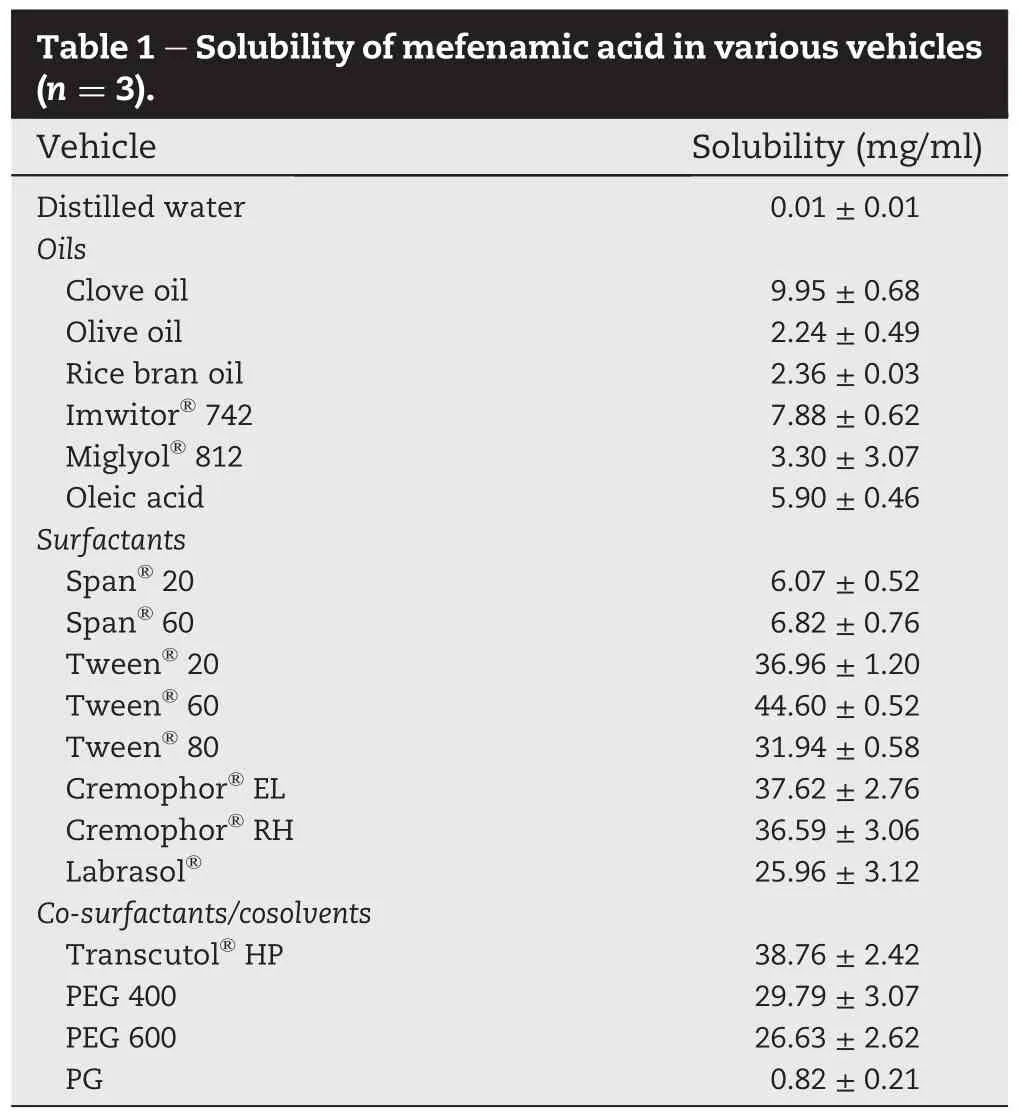
The solubility of mefenamic acid in each formulation was consequently determined(Table 2).It is clearly seen that the formulations with high concentration of surfactant(Cremophor?EL or Tween?60)and those with high concentration of oil(Imwitor?742)hada low drug loadingcapacity.Higher drug loading was obtained from the formulations with high concentrationofco-surfactant(Transcutol?HP).The resultsarein good agreement with those reported by Weerapol et al.[11]. Exception is for T7,which the appearance of the mixture changed to viscous gel-like appearance and the drug content was not determined.
The spontaneous emulsifying properties of selected formulations providing high drug loading,i.e.,C3,C8,T3 and T8,were observed visually as well as by droplet size analysis. It is noticeable that these formulations still remain as clear mixture after diluting with water(200 times).This is probablybecausethesurfactantandco-surfactantcan be adsorbed at the interface of oil and water and stabilized the formationofemulsions.Turbid,milkyemulsionswere observed as their droplet size was in micrometer range (Table 3).The droplet size of SEF containing mefenamic acid was larger than the SEF with no drug and with other drugs, prepared in our previous study[11].Additionally,small span values were obtained,indicating a narrow size distribution [12].
3.3. In vitro dissolution study
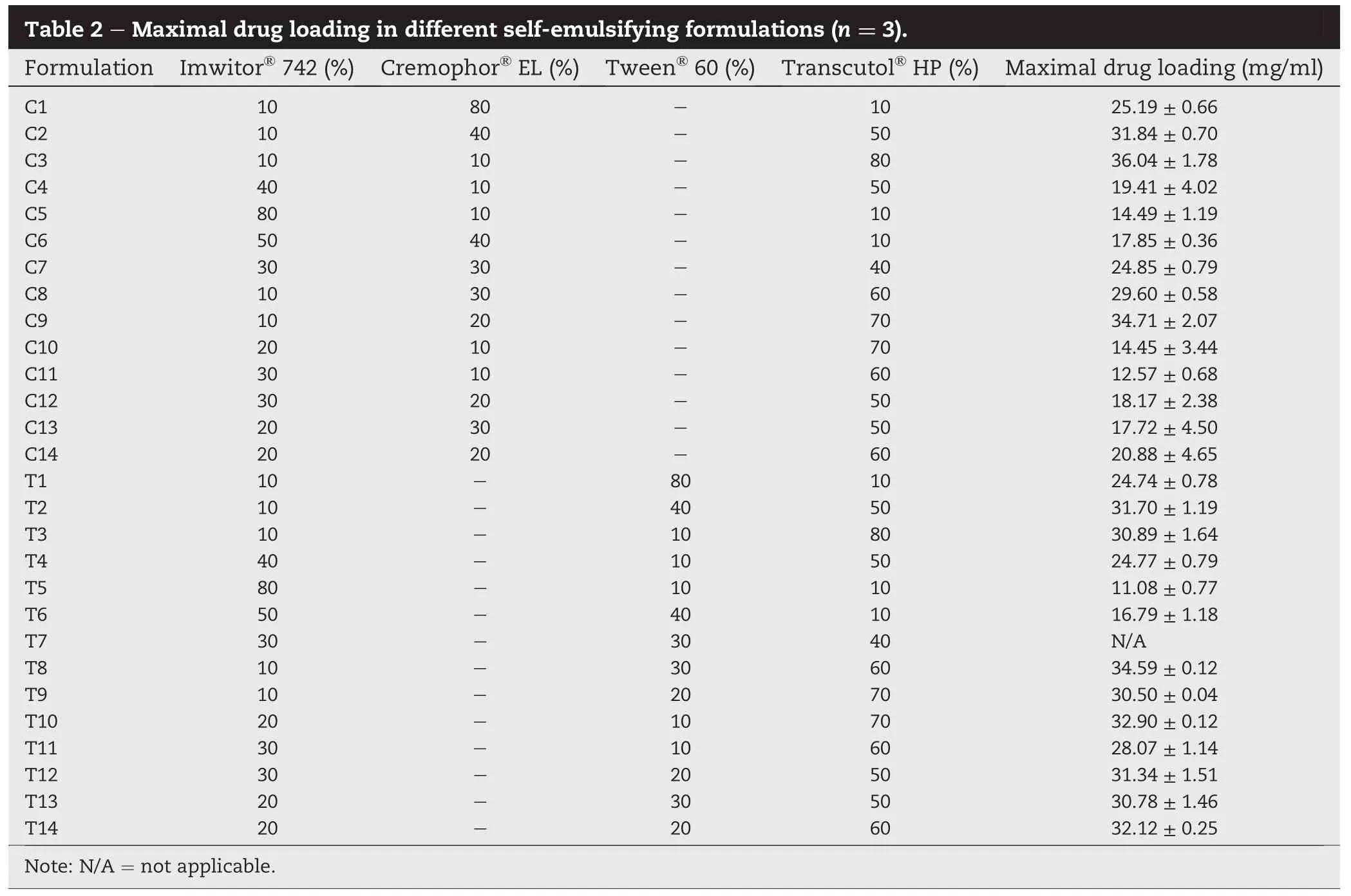
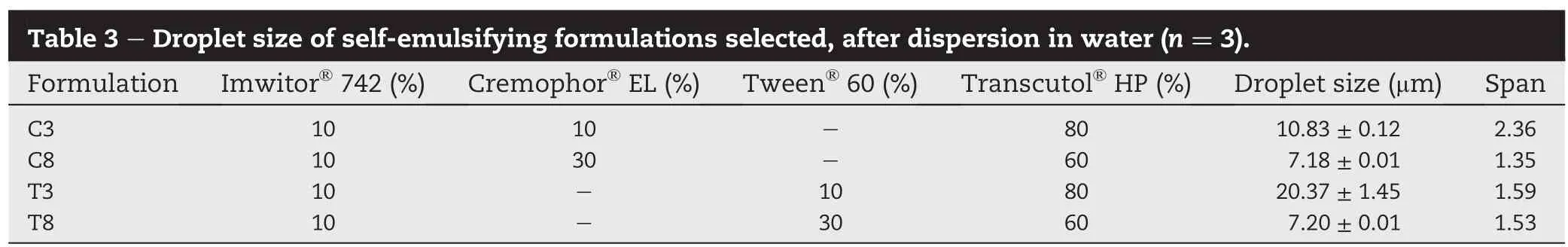
The dissolution of mefenamic acid,in 0.05 M Tris buffer(pH 9.0)with 1%(w/v)SLS according to USP monograph for mefenamic acid capsules,from SEF was compared with that of mefenamic acid powder and commercial product(Fig.2).As stated in the USP,the dissolution of mefenamic acid from the mefenamic acid capsules in 45 min should be not less than 80%.It is clearly seen that the dissolution,in 45 min,of mefenamic acid powder was about 57%while that of the commercial product conformed to the monograph(i.e.,about 80%dissolved).The dissolution from C3 and T8 demonstrated improved drug dissolution;the drug dissolution from these formulations seemed to reach nearly 100%immediately, suggesting the increased solubility and dissolution rate of mefenamic acid.This may result from a fast spontaneous emulsion formation and the small droplet size[13].Interestingly,the lower drug dissolution with a decline phase was observed from C8 and T3.It is possible that C8 and T3 formulations induced the generation of supersaturated solution of mefenamic acid.Brouwers et al.[14]suggested that the drugs in highly concentrated solution(including SEF)are thermodynamically unstable and have a tendency to precipitate in the medium.
The dissolution in pH 7.4 phosphate buffer showed different dissolution pro fi les(Fig.3);the dissolution of mefenamic acid powder was very low,less than 5%dissolved in 60 min.The drug dissolution,in pH 7.4 phosphate buffer,from commercial product was also low(only about 14%dissolved in 60 min).The results agreed with that reported by Vikram et al. [15],which the dissolution of commercial mefenamic acid tablets,performed in pH 7.4 phosphate buffer,shows a maximum drug dissolution of 51%in 45 min.The dissolution ofmefenamicacid from T3and C3(composingof oil:surfactant:co-surfactant of 10:10:80)was approximately 30-40%in 45 min while that from T8 and C8,which contained more surfactant(30%surfactant),was much higher(i.e., 60-80%in 45 min).The formation of fi ne emulsion droplets would be attributable to improved drug dissolution,which might partly explain the more rapid dissolution of SEF containing higher concentration of surfactant.Furthermore,the dissolution results showed that the SEF containing Tween?60 as surfactant presented a higher drug dissolution than that containing Cremophor?EL,at the same oil:surfactant:cosurfactantratio.Itislikelydueto thehigherhydrophilic-lipophilic balance(HLB)value of Tween?60(HLB 14.9), compared to Cremophor?EL(HLB 13).With the higher HLB values,hydrophilicity of surfactants was increased.An increase in water solubility and hydrophilicity of surfactants could increase the rate of drug dissolution and diffusion to the dissolution medium[16].
4. Conclusion
SEF composing of oil,surfactant and co-surfactant was used to improve the dissolution of mefenamic acid.The optimal concentration of components that provided high drug loading was determined using ternary phase diagram.The formulation containing Imwitor?742,Tween?60 and Transcutol?HP (10:30:60)can encapsulate high amount of mefenamic acid and demonstrated the highest drug dissolution in 45 min, resulting from a fast spontaneous emulsion formation and small droplet size.The developed formulations were very simple and can be used to improve drug dissolution and, hence,have a potential to enhance drug absorption and bioavailability.
Acknowledgement
Financial support from The Thailand Research Fund(grant number BRG5480013)is greatly acknowledged.Thanks to BASF(Thai)Co.,Ltd.(Thailand)and Gattefosse(France)who kindly donated samples.
REFERENCES
[1]Mudalip SKA,Bakar MRA,Jamal P,et al.Solubility and dissolution thermodynamic data of mefenamic acid crystals in different classes of organic solvents.J Chem Eng Data 2013;58:3447-3452.
[2]Rawashdeh NM,Najib NM,Jalal IM.Comparative bioavailability of two capsule formulations of mefenamic acid.Int J Clin Pharmacol Ther 1997;35:329-333.
[3]Shinkuma D,Hamaguchi T,Yamanaka Y,et al.Correlation between dissolution rate and bioavailability of different commercial mefenamic acid capsules.Int J Pharm 1984;21:187-200.
[4]Kawabata Y,Wada K,Nakatani M,et al.Formulation design for poorly water-soluble drugs based on biopharmaceutics classi fi cation system:basic approaches and practical applications.Int J Pharm 2011;420:1-10.
[5]Hintzen F,Perera G,Hauptstein S,et al.In vivo evaluation of an oral self-microemulsifying drug delivery system (SMEDDS)for leuprorelin.Int J Pharm 2014;472:20-26.
[6]Weerapol Y,Limmatvapirat S,Nunthanid J,et al.Selfnanoemulsifying drug delivery system of nifedipine: impact of hydrophilic-lipophilic balance and molecular structure of mixed surfactants.AAPS PharmSciTech 2014;15(2):456-464.
[7]Date AA,Desai N,Dixit R,et al.Self-nanoemulsifying drug delivery systems:formulation insights,applications and advances.Nanomedicine 2010;5:1595-1616.
[8]Solˋe I,Solans C,Maestro A,et al.Study of nano-emulsion formation by dilution of microemulsions.J Colloid Interface Sci 2012;376:133-139.
[9]Kohli K,Chopra S,Dhar D,et al.Self-emulsifying drug delivery systems:an approach to enhance oral bioavailability.Drug Discov Today 2010;15:958-965.
[10]Dahan A,Hoffman A.Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs.J Control Release 2008;129:1-10.
[11]Weerapol Y,Limmatvapirat S,Kumpugdee-Vollrath M,et al. Spontaneous emulsi fi cation of nifedipine-loaded selfnanoemulsifying drug delivery system.AAPS PharmSciTech 2015.http://dx.doi.org/10.1208/s12249-014-0238-0.
[12]Chew NY,Chan HK.Effect of powder polydispersity on aerosol generation.J Pharm Pharm Sci 2002;5:162-168.
[13]Kang JH,Oh DH,Oh YK,et al.Effects of solid carriers on the crystalline properties,dissolution and bioavailability of fl urbiprofen in solid self-nanoemulsifying drug delivery system(solid SNEDDS).Eur J Pharm Biopharm 2012;80:289-297.
[14]Brouwers J,Brewster ME,Augustijns P.Supersaturating drug delivery systems:the answer to solubility-limited oral bioavailability.J Pharm Sci 2009;98:2549-2572.
[15]Vikram A,Firoz S,Kishore D,et al.Formulation and eveluation of mefenamic acid tablets by using modi fi ed starch.Asian J Pharm Sci Technol 2012;2:46-53.
[16]Mohammadi-Samani S,Boostanian A.The effect of HLB on the release pro fi le of atenolol from ethyl cellulose-coated tablets.Iranian J Pharm Res 2004;3:145-148.
Pornsak Sriamornsaka,b,*,Sontaya Limmatvapirata,b, Suchada Piriyaprasartha,b,Punyanutch Mansukmaneea, Zongkang Huanga
aDepartment of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University, Nakhon Pathom 73000,Thailand
bPharmaceutical Biopolymer Group(PBiG),Faculty of Pharmacy,Silpakorn University, Nakhon Pathom 73000,Thailand
*Corresponding author.Department of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand.Tel.:+66 34 255800;fax:+66 34 255801.
E-mail address:sriamornsak_p@su.ac.th(P.Sriamornsak).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.10.003
1818-0876/?2014 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Mefenamic acid
Drug dissolution
 Asian Journal of Pharmacentical Sciences2015年2期
Asian Journal of Pharmacentical Sciences2015年2期
- Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- A developed HPLC method for the determination of Alogliptin Benzoate and its potential impurities in bulk drug and tablets
- Gradient high performance liquid chromatography method for simultaneous determination of ilaprazole and its related impurities in commercial tablets
- Mycosynthesis,characterization and antibacterial properties of AgNPs against multidrug resistant (MDR)bacterial pathogens of female infertility cases
- Can semipermeable membranes coating materials in fl uence in vivo performance for paliperidone tri-layer ascending release osmotic pump tablet: In vitro evaluation and in vivo pharmacokinetics study
- Design and evaluation of nicorandil extended-release tablet
