Investigation and corrosion performance of cast Mg-6Al-1Zn+XCa alloy under salt spray test(ASTM-B117)
*S.P.Kumresh BuS.Sundrrjn
aDepartment of Metallurgical and Materials Engineering,National Institute of Technology,Tirchirappalli,Tamil Nadu,India
bNational Institute of Technology,Tirchirappalli,Tamil Nadu,India
Investigation and corrosion performance of cast Mg-6Al-1Zn+XCa alloy under salt spray test(ASTM-B117)
S.Manivannana,*,P.Dinesha,S.P.Kumaresh Babua,S.Sundarrajanb
aDepartment of Metallurgical and Materials Engineering,National Institute of Technology,Tirchirappalli,Tamil Nadu,India
bNational Institute of Technology,Tirchirappalli,Tamil Nadu,India
The corrosion behavior of cast Mg-6Al-1Zn+XCa(Where X=0.5,1.0,1.5 and 2.0 wt.%Ca)magnesium alloy,aged at different temperatures of 180°C,200°C,220°C and 240°C was investigated in accelerated corrosion test chamber according to ASTM-B117 Standard(salt spray test).The exposed alloys were characterized by X-Ray Diffraction(XRD),Optical Microscopy(OM)and Scanning Electron Microscopy (SEM)techniques.The microstructural refnement due to calcium addition and ageing treatment results in the improvement of corrosion resistance of the experimental alloy.Corrosion rate decreases due to fne precipitation of the β(Mg17Al12)phase distributed along the α-grain boundaries during ageing.Intermetallic phases act as a barrier,which makes the alloy more resistant to corrosion.Results found that better corrosion resistance of the alloy was observed in Mg-6Al-1Zn+1.5 wt.%Ca with 180°C ageing temperature.The corrosion behavior of Mg-6Al-1Zn+XCa alloy exhibited by ASTM B117 salt spray testing and electrochemical polarization measurements showed similar trends concluding that the calcium addition and ageing temperature decreases the corrosion rate.
AZ61;Calcium;Precipitation;Corrosion rate;Polarization
1.Introduction
Magnesium alloy has interesting properties such as low density,high specifc strength and hardness,excellent castability,perfect electromagnetic interference shielding property,high thermal conductivity and good damping capability, which increases their use in microelectronic,automotive and aerospace industries[1-4].Many of cast alloys contains from 5 to 6%aluminum content in weight,with low amounts of zinc.Among these,AZ61 alloy has an attractive property which increases the use in automotive applications[3].The ternaryMg-Al-Zn magnesium alloy issusceptible to corrosion in high-salt,high temperature or humidity environment which limits their use in structural applications.Guangling Song investigated that the corrosion resistance of aged die cast AZ91D alloy,song et al.reported that the precipitation of the β-phase(Mg17Al12)occurs along the grain boundaries during ageing.Furthermore,above 45 h of ageing,the decreasing aluminium content of α grains makes the α-matrix more active,its decreases the corrosion resistance of the AZ91D alloy[5]and he also reported if the volume fraction of β-phase is small,β-phase mainly acts as a galvanic cathode and accelerates the corrosion process of the α-matrix.Also found that,if the alloy has high volume fraction of the β-phase may act as an anodic barrier to prevent the overall corrosion of the AZ magnesium alloy[6].B.S.You et al.investigated the effects of calcium additions on the oxidation behavior inmagnesium alloys.He reported that Ca additions to Mg alloy retards the oxidation rate during melting process by the formation of thin and dense CaO flm on the surface of the molten alloy.This implies that the rapid oxidation of magnesium alloys at elevated temperature can be suppressed by the additions of Ca[7].N.D.Nam et al.investigated the effect of calcium oxide on the corrosion behavior of AZ91 magnesium alloy.He reported that the pitting potential,pitting resistance and polarization resistance of AZ91 alloy increased with the addition of calcium oxide.Also he found that the CaO additions reduced the susceptibility to breakdown of passive flm in AZ91 alloy[8].However,there is a few research work have been studied extensively to investigates the infuence of rare earth in AZ61 magnesium alloy on electrochemical behavior. In this present research work,the effect of addition of calcium (Ca)with different ageing temperatures on corrosion behavior of AZ61 magnesium alloy was studied under(3.5 wt.%NaCl solution)accelerated corrosion test chamber according to ASTM B117[9]Standard(fog test)and electrochemical measurements like potentio-dynamic polarization(PDP)test, electrochemical impedance spectroscopy(EIS)techniques [10-12].
2.Experimental procedure
2.1.Experimental alloy
The Ca containing AZ61magnesium alloys were prepared by casting route.The experimental alloys were made by adding magnesium (99.999%),6.0 wt.% ofaluminim (99.999%)and1.0 wt.%of zinc(99.999%).The Ca addition was done in the form of Mg-Ca master alloy contains 70% magnesium and 30%calcium.This Mg-6Al-1Zn+XCa (X=0.5,1.0,1.5,2.0 wt.%Ca)alloy was melted in a Inconel 718 crucible by using electric resistance furnace and melt was completely protected by controlled gas mixtures of 99%Ar-1% SF6.The crucible,pouring system,die and the stirrer were coated with wolfram non Stick coating(capable to withstand -25°C-1200°C)and dried for 10 min at 300°C.The melt being homogenized by automatic stirring at 720°C,controlled gas was passed into a crucible at 0.25 m3/h and it has been maintained up to 750°°C.After 10 min,the Mg-Ca master alloy was added and then stirring was done,the molten metal was poured into a permanent mild steel mold.The chemical composition of the experimental alloys was confrmed by atomic emission spectroscopy(AES)technique,which is presented in Table 1.These casted specimens were solution heat treated at 400°C for 16 h and then aged at different temperatures of 180°C,200°C,220°C and 240°C for 24 h.
2.2.Specimen preparation
The samples were polished by emery papers(60-1500 emery sheet),alumina powder and then diamond cloth contains two different types of colors,green(3-5μ)and red color (1-2μ).Nital(2%HNO398%ethanol)etchant was used to reveal the microstructure by using DIC Leica optical microscope,model No:DM750M.
2.3.Salt spray test
The specimens were initially cleaned with acetone and then dried by compressed air.The initial(w0)weight of the specimen was calculated by using Mettler Toledo weighing balance,model no:XP504.The location and position of the specimens were placed in salt spray chamber(Ascott,UK, model No:Sis450)accordance with ASTM-B117 standard. After salt spraying test,the specimens were cleaned by acetone and dried by compressed air.After drying,the specimens were immersed in chromate acid to ensure that the corrosion products were completely removed.Then the fnal weights of the specimen(w1)were calculated and then the corrosion rate was measured[13].The corroded specimens were characterized by X-Ray Diffraction(XRD),Scanning Electron Microscopy(SEM),and Energy Dispersive Spectrometry(EDS)techniques.
2.4.Potentio-dynamic polarization(PDP)and electrochemical impedance spectroscopy(EIS)
Polarization curves and electrochemical impedance spectrum of the specimens were measured in an electrolytic cell, which contains a three electrode cell setup(ACM Gill2 instruments).The three electrode cell setup comprises of reference electrode (saturated calomel), counter electrode (platinum foil-10× 20 mm)and working electrode(specimen).The electrolytic cell contains 3.5 wt.%NaCl solution as the electrolyte and the exposed surface area of the specimen is 1 cm2.The polarization started from a potential of about -2000 mV relative to the corrosion potential and stopped at a potential 50 mV positive to the corrosion potential.The scanning rate was kept at 10 mV/min.When the magnesium electrode(specimen)immersed in electrolyte immediately cathodic polarization take place,because magnesium is tooactive in 3.5 wt.%NaCl solution.From this experiment,the plot between potential vs current can be obtained to explain the corrosion mechanism.In electrochemical impedance spectroscopy(EIS)test,the electrolyte and electrode remains same as used in PDP test.The amplitude of applied AC signal was 5 mV,and the measured frequency range was from 1 mHz to 1 kHz.
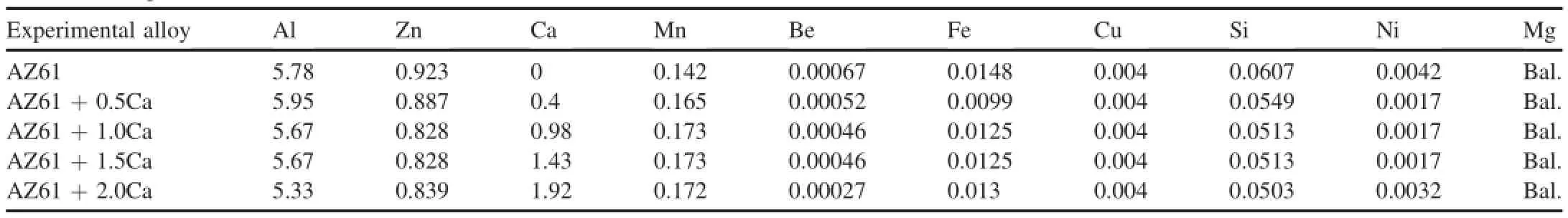
Table 1Chemical compositions of AZ6+xCa(X=0.5,1.0,1.5,2.0 wt.%Ca).
3.Results and discussion
3.1.Effect of microstructure
Fig.1 shows the typical microstructure of as cast AZ61 alloy at low and high magnifcation,respectively.This microstructure consists of α-phase and eutectic β-phase. Fig.1(a)illustrates the eutectic Mg phase surrounded by intermetallic phase of β(Mg17Al12).During solidifcation time the aluminum content increases towards the α-grain boundaries due to the coring effect.This β-phase is enriched with Al than primary α-phase,where the β-phase is discontinuous precipitates of inter-metallic compound of Mg17Al12[6].The corrosion barrier effect is mainly associated with volume fraction of β phase and its size and distribution.The β (Mg17Al12)phase is modifed and distributed along the α-grain boundaries through calcium addition and ageing treatment. The corrosion barrier effect of β(Mg17Al12)phase will explain the decrease in corrosion rate with calcium addition and ageing temperature.
Fig.2 show the optical microstructure of AZ61+xCa (x=0.5,1.0,1.5 and 2.0 wt.%Ca)magnesium alloy,reveals that the addition of calcium refnes the α-phase and also modify the intermetallic phase(β-phase).Based on the microstructural observations,high degree of grainrefnement obtained in 0.5 wt.%Ca addition.Fig.3 shows the XRD results for the as-cast AZ61+XCa(x=0.5,1.0,1.5&2.0 wt.% Ca addition)alloys.The XRD pattern confrms that the ternary alloy iscomposedofα-Mg,Mg2Ca,Ca2Mg6Zn3and β(Mg17Al12)phases.The addition levelof0.5 wt.% -1.5 wt.%Ca to the AZ61 Mg-alloy leads to the formation of intermetallic phases and the volume fraction of Mg2Ca,Al2Ca intermetallic phases increases with increasing Ca content. Furthermore,the grain sizes decreased with the addition of calcium into their respective alloys.This can be attributed to the pinning of grain boundaries by the increasing amount of Mg2Ca and Al2Ca second phase resulting in limited grain growth.The addition of calcium to AZ61 magnesium alloy reduces the grain size to serve as either nucleation site or obstacles to grain growth during solid state cooling.The results revealed that the addition of Ca(with increasing amount) into AZ61-magnesium alloy leads to an increase in the amount of Mg2Ca and Al2Ca phases,while the precipitation of Mg17Al12s phase reduces[14].The corrosion resistance of AZ alloys increases as the amount of β-phase increases,because the β-phase acts as a barrier.The amount of β-phase formation in ageing treatment(T6)is higher than the solution heat treatment(T4).The β precipitates were fnely distributed along the α grain boundaries,building up a certain degree of continuity in the barrier[6,15].Fig.2 shows the amount and the continuity of the β-phase along the grain boundaries increases with ageing treatment,the corrosion rate decreases with ageing treatment due to the barrier effect.The intermetallic β(Mg17Al12)phase appears in the form of discontinuous, enlarged and lamellar morphologies(Fig.4),The continuous network of β phase was obtained along the grain boundaries due to the precipitation reactions.Furthermore with extended ageing,the matrix becomes depleted of aluminum owing to precipitation reactions and moves towards the grain interiors. Fig.5 shows that the infuence of ageing temperature on the microstructure of AZ61+xCa(x=0.5,1.0,1.5 and 2.0 wt.% Ca)alloy reveals that the β precipitates nucleation and distribution rate.The corrosion barrier effect is having more signifcant role when the β precipitates are fnely distributed throughout the matrix,continuity of β phase are formed due to fne precipitates of β during ageing treatment.
3.2.After salt spray test
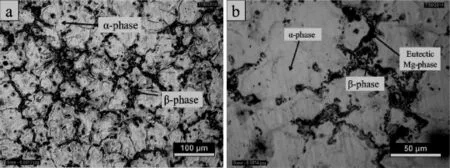
Fig.1.Representative optical micrographs showing the grain morphology of AZ61 alloy without calcium addition.
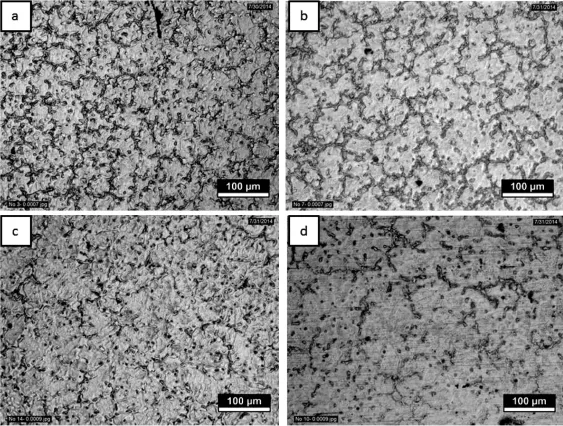
Fig.2.Representative optical micrographs showing the grain morphology of(a)AZ61+0.5 wt.%Ca,(b)AZ61+1.0 wt.%Ca,(c)AZ61+1.5 wt.%Ca,(d) AZ61+2.0 wt.%Ca.
Fig.6 show the corrosion rate of each specimen in 3.5 wt.% NaCl solution exposed to 48 h.Based on the curve,the lowest corrosion rate was observed in the specimens of 1.5 wt.%Ca content aged at180°C.It was found that the corrosion rate slowed down with increasing calcium addition and further ageing treatment.The corrosion rate increases further with increasing calcium content above which 1.5 wt.%addition. Fig.7(a)shows the corrosion rate against ageing temperature. Fig.7(b)shows the corrosion rate against wt.%of calcium addition for AZ61+XCa(x=0.0,0.5,1.0,1.5 and 2.0 wt.% Ca)alloy.These curves illustrate that the ageing temperature along with the calcium addition signifcantly improves the corrosion resistance of this experimental alloy.Fig.8 shows the macro images of AZ61+xCa(x=0.5,1.0,1.5 and 2.0 wt.%Ca)alloy with different ageing temperatures of 180, 200,220 and 240°C after the removal of corrosion products. In this observations,Fig.8(I)shows the better resistance against pitting corrosion after 48 h of exposure in 3.5 wt.% NaCl.Fig.8(P)shows the poor pitting corrosion resistance observed in 5.0 wt.%NaCl solution,it reveals that the AZ61+2.0 wt.%Ca magnesium alloy localized corrosion occurs frst,instead of the uniform corrosion.
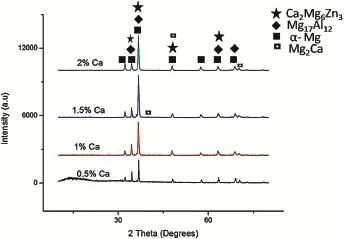
Fig.3.XRD results of AZ61+XCa(x=0.5,1.0,1.5&2.0 wt.%Ca addition) alloy.
From Fig.8,it is clear that the exposed surface area of the specimen after salt spraying test is rough and porous and exhibits the characteristic‘sand rose”[16]or‘sunfower’morphology.Bierwagen et al.reported that the morphology of magnesium hydroxide[Mg(OH)2]during corrosion of magnesium pigment in water and chloride environment[17].The conversion of magnesium to magnesium hydroxide in the presence of water is represented by Eq.(1)[17,18].
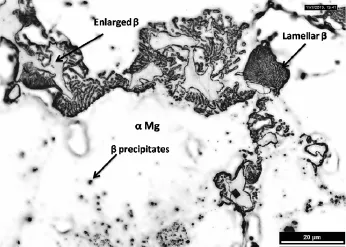
Fig.4.Infuence of the ageing on the microstructure of cast AZ61 alloy.The black arrow indicates a region of β phases with different morphologies such as lamellar,enlarged and discontinuous manner.
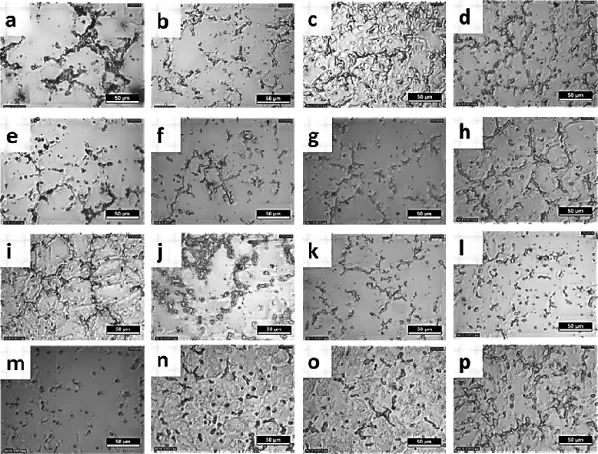
Fig.5.Microstructures of AZ61+xCa after ageing.(a)0.5Ca aged at 180°C(b)0.5Ca aged at 200°C(c)0.5Ca aged at 220°C(d)0.5Ca aged at 240°C(e)1.0Ca aged at 180°C(f)1.0Ca aged at 200°C(g)1.0Ca aged at 220°C(h)1.0Ca aged at 240°C(i)1.5Ca aged at 180°C(j)1.5Ca aged at 200°C(k)1.5Ca aged at 220°C(l)1.5Ca aged at 240°C(m)2.0Ca aged at 180°C(n)2.0Ca aged at 200°C(o)2.0Ca aged at 220°C(p)2.0Ca aged at 240°C.
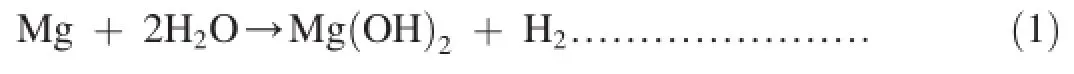
Fig.9 shows the SEM image of AZ61+1.5 wt.%Ca specimen aged at 180°C exposed to 3.5 wt.%NaCl solution at different time intervals under salt spray chamber.Fig.9.(a), (b),(c)and(d)shows that the specimens were exposed to different time intervals such as 12,24,36 and 48 h respectively.Fig.9(e)show the SEM and EDS analysis of 1.5 wt.% Ca exposed after 48 h in salt spraying.The enormous changes have been observed in corrosion rate of this alloy with the addition level upto 1.5 wt.%Ca.The corrosion rate mainly depends on the microstructures,when the alloy has more continuous network morphologies of β phase with fne precipitates of Mg2Ca and Al2Ca,which improves the corrosion resistance.This calcium added AZ61 magnesium alloy comprises of β(Mg17Al12)phase,Mg2Ca and Al2Ca phases.The α grain boundary decreases with increasing calcium content. Furthermore,the volume fraction of the β(Mg17Al12)phase increases the formation of network morphology.During ageing,Mg2Ca and Al2Ca precipitates are distributed along the grain boundaries[19].It was found that additions upto1.5 wt.%Ca increases the corrosion resistance of the AZ61 alloys.When adding above 1.5 wt.%calcium into AZ61 alloy, the large quantity of Al2Ca phases at the surface creates more micro galvanic sites.However,when increasing the Ca content up to 2 wt.%,the distribution of the phase near the surface area becomes more continuous,and the presence of Ca induces the formation of the less cathodic Al2Ca phase at the expanse of the more cathodic β phase so corrosion above 1.5 wt%calcium addition increase the corrosion rate of the AZ61 alloys.
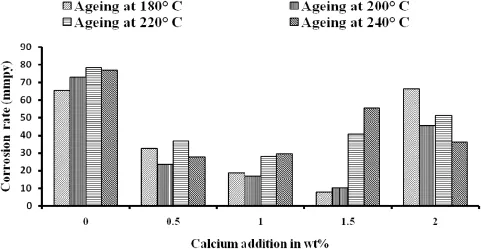
Fig.6.Corrosion rate of the AZ61+XCa(x=0.0,0.5,1.0,1.5 and 2.0 wt.%Ca)alloy.
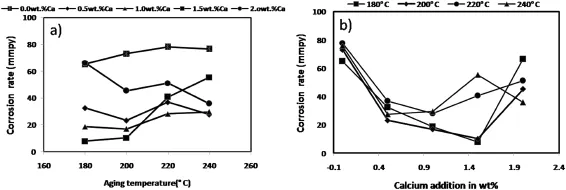
Fig.7.Corrosion rate of AZ61+XCa(x=0.0,0.5,1.0,1.5 and 2.0 wt.%Ca)alloy.(a)Corrosion rate Vs Ageing temperature(b)Corrosion rate Vs Ca addition.
3.2.1.Corrosion product analysis
The X-ray diffraction analysis was carried out to identify the corrosion products which are formed during 3.5 wt.%of NaCl solution under salt spray test.The specimens were tested in a salt spray chamber for 3 days.Fig.10 show the XRD pattern for AZ61+1.5 wt.%Ca aged at 180°C alloy exposed after salt spray test for 24 h,48 h,respectively.It was confrmed that the main corrosion products were brucite (Mg(OH)2)and CaO in 3.5 wt.%of NaCl solution.In addition, the β(Mg17Al12)phase and the α-Mg were also found in this pattern.
3.3.Electrochemical measurements
3.3.1.Electrochemical impedance spectroscopy

Fig.8.Macrographic images of alloys exposed in 3.5wt%NaCl solution in salt spray after 48 h(A)0.5Ca aged at 180°C(B)0.5Ca aged at 200°C(C)0.5Ca aged at 220°C(D)0.5Ca aged at 240°C(E)1.0Ca aged at 180°C(F)1.0Ca aged at 200°C(G)1.0Ca aged at 220°C(H)1.0Ca aged at 240°C(I)1.5Ca aged at 180°C (J)1.5Ca aged at 200°C(K)1.5Ca aged at 220°C(L)1.5Ca aged at 240°C(M)2.0Ca aged at 180°C(N)2.0Ca aged at 200°C(O)2.0Ca aged at 220°C(P) 2.0Ca aged at 240°C.
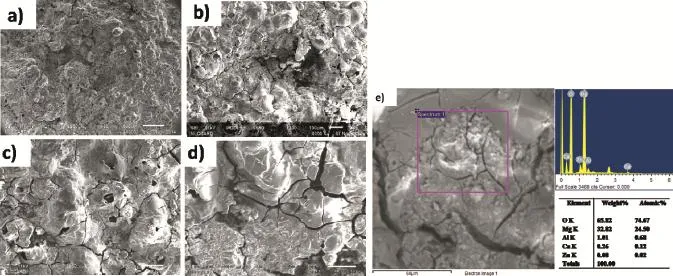
Fig.9.SEM micrographs of AZ61+1.5 wt.%Ca alloy aged at 180°C exposed to salt spray(a)after 12 h,(b)after 24 h,(c)after 36 h,(d)after 48 h and(e)SEM and EDS of after 48 h.
The corrosion resistances for different compositions of calcium added AZ61 alloy and CaO thin flm were evaluated by electrochemical impedance spectroscopy in 3.5 wt%NaCl solution.Electrochemical impedance spectroscopy measurements,which provide a quantitative estimation of alloy surface degradation and corrosion processes,were carried out in the different composition of calcium added AZ61 alloy samples in 3.5 wt%NaCl solution.Fig.11 Shows the impedance curve for 180°C aged specimens of AZ61+1.5 wt.%Ca, AZ61+2 wt.%Ca and cast AZ61 alloy.AZ alloy tends to form an oxide/hydroxide layer in a neutral aqueous solution,which is porous and does not cover the whole surface.Theoretically, impedance spectroscopy is consisted by a high-frequency and a low-frequency capacitive loop.The red color curve indicates that the high-frequency capacitive loop was attributed due to magnesium oxide/hydroxide flm on this alloy surface.Black color curve shows that the capacitive loop at low frequency range is related to the metal dissolution through the pores and defects of this protective layer.In all the cases,single capacitive loops are observed in AZ61+1.5 wt.%Ca aged at 180°C having much larger impedance loop compared to AZ61+2 wt.%Ca aged at 180°C and AZ61-as cast alloy. Fig.12(a)shows the Nyquist plots obtained from the AZ61with 1.5 wt.%Ca aged at 180°C alloy after different timings of immersion in 3.5 wt%NaCl.The impedance diagrams of the 1.5 wt.%Ca-containing specimen immersed in 3.5 wt%NaCl solution upto 10 h shown much larger impedance than the initial time of immersion.These results suggest that the addition of Ca promotes the formation of a passive flm.Equivalent circuit diagram of part of electrode process is obtained through ftting by software which is shown in Fig.12(b).The equivalent circuit consists of RS,RP,CPE.CPE is the constant phase element,Rs,and Rp represents the solution and polarization resistances,respectively.The Z-view software program was used to ft the EIS data to determine the optimized values for the resistance parameters RP,RS.RP,RSvalues are mentioned in Table 2.It shows the polarization resistances(Rp)with the immersion time for AZ61+1.5 wt% Ca aged at 180°C alloy.Resistance polarization(Rp)valueincreased strongly with increasing Ca content upto 1.5 wt% indicating better corrosion resistance.

Fig.10.XRD patterns of the corrosion products after salt spray of 180°C aged AZ61+1.5 wt.%Ca.
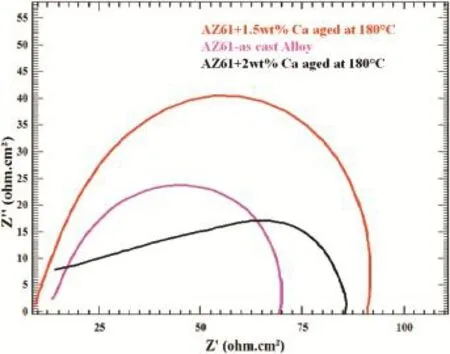
Fig.11.Nyquist plots of AZ61+1.5 wt%Ca aged at 180°C alloy,AZ61-as cast alloy and AZ61+2 wt%Ca aged at 180°C alloy.
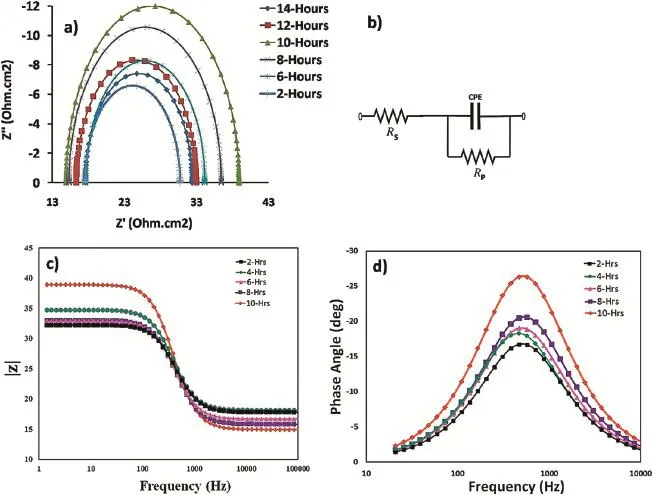
Fig.12.(a)Nyquist plots of AZ61+1.5 wt%Ca aged at 180°C alloy,(b)Equivalent circuit,(c)Bode plot of Frequency vs.|Z|and(d)Bode plot of Frequency vs. angle.
Fig.12(c)presents evolution of the impedance spectra of AZ61+1.5 wt%Ca aged at 180°C alloy during immersion timing upto 10 h.It clearly shows the impedance magnitude (|z|)increases with time,from 2 h to 10 h due to formation of passive flm on the surface of the AZ61+1.5 wt%Ca aged at 180°C alloy.For long immersion time impedance magnitude (|Z|)decreases because of degradation in passive flm.This result shows 1.5 wt%of Ca addition in AZ61 alloy acts as an effective barrier to prevent the penetration of the chlorine ions and increases the corrosion resistance.Fig.12(d)shows the impedance spectra of AZ61+1.5 wt%Ca aged at 180°C alloy in the form of Bode plots(phase angle vs.frequency) during immersion timing upto 10 h.The aperture of the phase angles increased with improvement of a surface flm.So addition of AZ61+1.5 wt%Ca aged at 180°C alloy having good improvement in surface flm formation.These resultssuggest that Ca addition promotes passive flm formation in AZ61 alloy.When α-Mg grains are corroded,the precipitates will be in the top layer as shown in pitting initiation and starts dissolving.This will result in the increase of the number of active atoms on the surface,accelerating the formation of the protective layer.This data illustrates the electrochemical principle of passive and breakdown of the passive flm.

Table 2Fitting results of EIS of AZ61+1.5 wt%Ca aged at 180°C alloy.
3.3.2.Potentio-dynamic polarization-results
The potentio-dynamic polarization test is a powerful tool for estimating the kinetics of the corrosion process and to determine the effectiveness of the flm on corrosion protection. In this study,potentio-dynamic polarization measurements were also employed in 3.5 wt%NaCl solution at room temperature for comparing base material with best sample chosen from salt spray test.The apparent changes in the plot caused by adding 1.5 wt.%Ca into AZ61 alloy aged at 180°C polarization curve is shifted to more positive potentials ascompared to the AZ61 base alloy.It was observed that the curve behaves more cathodic reaction in 1.5 wt.%Ca aged at 180°C sample side compared with the base alloy.Increased rates of cathodic reaction lead to a higher icorr and Ecorr value,which is confrmed by Tafel plotting.Corresponding Ecorr versus icorr values are presented in Table 3.This is could be evident that the calcium addition upto 1.5 wt.%aged at 180°C improves corrosion resistant of AZ61 magnesium alloy.Based on the curve,potential slightly increases above the corrosion potential of the specimen in 3.5 wt%NaCl solution caused the localized corrosion(pitting corrosion)of this alloy[5].During PDP test,potential is starting from cathodic potential and continuously increased by the rate of 5 mV and sudden drop in corrosion current take place which is followed by anodic current near the corrosion potential.Corresponding potential to the sudden drop in corrosion current is called pitting potential“Epitting”.The localized corrosion was started at pitting potential and hydrogen evolution takes place.This hydrogen evolution is easily visible on the surface of the specimen during the test.Pitting potential is very important parameter in PDP test.““Epitting”indicates the tendency of localized corrosion(Fig.13).Table 3 shows the values of corrosion current density(Icorr),corrosion potential(Ecorr). From Table 3.AZ61+1.5 wt%Ca aged at 180°C shows lower corrosion current density as compared with AZ61 base alloy which tends to reduce the localized corrosion.It was found that the alloy AZ61+1.5 wt%Ca aged at 180°C having better corrosion protective properties as compared to AZ61 base alloy.The relevant electrochemical parameters calculated from the Tafel plots.
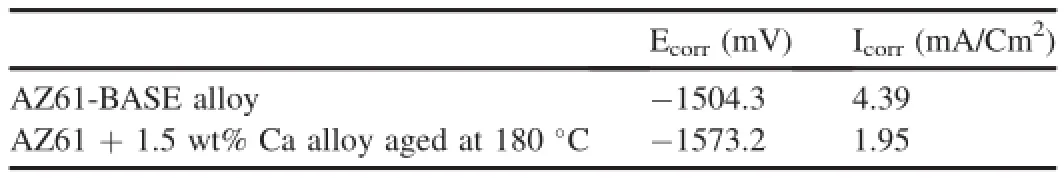
Table 3The relevant electrochemical parameters Ecorr(mV)and Icorr(mA/Cm2)values were calculated from the Tafel plots.

Fig.13.Potentio-dynamic polarization curves tested in 3.5 wt%NaCl solution for AZ61+1.5 wt%Ca aged at 180°C alloy,AZ61-base alloy.
4.Conclusion
An interesting observation was found in this study,there is a relationship between calcium addition and ageing treatment which improves corrosion resistance for the AZ61 alloy.The calcium addition upto 1.5 wt.%aged at 180°C decreases the corrosion rate and increases further with above addition.This relationship shows that the calcium addition and ageing treatment signifcantly decreases the corrosion rate of AZ61 alloy.The calcium addition and ageing treatment increased the amount of intermetallic phases,which results in the formation of continuous network of β phase.In terms of electrochemical studies,it was found that the addition of calcium and ageing treatment increased impedance and decreased corrosion rate.
Acknowledgments
This research work was supported by the department of Metallurgical and Materials Engineering,NITT,India.The author is grateful for the support of TEQIP,NITT,India for funding and facility provided throughout the research work. The authors wish to thank to Dr.S.Sundarrajan,director of NITT and Dr.S.P.Kumaresh Babu,Associate Professor, department of MME,NITT for their valuable supports in the experiments.
[1]F.Rosalbino,E.Angelini,S.De Negri,A.Saccone,S.Delfno,Intermetallics 14(2006)1487-1492.
[2]V.N.Balbyshev,L.S.Kasten,R.A.Mantz,Thin Solid Films 514(2006) 174-181.
[3]L.C.iz ek,M.Greger,L.Pawlica,L.A.Dobrza′nski,T.Tan ski,Mater. Process.Technol.157-158(2004)466-471.
[4]A.K.Dahle,S.Sannes,D.H.St John,H.Westengen,Light Met.1(2001) 99-103.
[5]Guangling Song,L.Amanda,Bowles,H.David,St John,Mater.Sci. Eng.A366(2004)74-86.
[6]Guangling Song,Andrej Atrens,Xianliang Wu,Bo Zhang,Corros.Sci. 40(1998)1769-1791.
[7]B.S.You,W.-W.Park,I.-S.Chung,Scr.Mater.42(2000)1089-1094.
[8]N.D.Nam,M.Z.Bian,M.Forsyth,M.Seter,M.Tan,K.S.Shin,Corros. Sci.64(2012)263-271.
[9]ASTM B117,Standard Practice for Standard Practice for Operating Salt Spray(Fog)Apparatus,2012.
[10]ASTM G1-03,Standard Practice for Preparing,Cleaning and Evaluating Corrosion Test Specimens,vol.03.02,2003.
[11]ASTM G102-89,Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements,2010.
[12]ASTM G59-97,Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements,2014.
[13]ASTM G31-72,Standard Practices for Laboratory Immersion Corrosion Testing of Metals,vol.03.02,2004.
[14]O.Lunder,J.E.Lein,T.K.Aune,K.Nisancioglu,Corros.Sci.45(1989) 741-748.
[15]Guangling Song,Andrej Atrens,Matthew Dargusch,Corros.Sci.41 (1999)249-273.
[16]Shashi S.Pathak,Michael D.Blanton,Sharathkumar K.Mendon,James W.Rawlins,Corros.Sci.52(2010)1453-1463.
[17]Gordon Bierwagen,Dante Battocchi,Alda Simoes,Anthony Stamness, Dennis Tallman,Prog.Org.Coat 59(2007)172-178.
[18]Rakel Lindstrom,Lars-GunnarJohansson,GeorgeE.Thompson, Peter Skeldon,Jan-Erik Svensson,Corros.Sci.(2004)1141-1158.
[19]G.Ben-Hamu,A.Eliezer,E.M.Gutman,Electrochim Acta 52(2006) 304-313.
Received 15 December 2014;revised 23 January 2015;accepted 2 February 2015 Available online 28 February 2015
*Corresponding author.
E-mailaddresses:manivannan.meta@gmail.com (S.Manivannan), dineshnehru92@gmail.com (P.Dinesh),babu@nitt.edu (S.P.K.Babu), sundar@nitt.edu(S.Sundarrajan).
Peer review under responsibility of National Engineering Research Center for Magnesium Alloys of China,Chongqing University.
http://dx.doi.org/10.1016/j.jma.2015.02.002.
2213-9567/Copyright 2015,National Engineering Research Center for Magnesium Alloys of China,Chongqing University.Production and hosting by Elsevier B.V.All rights reserved.
Copyright 2015,National Engineering Research Center for Magnesium Alloys of China,Chongqing University.Production and hosting by Elsevier B.V.All rights reserved.
 Journal of Magnesium and Alloys2015年1期
Journal of Magnesium and Alloys2015年1期
- Journal of Magnesium and Alloys的其它文章
- GUIDE FOR AUTHORS
- Optimization of mechanical and damping properties of Mg-0.6Zr alloy by different extrusion processing
- Synthesize of AZ31/TiC magnesium matrix composites using friction stir processing
- A two-step superplastic forging forming of semi-continuously cast AZ70 magnesium alloy
- Dry sliding wear behavior of an extruded Mg-Dy-Zn alloy with long period stacking ordered phase
- Evaluation of physical and mechanical properties of AZ91D/SiC composites by two step stir casting process
