過渡金屬改性的ZSM-5催化劑應用于甲硫醚轉化制甲硫醇
陳世萍 王偉明 劉迎偉 魏育才 袁成龍 方維平 楊意泉,*
(1廈門大學化學化工學院化學工程與生物工程系,福建廈門361005;2廈門大學化學化工學院化學系,福建廈門361005)
1 Introduction
The sulfur-containing compounds such as dimethyl sulfide(DMS),methanethiol(MT),and H2S are referred to as total reduced sulfur(TRS)compounds,and they all have malodorous odor.1,2Among them,MT is now used as an important chemical intermediate to produce organosulfur compounds such as methionine,widely used as feed additive.With increasing demand for methionine,the production of MT becomes more important.3Industrially,it is synthesized from methanol and hydrogen sulfide over alumina-supported metal oxide catalysts;and DMS,a major byproduct,is always formed along with methanethiol.4In the H2S atmosphere,DMS can be converted into MT in the presence of a catalyst,so as to boost the yield of methanethiol and lower the content of DMS in wastewater,which is economically as well as environmentally attractive for better carbon management.
Several solid acid catalysts like Al2O3,phosphorus promoted Al2O3,and WO3/ZrO2,have been studied for the synthesis of MT from DMS at 623-673 K with byproduct methane.5-12Besides,the effect of temperature,space velocity,and molar ratio of H2S to DMS was investigated in our previous study.6
There is still a constant search for the development of novel catalysts with high activity and selectivity for the conversion of DMS to MT.In this regard,less attention has been paid towards the ZSM-5 catalysts.Plaisance and Dooley13reported the production of DMS and MT by condensation of methanol and hydrogen sulfide in the presence of a kind of zeolite,and deduced that the zeolite with stronger acid sites can easily adsorb DMS and MT.Satokawa et al.14found that DMS was efficiently adsorbed on silver-exchanged Y zeolites(Ag/Na-Y)at room temperature.Hwang and Tai15have used Ag/ZSM-5,Mn/ZSM-5,and Ag-Mn/ZSM-5 as catalysts to catalyze the oxidation of DMS with ozone;they concluded that ion-exchanged ZSM-5 strengthened the adsorption and oxidation of DMS.
It is well known that transition metals(W,Ni,Co,Mo)have an ability to catalyze sulfurization.14-19However,to the best of our knowledge,these transition metals supported on ZSM-5 have not been systematically studied for the reaction of DMS with H2S.The aim of this work is to carry out a systematic comparison of the performance of ZSM-5-supported W,Ni,Co,and Mo catalysts for the reaction.The performance-structure correlation of different catalysts was discussed as well.
2 Experimental
2.1 Catalyst preparation
The catalysts were prepared by incipient wetness impregnation method.Ammonium metatungstate,nickel nitrate,cobalt nitrate,and ammonium molybdate(all are 99%of purity,Sinopharm Chemical Reagent Co.,Ltd.)were used as precursors of the said transition metals.Appropriate amount of transition metal salt was dissolved in distilled water to produce an aqueous solution,in which then the support material ZSM-5,(proton form,n(SiO2)/n(Al2O3)=38(molar ratio),Catalyst Factory of NanKai University)was soaked at room temperature.The impregnated sample was dried at 353 K for 24 h and then calcined in air at 773 K for 2 h.After pressing into wafer,crushing and sieving,the catalyst particles of 30-60 mesh were collected for use;the as-prepared catalysts are denoted as M/ZSM-5,(M=W,Ni,Co,Mo),the stoichiometric metal content was 2%(mass fraction).Besides,the used catalyst is marked as M/ZSM-5-A
2.2 Catalyst activity evaluation
DMS conversion reaction was conducted in a glass tubular fixed bed reactor with an internal diameter of 10 mm;typically,2.0 mL of the catalyst with 30-60 mesh was filled into the reactor,with a thin layer of glass fiber and a layer of quartz powder(30-60 mesh)covered on the catalyst bed.Before experiment,the catalyst was sulfurized with H2S for 1 h at 673 K to activate the catalyst;the H2S flow rate was maintained by mass flow controller(Beijing Seven star,D08-1F).Then the sulfurized catalyst was tested at 593,633,and 673 K in turn for 2 h,respectively,and the system pressure was held at 0.5 MPa with the aid of a back-pressure regulator.The DMS solution was injected into the catalyst bed by precision metering pump(Beijing Satellite Manufactory,2ZB-1L10).The outlet stream temperature was kept at 400 K with heater band and analyzed by an on-line gas-chromatograph equipped with a Porapak Q(2 m×Ф 3 mm)column connected to a thermal conductivity detector(TCD).
2.3 Catalyst characterization
XRD measurements were performed on a Panalytical X′pert PRO X-ray diffractometer utilizing monochromatic Cu Kαradiation(λ=0.15418 nm,tube voltage:40 kV,tube current:30 mA)in the 2θ range from 5°to 50°.Unmodified ZSM-5 zeolite sample was used as reference for crystallinity comparison.The degree of crystallinity of M/ZSM-5 was defined utilizing the main X-ray diffraction peak(2θ=22.0°-25.0°)by the following equation:

The surface areas of the catalysts were measured using nitrogen adsorption at 77 K with a Micromeritics Tristar 3000 surface area and pore analyzer.Prior to N2physisorption measurement,all samples were degassed at 393 K for 1 h and then evacuated at 573 K for 3 h to remove physically adsorbed impurities.The specific surface area(SBET)was determined by the Brunauer-Emmett-Teller(BET)method and the pore size distributions were calculated by Barrett-Joyner-Halenda(BJH)method according to the desorption branch of the isotherms.The Si/Al mole ratios and actual metal compositions of the M/ZSM-5 samples were determined by a Bruker S8 TIGER X-ray fluorescence(XRF)spectrometer.The contents of carbon and sulfur on the tested catalysts were measured on CHNS Equipment(Vario EL III elemental analyser)with the limit of detection(LOD)being 0.03-20 mg for carbon and 0.03-6 mg for sulfur.
CO2and NH3temperature-programmed desorption(TPD)measurements of the catalysts were conducted in a quartz tube reactor filled with 80 mg catalyst.For CO2-TPD experiment,the catalyst was pretreated in Ar at 673 K for 1 h,then cooled down to 323 K;carbon dioxide adsorption was performed for about 0.5 h in a CO2stream at a flow rate of 30 mL·min-1.Weakly adsorbed CO2was removed by Ar sweeping at 323 K,and then the temperature was increased to 1073 K at a heating rate of 10 K·min-1.The desorbed CO2component was monitored with a mass spectrometry(MS)signal of m/e=44 in multiple ion detection(MID)mode.So did the NH3-TPD experiment as CO2-TPD with NH3substituting for CO2.The NH3-TPD experiment was conducted from 323 to 873 K.Desorbed NH3and H2O were monitored with a MS signal of m/e=16,17 in MID mode,and a MS signal of m/e=18,respectively.
O2temperature-programmed oxidation(TPO)experiment for the used catalysts was performed in a quartz reactor.For each experiment,80 mg sample was pretreated in Ar at 323 K for 1 h,and then swept with 5%O2/Ar at a rate of 20 mL·min-1until the base line on the recorder remained unchanged.Finally,the sample was heated at a rate of 10 K·min-1in 5%O2/Ar.CO2and SO2formed were analyzed with a MS signal of m/e=44,64 in MID mode,respectively.
3 Results and discussion
3.1 Catalytic activity
The evaluation results of the catalysts as a function of temperature are shown in Fig.1;the activity data of the catalysts with different molar ratios of H2S to DMS at 593 K are summarized in Table 1.Earlier studies5-7indicated that the reaction of H2S with DMS to form MT is accompanied with by-product methane;two reactions,i.e.,CH3SH→CH4+S+C,and CH3SCH3→CH4+C2H6+S+C,led to the formation of methane at the expense of MT and DMS.
The data of DMS conversion and selectivities toward MT and methane are listed in Table 1.The conversions of DMS at 593 K for all catalysts are similar,and the selectivity toward MT is found to be higher than 98%for all catalysts.The modified ZSM-5 sample exhibits a relatively high activity,which may be due to the strong Lewis acid sites on ZSM-5.In the transition metal-modified ZSM-5 catalysts,the Co/ZSM-5 sample shows the best conversion at both H2S/DMS mole ratio cases,followed by Mo-,Ni-,and W-modified samples in turn.Several lines of evidences verified that both DMS and MT were adsorbed on the Al3+cation of ZSM-5 by electronic pairs,20-22the above activity results show that the additive ions(W6+,Ni2+,Co3+,and Mo6+)are more efficient than Al3+in adsorbing DMS and MT.
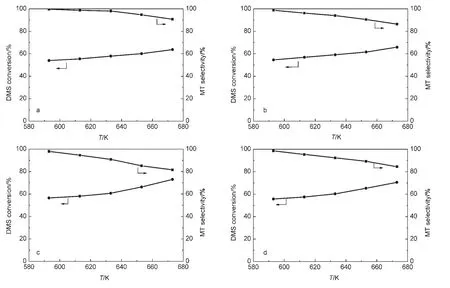
Fig.1 Conversion of DMS as a function of temperature over(a)W/ZSM-5,(b)Ni/ZSM-5,(c)Co/ZSM-5,(d)Mo/ZSM-5
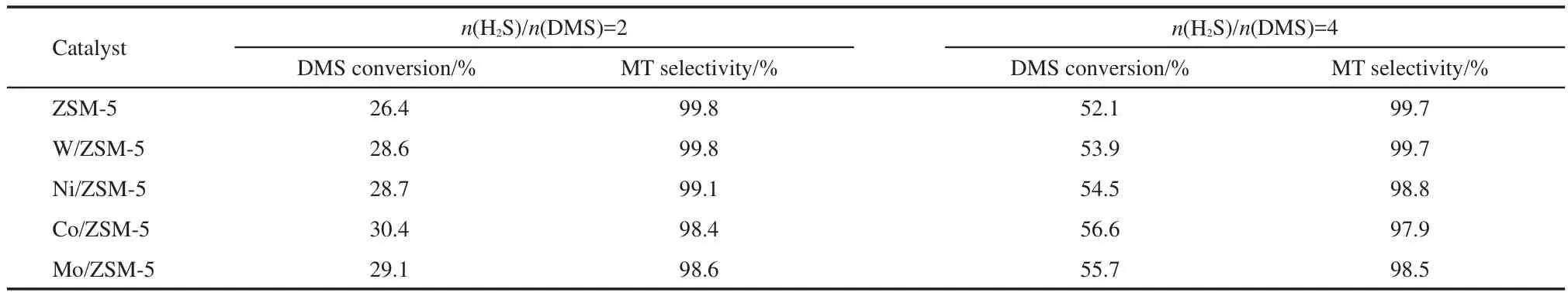
Table 1 Conversion of DMS at different mole ratios of H2S to DMS over the catalysts at 593 K
The shapes of conversion and selectivity curves for W-,Ni-,Co-and Mo-containing catalysts are similar(Fig.1).We observed that increasing in the reaction temperature led to the enhancement in the conversion of DMS and decline in the selectivity towards MT.It might be due to the inevitable decomposition of DMS and MT with the temperature increasing.23W/ZSM-5 exhibits the lowest conversion and the highest selectivity towards methanethiol as the increase of temperature with respect to the four transition metal-modified catalysts,whereas the Co/ZSM-5 catalyst is most active and the selectivity towards methanethiol severely decreases with temperature increasing.The decreasing rate of the selectivity towards methanethiol follows the sequence:Co/ZSM-5>Mo/ZSM-5>Ni/ZSM-5>W/ZSM-5.In other words,the transition metal-modified ZSM-5 catalysts not only strengthen the adsorption of DMS and MT,but also improve the decomposition of DMS and MT on active metal sites.Low selectivity towards MT of the Co-containing catalyst for this reaction is rather unexpected although the severe decomposition of DMS and MT may generate much carbon and sulfur deposition,which will clog the pore.When the amount of carbon accumulated has been over 20%(mass fraction)on the surface,the catalyst would be deactivated.5
For the four catalysts,the conversion of DMS is relative to the concentration of DMS in the feed.At H2S/DMS molar ratio of 4,the conversion of DMS is close to twice as many as that at H2S/DMS molar ratio of 2.This phenomenon is accordance with the result reported in the literature23for γ-Al2O3used in the reaction of DMS with H2S.
3.2 Catalyst characterization
3.2.1 Physicochemical properties
The XRD patterns of the metal-modified ZSM-5(M/ZSM-5)samples(both fresh and used samples)are shown in Fig.2.As can be observed from Fig.2a,all the fresh M/ZSM-5 samples exhibit typical peaks due to ZSM-5,indicating that the structure of the zeolite remained intact after metal loading.However,the crystallinity of different metal-modified catalysts drops to some extent(Table 2),possibly owing to the dealumination of the zeolite during the modification process(impregnating,drying,and calcining).The transition metal cations(W6+,Ni2+,Co3+,Mo6+)anchor to the negative framework charge held in the Al―O―(Si―O)2―Al cluster on the surface of ZSM-5 and replace for some of Al3+sites,24so the mole ratio of Si/Al for the modified catalysts exhibits a little increase.No diffraction peaks due to metal oxides(metal=W,Ni,Co,Mo)can be detected,indicating that the active metal component on the catalyst surface is highly dispersed or lower than the XRD detection limit.The XRD patterns of the used catalysts illustrate that the support ZSM-5 in all metal-modified ZSM-5 samples still preserves typical structure even under harsh reaction conditions,obviously,the crystallinity drops to varying degrees,which is estimated from Fig.2b;this may be attributed to carbon deposition on the surface.
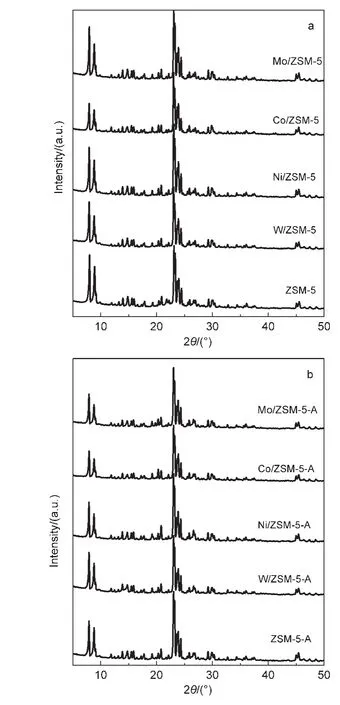
Fig.2 XRD patterns of the metal-modified ZSM-5 samples before and after using
The surface area,pore diameter,and pore volume of the M/ZSM-5 samples are found to be lower than those of ZSM-5 sample(Table 3).The surface area of Ni/ZSM-5 is lower than those of the others;the difference may be due to different particle sizes of these metal oxides and their different interactions with ZSM-5.It is observed that the porosity and specific surface area of the used catalyst reduce much;the losses in the sur-face area(compared with the surface area of the fresh catalyst)are 29.7%for W/ZSM-5,33.75%for Ni/ZSM-5,37%for Co/ASM-5,and 40.5%for Mo/ZSM-5.The distinct loss of the porosity and specific surface area may be ascribed to the deposition of carbon and sulfur on the surface,leading to blocking up the pore;these depositions caused by DMS conversion are subjected to oxidation treatment at above 773 K repeatedly to rejuvenate the catalyst in industrial process.6,7
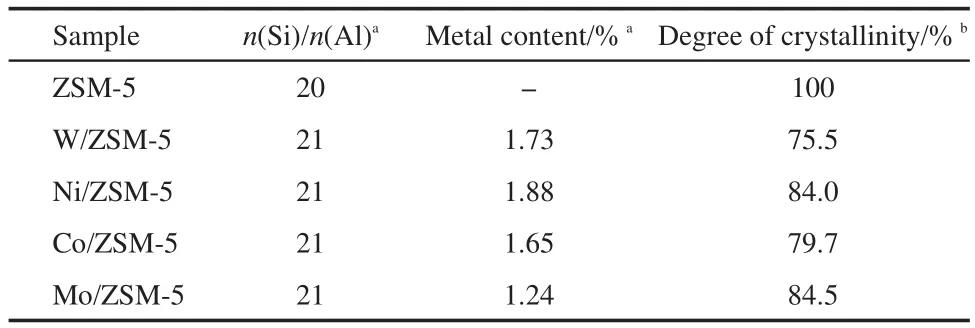
Table 2 Chemical composition of the different M/ZSM-5 samples

Table 3 Textural properties of M/ZSM-5 samples before and after using
3.2.2 Surface acid-base properties
The NH3-TPD and CO2-TPD measurement results are depicted in Figs.3 and 4.Two outstanding desorption peaks appear in the NH3-TPD patterns arising from the catalysts.A low temperature peak at near 420 K due to the ammonia species,which is desorbed from week acidic sites,in all catalysts appears;whereas a high temperature peak at near 730 K due to the ammonia species desorbed from strong acidic sites in W/ZSM-5,Ni/ZSM-5,Mo/ZSM-5 catalysts occurs.25,26Compared with ZSM-5 sample,the samples modified with M(M=W,Ni,Co,Mo)have a small shoulder peak at near 520 K in the NH3-TPD profile,indicating that small amounts of moderate acidic sites in all modified catalysts appear.In summary,the area below the curve increases as the addition of transition metal,this indicates that the total acidity of ZSM-5 is enhanced by the modification with transition metal;the addition of W,Ni,and Mo intensifies the strong acid of the catalysts,while Co makes the weak acidic sites increase.On the other hand,the intensities and quantities of basic sites on the modified catalysts are changed to some extent,especially in Co/ZSM-5 and Mo/ZSM-5.For Co/ZSM-5 catalyst,doping cobalt oxide results in the disappearance of the most of moderate basic sites with a CO2desorption peak occurring at 700 K,27and in the appearance of strong basic sites with a CO2desorption peak occurring at 800 K.A shoulder peak at 750 K appears in the profile for the Mo/ZSM-5 catalyst,indicating that Mo-modified ZSM-5 expresses more mild basicity.Weak basic sites shown by CO2desorption peak at 410 K do not exhibit significant change for all catalysts.

Fig.3 NH3-TPD profiles of M/ZSM-5 samples
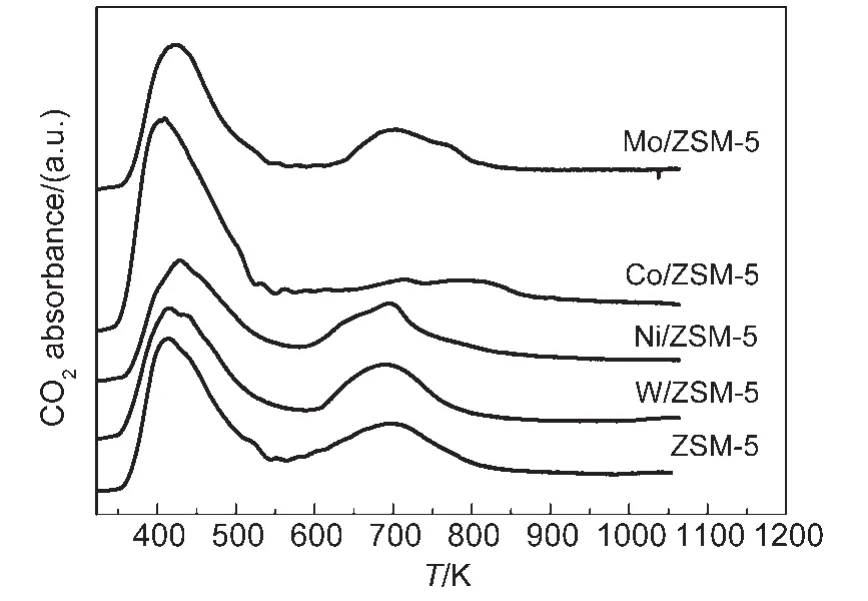
Fig.4 CO2-TPD profiles of M/ZSM-5 samples
The transformation of the acidities and basicities induced by doping transition metal oxides could be explained by the reaction of metal active sites and the acidic(basic)sites on the ZSM-5 surface.Therefore,the different metal-modified ZSM-5 zeolites result in various metal-sulfur interactions during the presulfurization with H2S.27The C―S bond is activated via acid site on the catalyst surface and cleaves to a methylthiolate group.14The increase of the acidity increases the capacity of the catalysts to carry out the C―S bond incision,28-31and subsequently improves the catalytic behaviors in converting DMS.The above catalyst activity test and characterization results strongly suggest that metal active sites and the acidic sites closely situated have a strong synergistic effect;therefore,the interactions of transition metals with DMS become stronger and the acid sites favor the cleavage of C―S bond.Furthermore,the MT selectivity decreases apparently with increasing in the surface basicity on Co/ZSM-5 and Mo/ZSM-5,this may be due to the decompositions of DMS and MT,which are easy to carry out on basic sites on the catalyst surface.
3.2.3 Investigation of C and S deposition measured by using O2-TPO
As we briefly mentioned above,the accumulation of carbon and sulfur on the surface may block up the pore,leading to the losses of porosity and specific surface area.The data of surface contents of C and S on used catalysts are listed in Table 3.DMS and MT decompositions are the main routes for coke and sulfur formation,resulting in the highest content of C and S on the Co/ZSM-5 catalyst owing to the strongest effect of Co3+on C―S bond incision.
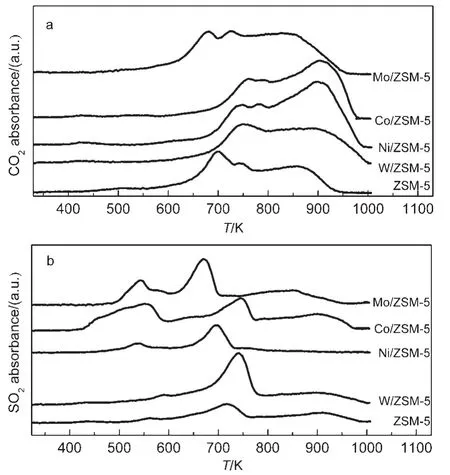
Fig.5 O2-TPO profiles of M/ZSM-5 samples
O2-TPO measurements for the used catalysts are depicted in Fig.5,the reaction includes the oxidation of the deposited carbon and sulfur along with the residual adsorbed TRS(DMS,MT,and H2S),resulting in the formation of CO2,SO2,and water,which are the complete oxidation products.It is evident that there are three regions of CO2formation with respect to the maximum peaks occurring at 690,750,and 860 K,respectively,which can be assigned to some carbonaceous deposits within the ZSM-5 zeolite channels.The M/ZSM-5 catalysts except Mo/ZSM-5 show a higher and stronger peak at 890 K,suggesting more carbon deposition existing,thus,higher temperature is needed when the reactivation of the catalyst is wanted.The release of SO2is more complicated,there is one apparent peak for ZSM-5 at 720 K,while,two small and broad peaks occur at 530 and 900 K for M/ZSM-5,whereas all the peaks of the M/ZSM-5 catalysts exhibit a small shift toward lower temperature for the oxidation of sulfur,The action of Co/ZSM-5 leads to producing largest amount of SO2,followed by that of Mo,Ni,W,indicating that the severest reaction occurs on Co/ZSM-5.
4 Conclusions
The reaction of H2S with DMS to form MT was studied over the transition metals(W,Ni,Co,Mo)modified ZSM-5 catalysts,the metal active sites and the acidic sites closely situated have a strong synergistic effect.The transition metal cations(W6+,Ni2+,Co3+,Mo6+)replace some of Al3+sites,since the transition metal cations are more efficient than Al3+in adsorbing DMS and MT,leading to more intense conversion of DMS.The total acidity of ZSM-5 was found to be enhanced by doping transition metal promoters,the addition of W,Ni,and Mo intensified the acidity of strong acid sites of the catalysts,while Co made the weak acidic sites increase.The increase of the acidity increases the capacity of the catalysts to carry out C―S bond incising,and subsequently improves the catalytic behavior in converting DMS.On the other hand,the MT selectivity decreases apparently with increasing in the surface basicity on Co/ZSM-5 and Mo/ZSM-5,which may be due to the fact that the decompositions of DMS and MT are easy to carry out on basic sites on the surface of the catalysts.
The used catalysts suffer from deactivation because of carbon and sulfur deposition on the surface;they cause distinct losses of the porosity and specific surface area,and subsequently block the pore and hinder the transport of reactants(H2S,DMS)to the surface,and,as a result,reduce the reaction rate.The oxidation treatment can efficiently rejuvenate the catalysts.
(1) Kastner,J.R.;Buquoi,Q.;Gangavaram,R.;Das,K.C.Envir.Sci.Technol.2005,39,1835.doi:10.1021/es0499492
(2) Demessie,E.S.;Devulapelli,V.G.Appl.Catal.B:Environ.2008,84,408.doi:10.1016/j.apcatb.2008.04.025
(3) Gutiérrez,O.;Kaufmann,C.;Hrabar,A.;Zhu,Y.;Lercher,J.J.Catal.2011,280,264.doi:10.1016/j.jcat.2011.03.027
(4) Chandra,S.;Soni,K.;Bunkar,R.;Sharma,M.;Singh,B.;Mahato,A.N.;Vijayaraghavan,R.Catal.Commun.2009,11,77.doi:10.1016/j.catcom.2009.08.014
(5) Beach,L.K.Preparation ofAlkyl Mercaptans.US Patent 2667515,1954-1-26.
(6)Chen,S.P.;Zhang,Y.H.;Wu,M.;Fang,W.P.;Yang,Y.Q.Appl.Catal.A 2012,431-432,151.
(7)Chen,S.P.;Wang,W.M.;Zhang,Y.H.;Wei,Y.C.;Fang,W.P.;Yang,Y.Q.J.Mol.Catal.A:Chem.2012,365,60.doi:10.1016/j.molcata.2012.08.009
(8) Chang,J.S.;Yu,H.B.;Jiang,X.D.;Ma,Y.Q.;Cheng,H.;Zhao,H.Ind.Catal.2005,13,32.[常俊石,于海斌,姜雪丹,馬月謙,成 宏,趙 虹.工業(yè)催化,2005,13,32.]
(9)Zhang,Y.H.;Chen,S.P.;Yuan,C.L.;Fang,W.P.;Yang,Y.Q.Chin.J.Catal.2012,33,317.[張元華,陳世萍,袁成龍,方維平,楊意泉.催化學報,2012,33,317.]
(10) Barth,J.O.Process for Preparing Methyl Mercaptan from Dialkyl Sulphides and Dialkyl Polysulphides.US Patent 7576243,2009-8-18.
(11) Mashkina,A.V.Petro.Chem.2009,49,441.
(12) Ziolek,M.;Kujawa,J.;Saur,O.;Lavalley,J.C.J.Phys.Chem.1993,97,9761.doi:10.1021/j100140a037
(13) Plaisance,C.P.;Dooley,K.M.Catal.Lett.2009,128,449.doi:10.1007/s10562-008-9772-2
(14) Satokawa,S.;Kobayashi,Y.;Fujiki,H.Appl.Catal.B:Environ.2005,56,51.doi:10.1016/j.apcatb.2004.06.022
(15) Hwang,C.L.;Tai,N.H.Appl.Catal.A 2011,393,251.doi:10.1016/j.apcata.2010.12.004
(16)Ding,L.H.;Zheng,Y.Catal Commun.2006,7,1035.doi:10.1016/j.catcom.2006.05.006
(17)Chen,A.P.;Wang,Q.;Li,Q.L.;Hao,Y.J.;Fang,W.P.;Yang,Y.Q.J.Mol.Catal.A:Chem.2008,238,69.
(18) Fan,X.L.;Liu,Y.;Du,X.J.;Liu,C.;Zhang,C.Acta Phys.-Chim.Sin.2013,29,263.[范曉麗,劉 燕,杜秀娟,劉 崇,張 超.物理化學學報,2013,29,263.]doi:10.3866/PKU.WHXB201211231
(19) Koranyi,T.I.;Moreau,F.;Rozanov,V.V.;Rozanova,E.A.J.Mol.Struct.1997,410,103.
(20) Hwang,C.L.;Tai,N.H.Appl.Catal.B 2010,93,363.doi:10.1016/j.apcatb.2009.10.009
(21) Maia,A.J.;Louis,B.;Lam,Y.L.;Pereira,M.M.J.Catal.2010,269,103.doi:10.1016/j.jcat.2009.10.021
(22) Garcia,C.L.;Johannes,A.L.J.Phys.Chem.1991,95,10729.doi:10.1021/j100179a040
(23) Mashkina,V.Y.Appl.Catal.A 1994,109,45.doi:10.1016/0926-860X(94)85002-X
(24) Sazama,P.;Dedecek,J.;Gábová,V.;Wichterlová,B.;Spoto,G.;Bordiga,S.J.Catal.2008,254,180.doi:10.1016/j.jcat.2007.12.005
(25)Luz,R.G.;Hermes,F.;Bertmer,M.;Enrique,R.C.;Antonio,J.L.;Simon,U.Appl.Catal.A 2007,328,174.doi:10.1016/j.apcata.2007.06.003
(26) Wang,W.L.;Liu,B.J.;Zeng,X.J.Acta Phys.-Chim.Sin.2008,24,2102.[王文蘭,劉百軍,曾賢君.物理化學學報,2008,24,2102.]doi:10.3866/PKU.WHXB20081128
(27)Seong,M.J.;Demoulin,O.;Grange,P.J.Mol.Catal.A:Chem.2005,236,94.doi:10.1016/j.molcata.2005.03.028
(28) Pecoraro,T.A.;Chianelli,F.R.J.Catal.1981,67,430.doi:10.1016/0021-9517(81)90303-1
(29) Mashkina,A.V.;Gruncald,V.R.;Borodin,B.P.;Nasteka,V.I.;Yakovleva,V.N.;Khairulina,L.N.React.Kinet.Catal.Lett.1991,43,361.doi:10.1007/BF02064698
(30) Koshelev,S.N.;Paukshtis,E.A.;Sagitullin,R.S.;Bezrukov,A.V.;Mashkina,A.V.React.Kinet.Catal.Lett.1985,27,387.doi:10.1007/BF02070480
(31) Ziolek,M.;Kujawa,J.;Saur,O.;Lavalley,J.C.J.Mol.Catal.A 1995,97,49.doi:10.1016/1381-1169(94)00068-9

