Effects of Promoters on the Ignition Process over NiO/Al2O3Catalyst for Autothermal Reforming of Methane to Hydrogen
Cai Xiulan; Li Guangyan; Lin Weiming
(1. Guangdong Pharmaceutical University, Guangzhou 510006; 2. South China University of Technology, Guangzhou 510640)
Effects of Promoters on the Ignition Process over NiO/Al2O3Catalyst for Autothermal Reforming of Methane to Hydrogen
Cai Xiulan1; Li Guangyan1; Lin Weiming2
(1. Guangdong Pharmaceutical University, Guangzhou 510006; 2. South China University of Technology, Guangzhou 510640)
The catalysts Ni/Al2O3, Ni/ZrO2-CeO2-Al2O3and Ni/CuO-ZrO2-CeO2-Al2O3were prepared by the co-precipitation method at a pH of 9 using Na2CO3as the precipitant. The Ni loading (mass fraction) of the catalysts was 10%. The ignition process on the catalysts for the autothermal reforming of methane to hydrogen was investigated and the surface properties of the catalysts were characterized by XPS. The results showed that the Ni/Al2O3catalyst could not ignite the process of autothermal reforming of methane to hydrogen. However, the Ni/CuO-ZrO2-CeO2-Al2O3catalyst could ignite the process of autothermal reforming of methane to hydrogen at lower reaction temperature (650 ℃) with the conversion of methane reaching 76%. The result of XPS analysis indicated that the promoters could change the binding energy (BE) of Ni2p3/2 obviously. The species of Cu in the Ni/CuO-ZrO2-CeO2-Al2O3catalyst comprised Cu2O and Cu2+. The formation of ZrO2-CeO2solid solution and a large amount of Cu2O might be the reason leading to good oxygen storage capacity and mobility of lattice oxygen of the Ni/CuO-ZrO2-CeO2-Al2O3catalyst, which could ignite the process of autothermal reforming of methane to hydrogen at lower reaction temperature.
methane; autothermal reforming; hydrogen production; ignition process
1 Introduction
In recent years, autothermal reforming (ATR) of methane is regarded as an important way to convert methane to hydrogen[1-11]. The practical application of ATR of methane needs a suitable and active catalyst. The Ni/Al2O3-based catalysts have a good application perspective because they are more economical than precious metals. But the Ni/Al2O3-based catalysts are more sensitive to coke formation and they cannot ignite autothermal reforming of methane at lower temperature because of the active sites of Ni0is easily re-oxidized by O2to form NiO in the course of reaction. The NiO species can hardly ignite autothermal reforming of methane. Only when the reaction temperature is higher than 770 ℃, the Ni/Al2O3-based catalysts can gradually ignite the methane reforming reaction. The research on process for igniting the autothermal reforming of methane has two major advantages. Firstly, reductive treatment of the Ni-based catalyst can be omitted before it is used; secondly, ignition of autothermal reforming process at lower temperature can improve the safety of reaction process. According to the related literature[12], the precious metal has a very strong ability of activating hydrogen and when the active hydrogen atoms overflow to the Ni based catalyst, the NiO reduction rate can be increased. Usually, the method for reducing the ignition temperature on the Ni based catalyst can be realized by adding precious metals (such as Pt, Pd and Ru) to the Ni based catalysts.
Many studies have shown[3-11]that there is a metalsemiconductor interaction between CeO2and active sites of Ni. The metal-semiconductor interaction can hinder the moving of Ni species on the surface of CeO2and improve the dispersion of nickel. Our research group has studied the effect of rare earth and transition metals (such as La, Ce, Zr, Fe, Co and Cu) on the catalytic performance of Ni/Al2O3for autothermal reforming of methane to hydrogen[13-15]. The effects of CeO2and ZrO2solid solution on the structure and performance ofNi/Al2O3were studied. The studies showed that the rare earth metal Ce and the transition metal Zr can greatly improve the catalytic performance of Ni/Al2O3catalyst. The conversion of methane on the Ni/ZrO2-CeO2-Al2O3catalyst (withm(Al):m(Ce):m(Zr)=70:20:10) was 93% at 750 ℃ and 100% at 800 ℃. The results of XRD analysis indicated that oxides of Ce and Zr can form CeO2-ZrO2solid solution. The result of SEM analysis showed that the dispersion of NiO or Ni of the Ni/ZrO2-CeO2-Al2O3catalyst was better than the Ni/CeO2-Al2O3catalyst because of the interaction between CeO2and ZrO2. The addition of Cu to the Ni/ZrO2-CeO2-Al2O3catalyst can further enhance the activity of the catalyst at low temperature. The methane conversion on the Ni/CuO-ZrO2-CeO2-Al2O3catalyst (withm(Al):m(Ce):m(Cu):m(Zr)=65:20:10:5) reached 82% at 650 ℃and 100% at 750 ℃. So in this paper, the effects of Zr, Ce and Cu on the ignition by the Ni/Al2O3catalyst for autothermal reforming of methane to hydrogen were discussed.
2 Experimental
2.1 Preparation of support
The supports Al2O3, ZrO2-CeO2-Al2O3and CuO-ZrO2-CeO2-Al2O3were prepared by mean of co-precipitation during the reaction of 1 mol/L of Na2CO3solution under vigorous stirring upon the aqueous solution of Al(NO3)3, ZrO(NO3)2, Ce(NO3)3, and Cu(NO3)2, respectively. The Al solution was simultaneously added at a constant rate of 50 mL/h. The addition of the precipitating agent (Na2CO3) was monitored using a stationary pH-meter, in order to maintain the pH value of the solution at 9.0 during precipitation. The temperature was kept at 80 ℃. The precipitate obtained thereby was subjected to ageing for 2 h with the mother liquor being under vigorous stirring at the reaction temperature. Then the precipitate was filtered out, washed with deionized water, dried at 120 ℃ for 12 h, and further calcined in air at 800 ℃ for 4 h. After this treatment, the support was ready for further experiments.
2.2 Preparation of catalyst
The Ni/Al2O3, Ni/ZrO2-CeO2-Al2O3and Ni/CuO-ZrO2-CeO2-Al2O3catalysts (with 10 m% of Ni loading apiece) were prepared via wet impregnation of the supports Al2O3, ZrO2-CeO2-Al2O3and CuO-ZrO2-CeO2-Al2O3by a Ni(NO3)2solution at room temperature for 12 h. The resulting materials were dried at 120 ℃ for 10 h and subsequently calcined in air at 650 ℃ for 6 h.
2.3 Test of catalytic performance
Autothermal reforming of methane (ATRM) reaction was carried out in a continuous flow fixed bed reactor at different temperatures (from 650 ℃ to 850 ℃) under atmospheric pressure. The reactor temperature was measured by a K-type thermocouple with a thermowell placed in the center of the catalyst bed. Two sets of mass flow meters (D07, China) were used to control the flow rates of reactants (i.e., CH4, O2). Deionized water was fed by a liquid micro-pump (SY-02A, China) and then, through a water evaporator. One mL of catalyst charge was used in a typical experiment and the particle size of catalyst was in the range of 20—40 mesh. Before catalytic reaction, the catalyst was reduced by a gas mixture consisting of 20% of H2and 80% of N2at 800 ℃ for 2 h. A gas mixture of CH4, O2and H2O with a molar ratio of 1:0.5:2.5 was introduced with a gas hourly space velocity of 4 800 h-1. The water in the tail gas was separated by a cold trap, and the product including H2, CH4, CO, and CO2was analyzed by one set of gas chromatograph equipped with a TDX-01 column and a thermal conductivity detector (TCD).
Conversion of CH4=[n(CH4)in-n(CH4)out]/n(CH4)in×100%
2.4 Characterization
X-ray photoelectron spectroscopy (XPS) analysis was carried out on a VG MuiltiLab 2 000 photoelectron spectrometer at room temperature under a vacuum of 10-8—10-9torr (1 torr=133 Pa), using Mg Kα radiation, powered at 10 keV and 20 mA. The binding energies (B. E.) of Ni2p3/2 were calibrated to the carbon with a C1s band at 284.6 eV.
3 Results and Discussion
3.1 Effect of reductive pretreatment on catalytic performance of Ni based catalysts
The effect of reductive pretreatment (with reductive pretreatment by H2or without reduction by H2) on catalytic performance of catalysts covering Ni/Al2O3, Ni/ZrO2-CeO2-Al2O3[m(Al):m(Ce):m(Zr) =70:20:10] and Ni/ CuO-ZrO2- CeO2-Al2O3[m(Al):m(Ce):m(Cu):m(Zr)= 65:20:10:5] for autothermal reforming of methane tohydrogen was studied to verify the CH4conversion. The reductive pretreatment of the catalysts by H2was carried out as follows. Before catalytic reforming reaction, the catalyst was reduced by a gas mixture composed of 20% of H2and 80% of N2at 800 ℃ for 2 h and then heated to 850 ℃ in N2. A gas mixture consisting of CH4, O2and H2O at a molar ratio of 1:0.5:2.5 was introduced at a gas hourly space velocity of 4 800 h-1.
The pretreatment of catalysts without reduction by hydrogen gas was carried out as follows. The catalyst was heated to 850 ℃ in the N2atmosphere. A gas mixture composed of CH4, O2and H2O at a molar ratio of 1:0.5:2.5 was introduced at a gas hourly space velocity of 4 800 h-1. The results are shown in Figure 1 and Figure 2.

Figure 1 Effect of pretreatment of Ni-based catalysts without reduction by hydrogen on CH4conversion
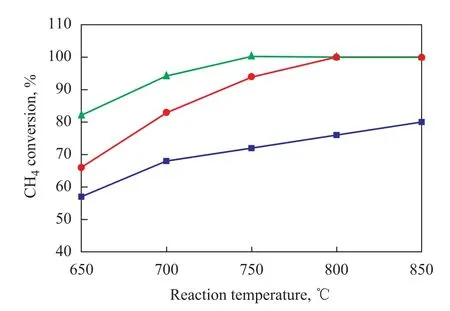
Figure 2 Effect of reductive pretreatment of Ni-based catalysts on CH4conversion
According to the results presented in Figure 1 and Figure 2, the reductive pretreatment had a great influence on the catalytic performance of Ni/Al2O3catalyst and had little influence on the catalytic performance of Ni/ZrO2-CeO2-Al2O3and Ni/CuO-ZrO2-CeO2-Al2O3catalysts. Compared with the performance of Ni/Al2O3catalyst after reductive pretreatment by H2, the Ni/Al2O3catalyst without reductive pretreatment showed very low catalytic activity. According to the results referred to in the literature[16], metallic Ni particles are the center of methane-splitting activity and Ni species on Ni/Al2O3catalyst without being reduced by H2were in the form of oxidation state in the initial stage of the reaction. In the programmed heating process, there exists competitive adsorption between CH4and O2in the central active sites of metallic nickel. Before the reaction temperature reaches the decomposition temperature of CH4, the active sites of metallic nickel will be occupied by O2that will completely oxidize Ni to NiO because the adsorption capacity of O2is stronger than CH4, and CH4hereupon cannot be decomposed by NiO. Therefore, the oxygen storage capacity and mobility of lattice oxygen on the surface of Al2O3support might be the main reasons denoting that the reductive pretreatment had a great influence on the catalytic performance of the Ni/Al2O3catalyst.
With the increase in reaction time, H2production will increase and H2produced in the reaction will reduce Ni species from NiO to Ni, resulting in more active sites of nickel, and the methane conversion on the Ni/Al2O3catalyst that had not been subjected to reductive pretreatment would gradually restore its activity after transformation of NiO to Ni by the nascent hydrogen.
Compared with the performance of the catalysts pretreated by H2during reduction, the Ni/ZrO2-CeO2-Al2O3and Ni/CuO-ZrO2-CeO2-Al2O3catalysts had almost the same catalytic activity, especially with respect to the Ni/CuOZrO2-CeO2-Al2O3catalyst. At 650 ℃, the Ni/CuO-ZrO2-CeO2-Al2O3catalyst without being subjected to reductive pretreatment by hydrogen showed an 82% of CH4conversion, which was equal to the CH4conversion achieved by the same catalyst that had been pretreated by H2during reduction. According to the results referred to in the literature[13-14], inside the Ni/ZrO2-CeO2-Al2O3and Ni/CuOZrO2-CeO2-Al2O3catalysts, the ZrO2species enter theCeO2cells to form a CeO2-ZrO2solid solution. Studies had found out[17]that the formation of CeO2-ZrO2solid solution can improve the oxygen storage capacity of CeO2and enhance the mobility of lattice oxygen. Therefore, the oxygen storage capacity and mobility of lattice oxygen of CeO2-ZrO2solid solution in the supports Ni/ZrO2-CeO2-Al2O3and Ni/CuO-ZrO2-CeO2-Al2O3may be the main reason showing that the reductive pretreatment had little effect on the activity of the Ni/ZrO2-CeO2-Al2O3and the Ni/CuO-ZrO2-CeO2-Al2O3catalysts.
3.2 Effect of reaction temperature sequence on catalytic performance of Ni based catalysts
The experimental data indicated that the Ni/Al2O3catalyst showed almost no activity during the heating process. So the effect of temperature sequence on catalytic performance of the Ni/Al2O3catalyst for autothermal reforming of methane was not studied in this paper. It can be seen from the data of Figures 1 and 2 that the Ni/CuO-ZrO2-CeO2-Al2O3catalyst exhibited a best catalytic activity. Therefore, the effect of temperature sequence on catalytic performance of the Ni/CuO-ZrO2-CeO2-Al2O3catalyst for autothermal reforming of methane was studied in this paper. The results are represented in Figures 3—5.
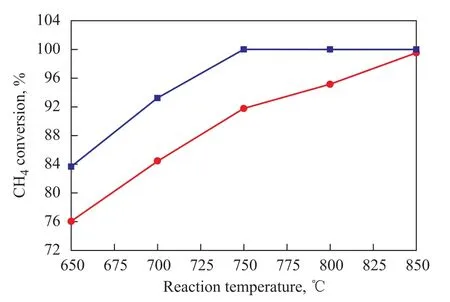
Figure 3 Effect of reaction temperature sequence on CH4conversion on Ni/CuO-ZrO2-CeO2-Al2O3catalyst
The methane conversion on the Ni/CuO-ZrO2-CeO2-Al2O3catalyst during the heating and cooling processes which varied with the reaction temperature was measured and the results are shown in Figure 3. Figure 3 indicates that the Ni/CuO-ZrO2-CeO2-Al2O3catalyst had good catalytic activity during the cooling and heating processes and the Ni/CuO-ZrO2-CeO2-Al2O3catalyst could ignite the process of autothermal reforming of methane to hydrogen at lower reaction temperature (650 ℃) with the conversion of methane reaching 76% at 650 ℃.
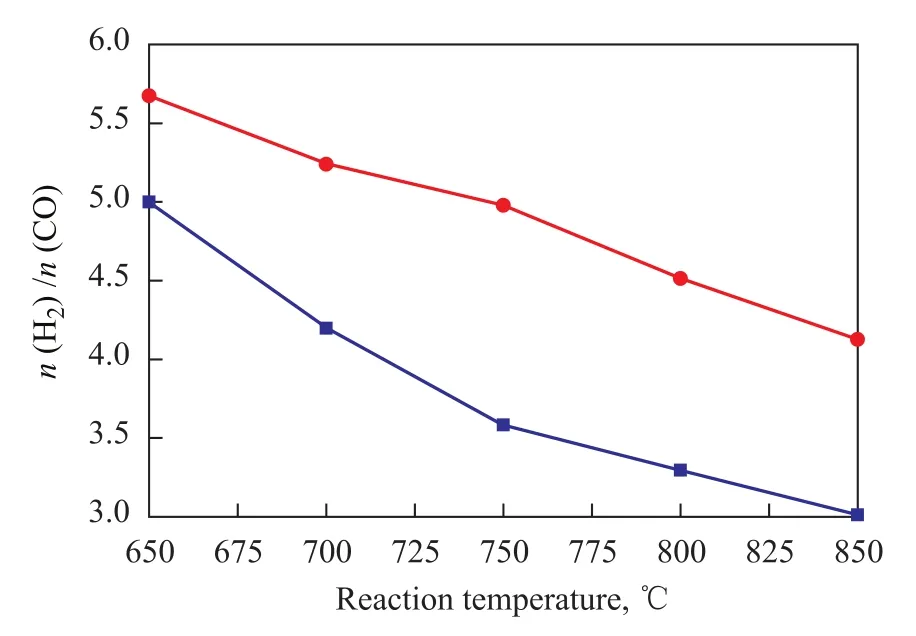
Figure 4 Effect of reaction temperature sequence of Ni/ CuO-ZrO2-CeO2-Al2O3catalyst on H2/CO molar ratio
The effect of reaction temperature sequence on H2/CO molar ratio on the Ni/CuO-ZrO2-CeO2-Al2O3catalyst was studied, with the result presented in Figure 4. The data in Figure 4 indicate that with the increase in the reaction temperature from 650 ℃ to 850 ℃, the H2/CO molar ratio on the Ni/CuO-ZrO2-CeO2-Al2O3catalyst showed a decreasing trend in the heating and cooling processes, but the H2/CO molar ratio on the Ni/CuO-ZrO2-CeO2-Al2O3catalyst was significantly higher during the heating process than that identified during the cooling process.
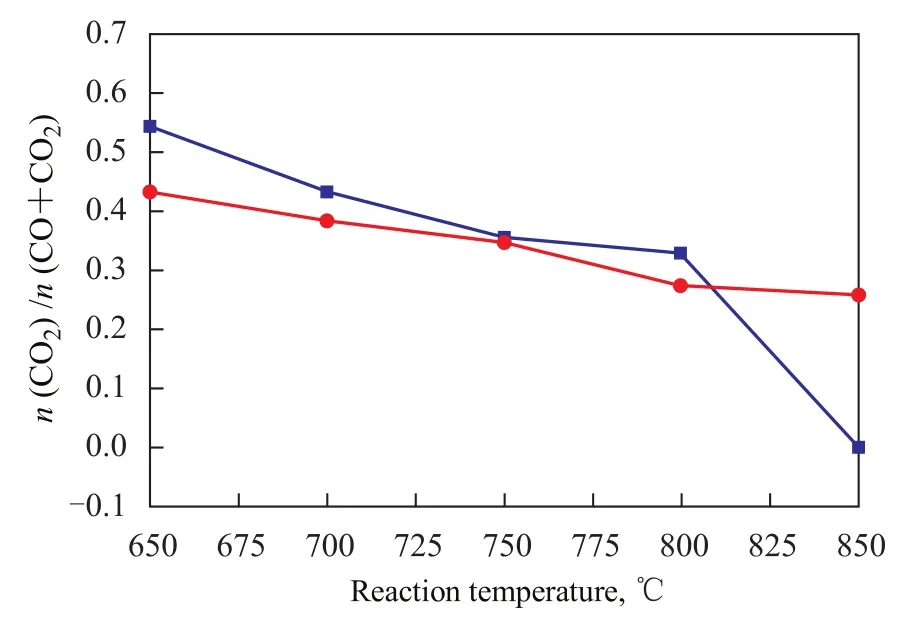
Figure 5 Effect of reaction temperature sequence of Ni/CuOZrO2-CeO2-Al2O3catalyst on CO2/ (CO+CO2) molar ratio
The effect of reaction temperature sequence of the Ni/ CuO-ZrO2-CeO2-Al2O3catalyst on CO2/(CO+CO2) mo-lar ratio was studied, with the results shown in Figure 5. The data in Figure 5 indicate that with the increase in the reaction temperature from 650 ℃to 850 ℃, the CO2/ (CO+CO2) molar ratio on the Ni/CuO-ZrO2-CeO2-Al2O3catalyst showed a decreasing trend during the heating and cooling processes, but the CO2/(CO+CO2) molar ratio on the Ni/CuO-ZrO2-CeO2-Al2O3catalyst was significantly higher during the cooling process than that achieved during the heating process in the temperature range of between 650 ℃ to 800 ℃.
Judging from the results of Figures 3 to 5, it can be found out that the catalytic performance of the Ni/CuO-ZrO2-CeO2-Al2O3catalyst could be affected by the reaction temperature sequence. Compared with the relevant data achieved during the cooling process, the methane conversion was less, the H2/CO molar ratio was relatively higher, and the CO2/(CO+CO2) molar ratio was also less during the heating process.
During the heating process, the Ni species in the Ni/CuOZrO2-CeO2-Al2O3catalyst can be oxidized to NiO which does not possess the active sites in this reaction system and the presence of NiO species does not lead to excessive oxidation of CO into CO2. This may be the reason which causes the reduction of catalytic activity and CO2/(CO+CO2) molar ratio. In addition, the increase of H2/CO molar ratio during the heating process has revealed the increase in the extent of water gas shift reaction, which is favorable for H2production.
3.3 Binding energy of Ni2p on the surface of catalysts
The binding energy change of each species on the surface of catalyst has a great influence on the performance of catalyst for autothermal reforming of methane. Therefore, the X-ray photoelectron spectroscopy (XPS) characterization was used to study the binding energy of each species on the surface of catalyst, which had a very important significance for studying the reaction mechanism. In this paper, the Ni/Al2O3, Ni/ZrO2-CeO2-Al2O3and Ni/CuOZrO2-CeO2-Al2O3catalysts were characterized by XPS, in order to find out the effect of Cu, Ce and Zr on the binding energy of Ni2p.
The XPS analysis of the Ni/Al2O3, Ni/ZrO2-CeO2-Al2O3and Ni/CuO-ZrO2-CeO2-Al2O3catalysts is shown in Figure 6. According to the results of XRD and TPR described in the literature[12-13], Ni species on the Ni/Al2O3catalyst exist mainly in the form of highly dispersed NiO particles and NiAl2O4phase. Usually, the binding energy of Ni2p is 853.6 eV in NiO and 856.6 eV in NiAl2O4[18]. The results of Figure 6 indicate that the binding energy of Ni2p in the Ni/Al2O3catalyst is 855.9 eV, which is between 853.6 eV and 856.6 eV. This shows that the Ni species exist in the Ni/Al2O3catalyst mainly in the form of NiO and NiAl2O4which is in agreement with the results of XRD and TPR analysis. In addition, there is a weak intensity peak at the position of 852.6 eV, which is attributed to the peak of metallic state of Ni2p3/2.
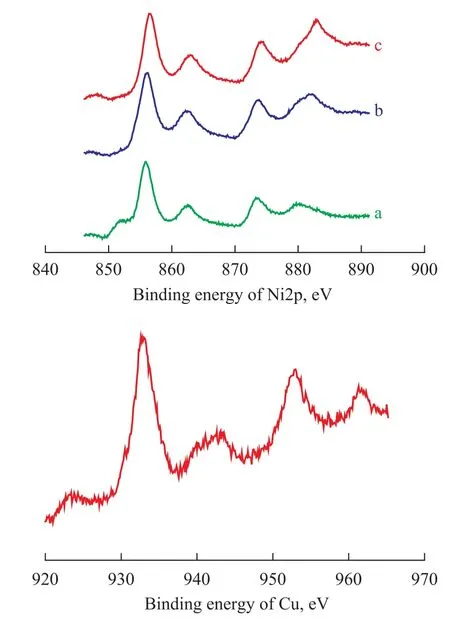
Figure 6 XPS profiles of Ni2p in the catalysts
Compared with the Ni/ZrO2-CeO2-Al2O3catalyst, the binding energy of Ni2p in the Ni/CuO-ZrO2-CeO2-Al2O3catalyst is 855.4 eV, which is lower by 0.5 eV as compared to the case of Ni/Al2O3catalyst. This shows that the electron cloud density in the outer layer of Ni2p in the Ni/ CuO-ZrO2-CeO2-Al2O3catalyst has somewhat increased. The increase in the electron cloud density of Ni atoms is good for strengthening the M—C bond and weakening the C—H bond which can make CH4easier activated during autothermal reforming of methane. Compared withthe Ni/ZrO2-CeO2-Al2O3catalyst, the intensity and shape of Ni2p3/2peak in the Ni/CuO-ZrO2-CeO2-Al2O3catalyst almost show no change, which indicates that the dispersion of Ni species on the Ni/ZrO2-CeO2-Al2O3and the Ni/CuOZrO2-CeO2-Al2O3catalysts has no significant difference.
The results of the Cu2p spectrum in Figure 6 show that the binding energy of Cu2p in the Ni/CuO-ZrO2-CeO2-Al2O3catalyst is 932.6 eV, which is equal to the standard binding energy of Cu2O. At the peak of 943.1 eV, there is a weak characteristic peak of Cu2+. The intensity ratio of the peak at 932.6 eV and 943.1 eV can reflect the change of the chemical state of Cu species in the Ni/CuO-ZrO2-CeO2-Al2O3catalyst. Judging from the above analysis, we can suppose that Cu species exist in the form of Cu2O and Cu2+(especially Cu2O) in the Ni/CuO-ZrO2-CeO2-Al2O3catalyst. The presence of Cu2O species may be the reason indicating that the reaction on the Ni/CuO-ZrO2-CeO2-Al2O3catalyst can be initiated at low temperature.
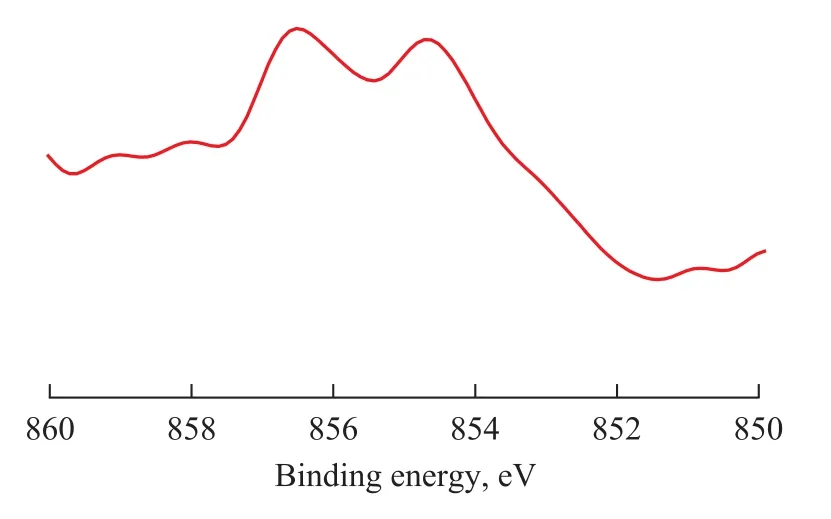
Figure 7 XPS profile of Ni/CuO-ZrO2-CeO2-Al2O3catalyst (Ni2p) after reaction for 4 h
The XPS profile of Ni/CuO-ZrO2-CeO2-Al2O3catalyst (Ni2p) after the reaction has proceeded for 4 h is shown in Figure 7. Compared with the value of 855.4 eV, the binding energy of the Ni/CuO-ZrO2-CeO2-Al2O3catalyst (Ni 2p3/2) after reaction for 4 h is 855.66 eV, which shows little change. This indicates that Ni species of the Ni/ CuO-ZrO2-CeO2-Al2O3catalyst after reaction for 4 h are mainly composed of NiO, and Ni species show a good stability, which denotes that the Ni/CuO-ZrO2-CeO2-Al2O3catalyst has good oxygen storage capacity and mobility of lattice oxygen.
4 Conclusions
The results presented in this paper showed that the reductive pretreatment of catalysts has a great influence on the catalytic performance of Ni/Al2O3catalyst, while little influence on the performance of Ni/ZrO2-CeO2-Al2O3and the Ni/CuO-ZrO2-CeO2-Al2O3catalysts is identified. Therefore, the reductive pretreatment of Ni/ZrO2-CeO2-Al2O3and Ni/CuO-ZrO2-CeO2-Al2O3catalysts can be omitted. In addition, the Ni/CuO-ZrO2-CeO2-Al2O3catalyst has good oxygen storage capacity and mobility of lattice oxygen and can ignite the process of autothermal reforming of methane to hydrogen at lower reaction temperature (650 ℃), with the conversion of methane reaching 76%. The XPS measurements have indicated that Cu species exist in the form of Cu2O and Cu2+(especially Cu2O) in the Ni/CuO-ZrO2-CeO2-Al2O3catalyst. The presence of Cu2O species may be the reason that the Ni/CuO-ZrO2-CeO2-Al2O3catalyst can ignite the autothermal reforming of methane at low temperature.
Acknowledgements:This work was supported by the Guangdong Provincial Natural Science Foundation (030514), the Science and Technology Plan of Guangdong Province of China (2004B33401006) and the Doctoral Startup Foundation of Guangdong Pharmaceutical University.
[1] Dias J A C, Assaf J M. Autoreduction of promoted Ni/ γ-Al2O3during autothermal reforming of methane[J]. Journal of Power Sources, 2005, 139(1): 176-181
[2] D ias J A C, Assaf J M. Autothermal reforming of methane over Ni/γ-Al2O3promoted with Pd: The effect of the Pd source in activity, temperature profile of reactor and in ignition[J]. Applied Catalysis A: General, 2008, 334(1): 243-250
[3] Takeguchi T, Furukawa S N, Inoue M, et al. Autothermal reforming of methane over Ni catalysts supported over CaO–CeO2–ZrO2solid solution[J]. Applied Catalysis A: General, 2003, 240(1): 223-233
[4] Souza M M V M, Schmal M. Autothermal reforming of methane over Pt/ZrO2/Al2O3catalysts[J]. Applied Catalysis A: General, 2005, 281(1): 19-24
[5] Laosiripojana N, Kiatkittipong W, Assabumrungrat S. Partial oxidation of palm fatty acids over Ce-ZrO2: Roles of catalyst surface area, lattice oxygen capacity and mobility[J]. AIChE Journal, 2011, 57(10): 2861-2869
[6] Cao L, Ni C, Yuan Z, et al. Correlation between catalyticselectivity and oxygen storage capacity in autothermal reforming of methane over Rh/Ce0.45Zr0.45RE0.1catalysts (RE= La, Pr, Nd, Sm, Eu, Gd, Tb)[J]. Catalysis Communications, 2009, 10(8): 1192-1195
[7] Lisboa J S, Terra L E, Silva P R J, et al. Investigation of Ni/Ce–ZrO2catalysts in the autothermal reforming of methane[J]. Fuel Processing Technology, 2011, 92(10): 2075-2082
[8] Escritori J C, Dantas S C, Soares R R, et al. Methane autothermal reforming on nickel–ceria–zirconia based catalysts[J]. Catalysis Communications, 2009, 10(7): 1090-1094
[9] Sadykov V, Muzykantov V, Bobin A, et al. Oxygen mobility of Pt-promoted doped CeO2–ZrO2solid solutions: Characterization and effect on catalytic performance in syngas generation by fuels oxidation/reforming[J]. Catalysis Today, 2010, 157(1): 55-60
[10] Yuan Z, Ni C, Zhang C, et al. Rh/MgO/Ce0.5Zr0.5O2supported catalyst for autothermal reforming of methane: The effects of ceria–zirconia doping[J]. Catalysis Today, 2009, 146(1): 124-131
[11] Ferreira A P, Zanchet D, Araújo J C S, et al. The effects of CeO2on the activity and stability of Pt supported catalysts for methane reforming, as addressed by in situ temperature resolved XAFS and TEM analysis[J]. Journal of Catalysis, 2009, 263(2): 335-344
[12] Zhou Z H, Zhang A L, Gong M C. Effects on calcination temperature of nickel-alumina catalysts [J]. Chemical Research and Application, 2000, 12(5): 521-524
[13] Cai X, Cai Y, Lin W. Autothermal reforming of methane over Ni catalysts supported over ZrO2-CeO2-Al2O3[J]. Journal of Natural Gas Chemistry, 2008, 17(2): 201-207
[14] Cai X, Dong X, Lin W. Autothermal reforming of methane over Ni catalysts supported on CuO-ZrO2-CeO2-Al2O3[J]. Journal of Natural Gas Chemistry, 2006, 15(2): 122-126
[15] Cai X L, Dong X F, Lin W M. Study of Ni catalyst for autothermal reforming of methane to produce hydrogen [J]. Journal of South China University of Technology (Natural Science Edition), 2006, 34(12): 68-71 (in Chinese)
[16] Zhang Z B, Yu C C, Shen S K. Partial oxidation of CH4to syngas on La2O3promoted Ni/MgAl2O4[J]. Chinese Journal of Catalysis, 2000, 21(1): 14-18 (in Chinese)
[17] Zhong S H, Wang X T, Bi L X, et al. Catalytic performance of ZrO2-SiO2supported Ni-Cu alloy catalysts for CO2hydrogenation[J]. Journal of Molecular Catalysis (China), 2001, 15(3): 170-174 (in Chinese)
[18] Roh H S, Jun K W. Low temperature methane steam reforming for hydrogen production for fuel cells[J]. Bull Korean Chem Soc, 2009, 30(1): 153-156
Received date: 2014-02-21; Accepted date: 2014-08-08.
Dr. Cai Xiulan, Telephone: +86-20-39352122; E-mail: caixiulan78@126.com .
- 中國(guó)煉油與石油化工的其它文章
- Solvothermal Synthesis of V2O3Catalysts for Oxidative Desulfurization of Dibenzothiophene
- Identification and Characterization of Sulfur Compounds in Straight-Run Diesel Using Comprehensive Two-Dimensional GC Coupled with TOF MS
- Oligomerization of 1-Decene: Catalyzation by Immobilized AlCl3/γ-Al2O3Catalyst in Fixed-bed Reactor
- Study on Tribological Properties of CVT Fluid Containing Inert and Active Functional Elements
- Application of Modified Attapulgite Clay as the Adsorbent in Gasoline Desulfurization
- Study on the Influence of Ni Modifying on Phase Transformation and Photocatalytic Activity of TiO2

