Solvothermal Synthesis of V2O3Catalysts for Oxidative Desulfurization of Dibenzothiophene
Liu Ni; Zhang Minghui; Wang Danhong
(Key Laboratory of Advanced Energy Materials Chemistry (MOE), College of Chemistry, Nankai University, Tianjin 300071)
Solvothermal Synthesis of V2O3Catalysts for Oxidative Desulfurization of Dibenzothiophene
Liu Ni; Zhang Minghui; Wang Danhong
(Key Laboratory of Advanced Energy Materials Chemistry (MOE), College of Chemistry, Nankai University, Tianjin 300071)
V2O3nanoparticles with high surface area have been successfully prepared by a new solvothermal method without using any surfactant and template. The size of V2O3nanoparticles is mostly equal to 10 nm-30 nm. The highest surface area of obtained V2O3nanoparticles reaches 49 m2/g. Several kinds of V2O3catalysts were prepared by different methods. All these V2O3catalysts obtained thereby showed high catalytic activity for oxidative desulfurization (ODS) reaction by using tert-butyl hydroperoxide as the oxidant. The V2O3catalyst with a highest ODS activity was obtained under the following conditions: The catalyst was prepared upon using V2O5as the vanadium source, methanol as the solvent, and oxalic acid as the complexing reagent at a V2O5/oxalic acid molar ratio of 1:2. The process for ODS of dibenzothiophene was carried out under mild conditions (under atmospheric pressure and at a relatively low temperature). The highest ODS activity of the obtained V2O3nanoparticles can be attributed to their highest surface area.
vanadium sesquioxide; nanoparticles; oxidative desulfurization; solvothermal; dibenzothiophene
1 Introduction
Nowadays, sulfur content in the fuel oil is being continuously regulated to lower levels due to environmental requirements[1-2]. Hydrodesulfurization (HDS) is a currently adopted technology for sulfur removal in the petroleum and petrochemical industries. HDS is highly efficient for the removal of thiols, sulfides, and disulfides. However, it is difficult to reduce refractory sulfur compounds such as dibenzothiophene (DBT). The HDS process requires high temperature and H2pressure, making HDS a very expensive pathway for deep desulfurization[3-4]. In recent years, a lot of researchers have explored several alternative technologies to remove these refractory sulfur compounds, such as adsorption[5], extraction[6], oxidation[7]and bioprocesses[8]. Among them, the ODS process seems to be very promising and is receiving increasing attention, because the sulfur compounds (DBTs) that are the most difficult to be removed by HDS are the most reactive in the ODS process. Moreover, the ODS process can be carried out under very mild conditions compared with severe conditions used in HDS process[9]. During the oxidative desulfurization process, sulfur compounds can be oxidized to sulfoxides and sulfones. The chemical and physical properties of sulfoxides and sulfones are very different from other hydrocarbons contained in fuel oil. They can be removed by distillation, solvent extraction, or adsorption. The DBTs are easily oxidized under mild reaction conditions (under atmospheric pressure and at a relatively low temperature) without using expensive hydrogen[9-12].
Lots of catalysts for ODS have been discussed in previous publications[13-16]. Recently, the supported vanadiumcontaining compounds have been researched in the ODS reaction[17-21]. For instance, V2O5/Al2O3, V2O5/TiO2and V2O5/SiO2-Al2O3catalysts were introduced in oxidative desulfurization process. V2O5catalyst is well known for its catalytic activity in the ODS process. But the catalytic activity of other vanadium oxides is still rarely studied. Actually there are plenty of publications about other vanadium oxides, such as V2O3, and VO2. Most of thesepublications have paid attention to the technological applications except for their catalytic property[22-24]. Over two decades, many techniques and different methods have been reported to synthesize V2O3powder. For example, the sphere-like V2O3particles were prepared by using O2-H2flame fusion of V2O5at 2 273 K. Spherelike V2O3particles were synthesized by reduction of V2O5in H2atmosphere at 1 123 K for 6 h. In addition, V2O3powders were formed by pyrolyzing the hydrazine containing vanadium salt and reducing the V2O5gel under H2atmosphere[25]. There are some other synthetic methods, such as the laser-induced vapor-phase reaction and ultrashort pulsed deposition of vanadium oxides[26]. However, there are several disadvantages in these systems, such as complicated process, high temperature, unavailability to dispersed nanoparticles, and expensive production costs. Only micrometer-scale powders are obtained by these methods. Few preparation methods of the V2O3nanoparticles have been reported.
In this paper, our group has developed a simple solvothermal approach for synthesis of V2O3nanoparticles by using V2O5, methanol and oxalic acid as reaction reagents. The application of this reaction is particularly attractive and the obtained V2O3nanoparticles are phase-pure. Besides, the process is rather simple and the reaction temperature is relatively low, which would make it much easier to achieve large-scale production. The obtained V2O3nanoparticles showed very high catalytic activity in oxidative desulfurization process. In addition, there was no contamination during the production with other inorganic compounds, such as halides or alkaline ions.
2 Experimental
2.1 Materials
In this synthesis procedure, we use V2O5as the source of vanadium. In a typical synthesis procedure, V2O5powder and oxalic acid in a definite proportion was added to 30 mL of aqueous solution under stirring, until the color of the solution changed from yellow to dark blue. This procedure was carried out in about 3 hours. The mixture was transferred into a teflon-lined autoclave. The autoclave was sealed and heated to 453 K for 16 hours. Then the autoclave was cooled to room temperature and the precipitate was separated by filtration, and then washed with deionized water and ethanol. The product was dried in a vacuum oven at 333 K for 3 h. The obtained black powder precursor was proved to be metastable VO2(B). The monoclinic VO2(M) was obtained by crystallizing the precursor at 773 K for 4 h in the Ar atmosphere[27-29]. When the precursor was crystallized at 873 K for 4 h in the Ar atmosphere, V2O3was formed. As shown in Table 1, we marked this V2O3product as V2O3(1). In a typical synthesis process, one gram of V2O5was directly added into a teflon-lined autoclave with the addition of excess methanol. The autoclave was sealed and heated to 453 K for 12 hours. Next steps were similar to those for preparation of V2O3(1). Similarly, V2O3was formed when the precursor was crystallized at 873 K for 4 h in the Ar atmosphere. As shown in Table 1, we marked this V2O3product as V2O3(2). V2O3nanoparticles can be directly synthesized by the solvothermal method. V2O5powder and oxalic acid in a def inite proportion with excess methanol being added into a teflon-lined autoclave. The autoclave was sealed and heated to 453 K for 12 hours. Then the autoclave was cooledto room temperature. The precipitate was separated by filtration and washed with deionized water and ethanol. The V2O3was formed when the precursor was crystallized at 673 K for 4 h in the Ar atmosphere. As shown in Table 1, we marked the obtained V2O3product as V2O3(3) when the molar ratio of V2O5to oxalic acid was 1:1. Likewise, we marked the obtained V2O3product as V2O3(4) when the molar ratio of V2O5to oxalic acid was 1:2 and labeled the product as V2O3(5) when the V2O5/oxalic acid molar ratio was equal to 1:3.
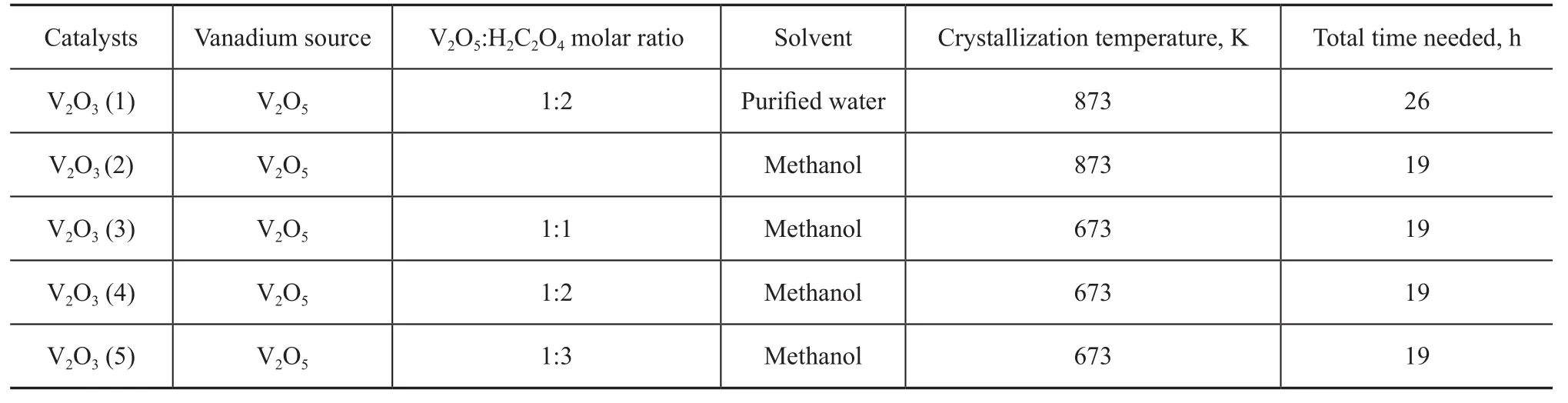
Table 1 Different experimental conditions for synthesis of V2O3particles
2.2 Catalysts preparation
A mixture of 0.20 g of V2O3black powder and 0.80 g of macroporous silica gel was ground, pressed, crushed, and screened. 0.10 g of sieved catalyst with a grain size of 40—60 mesh was used for oxidative desulfurization process.
2.3 Catalytic performance test
The obtained vanadium sesquioxide particles were used as the catalyst for oxidative desulfurization of dibenzothiophene. Oxidative desulfurization of DBT was carried out in a fixed-bed reactor. A simulated model diesel (MD500) was prepared as follows: 0.05 g of DBT was dissolved in 99.95 g of decalin to obtain a solution containing 500 mg/g of DBT. Since the molar mass of DBT is about 3 times that of TBHP, so 0.112 8 g of 65% TBHP was dissolved in 99.9 g of decalin to obtain a solution containing 500 mg/g of TBHP.
A typical oxidative desulfurization process was carried out in a stainless steel and temperature-controlled fixedbed reactor (with an i. d. of 3 mm). 0.10 g of sieved catalyst was loaded in the reactor and heated up to a specified temperature. Then the feed (MD500) was introduced by a peristaltic pump under atmospheric pressure at a WHSV of 40 h-1. After the effluent was maintained at each temperature for one hour, the reaction solution at that temperature was collected for half an hour to be analyzed by a gas chromatograph (GC-FID).
2.4 Characterization methods
Powder X-ray diffraction (XRD) patterns were obtained on a Rigaku D MAX diffractometer using CuKα radiation (at a tube voltage of 40 kV and a tube current of 100 mA) from 10° to 80° (2θ) with a scanning rate of 12(°)/min. N2adsorption–desorption analysis was carried out at 77 K on a Micromeritics TriStar 3000 apparatus. Transmission electron microscopy (TEM) experiments were carried out on a JEM-100CXII transmission electron microscope with an accelerating voltage of 100 kV. Scanning electron microscopy (SEM) images were collected by employing a Quanta 200 scanning electron microscope operated at 30 kV. Oxidation products were analyzed by a gas chromatograph, which was equipped with a SGE AC10 capillary column, 0.25 mm in diameter and 30 m in length. The products were identified by checking the retention time with standard materials.
3 Results and Discussion
3.1 Characterization of V2O3nanoparticles
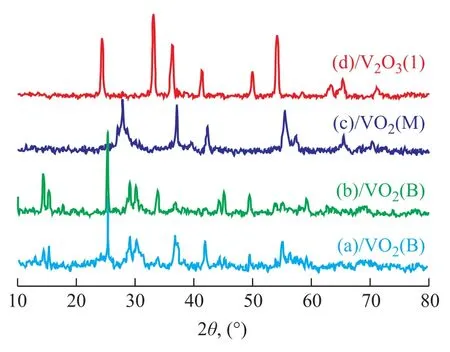
Figure 1 XRD patterns of vanadium oxides synthesized by using deionized water as the solvent and crystallized at different temperatures at a V2O5/H2C2O4molar ratio of 1∶3
Crystal structure of the as-synthesized V2O3samples prepared at different temperatures was characterized by wide-angle XRD as shown in Figure 1. V2O3(1) was obtained using deionized water as the solvent and was crystallized in the Ar atmosphere at 873 K. VO2(M) was obtained at 773 K and VO2(B) was obtained either without crystallization or through crystallization at 673 K. As shown in Figure 2, V2O3(2) was obtained using methanol as the solvent and was crystallized in the Ar atmosphere at 873 K. VO2(M) was obtained at 773 K and a mixture of VO2(B) and VO2(M) was obtained either without crystallization or through crystallization at 673 K. Figure 3 shows the XRD patterns of V2O3samples synthesized under dif-ferent experimental conditions. V2O3(3) was synthesized using methanol as the solvent and oxalic acid as the complexing agent (with the molar ratio of V2O5and oxalic acid equating to 1:1), and was crystallized in the Ar atmosphere at 673 K consequently. The addition of oxalic acid obviously decreased the reduction temperature upon comparing the conditions for synthesis of V2O3(3) with those of V2O3(2). Similarly, V2O3(4) was obtained at 673 K when the molar ratio of V2O5/oxalic acid was 1:2. However, the obtained product V2O3(5) was not pure when the V2O5/oxalic acid molar ratio was 1:3, because diffraction peaks of VO2(M) were identified, indicating that excessive amount of oxalic acid inhibited the reduction of V2O5to V2O3. Except for V2O3(5), all of the diffraction peaks can be assigned to the Karelianite phase of V2O3, which is consistent with the values given in the standard card (JCPDS no. 34-0187). No peaks of any other phases were detected, indicating to the excellent purity of the products. The XRD peaks are strong and narrow, indicating to the good crystallinity of the as-synthesized V2O3.The synthesized V2O3samples were characterized by the BET method using N2adsorption/desorption technique. As shown in Table 1 and Table 2, the BET surface area, pore size and pore volume of the synthesized V2O3samples increased with the increase in molar ratio of V2O5to H2C2O4in the methanol solvothermal systems. The obtained V2O3(4) sample showed a highest surface area of 48.7 m2/g. The morphology of the V2O3samples was examined by SEM. As shown in Figure 4, the synthesized V2O3seemed to be stacked with V2O3flakes. The size of V2O3flakes increased with an increasing amount of oxalic acid in the methanol solvothermal systems. The TEM image of V2O3(4) sample revealed that the size of V2O3nanoparticles was mostly around 10—30 nm as shown in Figure 5.
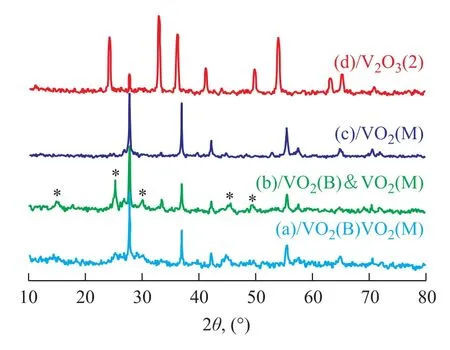
Figure 2 XRD patterns of samples synthesized by using methanol as the solvent and crystallized at different temperatures without oxalic acid
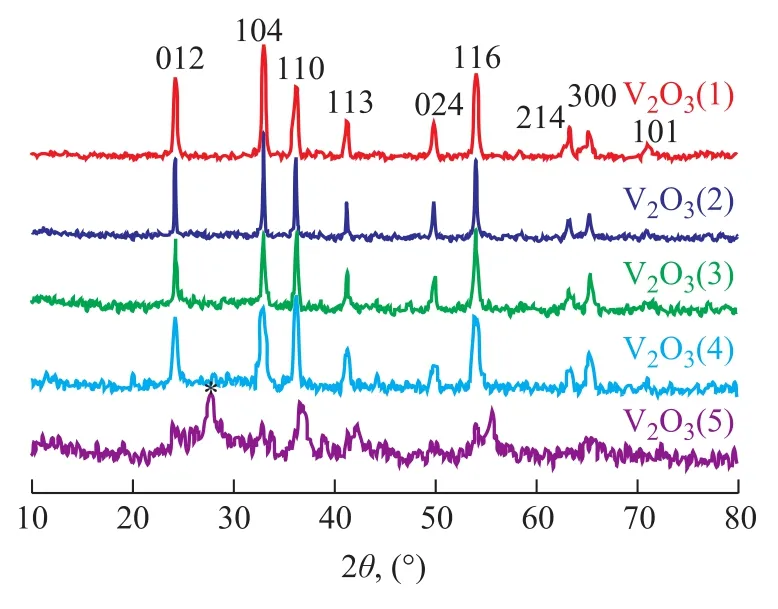
Figure 3 The XRD pattern of V2O3samples obtained under different experimental conditions
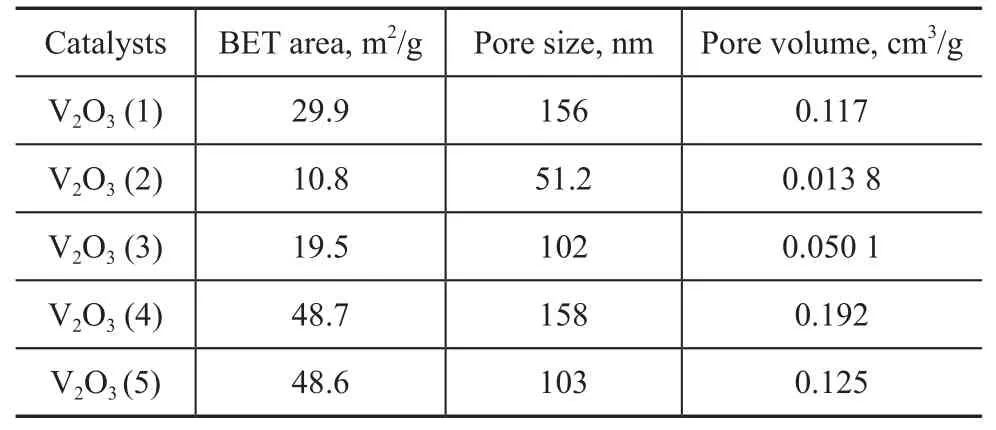
Table 2 Textural properties of these synthesized catalysts

Figure 4 SEM images of synthesized V2O3samples
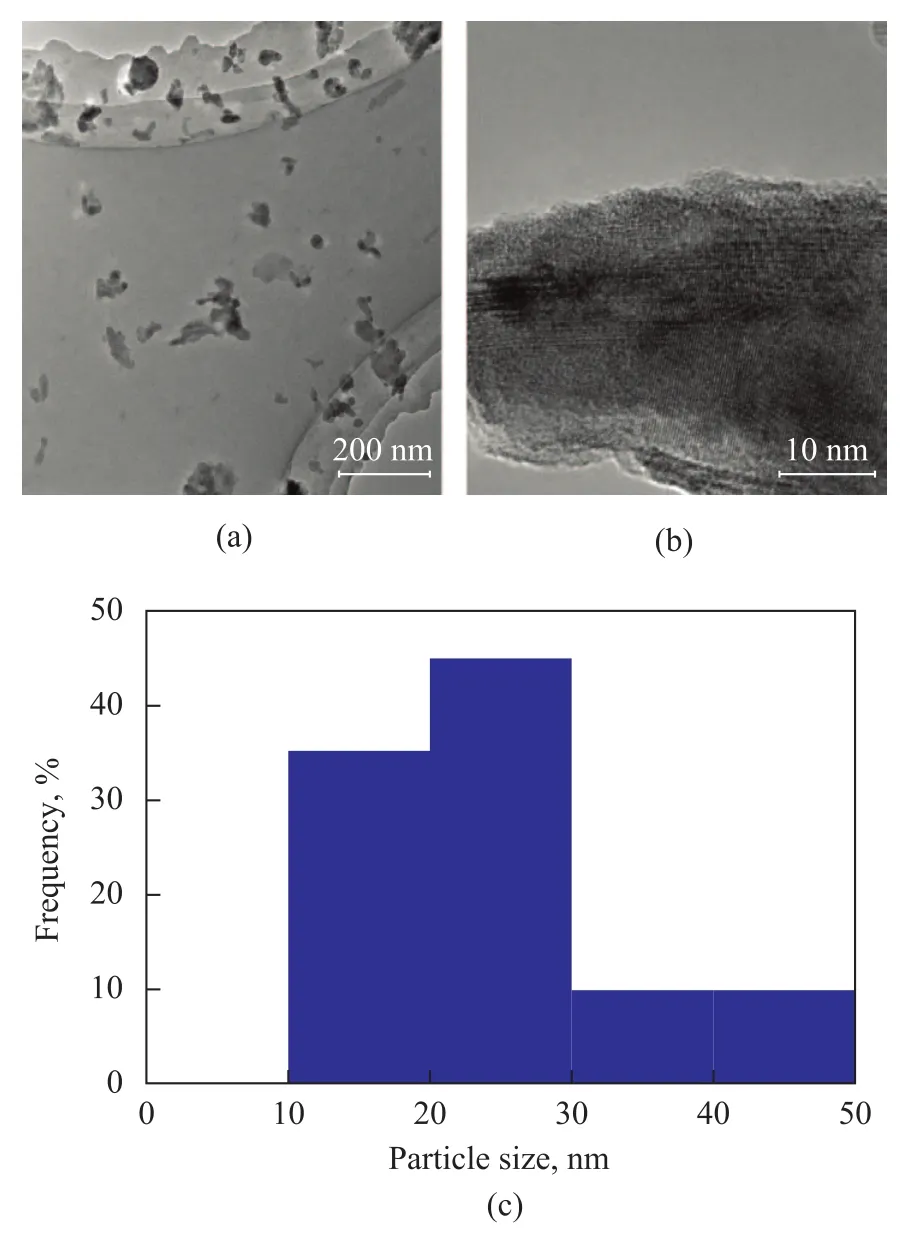
Figure 5 TEM images(a), HRTEM images(b) and particle size distribution graph(c) of V2O3(4) sample
3.2 Catalytic performance
The ODS activity of the synthesized V2O3catalysts is summarized in Figure 6. All V2O3catalysts showed high DBT conversion, which decreased at 343 K in the following order: V2O3(4)>V2O3(3)>V2O3(2)>V2O3(1)>V2O3(5). The activity of V2O3(4),V2O3(3) and V2O3(2) is obviously higher than that of V2O3(1). The reason is that they were synthesized by using different solvents as shown in Table 1. In addition, methanol as the solvent could significantly reduce the crystallization temperature upon comparing the synthesis of V2O3(1) with that of V2O3(4). As compared to the use of purified water, methanol used as the solvent has great advantages. In regard to V2O3(4), V2O3(3) and V2O3(2) catalysts, the ODS activity decreased with a decreasing surface area. The highest ODS activity of V2O3(4) sample can be attributed to its highest surface area. Despite the high surface area, V2O3(5) catalyst showed low ODS activity, which could be attributed to the existence of VO2(M). For methanol solvothermal system, the BET surface areas of synthesized V2O3catalysts increased with the addition of oxalic acid. Larger BET area leads to higher ODS catalytic activity. It is obvious that the appropriate molar ratio of V2O5to H2C2O4for the methanol solvothermal system is 1:2.
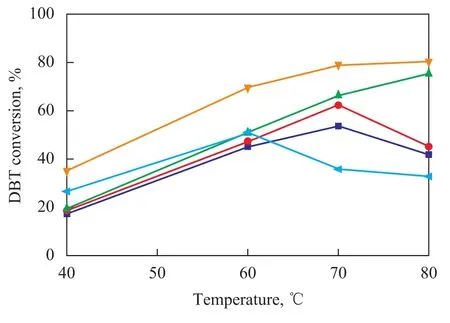
Figure 6 ODS of DBT under different temperature on V2O3catalysts
3.3 Reaction mechanism of V2O3catalyst
The chemical reactions involved in the methanol solvothermal processes are briefly proposed as follows:
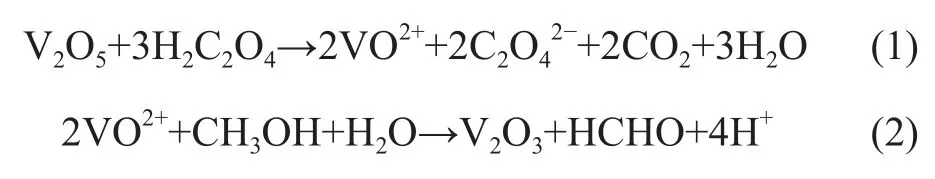
As described in Equation (1), VO2+with a characteristic sapphire color was produced by reducing vanadium pentaoxide with oxalic acid during the preparation of the precursor (VOC2O4), which was consistent with the result reported by Liu Xinghai[30]. Oxalic acid acts not only as a reducing reagent but also as a complexing reagent in the solvothermal process. VOC2O4was suggested to be formed in Eq. (1). It was reported[31]that hydroxide nanoflakes were synthesized by hydrothermal reaction with divalent cations (Ni2+, Co2+) and H2C2O4. The C2O42-ions acted as a complexing reagent in the formation of two-dimensional large plane and could control the reaction speed. Equation (2) was the key step for preparing V2O3. It was reported that ethanol can serve as the reducing reagent and aldehyde was detected in the solvothermal process[32].
4 Conclusions
In summary, a simple, safe, and facile method to obtain V2O3nanoparticles with diameters ranging from 10 nm to 30 nm by the solvothermal reduction of V2O5was studied.The obtained V2O3nanoparticles show very high catalytic activity in the oxidative desulfurization process. We believe that the V2O3nanoparticles catalyst would have a good application prospect in the oxidative desulfurization process.
Acknowledgment:This work was supported by the National Nature Science Foundation of China (21303088).
[1] Ito E, van Veen J A R. On novel processes for removing sulphur from refinery streams[J]. Catal Today, 2006, 116(4): 446-460
[2] Torres-García E, Galano A, Rodriguez-Gattorno G. Oxidative desulfurization (ODS) of organosulfur compounds catalyzed by peroxometallate complexes of WOx-ZrO2: Thermochemical, structural, and reactivity indexes analyses[J]. J Catal, 2011, 282(1): 201-208
[3] Rothlisberger A, Prins R. Intermediates in the hydrodesulfurization of 4,6-dimethyl-dibenzothiophene over Pd/ gamma-Al2O3[J]. J Catal, 2005, 235(1): 229-240
[4] Oyama S T, Zhao H, Freund H J, et al. Unprecedented selectivity to the direct desulfurization (DDS) pathway in a highly active FeNi bimetallic phosphide catalyst[J]. J Catal, 2012, 285(1): 1-5
[5] Jiang Z X, Liu Y, Sun X P, et al. Activated carbons chemically modified by concentrated H2SO4for the adsorption of the pollutants from wastewater and the dibenzothiophene from fuel oils[J]. Langmuir, 2003, 19(3): 731-736
[6] Shiraishi Y, Tachibana K, Hirai T, et al. Desulfurization and denitrogenation process for light oils based on chemical oxidation followed by liquid-liquid extraction[J]. Ind Eng Chem Res, 2002, 41(17): 4362-4375
[7] Lü H Y, Gao J B, Jiang Z X, et al. Ultra-deep desulfurization of diesel by selective oxidation with [C18H37N(CH3)3]4[H2NaPW10O36] catalyst assembled in emulsion droplets[J]. J Catal, 2006, 239(2): 369-375
[8] Villasenor F, Loera O, Campero A, et al. Oxidation of dibenzothiophene by laccase or hydrogen peroxide and deep desulfurization of diesel fuel by the latter[J]. Fuel Process Technol, 2004, 86(1): 49-59
[9] Otsuki S, Nonaka T, Takashima N, et al. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction[J]. Energy Fuels, 2000, 14(6): 1232-1239
[10] Zhang J, Wang A, Li X, et al. Oxidative desulfurization of dibenzothiophene and diesel over [Bmim]3PMo12O40[J]. J Catal, 2011, 279(2): 269-275
[11] Wang D H, Qian E W H, Amano H, et al. Oxidative desulfurization of fuel oil - Part I. Oxidation of dibenzothiophenes using tert-butyl hydroperoxide[J]. Appl Catal A, 2003, 253(1): 91-99
[12] Ishihara A, Wang D H, Dumeignil F, et al. Oxidative desulfurization and denitrogenation of a light gas oil using an oxidation/adsorption continuous flow process[J]. Appl Catal A, 2005, 279(1/2): 279-287
[13] Qian E W. Development of novel nonhydrogenation desulfurization process—Oxidative desulfurization of distillate[J]. J Jpn Pet Inst, 2008, 51(1): 14-31
[14] Chica A, Corma A, Domine M E. Catalytic oxidative desulfurization (ODS) of diesel fuel on a continuous fixedbed reactor[J]. J Catal, 2006, 224(2): 299-308
[15] Prassad V V D N, Jeong K, Chae H, et al. Oxidative desulfurization of 4,6-dimethyl dibenzothiophene and light cycle oil over supported molybdenum oxide catalysts[J]. Catal Commun, 2008, 9(10): 1966-1969
[16] Hulea V, Fajula F, Bousquet J. Mild oxidation with H2O2over Ti-containing molecular sieves - A very efficient method for removing aromatic sulfur compounds from fuels[J]. J Catal, 2001, 198(2): 179-186
[17] Gregori F, Nobili I, Bigi F, et al. Selective oxidation of sulfides to sulfoxides and sulfones using 30% aqueous hydrogen peroxide and silica-vanadia catalyst[J]. J Mol Catal A: Chem, 2008, 286(1/2): 124-127
[18] Long J, Zhu Y X, Liu Y J, et al. Effects of vanadium oxidation number on desulfurization performance of FCC catalyst[J]. Appl Catal A: Gen, 2005, 282(1/2): 295-301
[19] Caero L C, Hernandez E, Pedraza F, et al. Oxidative desulfurization of synthetic diesel using supported catalysts-Part I. Study of the operation conditions with a vanadium oxide based catalyst[J]. Catal Today, 2005, 107-108: 564-569
[20] Caero L C, Navarro J F, Gutierrez-Alejandre A. Oxidative desulfurization of synthetic diesel using supported catalysts: Part II. Effect of oxidant and nitrogen-compounds on extraction-oxidation process[J]. Catal Today, 2006, 116(4): 562-568
[21] Cede-no-Caero L, Gomez-Bernal H, Fraustro-Cuevas A, et al. Oxidative desulfurization of synthetic diesel using supported catalysts - Part III. Support effect on vanadiumbased catalysts[J]. Catal Today, 2008, 133: 244-254
[22] Qazilbash M, Schafgans A, Burch K, et al. Electrodynam-ics of the vanadium oxides VO2and V2O3[J]. Physical Review B, 2008, 77(11): 115
[23] Piccirillo C, Binions R, Parkin I P. Synthesis and functional properties of vanadium oxides: V2O3, VO2, and V2O5deposited on glass by aerosol-assisted CVD[J]. Chemical Vapor Deposition, 2007, 13(4): 145-151
[24] Sass B, Tusche C, Felsch W, et al. Structural and electronic properties of epitaxial V2O3thin films[J]. Journal of Physics: Condensed Matter, 2004, 16(1): 77-87
[25] Piao J, Takahashi S, Kohiki S. Preparation and characterization of V2O3powder and film[J]. Japanese Journal of Applied Physics: Part 1, 1998, 37(12A): 6519-6523
[26] Teghil R, D’Alessio L, De Bonis A, et al. Nanoparticles and thin film formation in ultrashort pulsed laser deposition of vanadium oxide[J]. Journal of Physical Chemistry A, 2009, 113(52): 14969-14974
[27] Kam K C, Cheetham A K. Thermochromic VO2nanorods and other vanadium oxides nanostructures[J]. Materials Research Bulletin, 2006, 41(5): 1015-1021
[28] Popuri S R, Miclau M, Artemenko A, et al. Rapid Hydrothermal Synthesis of VO2(B) and Its Conversion to Thermochromic VO2(M1)[J]. Inorg Chem, 2013, 52(9): 4780-4785
[29] Li Ming, Li Dengbing, Pan Jing, et al. Selective Synthesis of Vanadium Oxides and Investigation of the Thermochromic Properties of VO2by Infrared Spectroscopy[J]. Eur J Inorg Chem, 2013, (7): 1207-1212
[30] Liu Xinghai, Zhang Yifu, Yi Shengping, et al. Preparation of V2O3nanopowders by supercritical fluid reduction[J]. Journal of Supercritical Fluids, 2011, 56: 194-200
[31] Li Xiaolin, Liu Junfeng, Li Yadong. Low-temperature conversion synthesis of M(OH)2(M = Ni, Co, Fe) nanoflakes and nanorods[J]. Materials Chemistry and Physics, 2003, 80(1): 222-227
[32] Liu X, Xie G, Huang C, et al. A facile method for preparing VO2nanobelts[J]. Materials Letters, 2008, 62(12/13): 1878-1880
Received date: 2014-03-24; Accepted date: 2014-08-05.
Dr. Wang Danhong, Telephone: +86-22-23507730; E-mail: dhwang@nankai.edu.cn.
- 中國煉油與石油化工的其它文章
- Identification and Characterization of Sulfur Compounds in Straight-Run Diesel Using Comprehensive Two-Dimensional GC Coupled with TOF MS
- Effects of Promoters on the Ignition Process over NiO/Al2O3Catalyst for Autothermal Reforming of Methane to Hydrogen
- Oligomerization of 1-Decene: Catalyzation by Immobilized AlCl3/γ-Al2O3Catalyst in Fixed-bed Reactor
- Study on Tribological Properties of CVT Fluid Containing Inert and Active Functional Elements
- Application of Modified Attapulgite Clay as the Adsorbent in Gasoline Desulfurization
- Study on the Influence of Ni Modifying on Phase Transformation and Photocatalytic Activity of TiO2

