Identification and Characterization of Sulfur Compounds in Straight-Run Diesel Using Comprehensive Two-Dimensional GC Coupled with TOF MS
Niu Luna; Liu Zelong; Tian Songbai
(Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
Identification and Characterization of Sulfur Compounds in Straight-Run Diesel Using Comprehensive Two-Dimensional GC Coupled with TOF MS
Niu Luna; Liu Zelong; Tian Songbai
(Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
The solid-phase extraction using Pd-Al2O3as the stationary phase was employed to pre-separate the sulfur compounds in straight-run diesel. The isolating effect was evaluated quantitatively by gas chromatography with a sulfur chemiluminescence detector to harvest a satisfactory result. The identification of the structure of sulfur compounds by comprehensive two-dimensional gas chromatography coupled with the time-of-flight mass spectrometry indicated that cyclo-sulfides, benzothiophenes, dibenzothiophenes, dihydro-benzothiophenes and tetrahydro-dibenzothiophenes were included in straightrun diesel obtained from the Arab medium crude (AM). A total of 259 individual compounds were detected and their molecular structures were identified. The analytical method was approved as an effective way to characterize the composition of sulfur compounds, which reduced the interference of other compounds, facilitated the data presentation and provided more detailed information about molecular composition of sulfur compounds.
sulfur compounds; straight-run diesel; solid-phase extraction (SPE); comprehensive two-dimensional gas chromatography coupled with the time-of-flight mass spectrometry (GC×GC-TOF MS)
1 Introduction
In order to improve the efficiency of industrial processing and produce high quality low-sulfur diesel products, demanded by the environmental protection regulations, the knowledge of their origin and detailed molecular structure is needed. Currently, the organic sulfur compounds which exist in the form of aliphatic or aromatic thiols, sulfides and sulfur heterocycles have been proved to be more difficult to remove than inorganic sulfur compounds[1]. Many researchers have investigated in analyzing the components and distribution of organic sulfur compounds, especially polycyclic aromatic sulfur heterocycles (PASHs), but are less concentrating on the detailed characterization and identification such as analysis of molecular structure of sulfur compounds[2-5].
Among the many analytical techniques, gas chromatography (GC) coupled with the selective detector or mass spectrometry (MS) are extensively utilized in separating and characterizing sulfur compounds in diesel samples[6-13]. In detail, sulfur chemiluminescence detector (SCD) is selective and sensitive for sulfur response, which is valuable for analysis of sulfur-containing compounds. Gas chromatography-mass spectrometry (GCMS) is fundamental in qualitative analysis, but it shows the drawback that all compounds with the samem/zratio can interfere with identification and quantification of PASHs. Comprehensive two-dimensional gas chromatography coupled with the time-of-flight mass spectrometry (GC×GC-TOF MS) can provide more analytical information than GC-MS about the large number of complex sulfur compounds thanks to its higher peak capacity and resolution[14]. Light petroleum fractions like kerosene and diesel are usually subjected to the direct injection analysis without pre-separation to date[15-16]. However, diesel contains not only organic sulfur compounds but also polycyclic aromatic hydrocarbons (PAHs)[17]. PASHs and PAHs have similar physico-chemical properties and cannot be separated effectively by conventional analytical instruments[18]. Moreover, PASHs are in low concentration, and are prone to be interfered by PAHs, leading to inaccuracy of quali-tative and quantitative results. In other words, separating PASHs from aromatic fraction before analysis to reduce the complexity of sample is quite necessary. Among various kinds of separation methods, chromatography is widely used because its procedure is relatively easy and effective[19]. Studies have indicated that some metal ions with vacant orbitals have strong interaction with the electron cloud of sulfur atom, which is called π-complexation. It is much easier for aromatic sulfur compounds to form π-complexation than for aromatic hydrocarbons. Recent studies have noted that ligand exchange chromatography with a stationary phase containing Pd (II)-complex had been proved to be available for the separation process[20-21]. However, this method may consume large amount of solvents and Pd (II)-complex stationary phase, as well as operating time. Solid phase extraction, as a newly developed efficient separation technique, has been deduced in pretreatment of diesel fraction[22-23].
In this paper, separation of PASHs and PAHs from diesel fraction via the Pd (II) solid phase extraction (Pd2+-SPE) was introduced. The gas chromatography-flame ionization detector-sulfur chemiluminescence detector (GC-FIDSCD) and GC×GC-TOF MS were applied to characterize the PASHs fraction. By combining the solid phase extraction with the selective detector and two-dimensional gas chromatography, a sulfur-containing sample with little interference by other compounds and more detailed information at the molecular level were obtained.
2 Experimental
2.1 Materials
Raw material used in the experiments was straight-run diesel (180—350 ℃) with a total sulfur content of 0.98%, derived from Arab medium crude (AM).
2.2 Methods
2.2.1 Synthesis of Pd-Al2O3
Neutral alumina (dried at 550 ℃ for 5h) was mixed completely with a palladium chloride solution and kept still for 12 h in order to facilitate uniform distribution on the surface of alumina, then the product was dried at 150 ℃for 5 h to obtain the Pd-Al2O3(with a Pd loading of 5%). This stationary phase was kept in a dryer.
2.2.2 Separation process
2 grams of diesel sample was fractionated into saturates and aromatics by column adsorption chromatography on silica gel (which was heated at 150 ℃ for 5 h). The saturates were eluted with 70 mL ofn-pentane and the aromatics with 70 mL of dichloromethane and then 50 mL of mixed dichloromethane/ethanol (5:1 v/v). Then the aromatic fraction was recovered after evaporation of the solvent. Approximately 3 g of the Pd-Al2O3stationary phase was introduced into a solid phase extraction cartridge. 3 mL ofn-pentane were used to rinse the stationary phase and 20 μL of aromatics, which had been separated from the diesel sample, was added from the top of the cartridge. Thereafter, the PAHs fraction (A1) was subsequently eluted with 8 mL of mixedn-pentane/dichloromethane (95:5 v:v) and 4 mL of mixedn-pentane/dichloromethane (1:1 v:v), then 3 mL of dichloromethane was applied for solvent substitution. The PASHs fraction (A2) was washed with 15 mL of dichloromethane, and 10 mL of mixed dichloromethane/ethanol (5:1 v:v) were used to guarantee the recovery of PASHs. This process inevitably led to a small amount of Pd-PASH complexes to be eluted. However, a small amount of diethylamine could be added to replace the PASHs and form a strong ligand with Pd. The solvent volume was reduced by evaporation before analysis. 1 mL of 3-n-octadecylthiophene was used as the internal standard. The two separated fractions were analyzed by GC-FID-SCD and GC-MS to determine the isolating effect and the detailed characterization of PASHs was performed by GC×GC-TOF MS.
2.3 Instrumental conditions
2.3.1 GC-MS
An Agilent 7890A gas chromatograph provided with a 5975 type mass spectrometry detector was used. It was equipped with a DB-5MS column (manufactured by Agilent Technologies, 30 m×0.25 mm×0.25 μm). The initial GC oven temperature was maintained at 60℃ for 2 min and then increased at a heating rate of 2 ℃/min to 300 ℃, which was maintained for 4 min. 1 μL of sample was injected into the instrument operating at 300 ℃ in the splitless mode. The ion source temperature of MS was maintained at 220 ℃ with an ionizing energy of 70 eV.
2.3.2 GC-FID-SCD
An Agilent 7890A gas chromatograph coupled with a FIDand a Sievers 355 SCD was used. It was equipped with a HP-5 column (manufactured by Agilent Technologies, 30 m×0.32 mm×0.25 μm). The GC oven temperature was initially maintained at 60 ℃ for 2 min and then increased at a heating rate of 2 ℃/min to 300 ℃, which was maintained for 4 min. 1 μL of sample was injected into the instrument operating at 300 ℃ in the split mode (5:1). The SCD burner temperature was 800 ℃ and the pressure in the reaction cell was 8 torr.
2.3.3 GC×GC-TOF MS
A GC×GC-TOF MS Pegasus 4D system manufactured by the LECO Corporation was used. It was equipped with a HP-PONA column (manufactured by Agilent Technologies, 50 m×0.20 mm×0.50 μm) in the first dimension and a BPX50 column (manufactured by SGE Analytical Science, 1.2 m×0.1 mm×0.10 μm) in the second dimension. The temperature program of the first column was maintained at 100 ℃ for 1 min and then increased at a heating rate of 1.5 ℃/min to a final temperature of 280 ℃, which was maintained for 5 min. The secondary oven temperature offset was 5 ℃. The carrier gas was helium which was introduced at a constant flow rate of 1.5 mL/min and the volume of sample injected was 1 μL in the splitless mode. The electron impact ionization source was operated at 250 ℃ with an ionizing energy of 70 eV. The detector voltage was 1 650 V and the mass range was 35—500 amu at an 100 Hz data acquisition rate. Data processing was achieved by using the LECO Chroma TOF 4.0 software.
The confirmation of compounds was made by comparing the mass spectra between the unknown spectrum and the NIST library spectrum. The well-structured chromatogram with a superior structural effect in the separation space and roof-tile effect was employed to achieve tentative identif ication of compounds. The retention data of standard compounds were also used to identify a part of isomers.
3 Results and Discussion
3.1 Isolating effect for PASHs
The efficiency of Pd (II) solid phase extraction in separating PAHs and PASHs was evaluated by GC-FID-SCD. Comparison of chromatograms detected by FID (or MS) and sulfur-selective detector (e.g. SCD, flame photometric detector or atomic emission detector) was an universal method to judge the efficiency of separation, but no attempt was mentioned on describing the overlap of PAHs and PASHs quantitatively[24-26]. For corroboration of the separation efficiency, the SCD and FID were used to quantify the proportion of sulfur compounds eluted from each fraction. The quantitative analysis was performed by adding 1 mL of 3-n-octadecylthiophene serving as the internal standard to the separated fractions. Calculation of the concentration of each compound was based on the area of internal standard. Previous study indicated that the SCD connected with the GC-FID response value for sulfur compounds was independent of molecular structure[27], thus the total content of sulfur compounds could be determined by integrating all peaks while excluding that of the internal standard in the GC-SCD chromatogram. Similarly, peaks in GC-FID chromatogram were used to calculate the content of all hydrocarbons in the corresponding fraction. The proportion of sulfur compounds in each fraction was obtained by comparing the SCD and FID quantification results.
Since the sulfur chemiluminescence detector has the equal-mole response, it is necessary to identify peaks beforehand according to retention times through comparison between standard compounds and GC-MS. Figure 1 and Table 1 summarize the identification and proportion of different classes of sulfur compounds via comparing the retention time. Only 5.93% of sulfur compounds are contained in PAHs fraction and 97.98% in PASHs, respectively, indicating that the separating effect is desirable. Based on the above results, it is desirable to separate PAHs and PASHs by the Pd (II) solid phase extraction for achieving more accurate qualitative and quantitative analyses. The relatively pure and simple PASHs sample was used for detailed analysis by GC×GC-TOF MS.
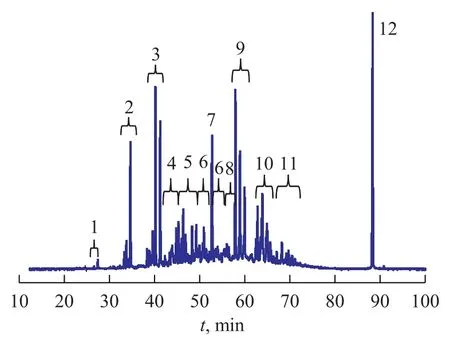
Figure 1 Gas chromatogram with SCD detection and identification of PASHs in straight-run diesel sample
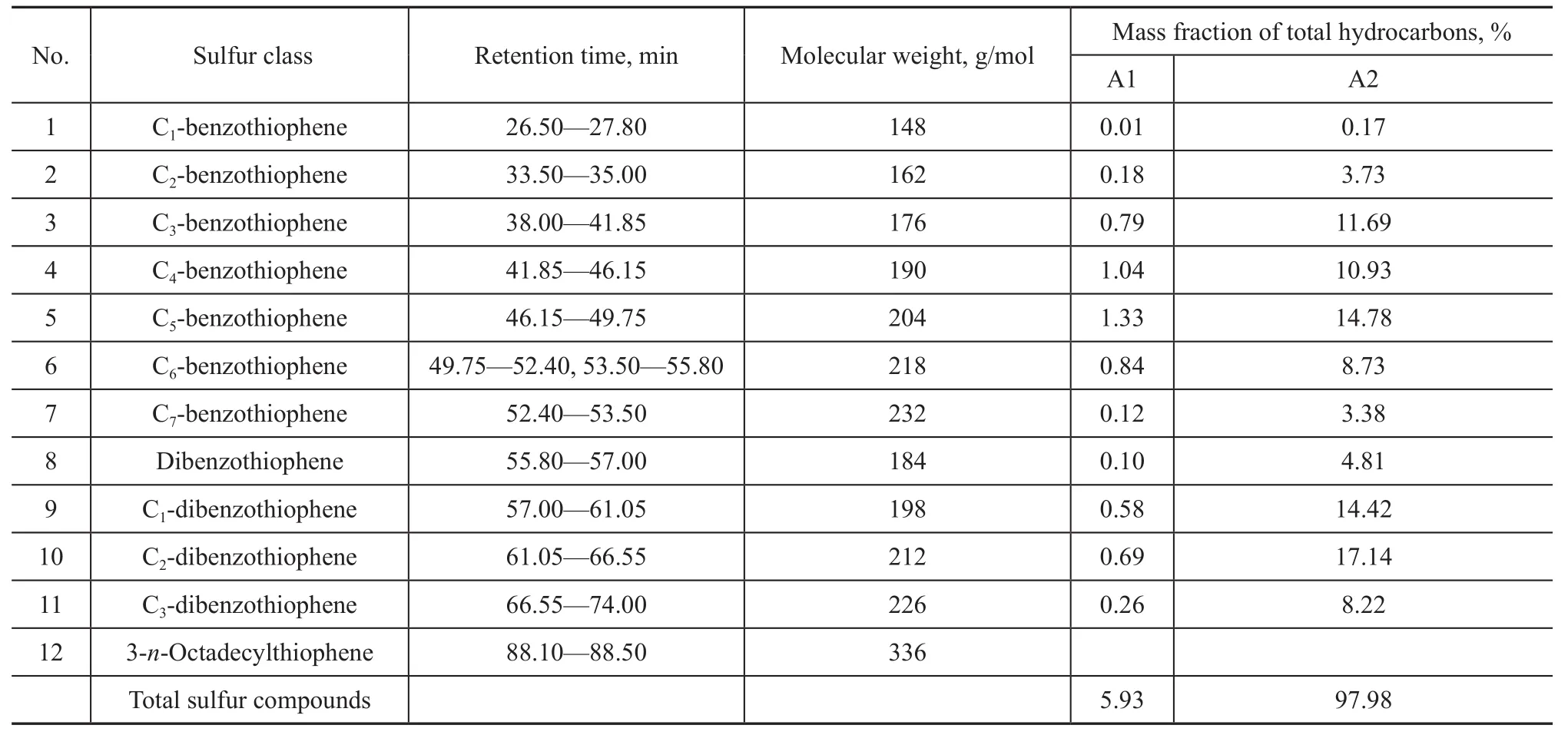
Table 1 Sulfur compounds in AM straight-run diesel sample belonging to different classes as defined by the retention time
3.2 Qualitative analysis of PASHs fraction
The Pd (II) solid phase extraction for pre-separating the PASHs from diesel has reduced the interference originated from PAHs to a large extent. However, it was reported that a large number of alkylated aromatic sulfur species might be co-eluted when using one-dimensional gas chromatography (1D-GC), especially when treating aromatic sulfur species with high carbon numbers[28]. Thanks to the increased peak capacity, greater sensitivity and the organized arrangement of chromatographic procedure, GC×GC was utilized for the purpose of avoiding co-elution problem.
Figure 2 is the two dimensional contour plot of PASHs, presenting a well-arranged color plot with clear roof-tile effect and structural effect. Each black dot represents a compound with a signal to noise ratio (S/N) of 50 and the total number of sulfur compounds detected was 259. The whole chromatogram was divided into five parts which are identified as five different classes, viz.: cyclo-sulfides (CS), dihydro-benzothiophenes (DH-BT), benzothiophenes (BT), tetrahydro-dibenzothiophenes (TH-DBT), and dibenzothiophenes (DBT). It was worth mentioning that the sub-class alkyl substituents are identified by detailed analysis of mass spectra and roof-tile effect.
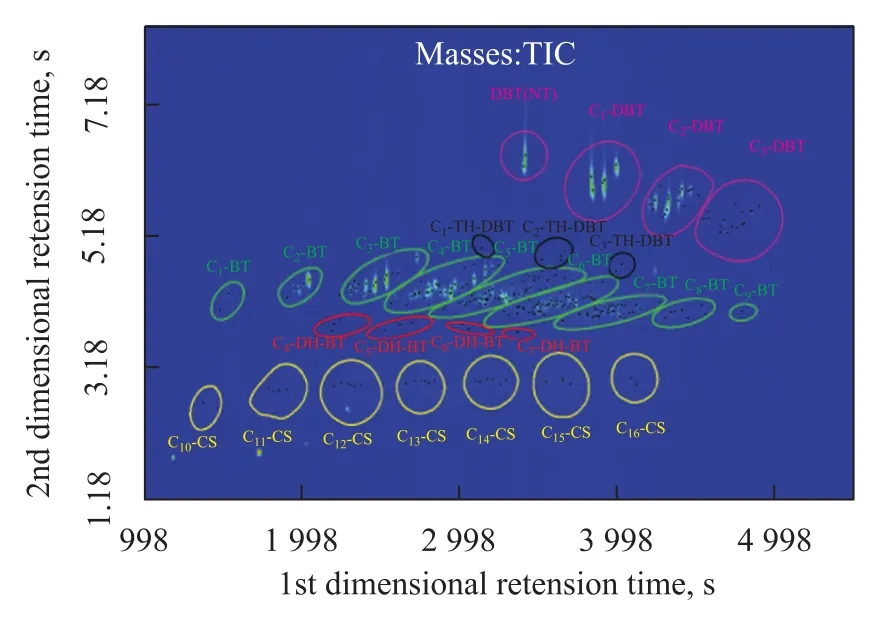
Figure 2 Two-dimensional contour plot of PASHs fraction obtained after Pd2+-SPE
The original aromatics sample including PAHs and PASHs was also analyzed by repeating the same instrumental conditions and the chromatogram is shown in Figure 3 (a). Even though thousands of chromatogram peaks were well ordered, the number of compounds was too large and compounds of different classes may cluster in the same region, leading to a more laborious and time-consuming analytical procedure. On the other hand, some compounds might be co-eluted in the first dimension and they might be separated by extra selectivity provided by the second dimension (taking C1-dibenzothiophene and C1-phenanthrene as an example, as shown in Figure 3 (b) ). These two comparison results confirmed the importance of preseparation and necessity of using GC×GC.
3.2.1 Cyclo-sulfides
Sulfur compounds in straight-run diesel consist of not only BT and DBT families, but also a substantial amountof sulfides[29]. Due to the limitation of one dimensional gas chromatography and low concentration of sulfides, very small amount of sulfides has been characterized. In this work, some cyclo-sulfides were detected successfully. This kind of sulfur compounds had a lower polarity and a shorter retention time in the second dimension.
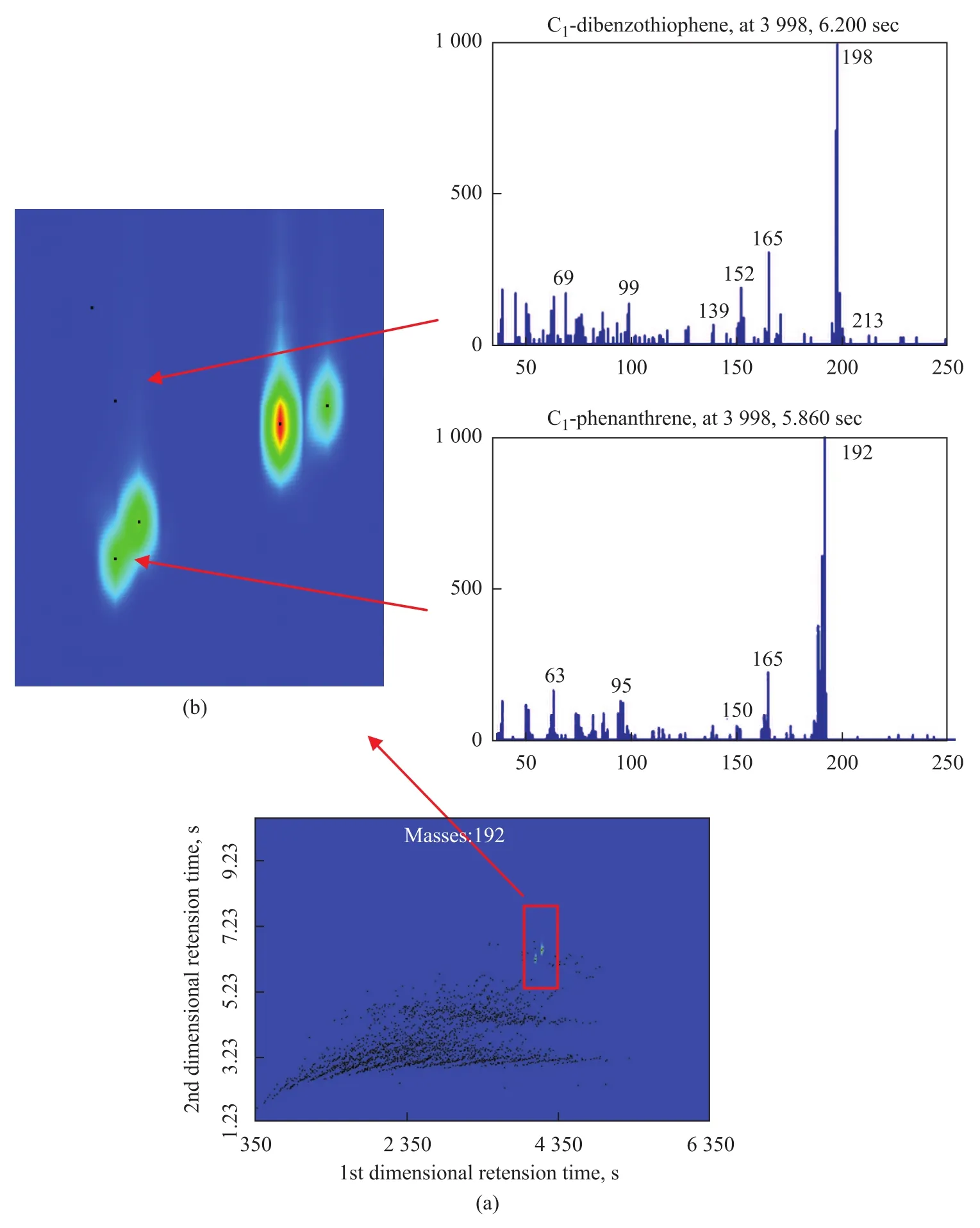
Figure 3 (a) Two-dimensional contour plot of aromatics fraction; (b) co-elution of C1-dibenzothiophene and C1-phenanthrene in the first dimension
Tetrahydrothiopyran and tetrahydrothiophene with alkyl substitution are the common type of cyclo-sulfides. Long side chain attached to the tetrahydrothiophene ring can be cleaved easily andm/z87 is the basepeak in mass spectra. When there is other alkyl substituents, the basepeak becomesm/z101+14n(n=0,1,2……) instead. Alkyltetrahydrothiopyrans present main ionization fragments withm/z101+14n(n=1,2,3……), causing difficulty in distinguishing these two classes. It was reported that tetrahydrothiopyran with two alkyl substituents was the main structure of this kind[30]. Since the largest alkyl radical followed the principle of being detached first of all, the alkyl group attached to tetrahydrothiopyran ring could be inferred tentatively through observing the basepeak in mass spectra. Thus, according to mass spectra together with the roof-tile effect, 29 cyclo-sulfides ranging fromC10to C16were identified in the PASHs fraction obtained after fractionation of AM-derived straight-run diesel with the Pd (II) stationary phase (Figure 4).
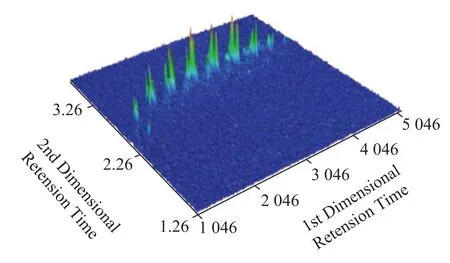
Figure 4 The chromatogram of cyclo-sulfides
3.2.2 Benzothiophenes and dibenzothiophenes
Some well-known troublesome sulfur compounds, such as BTs and DBTs, were also identified. The reason why they are recalcitrant seemed to be the difficulty in desulfurizing these compounds, especially alkyl dibenzothiophene in which the sulfur atom was condensed with benzo rings and shielded by alkyl substituents[31].
Selectingm/z134+14n(n=0,1,2……) as the characteristic ion fragments of BT family and 189 compounds were identified (peaks in Figure 5). The subclass covered from C1-BT to C9-BT, indicating to a wide carbon distribution. It seemed that the parent benzothiophene either was not contained in this diesel sample or had low concentration which could not be detected. One out of the two C1-BT isomers was identified as 3-methyl benzothiophene upon comparing with the standard compound. C2-BTs had the characteristic molecular ion fragment with a mass of 162. Two out of five isomers visible in the chromatogram were ethyl benzothiophene and the other three were dimethyl benzothiophene. Distinction between these different substituents was based on the characteristics of ion fragments in the mass spectra. The same method was applied to the identification of C3-BTs. The mass spectra of trimethyl benzothiophene (TriMe-BT), ethyl methyl benzothiophene (EtMe-BT) and propyl benzothiophene (Prop-BT) showed a molecular ion of mass equating to 176, but the intensity was not the same. TriMe-BT hadm/z176 as the basepeak and EtMe-BT showed a prominentm/z161, which indicates a loss of methyl group, whilem/z147 was ascribed to the basepeak when it came to Prop-BT, which was probably resulted from a loss of ethyl group. Thus, seventeen out of 62 possible isomers for this subclass were tentatively identified in straight-run diesel sample derived from AM. Due to the dramatic number of isomers which had similar mass spectra and lacked commercially available standard compounds, it was almost impossible to identify every compound for higher homologues of BT family. Some of benzothiophene compounds identified thereby are listed in Table 2.
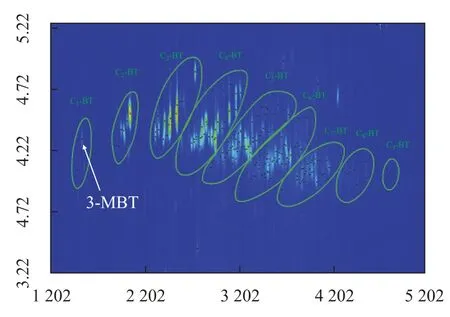
Figure 5 The chromatogram of benzothiophenes
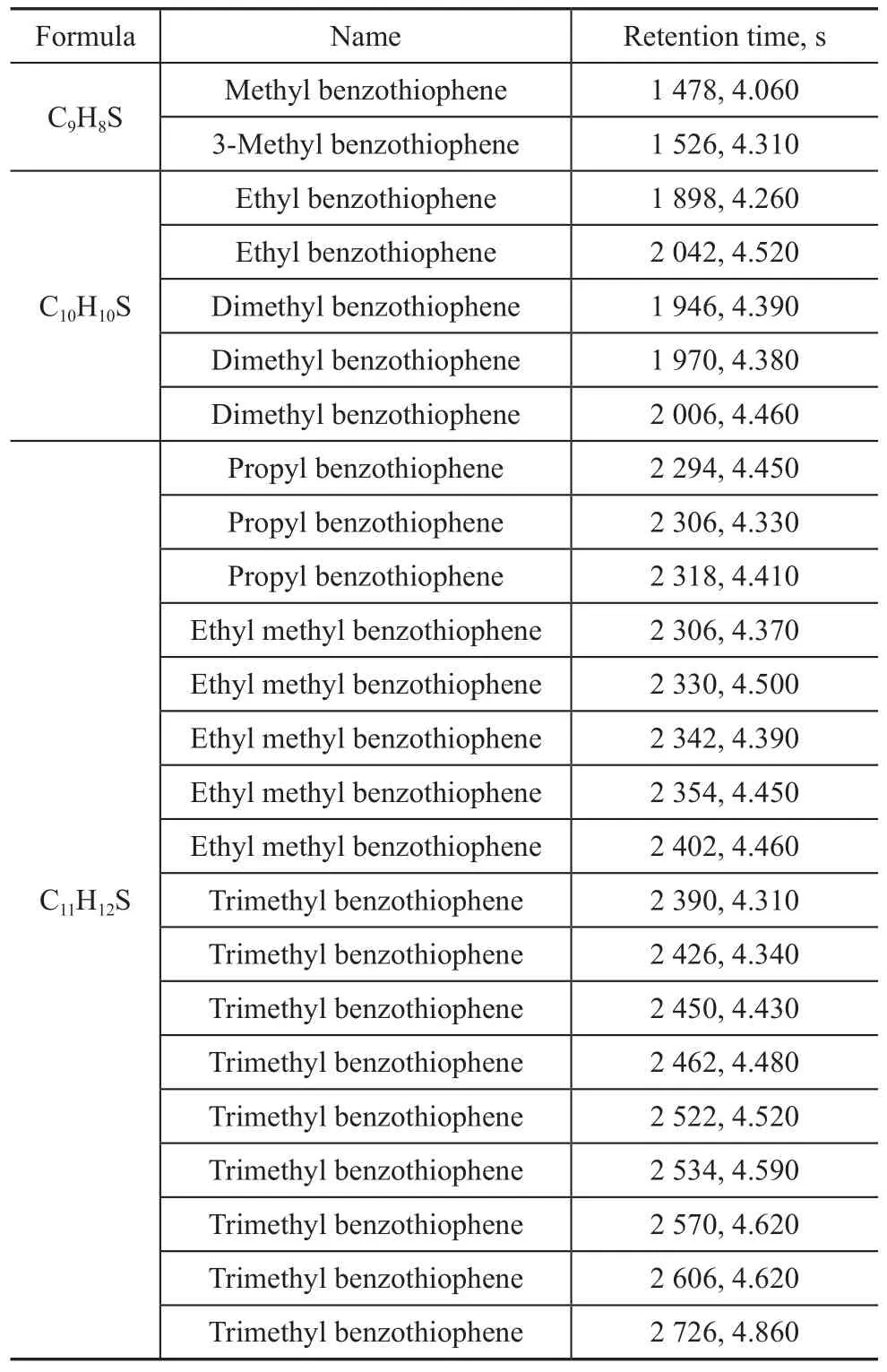
Table 2 Results of qualitatively identified benzothiophene compounds
Selectingm/z184+14n(n=0,1,2……) as the characteristic ion fragments of DBT family and 40 compounds ranging from C0-DBT to C3-DBT were identified (peaks in Figure 6). The parent dibenzothiophene was recognized by the standard compound dibenzothiophene. Another peak appeared in the same roof-tile was naphthothiophene (NT) that was less symmetric than dibenzothiophene, which showed a higher polarity and could be distinguished through the second dimensional separation. According to Machado and Caram?o[28], there was co-elution problem with DBT and NT in one dimensional gas chromatography although their mass spectra were similar, causing difficulty in the analytical process. In this paper, this problem was solved by the satisfactory result provided by GC×GC.
It can be seen from Figure 6 that all four C1-DBT isomers are arranged regularly in one slant line and one C1-NT compound has a relatively longer second dimensional retention time. Four C1-DBT peaks are regarded as 4-MeDBT, 2-MeDBT, 3-MeDBT and 1-MeDBT. In contrast, 2- and 3-MeDBT have similar retention time which could not be resolved in 1D-GC[32]. Among these four peaks, 3-MeDBT posesses the highest concentration.
Among all alkylated dibenzothiophene compounds, C2-DBTs are of particular interest for the hydrodesulfurization process. There are 20 possible C2-DBT isomers among which 15 have been detected. With the help of mass spectra, standard compounds and literature information[18,33], it is possible to tell the detailed molecular structure of each peak. C2-DBTs have the characteristic ion ofm/z212, and two types of isomers (dimethyl dibenzothiophene and ethyl dibenzothiophene) can be differentiated which havem/z212 and 197 as the basepeak, respectively. Depauw and Froment[32]indicated that the isomers with alkyl substituents next to sulfur atom had lower vapor pressure but two adjacent alkyl substituents could increase the elution time. García and Becchi[33]proposed correlations to predict the retention time of alkyl-DBT based on these influential facts. According to the correlations, all 15 compounds were identified. The retention time of 4,6-dimethyl dibenzothiophene and 2,8-dimethyl dibenzothiophene obtained thereby could match with that of standard compounds. Qualitative analytical results are shown in Table 3. Above all, a compound called thioxanthene in the C2-DBT region was found, which was seldom reported before.
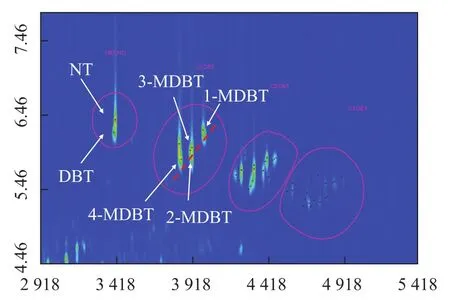
Figure 6 The chromatogram of dibenzothiophenes
C3+-DBTs might also be the hot issue in further work because some of these isomers were relatively prominent in amount after deeper hydrodesulfurization, especially isomers with substituents at the 1, 4 and/or 6 positions[8]. There were 64 possible C3-DBT isomers but only 17 were detected. What is worse, the commercial standard compounds were limited and the mass spectra of isomers were similar to a large extent, leading to difficulty in characterizing their accurate molecular structure.
3.2.3 Dihydro-benzothiophenes and tetrahydrodibenzothiophenes
These two groups of sulfur compounds have not been studied extensively to date. Selectingm/z163 as thecharacteristic ion of dihydrobenzothiophenes (DH-BT), it is possible to identify nine alkyl DH-BTs. The subclass covered from C4-DH-BT to C7-DH-BT, which had shorter retention time at the second dimension than benzothiophene groups. However, they were easily misclassifid into BT family since Cn-DH-BT and Cn-1-BT cluster closely and could be wrapped around in the same roof-tile. The same happened with tetrahydrodibenzothiophenes (TH-DBT) and DBTs. Seven compounds of TH-DBT family ranging from C1-THDBT to C3-TH-DBT were tentatively identified.
4 Conclusions
The Pd (II) solid phase extraction was employed to separate the polycyclic aromatic sulfur heterocycles from straight-run diesel where sulfur content is relatively high. It was proved to be a suitable method and a much purer sulfur-containing sample was obtained. Furthermore, a detailed qualitative study of sulfur compounds at molecular level using GC×GC-TOF MS was introduced. GC×GC-TOF MS showed superior advantages than GCMS, thanks to its higher peak capacity, better sensitivity and regular chromatograms. A total of 259 individual polycyclic aromatic sulfur heterocycles were detected in the AM-derived straight-run diesel with a S/N ratio of 50. It could be divided into five classes and each compound had been identified and determined.
This technique was approved as an effective way to characterize the composition of PASHs, which reduced the interference of other compounds and facilitated the data presentation of PASHs’ molecular composition. The detailed information obtained could be applied as a potential tool for optimizing the hydrodesulfurization process, as well as for guiding the development of new desulfurization catalysts.
Acknowledgement:This work was financially supported by the National Basic Research Program of China (973 Program) (2012CB224800).
[1] Borah D, Baruah M K, Haque I. Desulphurisation of organic sulphur by hydrogen peroxide in presence of metal ions[J]. Fuel, 2001, 80(10): 1475-1488
[2] Wang Z, Yang Y T. Distribution and change patterns of sulfur compounds in diesel oils[J]. Analytical Instrumentation, 2010(1): 70-73
[3] Pan N, Shi Q, Xu C M, et al. Synthesis and characterization of methylsulfonium salt in diesel fraction using electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry[J]. Chinese Journal of Analytical Chemistry. 2010, 38(3): 413-416 (in Chinese)
[4] Yang Y T, Wang Z, Yang H Y, et al. Determination of sulfur compounds in diesel oil by gas chromatography-flame ionization detector-sulfur chemiluminescence detector and data comparison of sulfur compounds by sulfur chemiluminescence detector and atomic emission detector[J]. Chinese Journal of Analytical Chemistry, 2005, 33(11): 1517-1521 (in Chinese)
[5] Panda S K, Schader W, Hajji A A, et al. Distribution of polycyclic aromatic sulfur heterocycles in three Saudi Arabian crude oils as determined by Fourier transform ion cyclotron resonance mass spectrometry[J]. Energy & Fuels, 2007, 21: 1071-1077
[6] Hua R, Li Y, Liu W, et al. Determination of sulfur-containing compounds in diesel oils by comprehensive twodimensional gas chromatography with a sulfur chemiluminescence detector[J]. Journal of Chromatography, A: 2003, 1019(1/2): 101-109
[7] Wang F C, Robbins W K, Di Sanzo F P, et al. Speciation of sulfur-containing compounds in diesel by comprehensive two-dimensional gas chromatography[J]. Journal of Chromatographic Science, 2003, 41: 519-523
[8] Schade T, Andersson J T. Speciation of alkylated dibenzothiophenes in a deeply desulfurized diesel fuel[J]. Energy & Fuels, 2006, 20: 1614-1620
[9] Lee I C, Ubanyionwu H C. Determination of sulfur contaminants in military jet fuels[J]. Fuel, 2008, 87(3): 312-318
[10] Chawla B, Di Sanzo F. Determination of sulfur components in light petroleum streams by high-resolution gas chromatography with chemiluminescence detection[J]. Journal of Chromatography A, 1992, 589(1/2): 271-279
[11] Link D D, Zandhuis P. The distribution of sulfur compounds in hydrotreated jet fuels: Implications for obtaining low-sulfur petroleum fractions[J]. Fuel, 2006, 85: 451-455
[12] Shi Q, Xu C M, Zhao S Q, et al. Characterization of heteroatoms in residue fluid catalytic cracking (RFCC) diesel by gas chromatography and mass spectrometry[J]. Energy & Fuels, 2009, 23, 6062-6069
[13] Yan X W. Sulfur and nitrogen chemiluminescence detection in gas chromatographic analysis[J]. Journal of Chromatography A, 2002, 976: 3-10
[14] Vendeuvre C, Fabrice B, Duval L, et al. Comparison of conventional gas chromatography and comprehensive twodimensional gas chromatography for the detailed analysis of petrochemical samples[J]. Journal of Chromatography A, 2004, 1056(1): 155-162
[15] Blomberg J, Riemersma T, van Zuijlen M, et al. Comprehensive two-dimensional gas chromatography coupled with fast sulphur-chemiluminescence detection: Implications of detector electronics[J]. Journal of Chromatography A, 2004, 1050(1): 77-84
[16] Nylén U, Delgado J F, J?r?s S, et al. Characterization of alkylated aromatic sulphur compounds in light cycle oil from hydrotreated vacuum gas oil using GC-SCD[J]. Fuel Processing Technology, 2004, 86(2): 223-234
[17] Bu J, Loh G, Gwie C G, et al. Desulfurization of diesel fuels by selective adsorption on activated carbons: competitive adsorption of polycyclic aromatic sulfur heterocycles and polycyclic aromatic hydrocarbons[J]. Chemical Engineering Journal, 2011, 166: 207-217
[18] M?ssner S G, Wise S A. Determination of polycyclic aromatic sulfur heterocycles in fossil fuel-related samples[J]. Analytical Chemistry, 1999, 71(1): 58-69
[19] Zhu G Q, Xia D H, Que G H. Progress in research of separation and enrichment of sulfur compounds in heavy petroleum distillates[J]. Journal of the University of Petroleum (China), 2000, 24(3): 112-115 (in Chinese)
[20] Nishioka M, Campbell R M, Lee M L, et al. Isolation of sulfur heterocycles from petroleum and coal-derived materials by ligand exchange chromatography[J]. Fuel, 1986, 65: 270-273
[21] Andersson J T. Retention properties of a palladium chloride/silica sorbent for the liquid chromatographic separation of polycyclic aromatic sulfur heterocycles[J]. Analytical Chemistry, 1987, 59: 2207-2209
[22] Wu M, Liu Z L, Tian S B, et al. Determination of saturates and aromatics of diesel fraction in wide-boiling cuts by solid extraction/gas chromatography[J]. Petroleum Processing and Petrochemicals, 2006, 37(7): 58-61 (in Chinese)
[23] Xu Y Q, Zhu X Y, Liu Z L, et al. Characterization of alkenes in diesel by solid phase extraction and gas chromatography field ionization time of flight high-resolution mass spectrometry[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2010, 26(3): 431-436 (in Chinese)
[24] Yan X L, Shi Q, Xu C M, et al. Separation and identification of sulfur compounds in Russia vacuum distillated oil by ligand exchange chromatography[J]. Journal of the University of Petroleum (China), 2004, 28(5): 108-112
[25] Willey C, Iwao M, Castle R N, et al. Determination of sulfur heterocycles in coal liquids and shale oils[J]. Analytical Chemistry, 1981, 53(3): 400-407
[26] Schade T, Roberz B, Andersson J T. Polycyclic aromatic sulfur heterocycles in desulfurized diesel fuels and their separation on a novel palladium (II)-complex stationary phase[J]. Polycyclic Aromatic Compounds, 2002, 22(3/4): 311-320
[27] Wang Z, Yang Y T. Determination of hydrocarbon compounds and sulfur compounds in gasoline by GC-FIDSCD[J]. Analytical Instrumentation, 2009(6): 62-65
[28] Machado M E, Caram?o E B, Zini C A. Investigation of sulphur compounds in coal tar using monodimensional and comprehensive two-dimensional gas chromatography[J]. Journal of Chromatography A, 2011, 1218(21): 3200-3207
[29] Gao L P, Liu P, Gu T, et al. Characterization of sulfur compounds in diesel fractions[J]. Journal of Fuel Chemistry and Technology, 2009, 37(2): 183-188
[30] Li W M. The sulphide constituents structure in the heavy quality oil of analysis[D]. Tianjin: Tianjin University, 2007
[31] Yin C L, Zhai X P, Zhao L Y, et al. Mechanism of hydrodesulfurization of dibenzothiophenes on unsupported NiMoW catalyst[J]. Journal of Fuel Chemistry and Technology, 2013, 41(8): 991-997
[32] Depauw G A, Froment G F. Molecular analysis of the sulphur components in a light cycle oil of a catalytic cracking unit by gas chromatography with mass spectrometric and atomic emission detection[J]. Journal of Chromatography A, 1997, 761: 231-247
[33] García C L, Becchi M, Grenier-Loustalot M F, et al. Analysis of aromatic sulfur compounds in gas oils using GC with sulfur chemiluminescence detection and highresolution MS[J]. Analytical Chemistry, 2002, 74(15): 3849-3857
Received date: 2014-03-22; Accepted date: 2014-09-18.
Prof. Tian Songbai, Telephone: +86-10-82368081; E-mail: tiansb.ripp@sinopec.com.
- 中國(guó)煉油與石油化工的其它文章
- Solvothermal Synthesis of V2O3Catalysts for Oxidative Desulfurization of Dibenzothiophene
- Effects of Promoters on the Ignition Process over NiO/Al2O3Catalyst for Autothermal Reforming of Methane to Hydrogen
- Oligomerization of 1-Decene: Catalyzation by Immobilized AlCl3/γ-Al2O3Catalyst in Fixed-bed Reactor
- Study on Tribological Properties of CVT Fluid Containing Inert and Active Functional Elements
- Application of Modified Attapulgite Clay as the Adsorbent in Gasoline Desulfurization
- Study on the Influence of Ni Modifying on Phase Transformation and Photocatalytic Activity of TiO2

