Support Effects on Thiophene Hydrodesulfurization over Co-Mo-Ni/Al2O3and Co-Mo-Ni/TiO2-Al2O3Catalysts*
Support Effects on Thiophene Hydrodesulfurization over Co-Mo-Ni/Al2O3and Co-Mo-Ni/TiO2-Al2O3Catalysts*
LIU Chao (劉超), ZHOU Zhiming (周志明)**, HUANG Yongli (黃永利), CHENG Zhenmin (程振民) and YUAN Weikang (袁渭康)
State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China
Hierarchically macro-/mesoporous structured Al2O3and TiO2-Al2O3materials were used as supports to prepare novel Co-Mo-Ni hydrodesulfurization (HDS) catalysts. A commercial Co-Mo-Ni/Al2O3catalyst without macroporous channels was taken as a reference. The catalysts were characterized by scanning electron microscope (SEM), transmission electron microscope (TEM), energy-dispersive spectrometry (EDS), N2adsorption-desorption, X-ray diffraction (XRD), and temperature programmed reduction (TPR). The apparent activities of the hierarchically porous catalysts for thiophene HDS were superior to those of the commercial catalyst, which was mainly ascribed to the diffusion-enhanced effect of the hierarchically bimodal pore structure. The addition of titania to alumina in the support helped to weaken the interaction between the active phase and the support, and as a result, the novel Co-Mo-Ni/TiO2-Al2O3catalyst with a low titania loading (28%, by mass) in the support exhibited high HDS activities, even without presulfiding treatment. However, the catalyst with a high titania loading (61%, by mass) showed much lower activities, which was mostly caused by its low surface area and pore volume as well as the non-uniform distribution of titania and alumina. The kinetic analysis further demonstrated the support effects on HDS activities of the catalysts.
hydrodesulfurization, support, hierarchically macro-/mesoporous structure, Al2O3, TiO2-Al2O3
1 INTRODUCTION
In the last two decades, increasing awareness of the effect of environmental pollution by automobiles and resultant stringent legislation for fuel quality in many countries have spurred considerable research efforts in sulfur removal from gasoline and diesel fractions through hydrodesulfurization (HDS) processes [1]. Among these research catalyst support plays an important role [2]. Although the most widely used commercial HDS catalysts have up to now been alumina-supported molybdenum promoted with cobalt or nickel, many investigations have been carried out to modify alumina or explore other supports in order to improve catalyst activity [2, 3]. The pore structure of support and the interaction between support and active phase are two important factors influencing the HDS performance of catalysts [4].
The commonly used alumina support possesses a unimodal mesopore size distribution, and such a pore structure unavoidably brings up problems such as mass transfer limitations of large sulfur-containing molecules from the outer catalyst surface to the active sites on inner surface. Our previous work proved that the effect of internal diffusion on the overall HDS rate is pronounced for a commercial Co-Mo-Ni/Al2O3catalyst with a mesoporous structure [5, 6]. One of the effective ways to improve the diffusion of large molecules is to substitute for unimodal mesoporous support with bimodal macro-/mesoporous one [7, 8]. Some studies demonstrated that the catalysts with macropores are superior to those without macropores in HDS of thiophene [9], dibenzothiophene [10] and vacuum gasoline [4]. Among many bimodal porous supports, hierarchically porous materials having parallel straight macrochannels with mesoporous walls are of particular interest, and in recent years have attracted much attention from researchers [11-16] due to their multiple applications in catalysis and separation processes [17-23]. Very recently, we prepared a hierarchically porous Co-Mo-Ni/Al2O3catalyst, which showed much higher apparent activity for thiophene HDS than a commercial catalyst [24].
Compared to Al2O3, TiO2presents a low metal-support interaction and excellent activities. For example, TiO2supported MoS2exhibits HDS activities several times higher than Al2O3supported one [2]. However, the relatively low surface area and the poor thermal stability of active anatase phase make TiO2unsuitable for industrial application [2, 3]. To overcome this problem, TiO2-Al2O3mixed oxides have been used as support to take advantage of beneficial features of both TiO2and Al2O3. Many investigations have showed that TiO2-Al2O3supports are suitable candidates for Al2O3in HDS processes [25-29]. However, almost all works to date are focused on mesoporous TiO2-Al2O3supports, and to the best of our knowledge no relevant results have been reported on hierarchically macro-/mesoporous structured TiO2-Al2O3as HDS catalyst supports. Considering the favorable properties of TiO2-Al2O3and the effective diffusivity of the hierarchically bimodal pore structure,one can speculate that a hierarchically porous TiO2-Al2O3supported molybdenum-nickel (cobalt) will be an ideal HDS catalyst. Recently we prepared a series of hierarchically macro-/mesoporous TiO2-Al2O3mixed oxides with different molar ratio of Ti to Al [15], laying a foundation for application of hierarchically porous TiO2-Al2O3supports to HDS reactions.
In the present work, novel Co-Mo-Ni catalysts supported on hierarchically macro-/mesoporous structured Al2O3and TiO2-Al2O3are systematically compared with a commercial Co-Mo-Ni catalyst. The comparison is made on physico-chemical properties of the catalysts and their thiophene HDS activities. In addition, a kinetic analysis is performed to evaluate support effects on HDS activities. The aim of this work is to (1) further elucidate the diffusion-enhanced effect of the hierarchically porous catalysts; and (2) understand the effect of titania addition to alumina on HDS activities of the catalysts.
2 EXPERIMENTAL
2.1 Catalyst preparation
The preparation methods for the hierarchically macro-/mesoporous metal oxides (Al2O3and TiO2-Al2O3) were described elsewhere [14, 15, 22]. As for the Co-Mo-Ni catalysts supported on these supports, we recently applied the conventional incipient wetness co-impregnation method to prepare a Co-Mo-Ni/Al2O3catalyst [24]. In the present work this method was further used to prepare two Co-Mo-Ni/TiO2-Al2O3catalysts with different Ti/Al molar ratios, one catalyst with the molar ratio being 0.2/0.8 and the other being 0.5/0.5. The hierarchically porous TiO2-Al2O3supports were calcined at 873 K for 5 h. Both catalysts were obtained by calcination of the impregnated supports at 773 K for 5 h. The detailed procedure for the catalyst preparation was available in our previous work [24]. The metal loading for each catalyst was kept constant (by mass) at about 5% Co, 17% Mo, and 3.5% Ni (determined by energy-dispersive spectrometry).
For simplicity, the commercial Co-Mo-Ni/Al2O3catalyst was denoted by CoMoNi/A(C), the hierarchically macro-/mesoporous structured Co-Mo-Ni/Al2O3by CoMoNi/A(N), and the hierarchically bimodal porous structured Co-Mo-Ni/TiO2-Al2O3with different Ti/Al molar ratios of 0.2︰0.8 and 0.5︰0.5 by Co-MoNi/T0.2A0.8(N) and CoMoNi/T0.5A0.5(N), respectively. The commercial CoMoNi/A(C) catalyst was supplied by the Chemical Research Institute of Lanzhou Petrochemical Corporation (LY-9802, prepared by impregnating Co, Mo and Ni on γ-Al2O3, with specific surface area of 228 m2·g?1and pore volume of 0.52 cm3·g?1). If the catalysts, e.g., CoMoNi/A(C) and CoMoNi/T0.2A0.8(N), were presulfided with a 1500 μg·g?1CS2in n-hexane (the presulfiding procedure was reported elsewhere [6]), the presulfided catalysts were named CoMoNi/A(C-S) and CoMoNi/T0.2A0.8(N-S), respectively.
2.2 Catalyst characterization
Brunauer-Emmett-Teller (BET) surface areas of the catalysts were obtained by N2adsorption measurement at 77 K on a Micromeritics ASAP 2010 instrument, and all samples were degassed at 463 K and 133 Pa for 6 h before analysis. The pore size distribution was determined from the desorption branch of the isotherm by the Barrett-Joyner-Halenda (BJH) method. X-ray diffraction (XRD) patterns were acquired on a Rigaku D/Max 2550 VB/PC diffractometer using a Cu Kα radiation source, and the intensity data were collected over a 2θ range of 10°-80°. The surface morphology was analyzed using a JEOL JSM 6360 LV scanning electron microscope (SEM). The elemental composition of catalyst surface was determined by energy-dispersive spectrometry (EDS, Falcon, EDAX) coupled to the SEM. Transmission electron microscopy (TEM) was recorded on a JEOL JEM 2100 microscope. Temperature programmed reduction (TPR) was performed on a Micromeritics AutoChem 2920 apparatus using 10% H2in Ar with a heating rate of 10 K·min?1up to final temperature of 1173 K, and the consumption of H2was monitored by a thermal conductivity detector (TCD).
2.3 Reaction tests
HDS of thiophene over the Co-Mo-Ni supported catalysts was conducted in a fixed-bed reactor (id=10 mm). The liquid feedstock was a mixture of thiophene and n-hexane (used as solvent), and the initial concentration of thiophene was 1500 μg·g?1. Detailed information on the reactor and the experimental procedure as well as the analytical method was reported in the previous study [24].
Kinetic experiments (45 runs for each type of catalyst) over fine powders (50-75 μm, 0.10 g) or relatively large particles (0.9-2.0 mm, 0.46 g) of the catalysts were carried out in the following experimental conditions: temperature (T) ranging from 543 to 623 K, total pressure (p) from 2.5 to 3.5 MPa, inlet liquid molar flow rate (0LF) from 3.07 to 5.37 mmol·min?1, inlet gas molar flow rate (0GF) from 10.71 to 13.79 mmol·min?1, molar ratio of gas to liquid (R) from 2.0 to 4.5. Preliminary tests had confirmed that the external diffusion limitation was eliminated by using the above flow rate, and that the intraparticle diffusion resistance was ignorable for the intrinsic kinetics by using fine powders (50-75 μm).
3 RESULTS AND DISCUSSION
3.1 Physico-chemical characterization

Figure 1 SEM images of CoMoNi/A(C) (a), CoMoNi/A(N) (b), CoMoNi/T0.2A0.8(N) (c) and CoMoNi/T0.5A0.5(N) (d) catalysts

Table 1 Textural properties of four catalysts
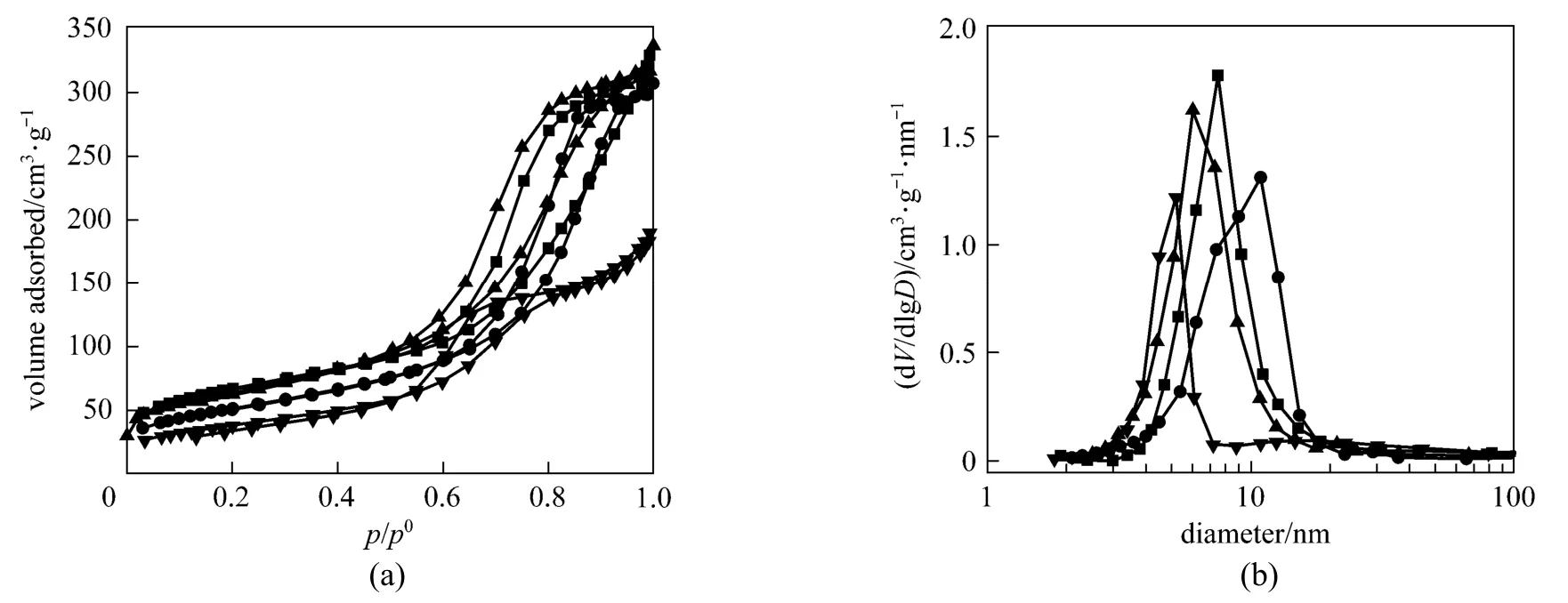
Figure 2 N2adsorption-desorption isotherms (a) and pore-size distribution curves (b) of four catalysts■ CoMoNi/A(C); ● CoMoNi/A(N); ▲ CoMoNi/T0.2A0.8(N); ▼ CoMoNi/T0.5A0.5(N)
The SEM images of four catalysts, i.e., Co-MoNi/A(C), CoMoNi/A(N), CoMoNi/T0.2A0.8(N) and CoMoNi/T0.5A0.5(N), are shown in Fig. 1. Different from the novel catalysts [Figs. 1 (b)-1 (d)] prepared in this work, which possess a parallel array of macroporous channels, the commercial catalyst has no macropores. As listed in Table 1, the average diameter of the macropores for the CoMoNi/A(N) catalyst is 0.50 μm, but this value is increased to 0.90-1.10 μm when titania is added into alumina. It is reasonable because the hierarchically macro-/mesoporous TiO2shows larger macropores after calcination at 773 K [14, 20].
The N2adsorption-desorption isotherms and the pore-size distribution curves of the above four catalysts are presented in Fig. 2. The textural properties (BET surface area, pore volume and average mesopore size) of these catalysts are summarized in Table 1. All the catalysts exhibit type IV isotherms and mesopore size distribution between 3 nm and 20 nm, indicating mesoporous walls separating the macrochannels for the hierarchically porous structured catalysts. The addition of titania to alumina deceases the mesopore size of the catalysts, which is probably caused by the high proportion of micropores in the pure titania sample [20]. Note that the surface area and the pore volume of the CoMoNi/T0.5A0.5(N) catalyst are smaller thanthose of other catalysts, which can be ascribed to the increased amount of titania in the support [15, 26].
Figure 3 shows the XRD patterns of different catalysts. Both the commercial CoMoNi/A(C) and the hierarchically porous CoMoNi/A(N) exhibit diffraction peaks (2θ=37°, 46°, 67°) belonging to the γ-Al2O3phase (JCPDS 10-0425). The CoMoNi/T0.2A0.8(N) catalyst also contains peaks assigned to γ-Al2O3, but the intensities of these peaks drop as a result of the titania addition. With respect to the CoMoNi/T0.5A0.5(N) catalyst, the γ-Al2O3crystalline peaks become much weaker owing to more titania added into the catalyst. In addition, CoMoNi/T0.5A0.5(N) displays a diffraction peak (2θ=25°) corresponding to the anatase phase (JCPDS 21-1272). An interesting phenomenon is that no Co or Mo or Ni-containing crystalline peaks are observed for all the catalysts in this work, indicating high dispersion of the active phase or very fine crystallites formed, beyond the detection capability of the XRD technique [30].
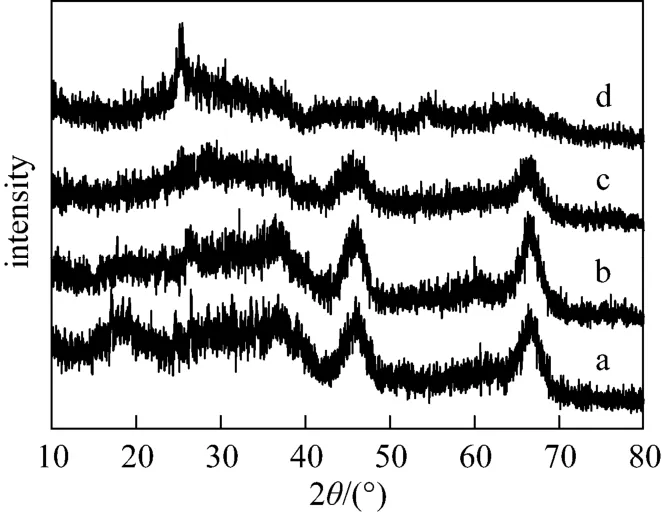
Figure 3 XRD patterns of four catalystsa—CoMoNi/A(C); b—CoMoNi/A(N); c—CoMoNi/T0.2A0.8(N); d—CoMoNi/T0.5A0.5(N)
The TEM images of the catalysts are displayed in Fig. 4. The CoMoNi/T0.2A0.8(N) catalyst (Fig. 4b) shows similar microstructure with CoMoNi/A(N) (Fig. 4a), implying the uniform distribution of titania and alumina. Further observations show that CoMoNi/ T0.2A0.8(N) has smaller crystallite size than CoMoNi/ A(N), which is consistent with the XRD analysis. In contrast, the microstructure of CoMoNi/T0.5A0.5(N) (Fig. 4c) is quite different from that of CoMoNi/ A(N) or CoMoNi/ T0.2A0.8(N). The spatial segregation between titania and alumina are clearly observed in CoMoNi/T0.5A0.5(N), indicating the non-uniform distribution of titania and alumina in the support. This result is also in accord with XRD and BET analysis, i.e., the presence of the anatase phase and the lower surface area and pore volume for CoMoNi/T0.5A0.5(N). Therefore, from the viewpoint of texture and structure, a small amount of titania in the support (T0.2A0.8) is favorable, which might lead to higher HDS activities.
The TPR profiles of the catalysts are shown in Fig. 5. For the catalysts without addition of titania (Figs. 5a, 5b), the first peak corresponds to the reduction of octahedral Mo species [26, 31]; the second small peak is due to the reduction of Ni2+species in the tetrahedral coordination [32]; the third peak is assigned to the second stage of reduction of octahedral Mo species and the reduction of the tetrahedral Mo species to lower oxide states [31, 33]. It has been widely accepted that octahedral Mo species are the precursors of the active phase MoS2. In this work, the temperature of the maxima for the first peak of the hierarchically porous structured CoMoNi/A(N) catalyst is about 20 K lower than that of the commercial CoMoNi/A(C), indicating the positive effect of the uniquely porous structure on the weakening of the interaction between octahedral Mo species and the support.
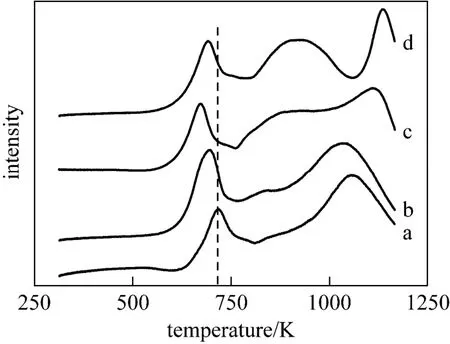
Figure 5 TPR patterns of four catalystsa—CoMoNi/A(C); b—CoMoNi/A(N); c—CoMoNi/T0.2A0.8(N); d—CoMoNi/T0.5A0.5(N)
In the presence of titania in the catalyst (Figs. 5c, 5d), the first peak shifts to lower temperature, and more importantly, the intensity of the second peak increases greatly, which is not only ascribed to the reduction of Ni2+, but also to the forward shift of the reduction of the tetrahedral Mo species (the third peak of CoMoNi/A(C) or CoMoNi/A(N)). Above observations demonstrate that the addition of titania to alumina can enhance reducibility of Mo species. Similar results were also obtained by Ramírez et al. [31] and Cede?o et al. [33]. The reason for the enhancement lies in the weakening of the metal-support interaction caused by a smaller polarization effect of Al3+ions [27]. In addition, the second peak includes contributions from reduction of titania [31, 33], which is evidenced by the increase of the peak intensity with the increasing amount of titania. The third peak is assigned to the difficult reduction of Mo species to metallic Mo [27], which is not observed for CoMoNi/A(C) and CoMoNi/A(N) catalysts owing to higher reduction temperature that is above the upper limit of the apparatus used.
3.2 Catalytic activities
The effect of the hierarchically macro-/mesoporous structure on the HDS activity of the catalyst is illustrated in Figs. 6 and 7. As shown in Fig. 6, the intrinsic activities (free of both external and internal diffusion limitations) are almost the same for the presulfided commercial CoMoNi/A(C-S) catalyst and the hierarchically porous structured CoMoNi/A(N-S) catalyst. It is expectable because both catalysts have similar physicochemical properties. However, when it comes to large catalyst particles (0.9-2.0 mm) in the presence of internal diffusion limitations, the apparent activities are quite different for the two catalysts. As indicated in Fig. 7, the hierarchically porous catalyst shows much higher HDS activities than the commercial catalyst, which suggests that the hierarchically porous structure can enhance diffusion of substances to the active sites of the catalyst.
Figure 7 Comparison of apparent HDS activities of novel and commercial Co-Mo-Ni/Al2O3catalysts (0.9-2.0 mm, 0.46 g)(Reaction conditions
The effect of titania addition to alumina on the HDS activity of the presulfided catalyst is shown in Fig. 8. The order of the HDS activity for the catalysts is CoMoNi/T0.2A0.8(N-S)>CoMoNi/A(N-S)>CoMoNi/ T0.5A0.5(N-S). Compared to the CoMoNi/A(N-S) catalyst without addition of titania in the support, the presence of a small amount of titania in CoMoNi/T0.2A0.8(N-S) improves the HDS activity, but a large proportion of titania in CoMoNi/T0.5A0.5(N-S) leads to a dramatic decrease of activity. As shown by the TPR profiles (Fig. 5) together with literature reports [34, 35], the interaction between Mo and TiO2-Al2O3is weaker than that between Mo and Al2O3, which is derived from the electronic promotion effect of TiO2[35, 36]. As a consequence, Mo species supported on TiO2-Al2O3are highly sulfided during the sulfidation process, resulting in an increase of the number of HDS active centers (coordinatively unsaturated sites), while the sulfidation of Mo is rather difficult on Al2O3due to the high metal-support interaction. For CoMoNi/T0.2A0.8(N-S), it possesses not only the lowest temperature for thereduction of octahedral Mo species, but also the largest surface area and pore volume as well as the uniform distribution of TiO2and Al2O3, and thus exhibits the highest HDS activity among the three catalysts. However, for CoMoNi/T0.5A0.5(N-S), both the non-uniform distribution of titania and alumina in the support and the lowest surface area and pore volume lead to a decrease in the dispersion of the active phase [37], which in turn counteracts the reduced metal-support interaction resulting from addition of titania, and consequently the HDS activity decreases sharply.

Figure 8 Comparison of HDS activities of Co-Mo-Ni/Al2O3and Co-Mo-Ni/TiO2-Al2O3catalysts (50-75 μm, 0.10 g)(Reaction conditions: (a) p=2.5 MPa, =4.60×10?3mol·min?1,R=3.0; (b) p=2.5 MPa,=5.37×10?3mol·min?1, R=2.0)CoMoNi/T0.5A0.5(N-S)
The effect of presulfidation on the HDS activity of the catalysts is shown in Figs. 9 and 10. As shown in Fig. 9, for the CoMoNi/A(N) catalyst without titania addition, the HDS activities of the presulfided CoMoNi/A(N-S) are much higher than those of the non-presulfided CoMoNi/A(N). The conversions of thiophene obtained with CoMoNi/A(N-S) are, depending on the reaction conditions, (30-77)% higher than those with CoMoNi/A(N). However, for the CoMoNi/T0.2A0.8(N) catalyst with titania addition, non-presulfided and presulfided catalysts exhibit similar HDS activities (shown in Fig. 10). The results clearly show that the non-presulfided CoMoNi/T0.2A0.8(N) catalyst still exhibits high HDS activities, whereas the CoMoNi/A(N) catalyst without presulfiding treatment shows low activities. Similar results are also found by Wei et al. [26], who reported that the capability of sulfur removal over a non-presulfided NiMo/TiO2-Al2O3catalyst was very high and even better than that over a presulfided commercial NiMo/Al2O3catalyst.
As mentioned above, the presence of titania on the alumina surface weakens the metal-support interaction, and as a result the non-presulfided CoMoNi/T0.2A0.8(N) catalyst could be readily sulfided during the initial stage of HDS reaction. On the contrary, it is very difficult to transform the non-presulfided CoMoNi/A(N) catalyst to the sulfided state during HDS reaction, which normally requires a relatively long time [26]. Therefore, the non-presulfided CoMoNi/A(N) catalyst shows much lower activities than the presulfided catalyst.

Figure 9 Comparison of HDS activities of presulfided and non-presulfided CoMoNi/A(N) catalysts (50-75 μm, 0.10 g)(Reaction conditions
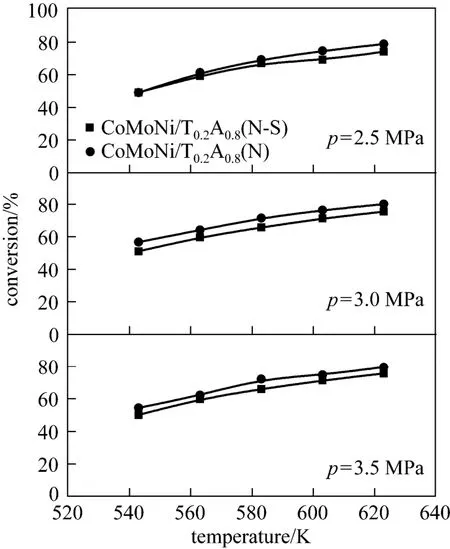
Figure 10 Comparison of HDS activities of presulfided and non-presulfided CoMoNi/T0.2A0.8(N) catalysts (50-75 μm, 0.10 g)(Reaction conditions
3.3 Kinetic analysis
In our very recent publication [24], a rigorous kinetic study was performed over the commercial CoMoNi/A(C)and the hierarchically porous structured CoMoNi/A(N) catalysts. Both intrinsic kinetics over fine powders (50-75 μm) and apparent kinetics over large particles (0.9-2.0 mm) were investigated. The intrinsic and apparent activation energies for the thiophene HDS reaction over CoMoNi/A(N) were 65.84 and 50.79 kJ·mol?1, respectively, while the corresponding values over CoMoNi/A(C) were 69.92 and 34.97 kJ·mol?1, respectively. These two catalysts had similar intrinsic activation energies, but the commercial catalyst had much lower apparent activation energy (34.97 kJ·mol?1) than the novel catalyst (50.79 kJ·mol?1). This result theoretically demonstrated the diffusion- facilitating effect of the hierarchically porous structure of the novel CoMoNi/A(N) catalyst. In this work, we extend the kinetic analysis to CoMoNi/T0.2A0.8(N) and Co-MoNi/T0.5A0.5(N) catalysts in order to further evaluate the effect of titania addition on the HDS activity of the catalyst from the viewpoint of kinetics.

Table 2 Estimated kinetic and adsorption parameters for different catalysts
Detailed information on the development of the kinetic model for thiophene HDS is reported elsewhere [24, 38]. The rate expression is given by

whereTp,2Hp and2HSp denote the partial pressures of thiophene, hydrogen, and hydrogen sulfide, respectively. The reaction and adsorption constants can be expressed in the usual Arrhenius form as follows,
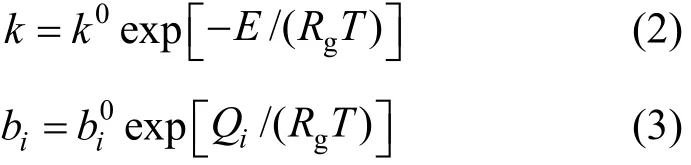
where E is the activation energy and Qiis the adsorption heat of species i. The kinetic and adsorption parameters involved in Eqs. (2) and (3) can be estimated by fitting the experimental data with the kinetic model. The parameter estimation procedure is described elsewhere [24].
The estimated intrinsic parameters for thiophene HDS over the sulfided CoMoNi/T0.2A0.8(N-S) and CoMoNi/T0.5A0.5(N-S) catalysts are shown in Table 2. For the purpose of comparison, the intrinsic parameters over the sulfided CoMoNi/A(N-S) catalyst, which have been reported in the previous work [24], are also listed here. Figs. 11 and 12 show comparison of the measured conversion of thiophene and the corresponding value predicted by the kinetic model over CoMoNi/ T0.2A0.8(N-S) and CoMoNi/T0.5A0.5(N-S), respectively. The average relative errors for the two catalysts are 6.0% and 5.7%, respectively, clearly indicating that the above model can describe the experimental result very well.

Figure 11 Comparison of experimental and calculated conversions of thiophene over the sulfided CoMoNi/T0.2A0.8(N-S) catalyst
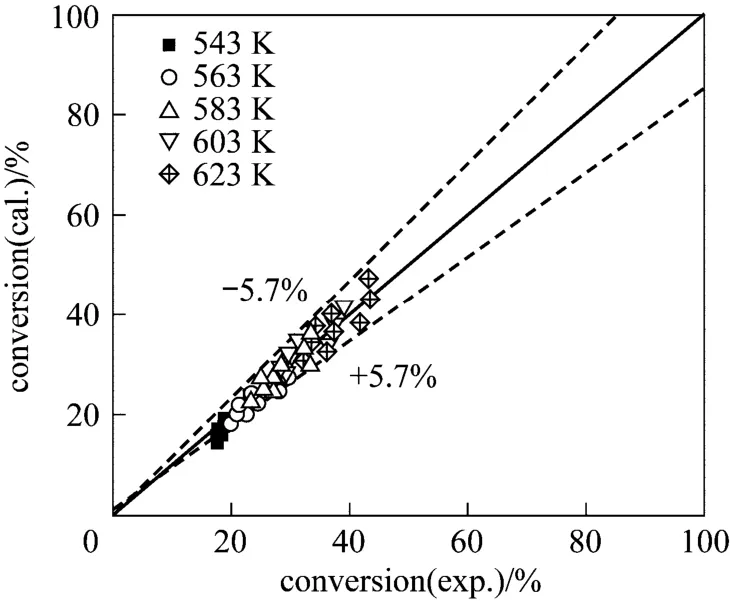
Figure 12 Comparison of experimental and calculated conversions of thiophene over the sulfided CoMoNi/T0.5A0.5(N-S) catalyst
The intrinsic activation energies for the three catalysts, CoMoNi/A(N-S), CoMoNi/T0.2A0.8(N-S) and CoMoNi/T0.5A0.5(N-S), are 65.8, 54.3 and 72.3 kJ·mol?1, respectively. CoMoNi/T0.2A0.8(N-S) that displays the highest HDS activity among the catalysts has the lowest activation energy, while CoMoNi/T0.5A0.5(N-S) with the lowest HDS activity has the highest activation energy. The order of the intrinsic activation energy of the catalysts is therefore consistent with that of their HDS activities. Hence, both experimental measurements and kinetic analysis reveal the effect of titania addition to alumina on the HDS activity of the catalyst: a low titania loading in the catalyst is favorable for anincrease in the HDS activity, but a high loading shows a negative effect.
4 CONCLUSIONS
In this work home made Co-Mo-Ni catalysts supported on hierarchically macro-/mesoporous structured Al2O3and TiO2-Al2O3exhibit higher activities in thiophene HDS compared to a commercial catalyst supported on unimodal mesoporous Al2O3. The straight macrochannels of the hierarchically porous supports play an important role, since they can greatly reduce the internal diffusion limitations of reactants and facilitate the diffusion of thiophene molecules from the outer surface to the active sites of the catalysts. For the hierarchically porous Co-Mo-Ni catalysts, the addition of titania to alumina in the support greatly influences HDS activities. The catalyst with a low titania loading shows very high HDS activities, even without presulfiding treatment. On the contrary, the catalyst with a high titania loading presents much lower activities. The difference in the textural properties of the catalysts with different titania loadings is the main reason. The present results indicate that hierarchically macro-/mesoporous structured TiO2-Al2O3mixed oxides with a low titania loading are good supports for Mo-based catalysts in thiophene HDS, and may be suitable for the deep HDS processes.
REFERENCES
1 Song, C.S., “An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel”, Catal. Today, 86, 211-263 (2003).
2 Breysse, M., Afanasiev, P., Geantet, C., Vrinat, M., “Overview of support effects in hydrotreating catalysts”, Catal. Today, 86, 5-16 (2003).
3 Dhar, G.M., Srinivas, B.N., Rana, M.S., Kumar, M., Maity, S.K.,“Mixed oxide supported hydrodesulfurization catalysts—A review”, Catal. Today, 86, 45-60 (2003).
4 Ling, T.R., Wan, B.Z., Lin, H.P., Mou, C.Y., “Desulfurization of vacuum gasoil by MCM-41 supported molybdenum-nickel catalysts”, Ind. Eng. Chem. Res., 48, 1797-1803 (2009).
5 Yang, D., Zhou, Z.M., Cheng, Z.M., Yuan, W.K., “Effect of internal diffusion on thiophene hydrodesulfurization in pyrolysis gasoline”, J. Fuel Chem. Technol., 36, 437-442 (2008).
6 Yang, D., Cheng, Z.M., Zhou, Z.M., Zhang, J.C., Yuan, W.K., “Pyrolysis gasoline hydrogenation in the second-stage reactor: reaction kinetics and reactor simulation”, Ind. Eng. Chem. Res., 47, 1051-1058 (2008).
7 Do?u, T., “Diffusion and reaction in catalyst pellets with bidisperse pore size distribution”, Ind. Eng. Chem. Res., 37, 2158-2171 (1998).
8 Silva, V.M.T.M., Rodrigues, A.E., “Adsorption and diffusion in bidisperse pore structures”, Ind. Eng. Chem. Res., 38, 4023-4031 (1999).
9 Hussain, M., Yun, J.S., Ihm, S.K., Russo, N., Geobaldo, F., “Synthesis, characterization, and thiophene hydrodesulfurization activity of novel macroporous and mesomacroporous carbon”, Ind. Eng. Chem. Res., 50, 2530-2535 (2011).
10 Liu, F., Xu, S.P., Cao, L., Chi, Y., Zhang, T., Xue, D.F., “A comparison of NiMo/Al2O3catalysts prepared by impregnation and coprecipitation methods for hydrodesulfurization of dibenzothiophene”, J. Phys. Chem. C, 111, 7396-7402 (2007).
11 Blin, J.L., Léonard, A., Yuan, Z.Y., Gigot, L., Vantomme, A., Cheetham, A.K., Su, B.L., “Hierarchically mesoporous/macroporous metal oxides templated from polyethylene oxide surfactant assemblies”, Angew. Chem. Int. Ed., 42, 2872-2875 (2003).
12 Yuan, Z.Y., Ren, T.Z., Azioune, A., Pireaux, J.J., Su, B.L.,“Self-assembly of hierarchically mesoporous-macroporous phosphated nanocrystalline aluminum (oxyhydr) oxide materials”, Chem. Mater., 18, 1753-1767 (2006).
13 Hakim, S.H., Shanks, B.H., “A comparative study of macroporous metal oxides synthesized via a unified approach”, Chem. Mater., 21, 2027-2038 (2009).
14 Zeng, T.Y., Zhou, Z.M., Cheng, Z.M., Yuan, W.K., “Effect of surfactant on the physical properties of hierarchically macro-mesoporous metal oxides”, Chem. Lett., 39, 680-681 (2010).
15 Zhou, Z.M., Zeng, T.Y., Cheng, Z.M., Yuan, W.K., “Preparation and characterization of titania-alumina mixed oxides with hierarchically macro-/mesoporous structures”, Ind. Eng. Chem. Res., 50, 883-890 (2011).
16 Yang, X.Y., Léonard, A., Lemaire, A., Tian, G., Su, B.L.,“Self-formation phenomenon to hierarchically structured porous materials: design, synthesis, formation mechanism and applications”, Chem. Commun., 47, 2763-2786 (2011).
17 Wang, X.C., Yu, J.C., Ho, C.M., Hou, Y.D., Fu, X.Z., “Photocatalytic activity of a hierarchically macro/mesoporous titania”, Langmuir, 21, 2552-2559 (2005).
18 Yu, J.G., Su, Y.R., Cheng, B., “Template-free fabrication and enhanced photocatalytic activity of hierarchical macro-/mesoporous titania”, Adv. Funct. Mater., 17, 1984-1990 (2007).
19 Tidahy, H.L., Hosseni, M., Siffert, S., Cousin, R., Lamonier, J.F., Abouka?s, A., Su, B.L., Giraudon, J.M., Leclercq, G., “Nanostructured macro-mesoporous zirconia impregnated by noble metal for catalytic total oxidation of toluene”, Catal. Today, 137, 335-339 (2008).
20 Zeng, T.Y., Zhou, Z.M., Zhu, J., Cheng, Z.M., Yuan, P.Q., Yuan, W.K., “Palladium supported on hierarchically macro-mesoporous titania for styrene hydrogenation”, Catal. Today, 147S, S41-S45 (2009).
21 Ma, T.Y., Zhang, X.J., Yuan, Z.Y., “Hierarchical meso-/macroporous aluminum phosphonate hybrid materials as multifunctional adsorbents”, J. Phys. Chem. C, 113, 12854-12862 (2009).
22 Zhou, Z.M., Zeng, T.Y., Cheng, Z.M., Yuan, W.K., “Preparation of a catalyst for selective hydrogenation of pyrolysis gasoline”, Ind. Eng. Chem. Res., 49, 11112-11118 (2010).
23 Zhou, Z.M., Zeng, T.Y., Cheng, Z.M., Yuan, W.K., “Diffusionenhanced hierarchically macro-mesoporous catalyst for selective hydrogenation of pyrolysis gasoline”, AIChE J., 57, 2198-2206 (2011).
24 Huang, Y.L., Zhou, Z.M., Qi, Y., Li, X., Cheng, Z.M., Yuan, W.K.,“Hierarchically macro-/mesoporous structured Co-Mo-Ni/γ-Al2O3catalyst for the hydrodesulfurization of thiophene”, Chem. Eng. J., 172, 444-451 (2011).
25 Yoshinaka, S., Segawa, K., “Hydrodesulfurization of dibenzothiophenes over molybdenum catalyst supported on TiO2-Al2O3”, Catal. Today, 45, 293-298 (1998).
26 Wei, Z.B., Yan, W.H., Zhang, H., Ren, T.L., Xin, Q., Li, Z.C., “Hydrodesulfurization activity of NiMo/TiO2-Al2O3catalysts”, Appl. Catal. A Gen., 167, 39-48 (1998).
27 Grzechowiak, J.R., Wereszczako-Zielińska, I., Rynkowski, J., Zió?ek, M., “Hydrodesulphurisation catalysts supported on alumina-titania”,Appl. Catal. A Gen., 250, 95-103 (2003).
28 Ramírez, J., Rayo, P., Gutiérrez-Alejandre, A., Ancheyta, J., Rana, M.S., “Analysis of the hydrotreatment of Maya heavy crude with NiMo catalysts supported on TiO2-Al2O3binary oxides: Effect of the incorporation method of Ti”, Catal. Today, 109, 54-60 (2005).
29 Huang, W.Q., Duan, A.J., Zhao, Z., Wan, G.F., Jiang, G.Y., Dou, T., Chung, K.H., Liu, J., “Ti-modified alumina supports prepared by sol-gel method used for deep HDS catalysts”, Catal. Today, 131, 314-321 (2008).
30 Reddy, B.M., Reddy, E.P., Srinivas, S.T., “Dispersion and activity of Molybdena-alumina catalysts prepared by impregnation and solid/solid wetting methods”, J. Catal., 136, 50-58 (1992).
31 Ramírez, J., Macías, G., Cede?o, L., Gutiérrez-Alejandre, A., Cuevas, R., Castillo, P., “The role of titania in supported Mo, CoMo, NiMo, and NiW hydrodesulfurization catalysts: analysis of past and new evidences”, Catal. Today, 98, 19-30 (2004).
32 Zepeda, T.A., Pawelec, B., Fierro, J.L.G., Olivas, A., Fuentes, S., Halachev, T., “Effect of Al and Ti content in HMS material on the catalytic activity of NiMo and CoMo hydrotreating catalysts in the HDS of DBT”, Micropor. Mesopor. Mat., 111, 157-170 (2008).
33 Cede?o, L., Ramírez, J., López-Agudo, A., Vrinat, M., Cordero, R.L.,“TPR and NO adsorption studies of Mo, CoMo and NiMo catalysts supported on Al2O3-TiO2mixed oxides”, Stud. Surf. Sci. Catal., 127, 369-372 (1999).
34 Segawa, K., Takahashi, K., Satoh, S., “Development of new catalysts for deep hydrodesulfurization of gas oil”, Catal. Today, 63, 123-131 (2000).
35 Saih, Y., Segawa, K., “Tailoring of alumina surfaces as supports for NiMo sulfide catalysts in the ultra deep hydrodesulfurization of gas oil: case study of TiO2-coated alumina prepared by chemical vapor deposition technique”, Catal. Today, 86, 61-72 (2003).
36 Wan, G.F., Duan, A.J., Zhang, Y., Zhao, Z., Jiang, G.Y., Zhang, D.Q., Liu, J., Chung, K., “NiW/AMBT catalysts for the production of ultra-low sulfur diesel”, Catal. Today, 158, 521-529 (2010).
37 Amezcua, J.C., Lizama, L., Salcedo, C., Puente, I., Domínguez, J.M., Klimova, T., “NiMo catalysts supported on titania-modified SBA-16 for 4,6-dimethyldibenzothiophene hydrodesulfurization”, Catal. Today, 107?108, 578-588 (2005).
38 Borgna, A., Hensen, E.J.M., van Veen, J.A.R., Niemantsverdriet, J.W., “Intrinsic kinetics of thiophene hydrodesulfurization on a sulfided NiMo/SiO2planar model catalyst”, J. Catal., 221, 541-548 (2004).
2012-10-30, accepted 2012-12-30.
* Supported by the National Natural Science Foundation of China (21276076), the Fundamental Research Funds for the Central Universities of China (WA1014003), and State Key Laboratory of Chemical Engineering (SKL-ChE-10C06).
** To whom correspondence should be addressed. E-mail: zmzhou@ecust.edu.cn
 Chinese Journal of Chemical Engineering2014年4期
Chinese Journal of Chemical Engineering2014年4期
- Chinese Journal of Chemical Engineering的其它文章
- PVC Membrane Selective Electrode for Determination of Cadmium(II) Ion in Chocolate Samples
- Determination and Correlation of Solubilities of Four Novel Benzothiazolium Ionic Liquids with6PF?in Six Alcohols*
- An Approach to Formulation of FNLP with Complex Piecewise Linear Membership Functions
- One Step Preparation of Sulfonated Solid Catalyst and Its Effect in Esterification Reaction*
- Impacts of Power Density on Heavy Metal Release During Ultrasonic Sludge Treatment Process*
- Determination and Correlation of Solubility for D-Xylose in Volatile Fatty Acid Solvents*
