Removal of Thiophenic Sulfur Compounds from Oil Using Chlorinated Polymers and Lewis Acid Mixture via Adsorption and Friedel-Crafts Alkylation Reaction*
SONG Hongyan (宋紅艷), GAO Jiajun (高家俊), CHEN Xingyu (陳星羽), HE Jing (何靜)and LI Chunxi (李春喜),**State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 0009, ChinaFaculty of Science, Beijing University of Chemical Technology, Beijing 0009, ChinaCollege of Chemical Engineering, Beijing University of Chemical Technology, Beijing 0009, China
Removal of Thiophenic Sulfur Compounds from Oil Using Chlorinated Polymers and Lewis Acid Mixture via Adsorption and Friedel-Crafts Alkylation Reaction*
SONG Hongyan (宋紅艷)1,2, GAO Jiajun (高家俊)1,3, CHEN Xingyu (陳星羽)1,3, HE Jing (何靜)1,2and LI Chunxi (李春喜)1,3,**
1State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China2Faculty of Science, Beijing University of Chemical Technology, Beijing 100029, China3College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, China
For efficient removal of thiophenic sulfur (S-) compounds from oils, a novel method is proposed here, i.e. one-pot alkylation desulfurization (OADS), in which oil insoluble chlorinated polymer such as polyvinyl chloride (PVC) is used as the alkylating regent with Lewis acid as catalyst. The aromatic S-compounds are grafted to the polymer through Friedel-Crafts reaction and removed facilely along with the polymer. The OADS mechanism is identified by scanning electron microscope and analyzer with surface area and pore size of the polymer. The influence of some factors on the OADS is studied, e.g. the type and amount of chlorinated polymers and Lewis acids. It is proved that thiophene and benzothiophene can be removed efficiently from oil by PVC+AlCl3mixture even in the presence of 25% (by mass) of benzene due to the synergetic effects of the adsorptive desulfurization of AlCl3and the alkylation desulfurization of PVC.
alkylation desulfurization, chlorinated polymer, thiophenic compound, Lewis acid, Friedel-Crafts reaction
1 INTRODUCTION
Thiophenic sulfur (S-) compounds, such as thiophene (T), benzothiophene (BT), dibenzothiophene (DBT) and their alkyl substituted derivatives [1], amount to about 70% of the total S-content in the fluidized catalytic cracking (FCC) naphtha, which are difficult to be removed by conventional catalytic hydrogenation desulfurization (HDS) process [2, 3]. To reduce the environmental pollution of the resulting SOx, the allowable S-content in the commercial oils decreases dramatically in recent years, e.g. the S-content of gasoline in Beijing is controlled within 10 μg·g?1. The conventional HDS shows some intrinsic demerits such as high capital cost, energy and hydrogen consumption, loss of octane number, and harsh operation conditions (temperature >573 K, pressure >5 MPa). Thereby, some alternative desulfurization methods have been explored, such as adsorptive desulfurization [4-8], extractive desulfurization [9-11], oxidative desulfurization [12, 13], condensation of thiophenic compounds [14], and olefin alkylation of thiophenic sulfur (OATS) [15, 16]. For a typical OATS process, thiophenic compounds are first alkylated by olefins with the help of some acidic catalysts [17-22] and the resulting alkylated S-compounds are separated by distillation due to their higher boiling points. This technology has been patented [23], but its commercialization is limited, which might be associated with the following problems. First, the reaction needs to be conducted at relatively higher S bond forms between thiophenic sulfur compounds and CH3I by SN2 nucleophilic substitution at room temperature, forming ionic sulfonium salts [24, 25]. The resulting salts are removed at 273 K through cooling. A synergic reactant AgBF4is necessary for facilitating the formation of sulfonium salts, which is too expensive to be applicable. Furthermore, good desulfurization is achieved only in the presence of CH2Cl2, which adversely introduces large amount of chlorine pollutant into the oil. As a result, the applicability of this process seems unrealistic.
To overcome the problems mentioned above, one-pot alkylation desulfurization (OADS) without distillation, cooling, and introduction of external pollutant is very important. Some chloro-containing polymers such as polyvinyl chloride (PVC) may be appropriate Friedel-Crafts alkylation regents, considering that (1) they are more reactive with thiophenic sulfur compounds than olefins, which makes the reaction achievable under mild conditions; (2) thiophenic sulfur compounds are grafted to PVC granules by the formation of C temperature (373-453 K) due to the lower reactivity of olefins for the Friedel-Crafts reaction. Second, the catalysts used are expensive and subject to deactivation. And finally, the energy consumption is high for the distillation separation of the alkylated S-compounds. To realize a facile desulfurization, a novel process is proposed by Shiraishi et al., in which the C
C bond, achieving an one-pot desulfurization by solid-liquid separation; (3) the PVC powder is not swollen and soluble in oil, which prevents theintroduction of foreign chemical pollutants to the oil. In this paper, the OADS performance of PVC is studied and compared with that of other chloro-containing polymers such as chlorinated PVC (CPVC) and chlorinated polypropylene (CPP). As a matter of fact, PVC is the most representative oil insoluble chlorinated polymers and can be used as a reactive reagent for the Friedel-Crafts reaction with the catalysis of Lewis acid AlCl3. Moreover, some chlorine atoms in the PVC chain present higher reactivity for the alkylation reaction than olefins, e.g. those connected to tert-butyl and allyl groups [26], forming S-containing PVC derivatives, as presented in Fig. 1. In this regard, the present OADS process is attractive and worthy of study. Three S-compounds, i.e. T, BT, and DBT, are selected as model thiophenic sulfur compounds in real oils.
2 EXPERIMENTAL
2.1 Chemicals
The chemical materials used were purchased from different companies, specifically, T (>99%, by mass) from Sinopharm Co. Ltd., BT (>97%, by mass) and DBT (>99%, by mass) from Acros Organics, PVC (SG-5, industrial grade) from Tangshan Alkali Co Ltd, CPVC (industrial grade) and CPP (industrial grade) from Shanghai Alkali Co. Ltd., AlCl3(>99%, by mass) and FeCl3(>99%, by mass) from Shantou Xilong Chemical Ltd., n-octane (analytical reagent) from Tianjin Guangfu Fine Chemical Industry Institute, and benzene (analytical reagent) from Beijing Chemical Works. The real oil is a heavy pyrolysis gasoline with sulfur content of 6234 μg·g?1, which is kindly supplied by Puyang oil refinery plant. All reagents were used as received.
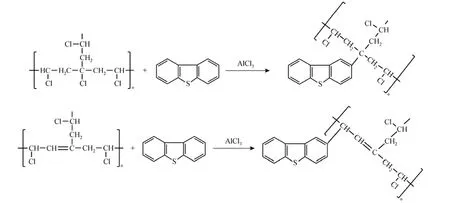
Figure 1 Alkylation desulfurization mechanism using PVC with catalysis of AlCl3
2.2 Alkylation desulfurization experiments
The model oil used is a binary mixture of n-octane and a thiophenic S-compound, namely, T, BT, or DBT, with its initial S-content being 1000 μg·g?1. To the model oil a certain amount of chlorinated polymer (PVC, CPVC or CPP) and Lewis acid (AlCl3or FeCl3) was added directly under magnetic stirring at a specific temperature (293, 313 or 333 K), and the samples were taken out by syringe with a microfiltration membrane head. The desulfurization experiment was generally controlled within 2 h. Moreover, benzene was chosen as a representative of aromatic compounds in real oil to explore its competitive alkylation with thiophenic compounds, for which the model oil with 25% (by mass) of benzene and 1000 μg·g?1S was prepared and used.
2.3 Analysis method
2.3.1 Sulfur content analysis
The S-contents with respect to T, BT and DBT in the oil phase were measured by sulfur and nitro analyzer (KY-3000SN, Jiangyan Keyuan Electronic Instrument Ltd, China) with its minimum detectable S-content being about 0.3 μg·g?1. For each sample, the analysis was repeated three times to obtain the average S-content. The maximum analysis errors of S-content were controlled within 1% (100-1000 μg·g?1), 3% (30-100 μg·g?1), 15% (10-30 μg·g?1), and 25% (<10 μg·g?1).
2.3.2 Characterization of PVC
To explore the removal mechanism of thiophenic S-compounds by PVC, the oil phase was poured out after a definite period of stirring, and then excessive amount of ethanol was added to the PVC residue to dissolve AlCl3and all S-compounds adsorbed physicallyor chemically. The resulting PVC was separated by vacuum filtration, and then washed three times by 50 ml of fresh ethanol for each. The used PVC as-pretreated was characterized by a scanning electron microscope (SEM, Zeiss Supra 55) with an energy dispersive X-ray detector (EDX) attachment (EDX Oxford Instruments Isis 350) and an automated surface area and pore size analyzer (Quadrasorbsi and Quantachrome). The above results were compared with that of PVC powder.
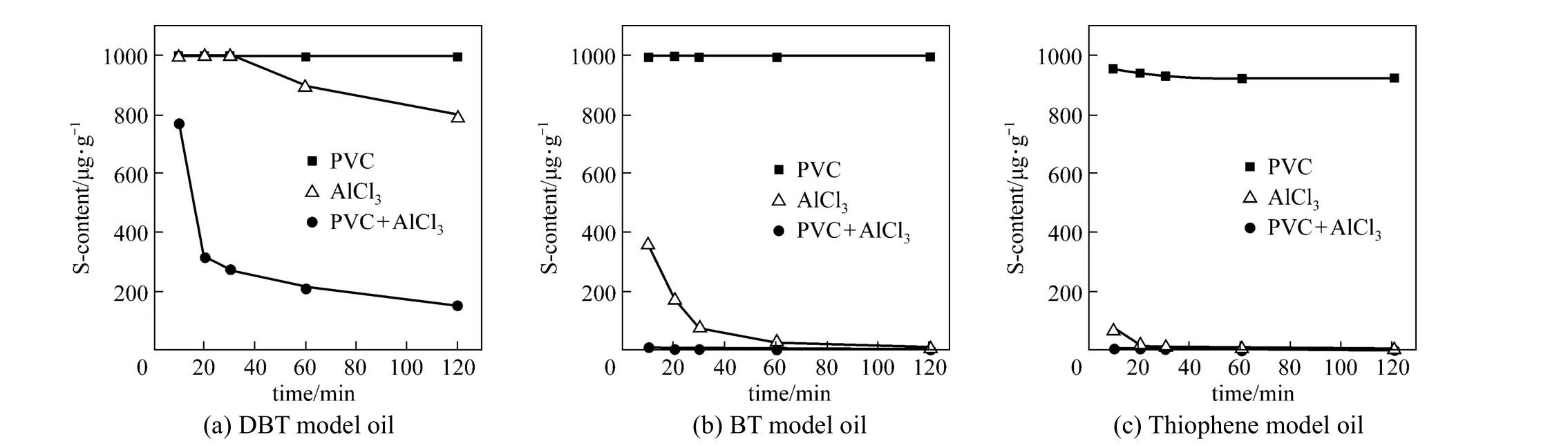
Figure 2 S-content of oil with reaction time for different model oils [AlCl3/S=5/1 (by mole); PVC/oil=0.3/10 (by mass); 333 K]
3 RESULTS AND DISCUSSION
3.1 Alkylation desulfurization using PVC and AlCl3
To study alkylation desulfurization activities of T, BT and DBT in model oil, the S-content in the resulting oil phase at 333 K is compared in Fig. 2. The desulfurization rates with PVC+AlCl3mixture are higher than those with AlCl3, especially for BT and DBT, while the PVC powder shows negligible desulfurization ability. For the removal of thiophene, the difference between the PVC+AlCl3mixture and AlCl3is small after 30 min, and the former is only slightly superior to the latter, which may be ascribed to the strong complexing interaction between AlCl3and T [7]. For BT, its removal is accelerated significantly by the alkylation reaction. With the PVC+AlCl3mixture, the S-removal at 10 min is 99%; with AlCl3, it is only 63%, and it needs 2 h to achieve 99% removal.
The good desulfurization ability of AlCl3here is attributed to its strong complexing interaction with BT, a fast process, as well as the oligomerization of BT with the catalysis of AlCl3, a slow process, which in return promotes the complexation greatly [7]. In contrast to T and BT, the PVC+AlCl3mixture shows much better desulfurization performance for DBT than AlCl3, and the adsorptive desulfurization of AlCl3for DBT is relatively weak due to the incompatible interaction between AlCl3, a hard and strong Lewis acid, and DBT, a soft and weak Lewis base. The above results indicate that the alkylation reaction of thiophenic compounds with PVC can be realized successfully under mild conditions with the catalysis of AlCl3, forming oil insoluble S-containing PVC derivatives, and separated facilely through simple decantation. Thus a new and efficient alkylation desulfurization process is proposed, with the S-removal above 99.5% for T and BT, and 84% for DBT.
3.2 Alkylation desulfurization mechanism
As seen from Fig. 1, the S-compounds are chemically grafted to the PVC chain in the presence of AlCl3. To verify this mechanism, the used PVC samples are pretreated following the procedure as described in Section 2.3.2, and characterized by SEM to justify the presence of S-element on the material surface. The results are shown in Fig. 3 and compared with that of fresh PVC powder. It is noted that S-element is absent in the fresh PVC, but the S-peak is detected in all three PVC samples used in the desulfurization of T, BT and DBT oils, indicating that the thiophenic compounds are grafted to PVC. It can be concluded that the desulfurization mechanism is mainly ascribed to the Friedel-Crafts alkylation reaction between PVC and aromatic S-compounds with the catalysis of the strong Lewis acid AlCl3. Moreover, the chlorine content of the used PVCs is much lower than that of the fresh PVC, which may be ascribed to the dehydrochlorination of PVC with the acidic catalytic interaction of AlCl3forming double bonds on the PVC chains.
The surface area (SBET) and pore size of the four PVCs are presented in Table 1. All the used PVC samples show larger surface area, average pore size and total pore volume (Vtotal) than the fresh PVC, implying the occurrence of the cross-linking of PVC chains due to the alkylation reaction between aromatic S-compounds and PVC molecules, i.e., one aromatic S-molecule reacts with two or more PVC molecules, as presented in Fig. 4. Some new and large pores form among PVC chains with the thiophenic S-compounds as nodes or wedges, resulting in larger average pore size, total pore volume and surface area. Moreover, the above three property values for the used PVCs show the order of DBT-PVC>BT-PVC>T-PVC, which is in line with sizes of the nodes, i.e. DBT>BT>T, that is, the larger the S-compound is, the larger the surface area and pore size of the corresponding used PVC.
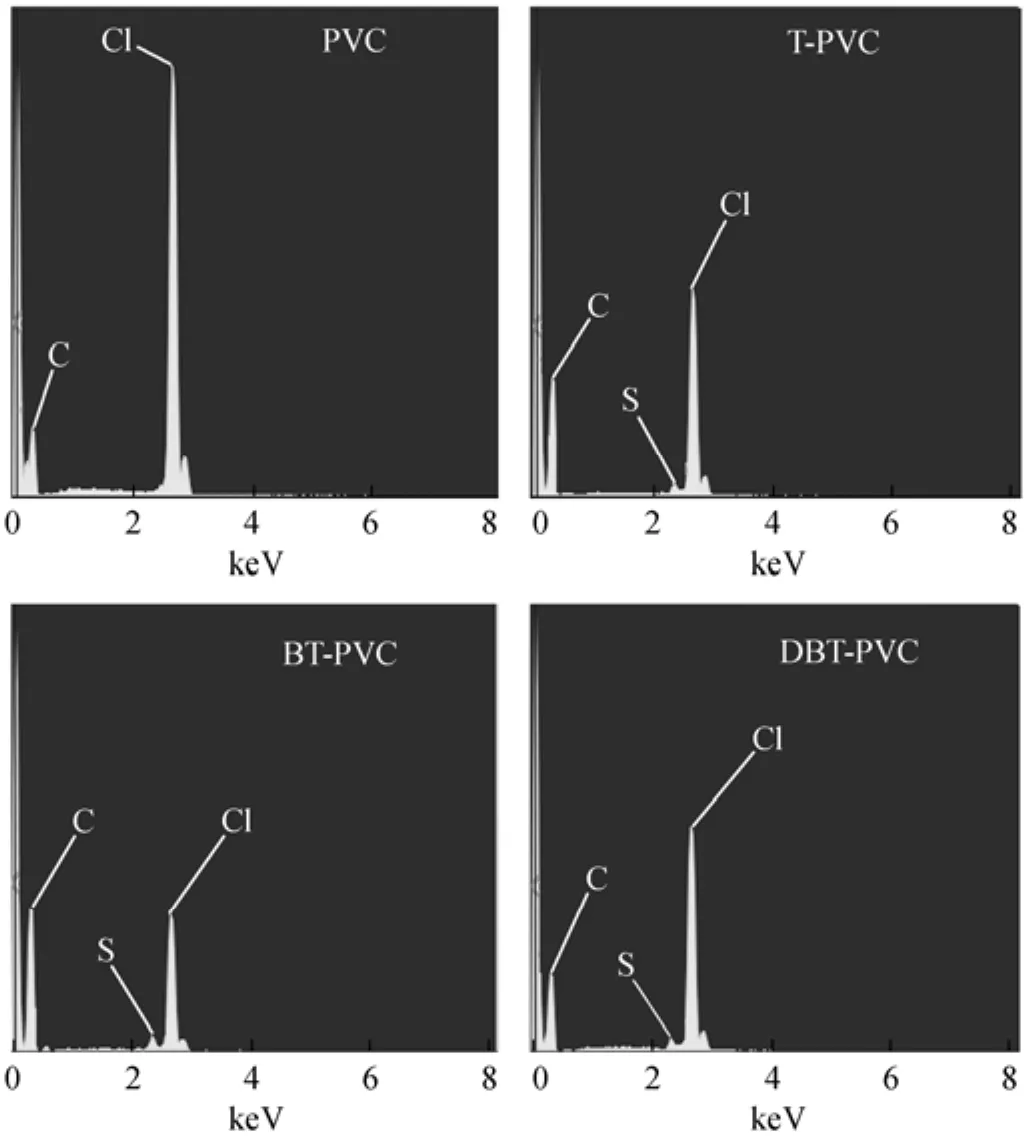
Figure 3 SEM results for fresh PVC and used PVCs
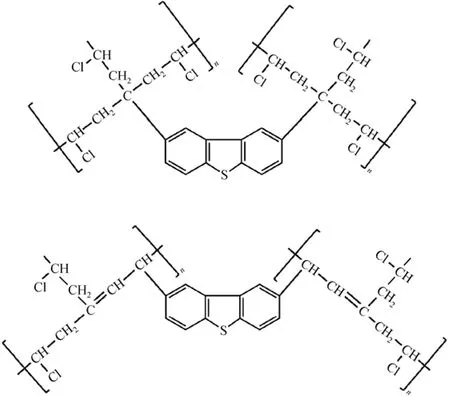
Figure 4 Cross-linking products formed in the alkylation desulfurization process
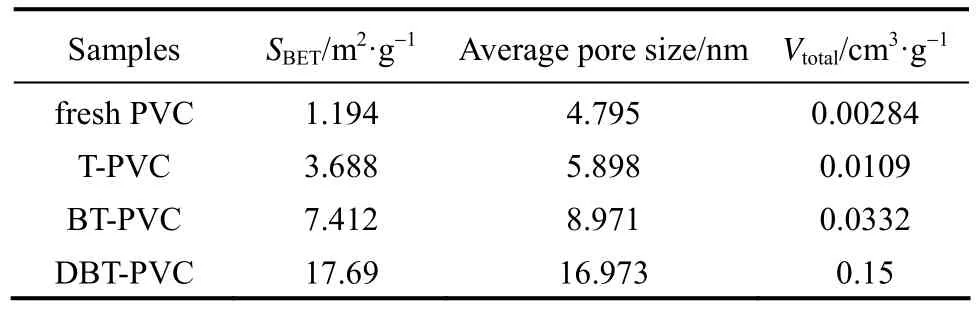
Table 1 Surface area and pore size for fresh PVC and used PVCs
3.3 Influence of Lewis acids and chlorinated polymers on desulfurization
The PVC+AlCl3mixture shows very good alkylation desulfurization performance at 333 K. To explore the applicability of other Lewis acids and chlorinated polymers, experiments are conducted under the same conditions. As seen from Fig. 5 (a), FeCl3shows weaker catalysis for the alkylation reaction of three thiophenic compounds than AlCl3due to its weaker Lewis acidity. Fig. 5 (b) compares the S-removal performance of three chlorinated polymers using AlCl3as catalyst. CPP shows the lowest alkylation activity for all three S-compounds, which may be attributed to its lowest chloride content (about 33%, by mass). However, alkylation activity of CPVC and PVC is virtually the same, though the chloride content of CPVC, about 67% (by mass), is a little higher than that of PVC [27]. Thus it can be inferred that it is the amount of the active chlorine rather than the total amount of chlorine that determines the alkylation reactivity of chlorinated polymers. In effect, some active allyl groups in the PVC molecules are destroyed with the chlorination of PVC to form CPVC, so that the amount of active chlorine in CPVC may be even lower than that in PVC, though excessive amount of chlorine in CPVC may compensate this negative effect to some extent. It should be pointed out that FeCl3is a weak oxidant even in the acidic medium, so it is unlikely to oxidize other useful components of oil, e.g. olefins, aromatics, and other components. It is only functioned as a Lewis acid catalyst for the alkylation reaction between thiophenic S-compounds and chlorine containing polymers.
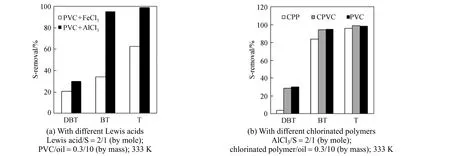
Figure 5 Comparison of S-removal for different Lewis acids and chlorinated polymers

Figure 6 S-removal with time at different temperatures [AlCl3/S=5/1 (by mole); PVC/oil=0.3/10 (by mass)]
3.4 Other influencing factors on OADS
Other factors, namely, temperature, and the amount of AlCl3and PVC, may also have important effect on the OADS. The results are presented in Fig. 6. The S-removal rates for the three thiophenic S-compounds, i.e. T, BT and DBT, increase remarkably with temperature, implying a high activation energy for the corresponding alkylation reactions. Moreover, the S-removal rate for DBT is very low at 293 and 313 K, but it increases sharply as the temperature rises to 333 K, which indicates that a higher temperature is necessary for the efficient removal of DBT or its homologues from oil through alkylation reaction with PVC. However, further increase of temperature from 333 K to 353 K only gives rise to a slight increase of S-removal rate. This may be ascribed to the inherent low alkylating reactivity of DBT as well as the availability of the active chlorines that are accessible to DBT molecules due to the limited porosity and specific surface area of the PVC powder. Besides, the slight increase of desulfurization rate from 63% to 66% with reaction time at 353 K may originate from the inward diffusion of DBT molecules from the PVC surface, and the resulting alkylation with the active chlorines wherein.
The influence of the amount of AlCl3on S-removal at fixed amount of PVC is presented in Fig. 7. The S-removal for T and BT increases significantly with the amount of AlCl3and then levels off, which may be attributed to the excellent adsorptive desulfurization performance of AlCl3for T and BT in addition to the alkylation desulfurization, while the S-removal for DBT increases steadily with the amount of AlCl3, since the adsorptive S-removal of AlCl3is quite low, as shown in Fig. 2, and the increase of S-removal is mainly related to the increasing catalysis as the amount of AlCl3increases. Further, the desulfurization activity of the PVC+AlCl3mixture for the S-compounds follows the order of T>BT>DBT, which may be ascribed to the synergetic effect of both adsorptive desulfurization and alkylation desulfurization.
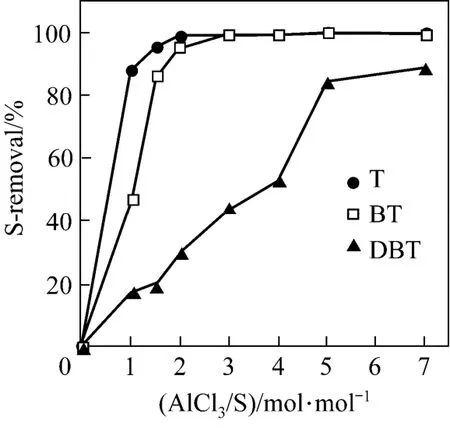
Figure 7 Relationship between S-removal and amount of AlCl3[PVC/oil=0.3/10 (by mass); 120 min; 333 K]
As a reacting regent with thiophenic S-compounds with the catalysis of AlCl3, the amount of PVC may also affect the alkylation reaction and the desulfurization rate to some extent depending on the overall heterogeneous reaction kinetics. The influence of the amount of PVC on the S-removal of T, BT and DBT at 333 K using AlCl3as catalyst is presented in Fig. 8. Both T and BT can be removed completely with different amounts of PVC, which implies that the adsorptive desulfurization of AlCl3plays a dominant role at the mole ratio of AlCl3/S being 5︰1, where an excellent desulfurization is achievable even in the absence of PVC, as shown in Fig. 2. However, the influence of PVC on the S-removal of DBT seems strange. The S-removal increases slightly as the amount of PVC increases to 0.3 g PVC per 10 g oil, and then decreases drastically. The weak dependence of the S-removal on the amount of PVC may be associated with the excessive usage of PVC in comparison with DBT on mole basis, which reduces the Lewis acidity and catalysis of AlCl3due to the strong acid-base complexation interaction between PVC and AlCl3.
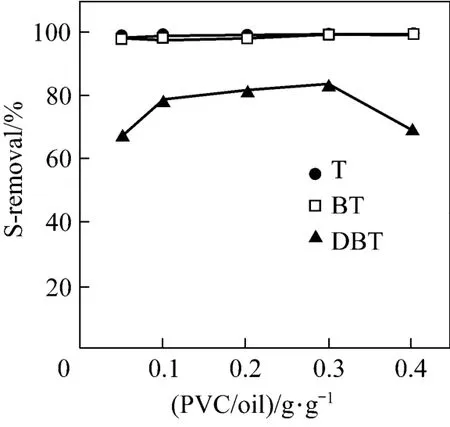
Figure 8 Relationship between S-removal and amount of PVC [AlCl3/S=5︰1 (by mole); 120 min; 333 K]
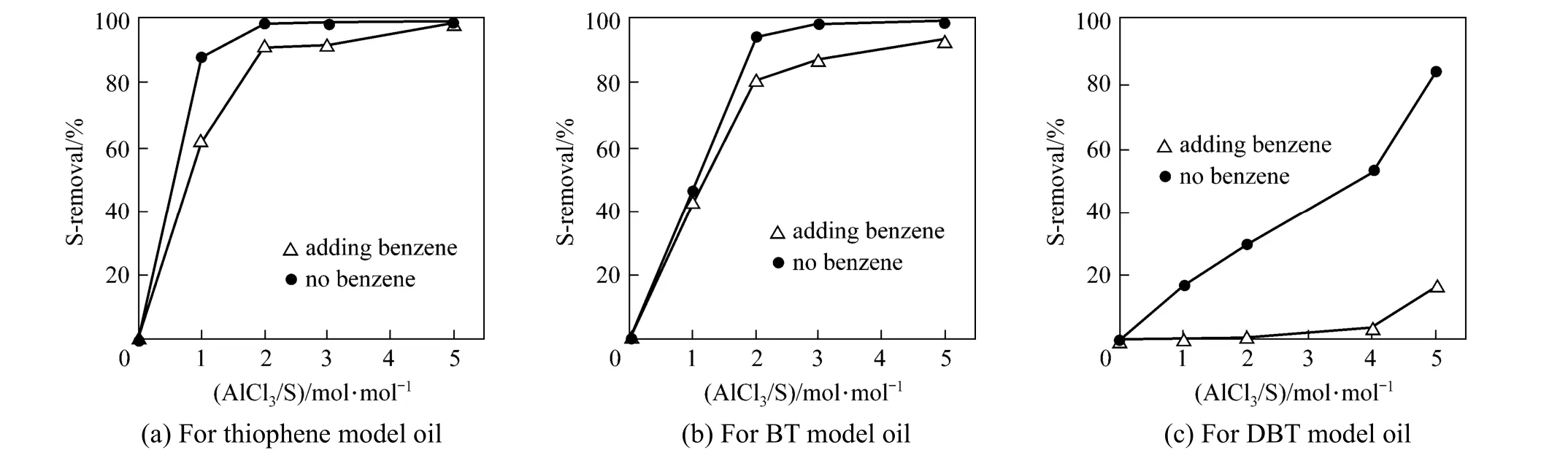
Figure 9 Comparison of S-removal between 25% (by mass) benzene and benzene-free systems at varying amount of AlCl3[PVC/oil=0.3/10 (by mass); 120 min; 333 K]
3.5 OADS selectivity
In the real oil, large amount of aromatics ranging from 20% to 45% (by mass) is present and may result in competitive alkylation with thiophenic S-compounds, decreasing the desulfurization. To assess such influence of aromatics in the oil, new model oils with 25% (by mass) benzene and 1000 μg·g?1S-content are prepared and used in the experiments. The S-removal results with and without benzene are compared with respect to T, BT and DBT at varying mole ratios of AlCl3/S, as shown in Fig. 9. The addition of benzene does reduce the S-removal for the three S-compounds, and T and BT still keep good alkylation activity in thepresence of excessive benzene. However, DBT encounters a strong competitive reaction of benzene with PVC, decreasing S-removal rate dramatically. As a whole, the OADS performance and selectivity of the present process for T and BT are satisfactory even in the presence of aromatics such as benzene but the desulfurization ability for DBT and its homologues needs to be enhanced further by screening other Lewis acid catalysts and halide-containing polymers under appropriate operation conditions. Therefore, the present process is worthy of further study for practical applications.
3.6 Desulfurization for real gasoline
The desulfurization performance of the present process for real oil was evaluated using a heavy pyrolysis gasoline. The distribution of S-compounds in the gasoline was analyzed by GC-flame photometric detector (GC-FPD) method, and compared with the standard S-compounds of thiophene (T), BT and DBT [28]. The results show that the S-compounds in the oil are mainly various alkylated derivatives of BT and DBT, being all the refractory ones in the conventional HDS process.
The OADS experiments were carried out at 298.15K and 333.15K within 120 min using 5-fold AlCl3/S (by mole) and 0.3 g PVC per 10 g oil. As shown in Fig. 10, the desulfurization rate at 298K is relatively low and increases steadily with time in the range of 20% to 40%, while the counterpart at 333K is quite high and increases little with time in the range of 63% to 66%. The results imply the effectiveness of the OADS process for the real oil, considering the mild desulfurization conditions, the extremely high S-content, 6234 μg·g?1, of the real oil, and the polycyclic aromatic attributes of the S-compounds, i.e. various alkyl substituted BTs and DBTs.
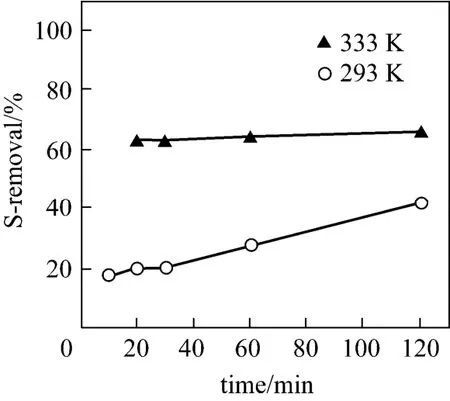
Figure 10 Desulfurization performance for real oil with time at different temperatures [AlCl3/S=5︰1 (by mole); PVC/oil= 0.3/10 (by mass)]
4 CONCLUSIONS
PVC, as an oil insoluble chlorinated polymer, can be used as an alkylating reagent for the thiophenic compounds with the catalysis of AlCl3, in which the aromatic S-compounds are grafted chemically to the polymer chain of PVC through Friedel-Crafts reaction under mild conditions, and removed facilely with the used PVC powder, forming a new desulfurization process, i.e. OADS. In the present OADS process, the catalysis of AlCl3is better than FeCl3, the reactivity of PVC and CPVC is comparable and higher than that of CPP, and the S-removal increases with temperature in the range of 293 to 333K. Benzene coexistent in the oil will deteriorate the OADS performance especially for DBT due to its competitive alkylation with thiophenic S-compounds. As a whole, the PVC+AlCl3mixture is quite effective for the removal of T and BT and maybe their homologues even in the presence of 25% (by mass) of benzene in the oil under appropriate conditions, which is ascribed to the synergetic effects of both adsorptive desulfurization of AlCl3and alkylation reaction between PVC and corresponding sulfuric compounds. The desulfurization ability of the present process for DBT needs to be enhanced further by screening other Lewis acid catalysts and halide-containing polymers, and optimizing the operation conditions.
REFERENCES
1 Song, C., “An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel”, Catal. Today, 86 (1-4), 211-263 (2003).
2 Wang, K., Yang, B., Liu, Y., Yi, C., “Preparation of Ni2P/TiO2-Al2O3and the catalytic performance for hydrodesulfurization of 3-methylthiophene”, Energy Fuels, 23 (9), 4209-4214 (2009).
3 Chen, T., Yang, B., Li, S., Wang, K., Jiang, X., Zhang, Y., He, G.,“Ni2P catalysts supported on titania-modified alumina for the hydrodesulfurization of dibenzothiophene”, Ind. Eng. Chem. Res., 50 (20), 11043-11048 (2011).
4 Seredych, M., Bandosz, T.J., “Removal of dibenzothiophenes from model diesel fuel on sulfur rich activated carbons”, Appl. Catal. B: Environ., 106 (1), 133-141 (2011).
5 Wang, L., Sun, B., Yang, F.H., Yang, R.T., “Effects of aromatics on desulfurization of liquid fuel by π-complexation and carbon adsorbents”, Chem. Eng. Sci., 73, 208-217 (2012).
6 Xiao, J., Song, C., Ma, X., Li, Z., “Effects of aromatics, diesel additives, nitrogen compound, and moisture on adsorptive desulfurization of diesel fuel over activated carbon”, Ind. Eng. Chem. Res., 51 (8), 3436-3443 (2012).
7 Gao, J., Li, H., Zhang, H., Lu, Y., Meng, H., Li, C., “Removal mechanism of thiophenic compounds in model oil by inorganic Lewis acids”, Ind. Eng. Chem. Res., 51 (12), 4682-4691 (2012).
8 Wang, L., Yang, R. T., Sun, C. L., “Graphene and other carbon sorbents for selective adsorption of thiophene from liquid fuel”, AIChE J., 59 (1), 29-32 (2013).
9 Chu, X., Hu, Y., Li, J., Liang, Q., Liu, Y., Zhang, X., Peng, X., Yue, W., “Desulfurization of diesel fuel by extraction with [BF4]?based ionic liquids”, Chin. J. Chem. Eng., 16 (6), 881-884 (2008).
10 Nie, Y., Li, C., Sun, A., Meng, H., Wang, Z., “Extractive desulfurization of gasoline using imidazolium-based phosphoric ionic liquids”, Energy Fuels, 20 (15), 2083-2087 (2006).
11 Schmidt, R., “[bmim]AlCl4ionic liquid for deep desulfurization of real fuels”, Energy Fuels, 22 (3), 1774-1778 (2008).
12 Asghar, M.D., Mohammad, A.S., Mohammad A. N., “Oxidative desulfurization of non-hydrotreated kerosene using hydrogen peroxide and acetic acid”, Chin. J. Chem. Eng., 17 (5), 869-874 (2009).
13 Zhang, H., Gao, J., Meng, H., Lu, Y., Li, C., “Catalytic oxidative desulfurization of fuel by H2O2in situ produced via oxidation of isopropanol”, Ind. Eng. Chem. Res., 51 (13), 4868-4874 (2012).
14 Dai, W., Zhou, Y., Wang, S., Su, W., Sun, Y., Zhou, L., “Desulfurization of transportation fuels targeting at removal of thiophene/benzothiophene”, Fuel Process. Technol., 89 (8), 749-755 (2008).
15 Belliere, V., Geantet, C., Vrinat, M., Ben-Taarit, Y., Yoshimura, Y.,“Alkylation of 3-methylthiophene with 2-methyl-2-butene over a zeolitic catalyst”, Energy Fuels, 18 (6), 1806-1813 (2004).
16 Arias, M., Laurenti, D., Geantet, C., Vrinat, M., Hideyuki, I., Yoshimura, Y., “Gasoline desulfurization by catalytic alkylation over silica-supported heteropolyacids: From model reaction to real feed conversion”, Catal. Today, 130 (1), 190-194 (2008).
17 Zheng, X., Dong, H., Wang, X., Shi, L., “Study on olefin alkylation of thiophenic sulfur in FCC gasoline using La2O3-modified HY zeolite”, Catal. Lett., 127 (1-2), 70-74 (2009).
18 Wang, R., Li, Y., “Preparation of MCM-41 supported phosphoric acid catalyst for thiophenic compounds alkylation in FCC gasoline”, Catal. Commun., 11 (8), 705-709 (2010).
19 Guo, B., Wang, R., Li, Y., “Gasoline alkylation desulfurization over Amberlyst 35 resin: Influence of methanol and apparent reaction kinetics”, Fuel, 90 (2), 713-718 (2011).
20 Liu, Y., Yang, B., Yi, C., Chen, T., Li, S., “Kinetics study of 3-methylthiophene alkylation with isobutylene catalyzed by NKC-9 ion exchange resin”, Ind. Eng. Chem. Res., 50 (16), 9609-9616 (2011).
21 Wang, R., Li, Y., Guo, B., Sun, H., “Effect of methanol on catalytic performance of HY zeolite for desulfurization of FCC gasoline by alkylation”, Ind. Eng. Chem. Res., 51 (18), 6320-6326 (2012).
22 Arias, M., Laurenti, D., Bellière, V., Geantet, C., Vrinat, M., Yoshimura, Y., “Preparation of supported H3PW12O40·6H2O for thiophenic compounds alkylation in FCC gasoline”, Appl. Catal. A: Gen., 348 (1), 142-147 (2008).
23 Nick, A., Colins, T.J.C., “Mobil oil corporation”, US Pat., 5599441 (1997).
24 Shiraishi, Y., Taki, Y., Hirai, T., Komasawa, I., “A novel desulfurization process for fuel oils based on the formation and subsequent precipitation of S-alkylsulfonium salts. 1. Light oil feedstocks”, Ind. Eng. Chem. Res., 40 (4), 1213-1224 (2011).
25 Shiraishi, Y., Tachibana, K., Taki, Y., Hirai, T., Komasawa, I., “A novel desulfurization process for fuel oils based on the formation and subsequent precipitation of S-alkyl-sulfonium salts. 2. Catalyticcracked gasoline”, Ind. Eng. Chem. Res., 40 (4), 1225-1233 (2001).
26 Vymazal, Z., Mastn?, L., Vymazalová, Z., “Study of the effect of the chloroallyl group content on the thermal dehydrochlorination of PVC”, Eur. Polym. J., 21 (8), 747-751 (1985).
27 Lu, W., Cao, T., Wang, Q., Cheng, Y., “Plasma-assisted synthesis of chlorinated polyvinyl chloride (CPVC) using a gas-solid contacting process”, Plasma Process. Polym., 8 (2), 94-99 (2011).
28 Gao, J., Chen, X., Ren, N., Wu, W., Li, C., Meng, H., Lu, Y., “Acylation desulfurization of oil via reactive adsorption”, AIChE J., 59 (8), 2966-2976 (2013).
2013-03-17, accepted 2013-10-19.
* Supported by the National Natural Science Foundation of China (21376011) and the Fundamental Research Foundation of Sinopec (X505015).
** To whom correspondence should be addressed. E-mail: Licx@mail.buct.edu.cn
 Chinese Journal of Chemical Engineering2014年6期
Chinese Journal of Chemical Engineering2014年6期
- Chinese Journal of Chemical Engineering的其它文章
- Unified Model of Purification Units in Hydrogen Networks*
- Photochemical Process Modeling and Analysis of Ozone Generation
- Roles of Biomolecules in the Biosynthesis of Silver Nanoparticles: Case of Gardenia jasminoides Extract*
- Phase Behavior of Sodium Dodecyl Sulfate-n-Butanol-Kerosene-Water Microemulsion System*
- Symbiosis Analysis on Industrial Ecological System*
- Modeling and Optimization for Short-term Scheduling of Multipurpose Batch Plants*
