Roles of Biomolecules in the Biosynthesis of Silver Nanoparticles: Case of Gardenia jasminoides Extract*
Lü Fenfen (呂芬芬), GAO Yixian (高藝), HUANG Jiale (黃加樂(lè)), SUN Daohua (孫道華)** and LI Qingbiao (李清彪)Department of Chemical and Biochemical Engineering, College of Chemistry and Chemical Engineering, Fujian Provincial Key Laboratory of Chemical Biology, Xiamen University, Xiamen 361005, China
Roles of Biomolecules in the Biosynthesis of Silver Nanoparticles: Case of Gardenia jasminoides Extract*
Lü Fenfen (呂芬芬), GAO Yixian (高藝), HUANG Jiale (黃加樂(lè)), SUN Daohua (孫道華)** and LI Qingbiao (李清彪)
Department of Chemical and Biochemical Engineering, College of Chemistry and Chemical Engineering, Fujian Provincial Key Laboratory of Chemical Biology, Xiamen University, Xiamen 361005, China
Rapid development of biosynthesis of metal nanoparticles using plants has attracted extensive interests to further investigate this novel and eco-friendly method. In the biosynthesis process, the plant may act as reducing agent, capping agent or shape directing agent. However, identifying specific roles of various components in the plant is challenging. In this study, we use biosynthesis of silver nanoparticles with Gardenia jasminoides Ellis extract to address this issue. The formation process of silver nanoparticles is investigated and the nanoparticles are characterized with the ultraviolet-visible spectroscopy, Fourier transform infrared spectra and scanning electron microscopy. The results indicate that the Gardenia jasminoides Ellis extract can reduce silver ions to form silver nanoparticles, stabilize the nanoparticles, and affect the growth of silver nanocrystal to form silver nanowires. Only geniposide in the extract exhibits good shape-directing ability for silver nanowires. It is found that bovine albumin is a potential capping agent, whereas rutin, gallic acid and chlorogenic acid possess reducing and capping ability.
biosynthesis, nanoparticle, nanowire, silver
1 INTRODUCTION
Synthesis of nanoparticles is of great importance and interest due to their particular physical and chemical properties, which provide a lot of opportunities for technical advances in the fields of mechanics, optics, electronics, chemical industry, medical science, and catalysis [1-5]. The synthesis of nanoparticles by various physical and chemical methods, such as laser radiation [6], ultrasound irradiation [7], evaporative cooling [8], chemical vapor deposition [9], explosion [10], impregnation [11], co-precipitation [12], sol-gel [13], and deposition-precipitation [14], has been reported extensively. Although these methods have successfully produced well-defined nanoparticles, they are environmental unfriendly and economically unsustainable. To overcome the problems, several studies have used biological resources available in nature, such as bacteria [15, 16], fungi [17], yeast [18], algae [19], and plants [20-22], for the synthesis of a variety of metal nanoparticles. Since the maintenance of cell cultures is elaborate, utilization of plant is well defined and established. Plant-mediated nanoparticle biosynthesis provides an important opportunity for straightforward, rapid and convenient approaches for the formation of metal nanoparticles. Plant-mediated biosynthesis of metal nanostructures only requires mild reaction conditions, and thus has many environmental and economic advantages. More importantly, the as-biosynthesized nanostructures possess high stability, which is one of the key issues for making nanoparticles. Therefore, the rapid development of the plant-mediated method has captured considerable attention in recent years.
Many kinds of plants have been successfully utilized for synthesizing silver nanoparticles. Important examples include plant leaf/seed extract of alfalfa [23], Capsicum annuum [24], Azadirachta indica [25], Lippia citriodora [26], Polyalthia longifolia [27], Stevia rebaudiana [28], Crossandra infundibuliformis [29] and Ficus benghalensis [30]. In the synthesis process, bioactive components in the plant, such as proteins, polyphenols, flavonoid and reducing sugars, play different roles. For example, Zhou et al. [31] have demonstrated that reducing sugars and flavonoids are important reductants responsible for the formation of gold nanoparticles, and the proteins are not reducing agent but capping agent. Raghunandan et al. [32] have found that flavonoids could be adsorbed on the metal surface by interaction with carbonyl groups or π-electrons, and the internal conversion mechanism of ketone group to carboxylic acid in flavonoids may influence the metal ion reduction. Shankar et al. [33] have reported that the formation of gold nanoparticles by lemongrass plant is a result of reduction of aqueous AuCby the reducing sugars and the aldehydes/ ketones bound to the nascent spherical nanoparticles. Huang et al. [34] have demonstrated that polyol components and water-soluble heterocyclic components are involved in the reduction of silver ions as well as the stabilization of nanoparticles. Bar et al. [35] have reported the roles of cyclic peptides as bio-reducing and encapsulating agents for the synthesis of silver nanoparticles by Jatropha curcas, and hypothesized that smaller particles are mostly stabilized by cyclic octapeptide.
It is clear that the formation of metal nanoparticles by plants usually involves several active components.In the synthesis process, the plant may act as reducing agent, capping agent or shape directing agent. Nevertheless, due to the complicated nature and enormous diversity of plants, identifying particular roles of different plant components responsible for the formation of silver nanoparticles is challenging. In this study, we obtain silver nanoparticles by using the extract of Gardenia jasminoides Ellis. The role of proteins, flavonoids, reducing sugars, polyphenols, geniposide and chlorogenic acids as the main components in the extract of Gardenia jasminoides Ellis are systematically investigated and clarified.
2 EXPERIMENTAL
2.1 Chemicals and materials
Sundried Gardenia jasminoides Ellis was purchased from Xiamen Jiuding Drugstore (China). Silver nitrate (AgNO3), rutin hydrate (C27H30O16·3H2O), gallic acid (C7H6O5), 3,5-dinitrosalicylic acid, phenol (C6H5OH), sulfuric acid (H2SO4), and D-glucose (C6H12O6) were purchased from Sinopharm Chemical Reagent Co. Ltd., China and used as received.
2.2 Preparation of extracts of Gardenia jasminoides Ellis
The Gardenia jasminoides Ellis was ground to powder before use. 1.0 g of such powder was dispersed in 250 ml conical flask with 100 ml deionized water in a water bath shaker for 12 h. The mixture was filtered to obtain the extract for further experiments.
2.3 Synthesis of silver nanoparticles
Biosynthesis of silver nanoparticles was carried out by a simple procedure by reducing AgNO3with the extract of Gardenia jasminoides Ellis. Specifically, a certain amount of aqueous AgNO3(100 mmol·L?1) was added into a 100 ml flask with 50 ml of extract. Then, the reaction flask was covered with aluminum foil and incubated in a water bath shaker (30 °C, 150 r·min?1).
2.4 Characterization of silver nanoparticles
UV-Vis spectroscopic analyses of silver nanostructures were carried out as a function of bioreduction time at room temperature using TU 1900 UV-Vis spectrophotometer (Pgeneral, China) at a resolution of 1 nm. Samples for scanning electron microscopy (SEM) were prepared by placing drops of silver hydrosol on silicon wafers and allowing water or ethanol to evaporate completely. Then, the silicon wafers with silver nanoparticles were analyzed by a LEO-1530 Electron Microscope (LEO, Germany). Fourier transform infrared spectroscopy (FTIR) analysis was performed in order to identify the biomolecules responsible for the reduction of silver ions in Gardenia jasminoides Ellis extract. Specifically, the extract before reaction and the resulting solution after biosynthesis of silver nanoparticles were dried at 50 °C. The dried samples were ground with KBr (dried at 120 °C) and analyzed by Nicolet IR200 spectrophotometer (Thermo Nicolet, USA).
2.5 Determination of components of Gardenia jasminoides Ellis
The contents of proteins, flavonoid, reducing sugar, polyphenol in the Gardenia jasminoides Ellis extract before and after the reaction were determined by the spectrophotometric analysis. The contents of geniposide and chlorogenic acid in samples were obtained by high performance liquid chromatography (HPLC) method.
The content of proteins was quantified by the coomassie brilliant blue colorimetric method [36]. Coomassie brilliant blue G-250 dye regent shows red color (the maximum UV-Vis absorption at 595 nm) in acidic state, while combined with protein, it shows blue color instead. 10 mg bovine albumin was dissolved in 100 ml water in 100 ml volumetric flask for linear assay to establish the calibration line.
The content of flavonoid was determined using rutin as a standard [37]. The absorbance at 510 nm was measured against the color-developing blank agent using UV-Vis spectrophotometer. Rutin of 50 mg was dissolved in 20 ml alcohol (95%, by volume) in a 250 ml volumetric flask, and the solution was diluted 10 times with deionized water. The diluted solution was employed to establish the calibration line.
The 3,5-dinitrosalicylic acid method was used for determination of reducing sugar [38]. Reducing sugar would reduce the nitro on 3,5-dinitrosalicylic acid to amino in the presence of sodium hydroxide and glycerol, and the ammonium compound would appear orange. The absorbance of samples at 540 nm was measured by UV-Vis spectrophotometer. D-Glucose of 3.0 g (dried at 105 °C) was dissolved in 1000 ml deionized water as standard solution to make the calibration line.
The Prussian blue method was used to determine the total polyphenols content [39]. A combination of 1.0 ml aqueous Prussian blue solution (100 mmol·L?1HCl, 1.6 mmol·L?1K3Fe(CN)6, and 20 mmol·L?1FeCl3) and 3.0 ml adjusted sample solution were vortex-mixed in a test tube of 10 ml. The mixture showed blue color in 15 min after vortex. 3.0 ml of H3PO4was added into the solution, agitated, and kept for 2 min at room temperature. Finally, aqueous Arabic gum (2 ml, 1%) was added into the mixture. The absorbance of mixture at 700 nm was measured with the UV-Vis spectrophotometer.
A straightforward, sensitive and specific HPLC method was employed to determined the content of geniposide and chlorogenic acid [40, 41], with anAgilent 1200 liquid chromatography system (Agilent Technologies, USA). For chromatographic analysis, a Hypersil ODS C18 column was used at a temperature of 30 °C. The mobile phase consisted of 100% methanol and 2% glacial acetic acid. The flow rate was 0.8 ml·min?1and the injection volume was 10 μl. Absorbance at 239 nm for geniposide and at 327 nm for chlorogenic acid was measured for quantification of the two compounds. The mixture solution of geniposide (100 mg·L?1) and chlorogenic acid (100 mg·L?1) was employed to establish the calibration line. The sample to be measured was filtered through a 0.45 μm membrane before injection for HPLC analysis.
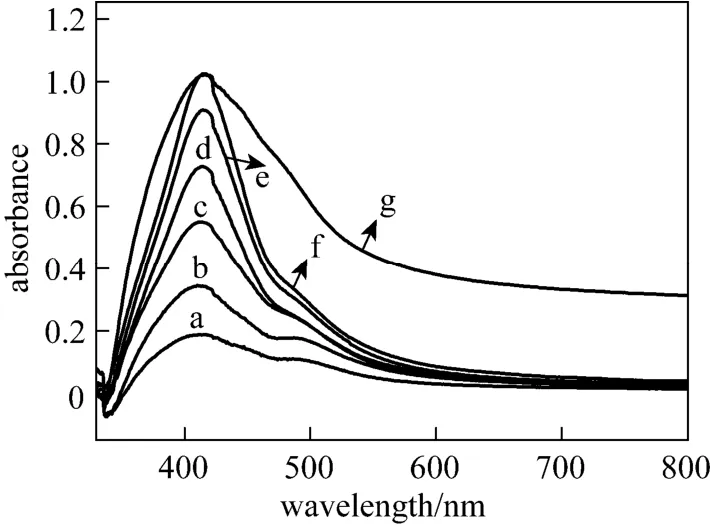
Figure 1 UV-Vis spectra of silver nanoparticles by reducing AgNO3with Gardenia jasminoides Ellis extract a—1d; b—2d; c—3d; d—4d; e—6d; f—7d; g—14d
3 RESULTS AND DISCUSSION
3.1 Preparation of silver nanoparticles with Gardenia jasminoides Ellis extract
The formation of silver nanoparticles was primarily characterized by UV-visible spectroscopy analysis, which is generally considered to be a reliable way to examine size and shape of nanoparticles in aqueous solution [33, 42]. Herein, reduction of aqueous AgNO3during exposure to the extract of Gardenia jasminoides Ellis could be easily monitored by UV-Vis spectroscopy. Generally, the surface plasmon band of silver nanoplates falls in the range of 450-870 nm, with nanoparticles in the range of 400-450 nm and nanowires in the range of 350-390 nm in aqueous solutions [43-46]. Fig. 1 shows the UV-Vis spectra recorded from the reaction mixture of aqueous AgNO3and Gardenia jasminoides Ellis extract as a function of reaction time. It clearly demonstrates that the maximum absorbance wavelength red shifts and the absorbance increases with time from one day to two weeks. The red shifts also indicate the increasing size of silver nanoparticles, which is confirmed by the SEM images (Fig. 2). It is worth to note that the peak on the 14th day is broadened, due to the formation of
O stretching vibration in aldehydes and cyclic compounds, respectively. The peaks at 1384 and 2927 cm?1is due to the bending vibration and stretching vibration of C silver nanowires [Fig. 2 (F)].
FTIR analysis was carried out to identify possible functional groups responsible for the formation of silver nanoparticles. The peaks at 1045, 1075, 1158, 1284, 1384, 1440, 1514, 1630, 1701, 1756, 2927, and 3410 cm?1are identified [Fig. 3 (a)] before the reaction. Specifically, the peaks at 1075 and 1045 cm?1correspond to the hemiacetal in carbohydrate; the peaks at 1158 and 1284 cm?1can be attributed to the C
C in aromatic compounds; the peaks at 1630 cm?1is attributed to the C H in alkanes; the peaks of 1440 and 1514 cm?1are associated with C
O stretching vibration in carbonyl compounds or carboxylic acids; and the peak at 3410 cm?1was assigned to O
O in carboxylates; the peaks at 1756 and 1701 cm?1are associated with C
H stretching vibration in alcohols and phenolic compounds. Through the peak identification, we are able to show that the compounds responsible for the formation of silver nanoparticles in Gardenia jasminoides Ellis extract may includesaccharides, polyphenols, aldehydes, alcohol ketones, and carbonyl compounds.
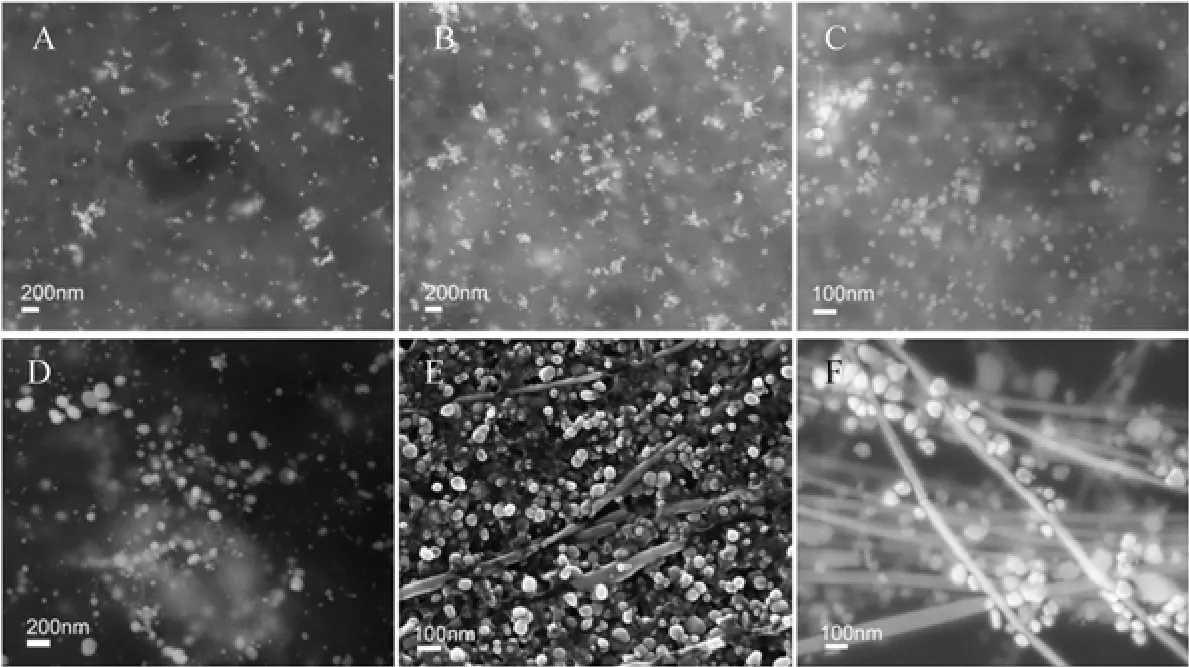
Figure 2 SEM images of silver nanostructures synthesized by aqueous silver nitrate reacted with Gardenia jasminoides Ellis extract A—1d; B—2d; C—4d; D—6d; E—7d; F—14 d
The FTIR spectra of the extract with AgNO3solution at 7 d and 14 d are presented as curves b and c. The peak at 1075 cm?1after reaction appears weaker than that before reaction, due to the reduction process with hemiacetal in carbohydrate [20]. The disappearance of the peaks at 1284 and 1514 cm?1is because of the reduction of silver ions by aldehydes and cyclic compounds. The peaks at 1756 and 1701 cm?1disappear after reaction. Earlier studies have demonstrated that polyvinyl pyrrolidone (PVP) protects palladium nanoparticles via the coordination of the C O group with particle surface [47]. Therefore, we speculate that the formation of silver nanoparticles is due to the reduction of aqueous AgNO3by the saccharides, carbonyl compounds or phenolic hydroxyl group, and the aldehydes ketones binding to silver nanoparticles act as protective group.
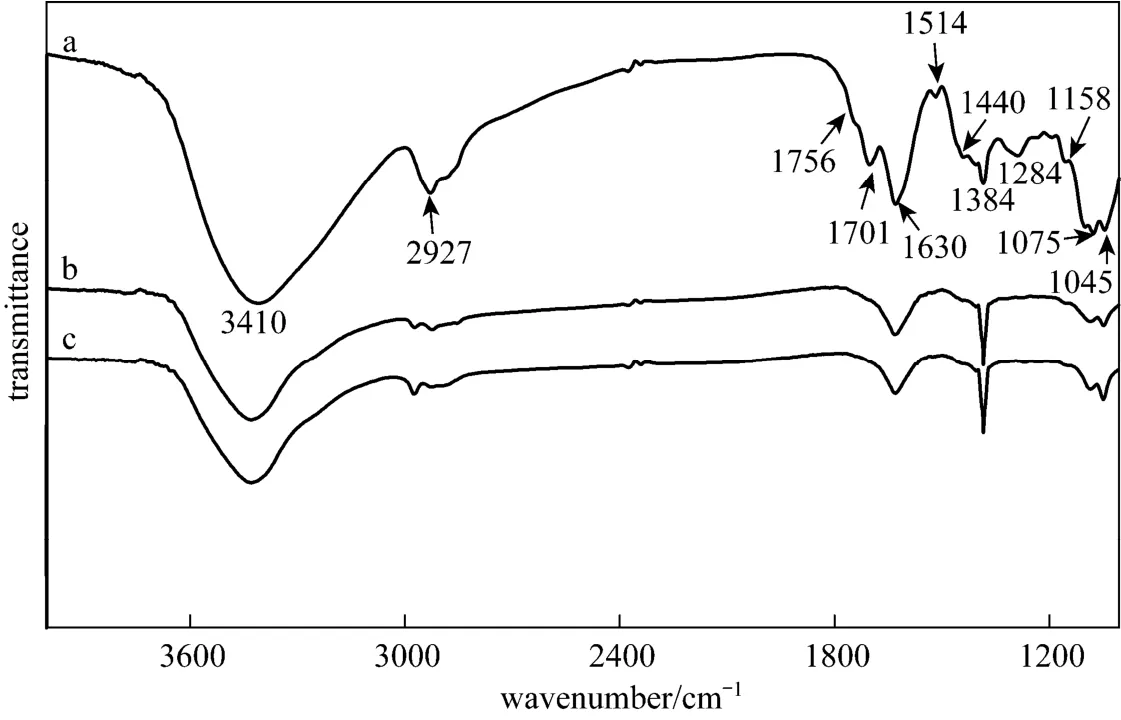
Figure 3 FTIR spectra of Gardenia jasminoides Ellis extract before bioreduction (a), after bioreduction for 7 days (b) and 14 days (c) of silver nitrate
3.2 Variance of main components in the extract before and after reaction
In order to identify the active biomolecules responsible for the formation of silver nanoparticles, the major biomolecule components [41, 48] of the extract before and after the reaction were tested and the results are showed in Table 1. It is clear that proteins, flavonoid, geniposide and chlorogenic acid presents noticeable changes after reaction among all the biomolecules, revealing that they should be responsible for the formation of silver nanoparticles. This result is consistent with that of the FTIR analysis.
To further confirm the role (reducing agent, capping agent, and shape-directing agent) of the biomolecule components in the Gardenia jasminoides Ellis extract during the biosynthesis of silver nanoparticles, six pure chemicals of these active components (bovine albumin, rutin, glucose, gallic acid, geniposide and chlorogenic acid) are employed for the following evaluation. Notably, bovine albumin, rutin, glucose and gallic acid are chosen to represent the protein, flavonoid, reducing sugars and polyphenols in the Gardenia jasminoides Ellis extract, respectively, since each of them includes a wide range of constituents.
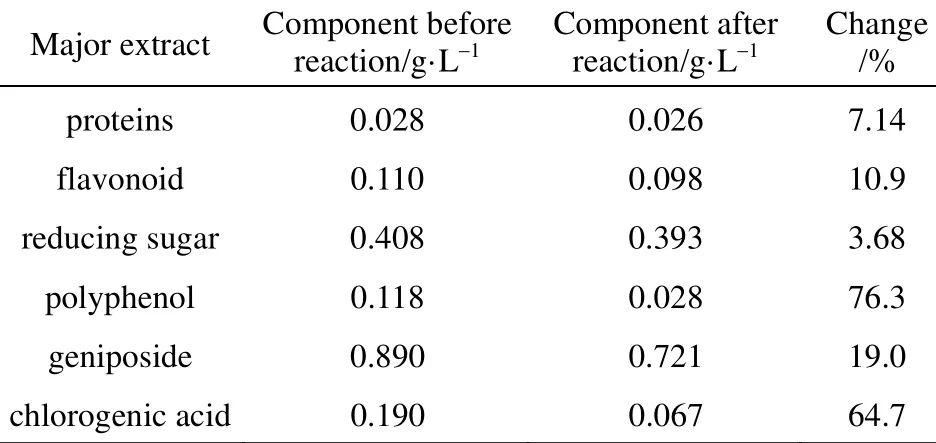
Table 1 Components of Gardenia jasminoides Ellis extract before and after the reaction
3.3 Identification of reducing agent in Gardenia jasminoides Ellis extract
Given constant amount of aqueous AgNO3(50 ml, 1 mmol·L?1), with PVP as typical capping agent, comparative amounts of six components were added to carry out possible reducing reaction in Gardenia jasminoides Ellis extract. The results are shown in Fig. 4. The fairly sharp absorbance at 400-450 nm is responsible for the formation of spherical silver nanoparticles [49]. No peak is observed with deionized water as a reference. Curves c, e and g show the resulting silver nanoparticles by the reactions of AgNO3with rutin, gallic acid and chlorogenic acid, respectively, which reveal that these three components have the reducing ability. Specially, the absorption peak intensity of silver nanoparticles by adding gallic acid is stronger than theother two, indicating that gallic acid is a stronger reducing agent.
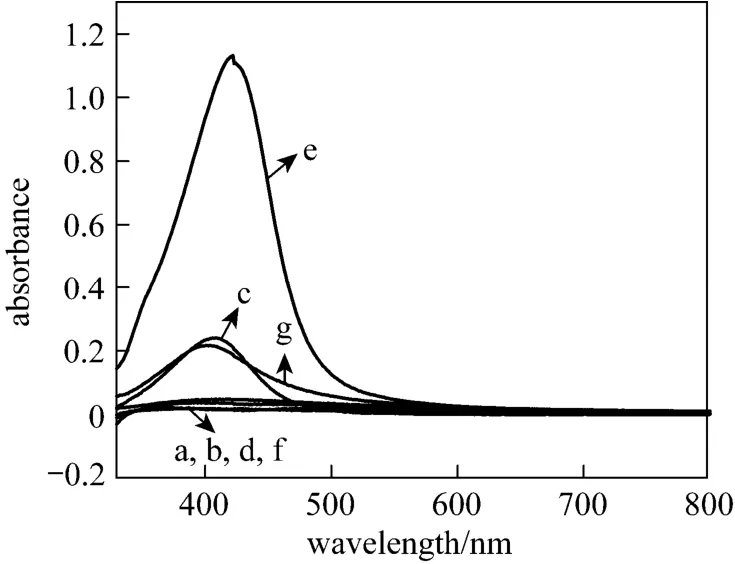
Figure 4 UV-Vis spectra of silver nanostructures synthesized by aqueous silver nitrate reacted with different components with PVP as protectant a—reference; b—bovine albumin; c—rutin; d—glucose; e—gallic acid; f—geniposide; g—chlorogenic acid
3.4 Identification of capping agent in Gardenia jasminoides Ellis extract
To confirm the potential capping agents in Gardenia jasminoides Ellis extract, the reactions of AgNO3with six components are presented in Fig. 5, with NaBH4added as typical reducing agent. No absorbance peak appears in the blank control sample and glucose (curves a and d), while geniposide (curve f) show minimal capping capacity in biosynthesis of silver nanoparticles. Obviously, bovine albumin, rutin, gallic acid and chlorogenic acid display peculiar capping capacity.
3.5 Identification of shape-directing agent in Gardenia jasminoides Ellis extract
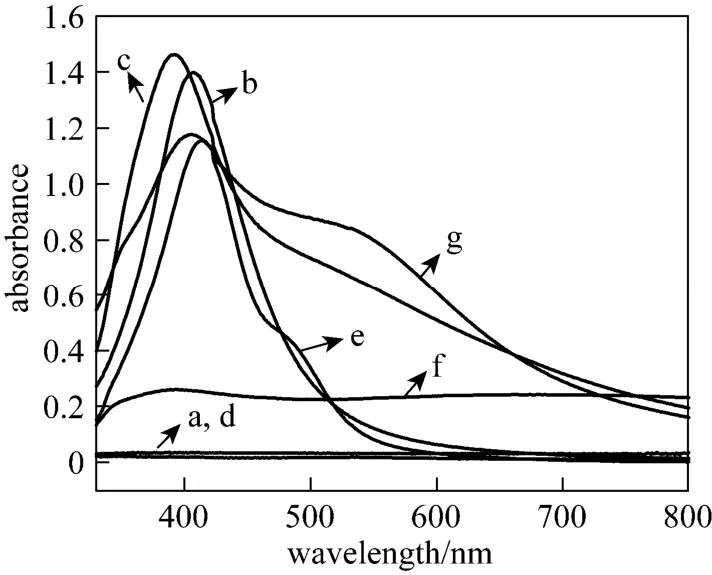
Figure 5 UV-Vis spectra of silver nanostructures synthesized by aqueous silver nitrate reacted with components with NaBH4as reductanta—reference; b—bovine albumin; c—rutin; d—glucose; e—gallic acid; f—geniposide; g—chlorogenic acid
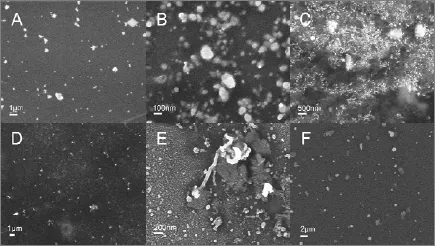
Figure 6 SEM images of silver nanostructures synthesized by aqueous silver nitrate reacted with different components with PVP as protectant and NaBH4as reductant A—bovine albumin; B—rutin; C—glucose; D—gallic acid; E—geniposide; F—chlorogenic acid
In order to identify possible shape-directing agents, NaBH4and PVP were added to the aqueous mixture of AgNO3and six components separately. Fig. 6 shows the SEM images of silver nanoparticles synthesized by aqueous AgNO3reduced by different components with PVP as a protectant and NaBH4as a reductant. Clearly, silver nanoparticles are present in all the samples. Especially, Fig. 6 (E) illustrates silver nanoparticles with different morphologies obtained through adding geniposide into the initial solution. The formation of as-synthesized silver nanorods indicates the shape-directing capacity of geniposide. The addition of geniposide in chemical reduction systems results in the appearance of silver nanorods, since NaBH4has strong deoxidizing ability, which may impose restriction on shape-directing capacity of geniposide. In order to further confirm the role of geniposide in the process of synthesizing silver nanoparticles, a milder agent, the extract of Cinnamomum camphora leaf, was used as the reducing and protecting agent. Fig. 7 (C, D) shows typical SEM image of silver nanowires, while spherical and plate-like silver nanoparticles, without any silver nanowires, are observed in Fig. 7 (A, B). This further indicates thatgeniposide serves as a shape-directing agent in this system to promote the formation of silver nanowires.
The above results are summarized in Table 2, which clearly shows that only geniposide in Gardenia jasminoides Ellis extract exhibits good shape-directing ability for plant-mediated biosynthesis of silver nanowires, but it barely shows any reducing and capping capacity. Rutin, gallic acid and chlorogenic acid possess both reducing and capping capacity, and gallic acid exhibits the strongest reducing capacity for synthesizing silver nanoparticles. Bovine albumin has the capping capacity, and early study has also proved that proteins might be protection agents in biosynthesis of gold nanoparticles [31].
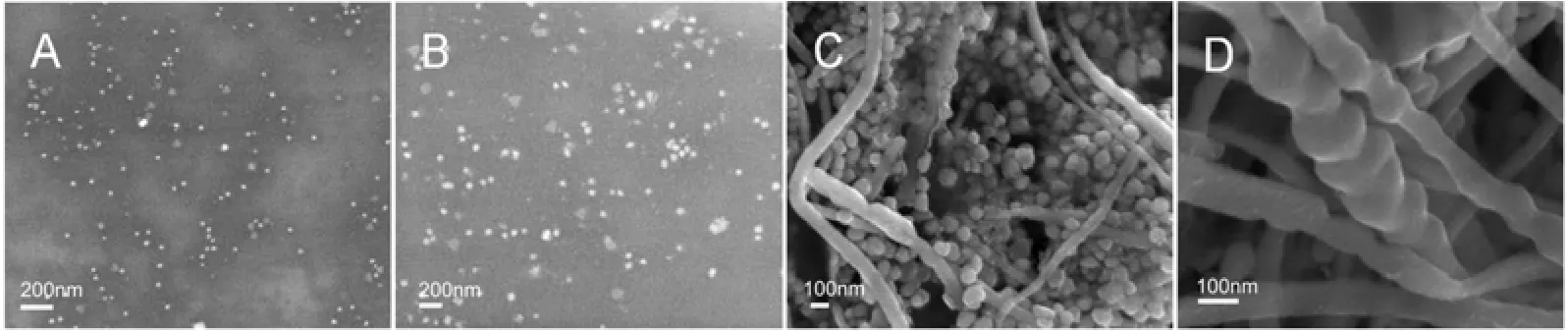
Figure 7 SEM images of silver nanostructures synthesized by aqueous silver nitrate reacted with water extract of Cinnamomum camphora leaf (A, B) and with 16 mg·L?1geniposide added (C, D)

Table 2 Summary of the effect of biomolecule components in the Gardenia jasminoides Ellis
4 CONCLUSIONS
Biosynthesis of silver nanoparticles by Gardenia jasminoides Ellis extract is used as a showcase to systematically study the specific roles of different plant components in the formation of nanoparticles. The component analysis shows that proteins, flavonoid, reducing sugar, polyphenol, geniposide and chlorogenic acid are important for the formation of silver nanoparticles. Especially, the content of flavonoid, polyphenols and chlorogenic acid has a significant drop after reaction. To further ascertain the role of the components in Gardenia jasminoides Ellis extract, pure chemicals of these active components are utilized and corresponding identifying experiments show that rutin, gallic acid and chlorogenic acid have reducing and protecting capacities. It is worth to note that geniposide exhibits good shape-directing capacity for the formation of silver nanowires.
REFERENCES
1 Iravani, S., “Green synthesis of metal nanoparticles using plants”, Green Chemistry, 13 (10), 2638-2650 (2011).
2 Schmid, G., “Large clusters and colloids metals in the embryonic state”, Chemical Reviews, 92, 1709-1727 (1992).
3 Colvin, V.L., Schlamp, M.C., Allvlsatos, A.P., “Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer”, Nature, 370 (4), 354-357 (1994).
4 Wang, R., Hashimoto, K., Fujishima, A., “Light-induced amphiphilic surfaces”, Nature, 388, 431-432 (1997).
5 Medintz, I.L., Uyeda, H.T., Goldman, E.R., mattoussi, H., “Quantum dot bioconjugates for imaging, labelling and sensing”, Nature Materials, 4, 435-446 (2005).
6 Sivakumar, M., Venkatakrishnan, K., Tan, B., “Synthesis of nanoscale tips using femtosecond laser radiation under ambient condition”, Nanoscale Research Letters, 5 (2), 438-441 (2009).
7 Kundu, S., Panigrahi, S., Praharaj, S., Basu, S., Ghosh, S.K., Pal, A., Pal, T., “Anisotropic growth of gold clusters to gold nanocubes under UV irradiation”, Nanotechnology, 18 (7), 075712 (2007).
8 Fisenko, S.P., Khodyko, J.A., “Low pressure evaporative cooling of micron-sized droplets of solutions and its novel applications”, Int. J. Heat and Mass Transfer, 52 (15-16), 3842-3849 (2009).
9 Li, Y.L., Kinloch, I.A., Windle, A.H., “Direct spinning of carbon nanotube fibers from chemical vapor deposition synthesis”, Science, 304 (5668), 276-278 (2004).
10 Wu, W., Zhu, Z., Liu, Z., Xie, Y., Zhang, J., Hu, T., “Preparation of carbon-encapsulated iron carbide nanoparticles by an explosion method”, Carbon, 41, 317-321 (2003).
11 Tsoncheva, T., Rosenholm, J., Linden, M., Kleitz, F., Tiemann, M., Ivanova, L., Dimitrov, M., Paneva, D., Mitov, I., Minchev, C.,“Critical evaluation of the state of iron oxide nanoparticles on different mesoporous silicas prepared by an impregnation method”, Microporous and Mesoporous Materials, 112 (1-3), 327-337 (2008).
12 Yang, H., Song, X., Zhang, X., Ao, W., Qiu, G., “Synthesis of vanadium-doped SnO2nanoparticles by chemical co-precipitation method”, Materials Letters, 57 (20), 3124-3127 (2003).
13 Lu, Y., Yin, Y., Mayers, B.T., Xia, Y., “Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol-gel approach”, Nano Letters, 2 (3), 183-186 (2002).
14 You, X., Chen, F., Zhang, J., Anpo, M., “A novel deposition precipitation method for preparation of Ag-loaded titanium dioxide”, Catalysis Letters, 102 (3-4), 247-250 (2005).
15 Taheri Otaqsara, S.M., “Biosynthesis of quasi-spherical Ag nanoparticle by Pseudomonas aeruginosa as a bio-reducing agent”, The European Physical Journal Applied Physics, 56 (3), 30402 (2011).
16 Fu, M.X., Li, Q.B., Sun, D.H., Lu, Y.H., He, N., Deng, X., Wang, H.X., Huang, J.L., “Rapid preparation process of silver nanoparticles by bioreduction and their characterizations”, Chin. J. Chem. Eng., 14 (1), 114-117 (2006).
17 Du, L.W., Xian, L.A., Feng, J.X., “Rapid extra-/intracellular biosynthesis of gold nanoparticles by the fungus Penicillium sp”, Journal of Nanoparticle Research, 13 (3), 921-930 (2011).
18 Subramanian, M., Alikunhi, N.M., Kandasamy, K., “In vitro synthesisof silver nanoparticles by marine yeasts from coastal mangrove sediment”, Advanced Science Letters, 3 (4), 428-433 (2010).
19 Sicard, C., Brayner, R., Margueritat, J., Hemadi, M., Coute, A., Yepremian, C., Djediat, C., Aubard, J., Fievet, F., Livage, J., Coradin, T., “Nano-gold biosynthesis by silica-encapsulated micro-algae: a‘living’ bio-hybrid material”, J. Mater. Chem., 20 (42), 9342-9347 (2010).
20 Zhang, G., Du, M., Li, Q., Li, X., Huang, J., Jiang, X., Sun, D.,“Green synthesis of Au-Ag alloy nanoparticles using Cacumen platycladi extract”, RSC Advances, 3 (6), 1878 (2013).
21 Lin, L., Wang, W., Huang, J., Li, Q., Sun, D., Yang, X., Wang, H., He, N., Wang, Y., “Nature factory of silver nanowires: plant-mediated synthesis using broth of Cassia fistula leaf”, Chem. Eng. J., 162 (2), 852-858 (2010).
22 Chang, X., Yang, Z., Zeng, R., Yang, G., Yan, J., “Production of chiral aromatic alcohol by asymmetric reduction with vegetable catalyst”, Chin. J. Chem. Eng., 18 (6), 1029-1033 (2010).
23 Gardea-Torresdey, J.L., Gomez, E., Peralta-Videa, J.R., Parsons, J.G., Troiani, H., Jose-Yacaman, M., “Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles”, Langmuir, 19 (4), 1357-1361 (2003).
24 Li, S.K., Shen, Y.H., Xie, A.J., Yu, X.R., Qiu, L.G., Zhang, L., Zhang, Q.F., “Green synthesis of silver nanoparticles using Capsicum annuum L. extract”, Green Chemistry, 9 (8), 852-858 (2007).
25 Tripathy, A., Raichur, A.M., Chandrasekaran, N., Prathna, T.C., Mukherjee, A., “Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves”, Journal of Nanoparticle Research, 12 (1), 237-246 (2009).
26 Cruz, D., Fale, P.L., Mourato, A., Vaz, P.D., Serralheiro, M.L., Lino, A.R.L., “Preparation and physicochemical characterization of Ag nanoparticles biosynthesized by Lippia citriodora (Lemon Verbena)”, Colloids and Surfaces B—Biointerfaces, 81 (1), 67-73 (2010).
27 Kaviya, S., Santhanalakshmi, J., Viswanathan, B., “Green synthesis of silver nanoparticles using Polyalthia longifolia leaf extract along with D-sorbitol: study of antibacterial activity”, Journal of Nanotechnology, 2011, 1-5 (2011).
28 Yilmaz, M., Turkdemir, H., Kilic, M.A., Bayram, E., Cicek, A., Mete, A., Ulug, B., “Biosynthesis of silver nanoparticles using leaves of Stevia rebaudiana”, Materials Chemistry and Physics, 130 (3), 1195-1202 (2011).
29 Kaviya, S., Santhanalakshmi, J., Viswanathan, B., “Biosynthesis of silver nano-flakes by Crossandra infundibuliformis leaf extract”, Materials Letters, 67 (1), 64-66 (2012).
30 Saxena, A., Tripathi, R.M., Zafar, F., Singh, P., “Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity”, Materials Letters, 67 (1), 91-94 (2012).
31 Zhou, Y., Lin, W.S., Huang, J.L., Wang, W.T., Gao, Y.X., Lin, L.Q., Li, Q.B., Lin, L., Du, M.M., “Biosynthesis of gold nanoparticles by foliar broths: roles of biocompounds and other attributes of the extracts”, Nanoscale Research Letters, 5 (8), 1351-1359 (2010).
32 Raghunandan, D., Basavaraja, S., Mahesh, B., Balaji, S., Manjunath, S.Y., Venkataraman, A., “Biosynthesis of stable poly-shaped gold nanoparticles from microwave-exposed aqueous extracellular antimalignant guava (Psidium guajava) leaf extract”, NanobioTechnology, 5 (1-4), 34-41 (2009).
33 Shankar, S.S., Rai, A., Ankamwar, B., Singh, A., Ahmad, A., Sastry, M., “Biological synthesis of triangular gold nanoprisms”, Nature Materials, 3 (7), 482-488 (2004).
34 Huang, J.L., Li, Q.B., Sun, D.H., Lu, Y.H., Su, Y.B., Yang, X., Wang, H.X., Wang, Y.P., Shao, W.Y., He, N., Hong, J.Q., Chen, C.X., “Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf”, Nanotechnology, 18 (10), 105104 (2007).
35 Bar, H., Bhui, D.K., Sahoo, G.R., Sarkar, P., De, S.R., Misra, A.,“Green synthesis of silver nanoparticles using latex of Jatropha curcas”, Colloids and Surfaces A—Physicochemical and Engineering Aspects, 339 (1-3), 134-139 (2009).
36 Hou, M., Food Anlysis, Chemical Industry Press, Beijing (2004). (in Chinese)
37 Sun, C., Huang, K., Chen, C., Zhang, D., “Extraction of flavonoids from Cinnamomum camphora leaves”, Appl. Chem. Ind., 35 (2), 142-143 (2006).
38 Wang, J., Zhang, S., Yang, B., Cheng, P., Wu, Z., Hu, J., “Application of 3,5-dinitrosalicylic acid (DNS) method to test the reducing sugar and water-soluble total sugar content in sugarcane internodes”, Sugarcane and Canesugar, 5, 45-49 (2008).
39 Graham, H.D., “Stabilization of the Prussian blue color in the determination of polyphenols”, Journal of Agricultural and Food Chemistry, 40 (5), 801-805 (1992).
40 He, M.L., Cheng, X.W., Chen, J.K., Zhou, T.S., “Simultaneous determination of five major biologically active ingredients in different parts of Gardenia jasminoides fruits by HPLC with diode-array detection”, Chromatographia, 64 (11-12), 713-717 (2006).
41 Ji, X., Bi, K., Wang, Y., Chen, X., “Simultaneous HPLC determination of chlorogenic acid, jasminoidin and puerarin in Kanggan Jiedu granules”, Chinese Journal of Pharmaceutical Analysis, 30 (1), 56-58 (2010).
42 Shankar, S.S., Ahmad, A., Pasricha, R., Sastry, M., “Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes”, J. Mater. Chem., 13 (7), 1822-1826 (2003).
43 Sun, Y., Gates, B., Mayers, B., Xia, Y., “Crystalline silver nanowires by soft solution processing”, Nano Letters, 2 (2), 165-168 (2002).
44 Zhang, W.C., Wu, X.L., Chen, H.T., Gao, Y.J., Zhu, J., Huang, G.S., Chu, P.K., “Self-organized formation of silver nanowires, nanocubes and bipyramids via a solvothermal method”, Acta Materialia, 56 (11), 2508-2513 (2008).
45 Kottmann, J., Martin, O., Smith, D., Schultz, S., “Plasmon resonances of silver nanowires with a nonregular cross section”, Physical Review B, 64, 235402 (2001).
46 Zhang, Q., Ge, J., Pham, T., Goebl, J., Hu, Y., Lu, Z., Yin, Y., “Reconstruction of silver nanoplates by UV irradiation: tailored optical properties and enhanced stability”, Angewandte Chemie, 48 (19), 3516-3519 (2009).
47 Nemamcha, A., Rehspringer, J.L., Khatmi, D., “Synthesis of palladium nanoparticles by sonochemical reduction of palladium(II) nitrate in aqueous solution”, Journal of Physical Chemestry B, 110 (1), 383-387 (2006).
48 Kim, H.J., Kim, E.J., Seo, S.H., Shin, C.G., Jin, C., Lee, Y.S., “Vanillic acid glycoside and quinic acid derivatives from Gardeniae fructus”, Journal of Natural Products, 69 (4), 600-603 (2006).
49 Vijayaraghavan, K., Nalini, S.P.K., Prakash, N.U., Madhankumar, D.,“Biomimetic synthesis of silver nanoparticles by aqueous extract of Syzygium aromaticum”, Materials Letters, 75, 33-35 (2012).
2013-07-30, accepted 2013-10-22.
* Supported by the National Natural Science Foundation of China (21036004, 21206140) and Science and Technology Program of Xiamen of Fujian Province, China (3502Z20133006).
** To whom correspondence should be addressed. E-mail: sdaohua@xmu.edu.cn
 Chinese Journal of Chemical Engineering2014年6期
Chinese Journal of Chemical Engineering2014年6期
- Chinese Journal of Chemical Engineering的其它文章
- Unified Model of Purification Units in Hydrogen Networks*
- Photochemical Process Modeling and Analysis of Ozone Generation
- Removal of Thiophenic Sulfur Compounds from Oil Using Chlorinated Polymers and Lewis Acid Mixture via Adsorption and Friedel-Crafts Alkylation Reaction*
- Phase Behavior of Sodium Dodecyl Sulfate-n-Butanol-Kerosene-Water Microemulsion System*
- Symbiosis Analysis on Industrial Ecological System*
- Modeling and Optimization for Short-term Scheduling of Multipurpose Batch Plants*
