KAI1 inhibits lymphangiogenesis and lymphatic metastasis of pancreatic cancer in vivo
Xu Liu, Xiao-Zhong Guo, Hong-Yu Li, Jiang Chen, Li-Nan Ren and Chun-Yan Wu
Shenyang, China
KAI1 inhibits lymphangiogenesis and lymphatic metastasis of pancreatic cancer in vivo
Xu Liu, Xiao-Zhong Guo, Hong-Yu Li, Jiang Chen, Li-Nan Ren and Chun-Yan Wu
Shenyang, China
BACKGROUND:Several studies have shown that KAI1 inhibits tumor metastasis, but its mechanism is not clear. The present study aimed to determine the role of KAI1 in lymphatic metastasis, specifically in pancreatic cancer.
METHODS:The KAI1 gene was transfected into the pancreatic cancer cell line MIA PaCa-2 and PANC-1 by using liposomes and selected by G418, and the protein was measured by Western blotting. After successful infection, the cell growth curve was studied by MTT, vascular endothelial growth factor C (VEGF-C) secretion by pancreatic cancer cell were measured by ELISA. The KAI1 and pCMV transfected MIA PaCa-2 cells were renamed as MIA PaCa-2-K and MIA PaCa-2-p. These two kinds of cells were injected into the subcuticular layer of nude mice; both tumor growth and metastasis through the lymphatic nodes were assessed. Lymphangiogenesis in tumors was measured by immunohistochemistry.
RESULTS:The VEGF-C secretion was significantly reduced in MIA PaCa-2 cells compared with PANC-1 cells after being transfected with the KAI1 gene. The growth rate of subcutaneous tumors was similar after the injection of MIA PaCa-2-K, MIA PaCa-2, and MIA PaCa-2-p. MIA PaCa-2-K tumors showed slower lymphangiogenesis and lymph node metastasis compared with MIA PaCa-2 and MIA PaCa-2-p tumors.
CONCLUSION:The overexpression of KAI1 inhibits the lymphangiogenesis and lymph node metastasis of MIA PaCa-2 pancreatic tumors. (Hepatobiliary Pancreat Dis Int 2014;13:87-92)
KAI1;
pancreatic cancer;
lymphatic metastasis; lymphangiogenesis
Introduction
Pancreatic cancer is the fourth most frequent cause of cancer death worldwide. Despite the advance in the diagnosis and management, the prognosis of patients with pancreatic cancer is very poor, the 5-year survival rate remains less than 5% and the median survival time after diagnosis is only 4-6 months.[1-3]The main factor contributing to the high mortality is metastasis. Early lymph node metastasis is common in patients with pancreatic cancer, and the presence of lymphatic metastases is an important indicator for poor prognosis. Thus, the search for novel treatment strategies to inhibit lymphatic metastases and therefore to improve the prognosis are an exigent task.
Lymphatic metastasis is a complex process that involves molecular mechanisms and multiple gene alterations. Metastasis suppressors, such as KAI1, may play a role in this process. Previous studies showed that KAI1 mRNA transcription was significantly lower in lymph node metastasis than in primary tumors. This phenomenon was observed in the cells of the pancreas, colon, lung, esophageal carcinoma, and gastric cancer.[4-7]
KAI1, known as CD82, is a metastasis suppressor. KAI1 has four hydrophobic, and presumably, transmembranous domains, two extracellular domains, and three short intracellular domains. It belongs to the family of tetraspanins. The downregulation of KAI1 is seen often in the advanced stages of many types of tumors.[8, 9]Experimental studies using a combination of in vitro and in vivo approaches have demonstrated that decreased KAI1 expression is associated with reduced homotypic cell adhesion, increased cell migration, and altered ability of tumor cells to bind specific extracellular proteins.[10-12]However, whether increasing KAI1 expression suppresses the process of lymphatic metastasis is not clear.
The present study was undertaken to examine the expression of the KAI1/CD82 protein in pancreatic cancer cell line transfected by the KAI1 gene. Asubcuticular pancreatic tumor model was developed by the injection of pancreatic cancer cells into BALB/c nude mice.
Methods
Cell culture
The human pancreatic cancer cell lines, MIA PaCa-2 and PANC-1, were kindly provided by Dr. Friess (University of Heidelberg, Heidelberg, Germany). The cells were grown as subconfluent monolayers in dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS) at 37 ℃ with 5% CO2. Cell lysates were harvested after 24 hours for Western blotting.
Transfection
Expression plasmids encoding KAI1 (pCMV-KAI1 DNA) was obtained from Dr. Dong (Emory University School of Medicine, Atlanta, Georgia, USA) as a gift. Plasmid transfection was carried out in accordance with the transfection guidelines provided with Lipofectamine 2000 (Gibco BRL, Gaithersburg, Maryland, USA). Briefly, 1×105MIA PaCa-2 and PANC-1 cells were plated in 96-well plates. When the cells reached 90%-95% confluence, 1.0 μg pCMV-KAI1 DNA or pCMV DNA, 1 μL Lipofectamine 2000 reagent, and 100 μL medium without serum (this mixture can be prepared in bulk for multiple wells) were mixed and incubated for 15 minutes at room temperature and then added to each well. The cells were incubated at 37 ℃ in a CO2incubator for 24 hours, and passaged at a ratio of 1:10 into fresh growth medium, then, 400 μg/mL G418 selection medium was added. The stable transfected cells were established in 4 weeks. The expression of the KAI1/CD82 protein was confirmed by Western blotting.
MTT assay
Cell viability was determined by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Briefly, 1×104cells were seeded into 96-well flatbottomed plates in triplicate and allowed to adhere for 12 hours. MTT assays were carried out 24, 48, 72 and 96 hours after seeding. At the time of the assay, the cells were stained with 20 μL MTT (Sigma, St Louis, MO, USA) at 37 ℃ for 4 hours and, after which, dimethyl sulfoxide (DMSO) was added. The microplate reader (Wako, Osaka, Japan) was used to measure the absorbance at 490 nm.
Western blotting analysis
Cells were harvested in a lysis buffer, and total protein was quantified using bicinchoninic acid (BCA) assay. The proteins (30 μg) were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were then incubated in blocking buffer for 2 hours at room temperature, followed by hybridization with anti-KAI1 antibody (1:100 dilution; BD Pharmingen, Heidelberg, Germany) at 4 ℃ for 12 hours. After three washes in Tris-buffered saline (TBS)/0.1% Tween-20, the membranes underwent hybridization with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:2000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for one hour at room temperature. After three washes in TBS/0.1% Tween-20, immunoreactive bands were visualized using an enhanced luminol reagent (Perkin-Elmer Life Sciences, Boston, MA, USA).
Enzyme-linked immunosorbent assay (ELISA)
1×105cells/mL were seeded into 12-well flat-bottomed plates and cultured for 12 hours. After medium exchange, the cells were cultured for 48 hours further. Culture media were collected and centrifuged at 1200 rpm for 6 minutes to remove particles. The supernatants were frozen at -80 ℃. Supernatant concentration of vascular endothelial growth factor C (VEGF-C) was measured using an ELISA kit (R&D systems, Minneapolis, USA) according to the manufacturer's instructions.
Animals and xenograft experiments
Single tumor cell suspensions with >95% viability, which were tested by trypan blue exclusion, were used for injections. 1×106cells in 0.06 mL phosphate buffered saline (PBS) were injected subcutaneously into the left flank region of 10 nude mice per group (20 g body weight; China Medical University Breeding Laboratories, Shenyang, China). Tumor size was monitored at day 7 after inoculation of tumor cells. Measurements of tumor size, with calipers twice a week, started immediately after the appearance of tumors. The volume of the tumor was calculated using the formula length×width × depth×π/6. Mice were sacrificed 7 weeks after tumor cell injection, and each tumor was harvested. The mice were necropsied for examination of left armpit regional lymph node metastases. Tumors and lymph nodes were processed for immunohistochemical analysis.
Immunohistochemistry
Primary tumors and lymph nodes were analyzed by a standard immunoperoxidase staining procedure.Briefly, cryosections of 7 mm thickness were fixed in 4% paraformaldehyde PBS (PH 7.0) for 20 minutes at room temperature. Treated with H2O2(0.3%) for 10 minutes, the sections were incubated with antimouse LYVE-1 antibody (BD Pharmingen, Heidelberg, Germany) for 1 hour at room temperature. After three washes in PBS, the sections were incubated with a biotin labeled secondary antibody. Immunoreactivity was detected with an avidin-biotin-peroxidase complex. The immunoreactive signal was developed by using diaminobenzidine as a substrate for 2 minutes. The sections were counterstained with hematoxylin-eosin (HE) staining. For quantitation of lymphatic vessel density, the average numbers of LYVE-1 positive vessels from three vascular hotspots (the areas of maximal vascular density) were counted.
Statistical analysis
All data were expressed as mean±SD from at least three independent experiments. Two-tailed Student's t test was used to analyze statistical significance, using SPSS 16.0 statistical software. P values <0.05 were considered statistically significant.
Results
Transfection and confirmation of KAI1 expression by pCMV plasmid in MIA PaCa-2 cells
To examine the expression of KAI1/CD82 on cell, the plasmid was transfected into highly metastatic MIA PaCa-2 and PANC-1 pancreatic cancer cells. Transfection was followed by G418 selection for 10 generations, the pCMV-KAI1-transfected cells were renamed as MIA PaCa-2-K and PANC-1-K. The cells transfected with pCMV were renamed as MIA PaCa-2-p and PANC-1-p. Western blotting analysis showed that the KAI1 protein was clearly visible at about 29.1 kDa in MIA PaCa-2-K and PANC-1-K cells, but was almost undetectable in MIA PaCa-2, MIA PaCa-2-p and PANC-1, PANC-1-p cells (Fig. 1).
Cell growth
Effects of KAI1 on proliferation of MIA PaCa-2 and PANC-1 cells in vitro were measured by MTT. An increase in cell numbers was observed in different cells in a time-dependent manner. pCMV-KAI1 transfected cells displayed a reducing trend of cell numbers, although this effect was not statistically significant (P>0.05; Fig. 2).
Inhibition of the expression of VEGF-C by KAI1
The VEGF-C secretion into medium was examined by ELISA. The levels of VEGF-C were reduced in medium for pCMV-KAI1-transfected cells. However, the reduction was significantly lower in PANC-1 cells than in MIA PaCa-2 cells (P<0.05; Fig. 3).

Fig. 1.KAI1 expression by Western blotting: there is no KAI1 expression in MIA PaCa-2 and PANC-1 cells. The plasmids pCMV-KAI1 and pCMV transfections significantly increased KAI1 expression of MIA PaCa-2 and PANC-1 cells.
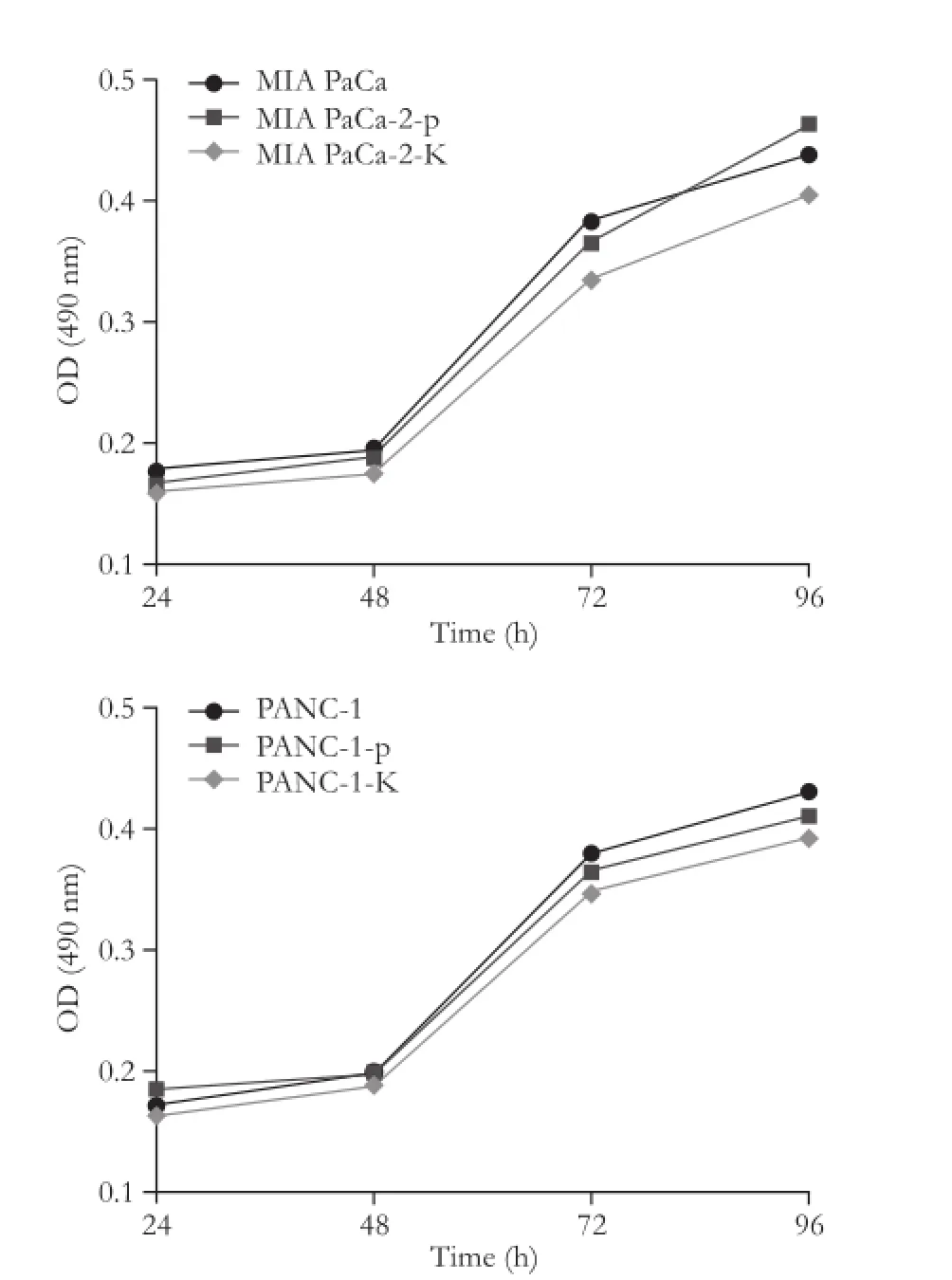
Fig. 2.Growth curves of MIA PaCa-2 and PANC-1 pancreatic cancer cells transfected with pCMV-KAI1 or pCMV and controls in vitro. There was no significant difference in cell growth in both MIA PaCa-2 and PANC-1 cells.
Tumor growth
We evaluated the in vivo impact of KAI1 on tumor growth and metastasis using a nude mice model. Subcutaneous tumors were found in all of the mice carrying MIA PaCa-2 and MIA PaCa-2-p cells, but only in 70% of the mice carrying MIA PaCa-2-K cells. No statistical difference was noted in tumor growth in the mice injected with MIA PaCa-2, MIA PaCa-2-p, and MIA PaCa-2-K cells (Fig. 4).
After 7 weeks, the mice were sacrificed and primary tumors were removed to measure their volume and weight. Mean primary weight was 1364.40±211.30, 1426.13±175.10 and 1294.37±195.40 mg in MIA PaCa-2-p and MIA PaCa-2 injected mice, respectively. However, a conspicuous variation of values in different groups was statistically significant. The transfection of KAI1 in pancreatic cancer cells did not delay subcutaneous tumor growth in vivo.
Lymph node metastasis and intratumor lymphangiogenesis in mice
On macroscopic inspection, the first occurrence of lymph node metastasis was observed at 35, 28, and 30 days respectively after injection of MIA PaCa-2-K, MIA PaCa-2 and MIA PaCa-2-p cells in mice. When the mice were sacrificed, metastatic lymph nodes were found in 3 of 7 mice in the MIA PaCa-2-K group, 8 of 10 in the MIA PaCa-2 group, and 7 of 10 in the MIA PaCa-2-p group, respectively. The mean number of metastatic lymph nodes in the three groups was 1.33, 2.38, and 2.14, respectively, and the difference was statistically significant (P<0.05, Fig. 5A).
To identify tumor-associated lymphatic vessels, immunohistochemistry was used to detect LYVE-1, a highly specific marker for mouse lymphatic vessels (Fig. 5B). The number of microlymphatic vesseldensities (MLVDs) per microscopic field was 12.20± 3.30, 28.30±5.22, and 30.50±4.68 in the three groups of mice, respectively, and the difference was statistically significant (P<0.05; Fig. 5C)
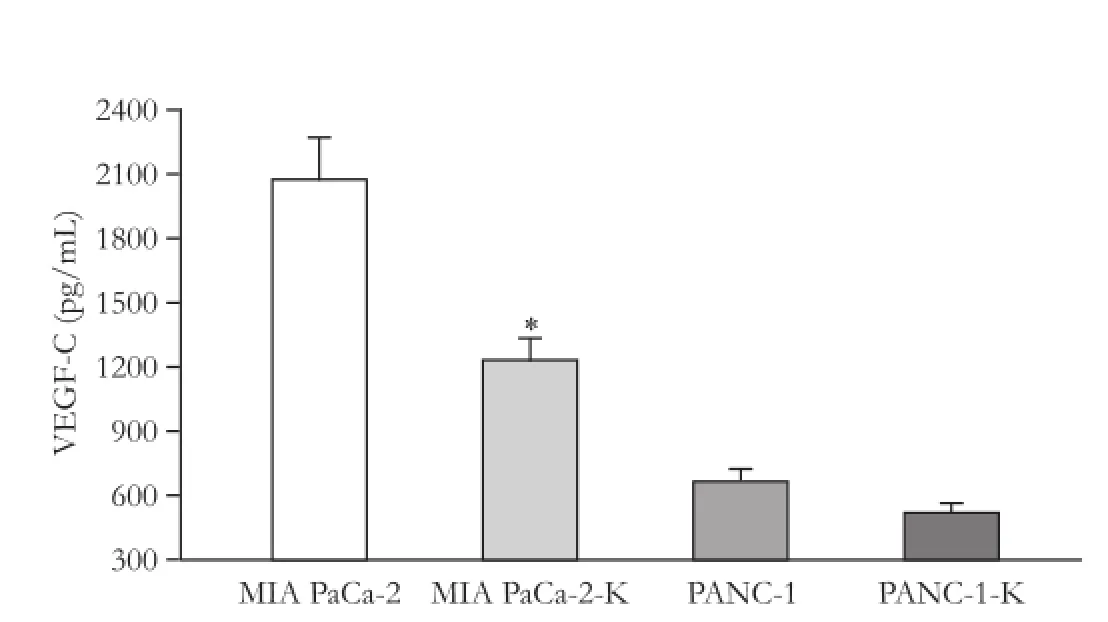
Fig. 3.Quantification of VEGF-C protein in cultured medium for MIA PaCa-2 and PANC-1 pancreatic cancer cells transfected with pCMV-KAI1. Values are expressed as mean±SD. VEGF-C secretion was significantly decreased in pCMV-KAI1-transfected cells in MIA PaCa-2 cell line (*: P<0.05).
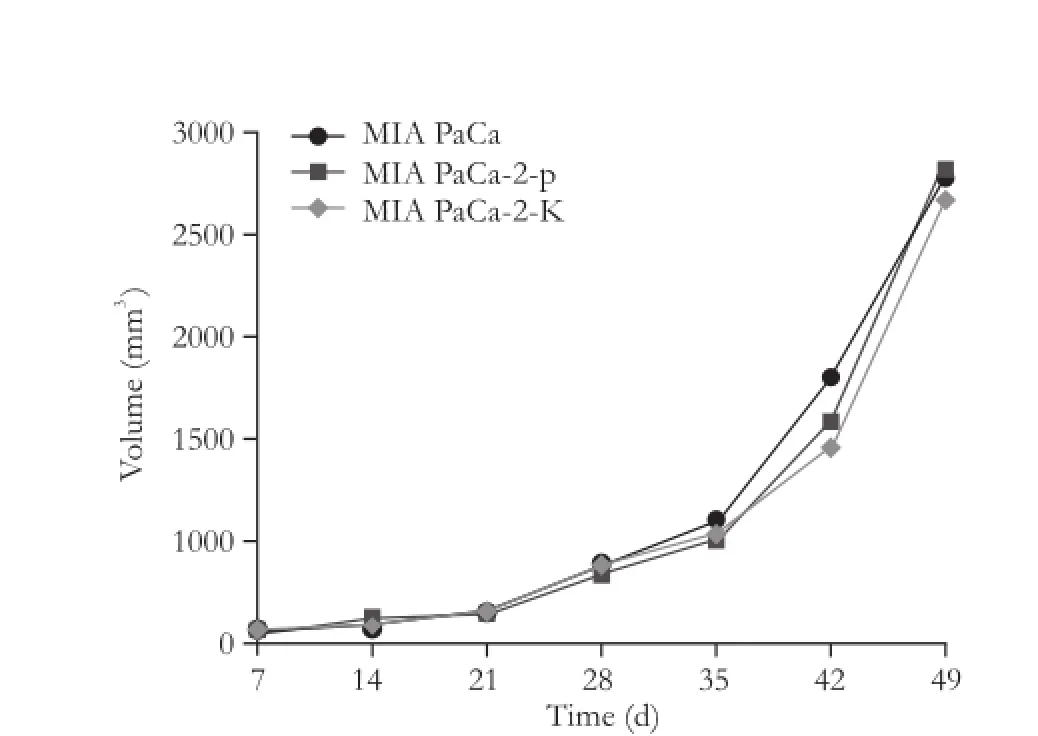
Fig. 4.The size of the subcutaneous tumors in each group. There was no significant change after KAI1 transfection compared with no transfected MIA PaCa-2 cell line.
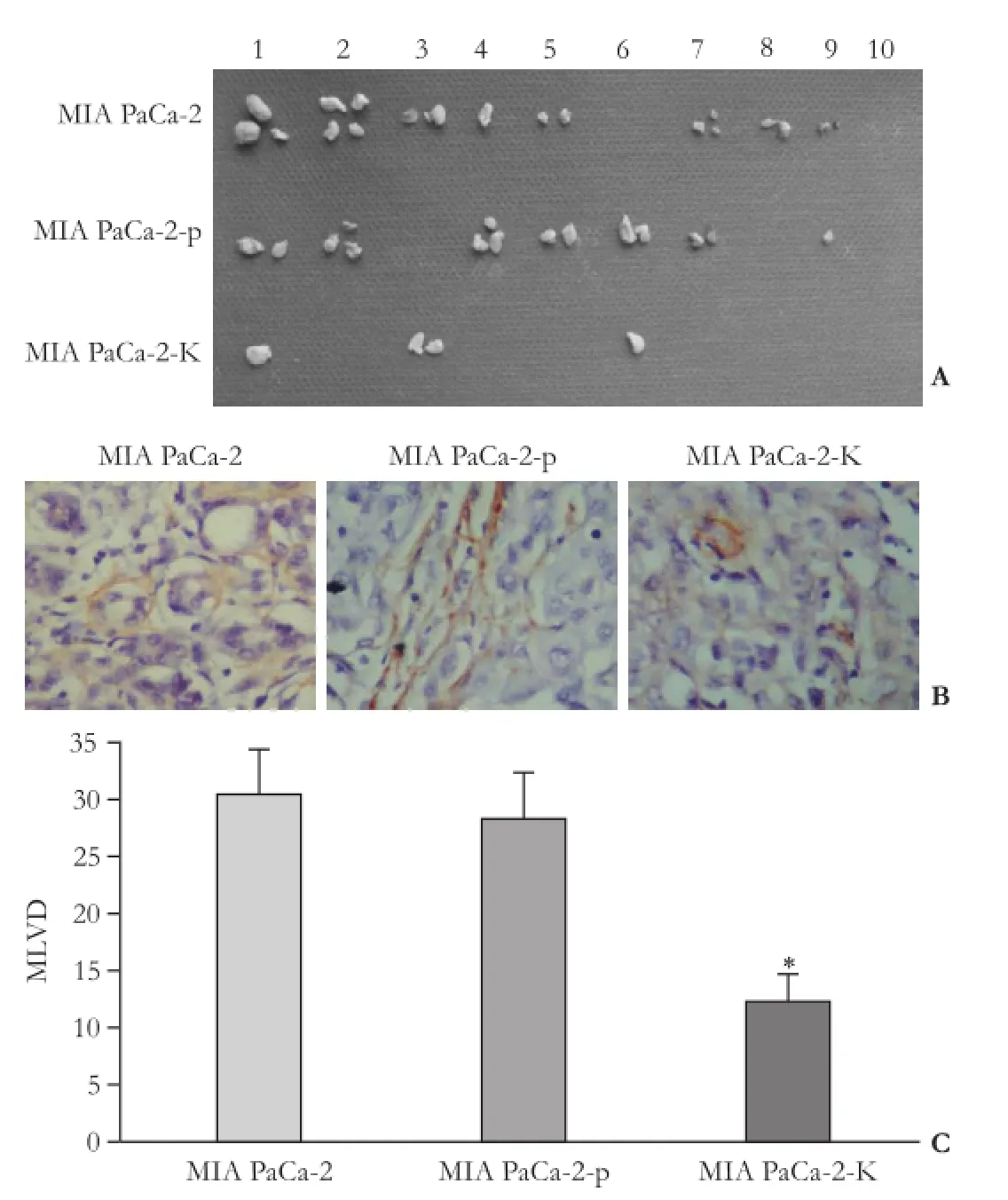
Fig. 5.The effect of KAI1 on intratumoral lymphangiogenesis and lymphatic metastasis.A: The number of the lymphatic metastasis was significantly lower in the MIA PaCa-2-K group than that in the MIA PaCa-2 group;B: Representative examples of LYVE-1 immunostains of the subcutaneous tumors in three groups of mice. The lymphatic vessels were less conspicuous in the tumor of MIA PaCa-2-K-injected mice than that of MIA PaCa-2 and MIA PaCa-2-p-injected mice;C: The number of MLVD was significantly decreased in MIA PaCa-2-K-injected mice compared with that in MIA PaCa-2 controls (*: P<0.05).
Discussion
The lymphatic route is an important initial dissemination of pancreatic cancer. Compared with the blood vasculature and angiogenesis, little is known about the biology of vasa lymphatica and lymphangiogenesis. More attentions have been paid to the mechanisms that determine the interaction of tumor cells with lymphatic vessels.[13]Some studies[14, 15]have found that peritumoral lymphatic vessels contribute to tumor metastasis, although it is unknown whether intratumoral lymphatics play any role in tumor metastasis. Thus, modulation of lymphatics may be promising for cancer diagnosis and treatment, but its application is clearly context-dependent. KAI1, a metastasis suppressor, is known to inhibit cancer metastasis without affecting primary tumorigenicity. The present study is the first to investigate the relationship between KAI1 and lymphatics in pancreatic cancer.
VEGF-C is the central regulator of lymphatics in pancreatic cancer.[16]Our study revealed the effects of KAI1 induction on the protein of VEGF-C secreted from MIA PaCa-2 and PANC-1 cells, and found the secretion levels of VEGF-C for PANC-1 cells were significantly lower than those for MIA PaCa-2 cells. Therefore, we hypothesized that KAI1 may influence the lymphatics of subcutaneous tumors in mice bearing MIA PaCa-2 cells.
Human MIA PaCa-2 pancreatic cancer cells, which have a high metastatic potential, VEGF-C high-secretion,[17]do not express KAI1/CD82 protein, and nude mice were used as the two main tools in this investigation. Our study showed that the size of subcutaneous tumors was similar, and increased at the same rate, in mice bearing MIA PaCa-2-K, MIA PaCa-2, and MIA PaCa-2-p cells. However, the development of lymph node metastasis was significantly reduced in the tumors of mice injected with MIA PaCa-2-K as compared with mice injected with MIA PaCa-2 and MIA PaCa-2-p cells. This result is consistent with the previous research that KAI1 can significantly reduce metastases without affecting the outcome and with no major effects on primary tumor growth.[18]
Further, we found that the LYVE-1 positive MLVD was significantly lower in the tumors of MIA PaCa-2-K injected mice than that in those of other groups. MLVD has a significantly positive relationship with lymphangiogenesis.[19]The previous evidence for lymphangiogenesis has raised the possibility that primary tumors cells can contribute actively to lymphatic dissemination through the induction of a lymphangiogenic process. In addition, lymphangiogenesis is associated with lymph vessel invasion and metastatic tumor features of pancreatic cancer.[20, 21]Therefore, overexpression KAI1 in MIA PaCa-2-K cells inhibits lymphatic metastasis, possibly through inhibition of tumor-associated lymphangiogenesis in vivo.
In summary, this study provides the first preclinical data that KAI1 has the potential to suppress tumorrelated lymph node metastasis and lymphangiogenesis. KAI1 is an important and VEGF-C upstream regulator of lymphangiogenesis in pancreatic cancer. So far, little is known about the consequences of KAI1 re-expression for more complex mechanism of lymphangiogenesis.
Contributors:LX proposed the study. LX and GXZ wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts. GXZ is the guarantor.
Funding:The study was supported by a grant from the National Nature Science Foundation of China (81071982).
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Coppola D. Molecular prognostic markers in pancreatic cancer. Cancer Control 2000;7:421-427.
2 Welsch T, Kleeff J, Friess H. Molecular pathogenesis of pancreatic cancer: advances and challenges. Curr Mol Med 2007;7:504-521.
3 Castellanos E, Berlin J, Cardin DB. Current treatment options for pancreatic carcinoma. Curr Oncol Rep 2011;13:195-205.
4 Wu CY, Yan J, Yang YF, Xiao FJ, Li QF, Zhang QW, et al. Overexpression of KAI1 induces autophagy and increases MiaPaCa-2 cell survival through the phosphorylation of extracellular signal-regulated kinases. Biochem Biophys Res Commun 2011;404:802-808.
5 Chen QY, Lin XM, Zhou JY, An Z, Yang L, Jiang ZY. Relationship between the levels of KAI1/CD82 and CD44 and the clinicopathological features of non-small cell lung cancer. Zhonghua Jie He He Hu Xi Za Zhi 2004;27:101-104.
6 Liu X, Guo XZ, Zhang WW, Lu ZZ, Zhang QW, Duan HF, et al. KAI1 inhibits HGF-induced invasion of pancreatic cancer by sphingosine kinase activity. Hepatobiliary Pancreat Dis Int 2011;10:201-208.
7 Guan-Zhen Y, Ying C, Can-Rong N, Guo-Dong W, Jian-Xin Q, Jie-Jun W. Reduced protein expression of metastasis-related genes (nm23, KISS1, KAI1 and p53) in lymph node and liver metastases of gastric cancer. Int J Exp Pathol 2007;88:175-183.
8 Liu WM, Zhang XA. KAI1/CD82, a tumor metastasissuppressor. Cancer Lett 2006;240:183-194.
9 Tonoli H, Barrett JC. CD82 metastasis suppressor gene: a potential target for new therapeutics? Trends Mol Med 2005;11:563-570.
10 Jee B, Jin K, Hahn JH, Song HG, Lee H. Metastasissuppressor KAI1/CD82 induces homotypic aggregation of human prostate cancer cells through Src-dependent pathway. Exp Mol Med 2003;35:30-37.
11 Takaoka A, Hinoda Y, Satoh S, Adachi Y, Itoh F, Adachi M, et al. Suppression of invasive properties of colon cancer cells by a metastasis suppressor KAI1 gene. Oncogene 1998;16: 1443-1453.
12 Yang JL, Jackson P, Yu Y, Russell PJ, Markovic B, Crowe PJ. Expression of the KAI1 metastasis suppressor gene in non-metastatic versus metastatic human colorectal cancer. Anticancer Res 2002;22:3337-3342.
13 Raica M, Ribatti D. Targeting tumor lymphangiogenesis: an update. Curr Med Chem 2010;17:698-708.
14 Gombos Z, Xu X, Chu CS, Zhang PJ, Acs G. Peritumoral lymphatic vessel density and vascular endothelial growth factor C expression in early-stage squamous cell carcinoma of the uterine cervix. Clin Cancer Res 2005;11:8364-8371.
15 Liu B, Ma J, Wang X, Su F, Li X, Yang S, et al. Lymphangiogenesis and its relationship with lymphatic metastasis and prognosis in malignant melanoma. Anat Rec (Hoboken) 2008;291:1227-1235.
16 Cheng P, Jin G, Hu X, Shi M, Zhang Y, Liu R, et al. Analysis of tumor-induced lymphangiogenesis and lymphatic vessel invasion of pancreatic carcinoma in the peripheral nerve plexus. Cancer Sci 2012;103:1756-1763.
17 Ochi N, Matsuo Y, Sawai H, Yasuda A, Takahashi H, Sato M, et al. Vascular endothelial growth factor-C secreted by pancreatic cancer cell line promotes lymphatic endothelial cell migration in an in vitro model of tumor lymphangiogenesis. Pancreas 2007;34:444-451.
18 Miranti CK. Controlling cell surface dynamics and signaling: how CD82/KAI1 suppresses metastasis. Cell Signal 2009;21: 196-211.
19 Zhang B, Zhao WH, Zhou WY, Yu WS, Yu JM, Li S. Expression of vascular endothelial growth factors-C and -D correlate with evidence of lymphangiogenesis and angiogenesis in pancreatic adenocarcinoma. Cancer Detect Prev 2007;31:436-442.
20 Sipos B, Klapper W, Kruse ML, Kalthoff H, Kerjaschki D, Kl?ppel G. Expression of lymphangiogenic factors and evidence of intratumoral lymphangiogenesis in pancreatic endocrine tumors. Am J Pathol 2004;165:1187-1197.
21 Schneider M, Büchler P, Giese N, Giese T, Wilting J, Büchler MW, et al. Role of lymphangiogenesis and lymphangiogenic factors during pancreatic cancer progression and lymphatic spread. Int J Oncol 2006;28:883-890.
Received August 30, 2012
Accepted after revision April 11, 2013
Author Affiliations: Department of Gastroenterology, General Hospital of Shenyang Military Area, Shenyang 110840, China (Liu X, Guo XZ, Li HY, Chen J, Ren LN and Wu CY)
Xiao-Zhong Guo, MD, Department of Gastroenterology, General Hospital of Shenyang Military Area, Shenyang 110840, China (Tel: 86-24-28897603; Fax: 86-24-28851113; Email: guoxiaozhong1962 @163.com)
? 2014, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60012-6
 Hepatobiliary & Pancreatic Diseases International2014年1期
Hepatobiliary & Pancreatic Diseases International2014年1期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Samaritan donor interchange in living donor liver transplantation
- lntrahepatic Glissonian approach and outflow vascular occlusion during partial hepatectomy
- Complex hepatic outflow reconstruction in domino liver transplantation
- Novel en-bloc resection of locally advanced hilar cholangiocarcinoma: the Rex recess approach
- Effect of CD74 on the prognosis of patients with resectable pancreatic cancer
- Blood group type antigens in pancreatic intraductal papillary mucinous neoplasms
