A matched-pair analysis of laparoscopic versus open pancreaticoduodenectomy: oncological outcomes using Leeds Pathology Protocol
Abdul R Hakeem, Caroline S Verbeke, Alison Cairns, Amer Aldouri, Andrew M Smith and Krishna V Menon
Leeds, UK
A matched-pair analysis of laparoscopic versus open pancreaticoduodenectomy: oncological outcomes using Leeds Pathology Protocol
Abdul R Hakeem, Caroline S Verbeke, Alison Cairns, Amer Aldouri, Andrew M Smith and Krishna V Menon
Leeds, UK
BACKGROUND:Laparoscopic pancreaticoduodenectomy (LPD) is a safe procedure. Oncological safety of LPD is still a matter for debate. This study aimed to compare the oncological outcomes, in terms of adequacy of resection and recurrence rate following LPD and open pancreaticoduodenectomy (OPD).
METHODS:Between November 2005 and April 2009, 12 LPDs (9 ampullary and 3 distal common bile duct tumors) were performed. A cohort of 12 OPDs were matched for age, gender, body mass index (BMI) and American Society of Anesthesiologists (ASA) score and tumor site.
RESULTS:Mean tumor size LPD vs OPD (19.8 vs 19.2 mm,P=0.870). R0 resection was achieved in 9 LPD vs 8 OPD (P=1.000). The mean number of metastatic lymph nodes and total number resected for LPD vs OPD were 1.1 vs 2.1 (P=0.140) and 20.7 vs 18.5 (P=0.534) respectively. Clavien complications grade I/II (5 vs 8), III/IV (2 vs 6) and pancreatic leak (2 vs 1) were statistically not significant (LPD vs OPD). The mean high dependency unit (HDU) stay was longer in OPD (3.7 vs 1.4 days,P<0.001). There were 2 recurrences each in LPD and OPD (logrank,P=0.983). Overall mortality for LPD vs OPD was 3 vs 6(log-rank,P=0.283) and recurrence-related mortality was 2 vs 1. There was one death within 30 days in the OPD group secondary to severe sepsis and none in the LPD group.
CONCLUSIONS:Compared to open procedure, LPD achieved a similar rate of R0 resection, lymph node harvest and longterm recurrence for tumors less than 2 cm. Though technically challenging, LPD is safe and does not compromise oncological outcome.
(Hepatobiliary Pancreat Dis Int 2014;13:435-441)
pancreaticoduodenectomy;
minimally invasive;
laparoscopic;
open;
oncological outcomes;
resection margins;
pathology
Introduction
Pancreaticoduodenectomy (PD) or Whipple's procedure is the standard treatment for ampullary and periampullary tumors.[1-4]However, most studies have reported significant morbidity and mortality in the range of 18%-54% and 1%-4%, respectively with the conventional open pancreaticoduodenectomy (OPD).[5]Minimally invasive techniques were employed for surgical resection of esophagogastric, colonic and other tumors with acceptable oncological outcomes compared to open techniques.[6-9]Despite its widespread use in other intra-abdominal malignancies, minimally invasive techniques were slow to be adopted for pancreatic diseases.[10,11]It was not until 1992 when Gagner and Pomp performed the first successful laparoscopic pancreaticoduodenectomy (LPD) on a patient with chronic pancreatitis and subsequently several reports and case series were published for pancreatic andperiampullary cancers.[12-17]
Seven decades after the first PD was performed, it still remains one of the most technically challenging operations.[18,19]PD has undergone various modifications both in terms of open technique and the introduction of minimally invasive alternatives like LPD and roboticassisted pancreaticoduodenectomy (RAPD).[20-22]Nevertheless, early reports of LPD received considerable skepticism from advocates of OPD due to technical complexity, similar morbidity and increased length of hospital stay in the former group.[23]Though the feasibility and safety of minimally invasive technique for PD have encouraged widespread acceptance of LPD over the past decade, there are still uncertainties over the oncological outcomes.[24,25]Some groups argued against minimally invasive procedures for PD because of risk of compromising surgical resection margins and potential inadequate removal of surrounding lymph nodes.[26]The ultimate goal of LPD is not only to expedite patient recovery, but also to achieve oncological results similar to those of OPD. Technical advances in minimally invasive surgery in addition to the experience of surgeons involved in performing laparoscopic pancreatic procedures have widely overcome the initial skepticism.[27]
The aim of this study was to compare the oncological outcomes of LPD with those of OPD using the previously validated Leeds Pathology Protocol (LEEPP) for histological analysis.[28,29]Comparison was done using a matchedpair method, so as to achieve balanced interpretation of outcomes between LPD and OPD.
Methods
Between November 2005 and April 2009, 12 LPDs were carried out in a tertiary referral center by a single surgeon (Menon KV). The clinical and oncological data were collected retrospectively from medical and pathology reports. The data were obtained from department database which are maintained after appropriate local research governance approval. Local National Health Service approval was obtained to conduct the case-matched analysis. Demographic data, perioperative data, pathology results and oncological outcomes were compared with a case-matched cohort of 12 patients who underwent OPD during the study period. On an average the unit currently performs around 80-90 OPDs per year, including pancreatic head tumors. The 12 consecutive LPD patients were case-matched from the series of 84 OPDs [ampullary and distal common bile duct tumors] performed during the study period, with matching done for age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) score and tumor origin. We did not match the tumor size or tumor stage. Histology was assessed using the previously validated LEEPP.[28,29]R1 was defined as tumor within one millimeter of the resection margin.
The patients were selected for LPD based on the site of tumor origin as ampullary and distal common bile duct and those that were resectable on the basis of radiology. We did not perform LPDs on patients with pancreatic head tumors. Also, the selection was not dependent on the size of the tumor based on radiology, as this could be quite variable and not a good indicator of actual tumor size.
The primary aim of this case-matched study was to compare the resection margin status and mean number of lymph nodes harvested between the LPD and the OPD groups. The secondary endpoints were comparison of postoperative complications, length of high-dependency unit stay, length of hospital stay, tumor recurrence, and recurrence-related mortality.
LPD procedure
LPD was performed as a total laparoscopic procedure including reconstruction. In brief, the patient was placed in a reverse Trendelenberg, Lloyd-Davis position. The procedure was performed using 5 laparoscopic ports and similar steps to the open approach for a standard PD. A harmonic scalpel (Ethicon Endosurgery Inc., Johnson & Johnson, USA) was used for dissection and endoscopic linear stapling devices (model ATW35, Ethicon Endosurgery Inc., Johnson & Johnson, USA) for bile duct and intestinal division. The resection specimen was placed in a bag and retrieved through a 5 cm Pfannenstiel incision.
Reconstruction was done on a single loop, two-layer interrupted duct-to-mucosa intra-corporeal sutures for pancreaticojejunostomy, single layer interrupted suture for hepaticojejunostomy and a stapled gastroenterostomy.
Statistical analysis
Categorical variables were expressed as percentages and continuous variables as mean±SD. Statistical associations between the categorical variables were assessed using Fisher's exact test or the Chi-square test, as appropriate. Student's t test or analysis of variance (ANOVA) was used to compare continuous variables. Kaplan-Meier survival curves with log-rank comparison were used to assess overall survival and disease-free survival. Statistical analysis was carried out using SPSS for Windows version 19.0 (SPSS Inc., Chicago, IL, USA). A P value less than 0.05 was considered as statistically significant.
Results
(25.0%) in both groups (P=1.000). All tumors were adenocarcinoma in both groups and tumor staging was done using TNM classification (7th Edition). The number of patients with T stage T2, T3, T4 were 6, 4, 2 for LPD vs 5, 7, 0 for the OPD group, respectively (P=1.000). The ampullary tumors were T2 (5), T3 (2) and T4 (2) in the LPD group and T2 (5), T3 (4) and T4 (0) in the OPD group. The clinical and pathological outcomes were elaborated in Tables 2 and 3. R0 resection was achieved in 9 (75.0%) LPD patients and 8 (66.7%) OPD patients (P=1.000). Based on LEEPP margin assessment, the posterior circumferential margin was involved in all 7 patients with R1 resection. The mean number of lymph nodes resected was higher in the LPD group than in the OPD group (20.7 vs 18.5), but this was not statistically significant (P=0.534). The mean number of metastatic nodes was higher in the OPD group (2.1) than in the LPD group (1.1), but this again was not statistically significant (P=0.140).
There were 2 patients with tumor recurrence in both the LPD and OPD groups (log-rank, P=0.983). The recurrences in the LPD patients occurred in the lung at 18.0 months and in segment 3 of the liver at 32.6
The median follow-up was 46.8 months (13.0-73.7) for the LPD group and 56.0 months (1.0-97.4) for the OPD group. The demographics as matched for LPD and OPD were shown in Table 1. There were 9 ampullary (75.0%) and 3 distal third common bile duct tumorsmonths. The patient with lung metastasis had palliative chemotherapy and died 24 months after diagnosis of the metastasis, whereas the patient with liver metastasis died 6 months after diagnosis. The two recurrences in the OPD patients were in segment 6 of the liver at 18.5 months and in the coeliac lymph nodes at 30.4 months. The patient with liver metastasis died two months following the diagnosis of recurrence, whereas the patient with coeliac node recurrence responded to chemotherapy and was alive at 61 months.
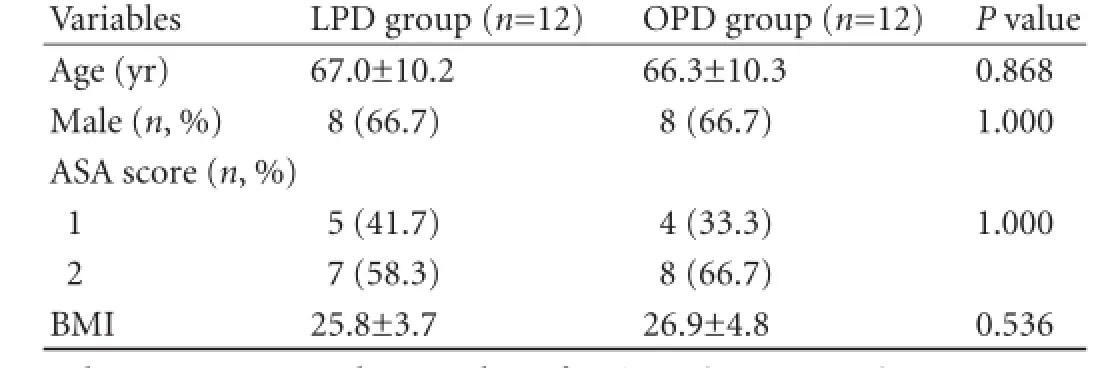
Table 1. Demographics of LPD and OPD groups
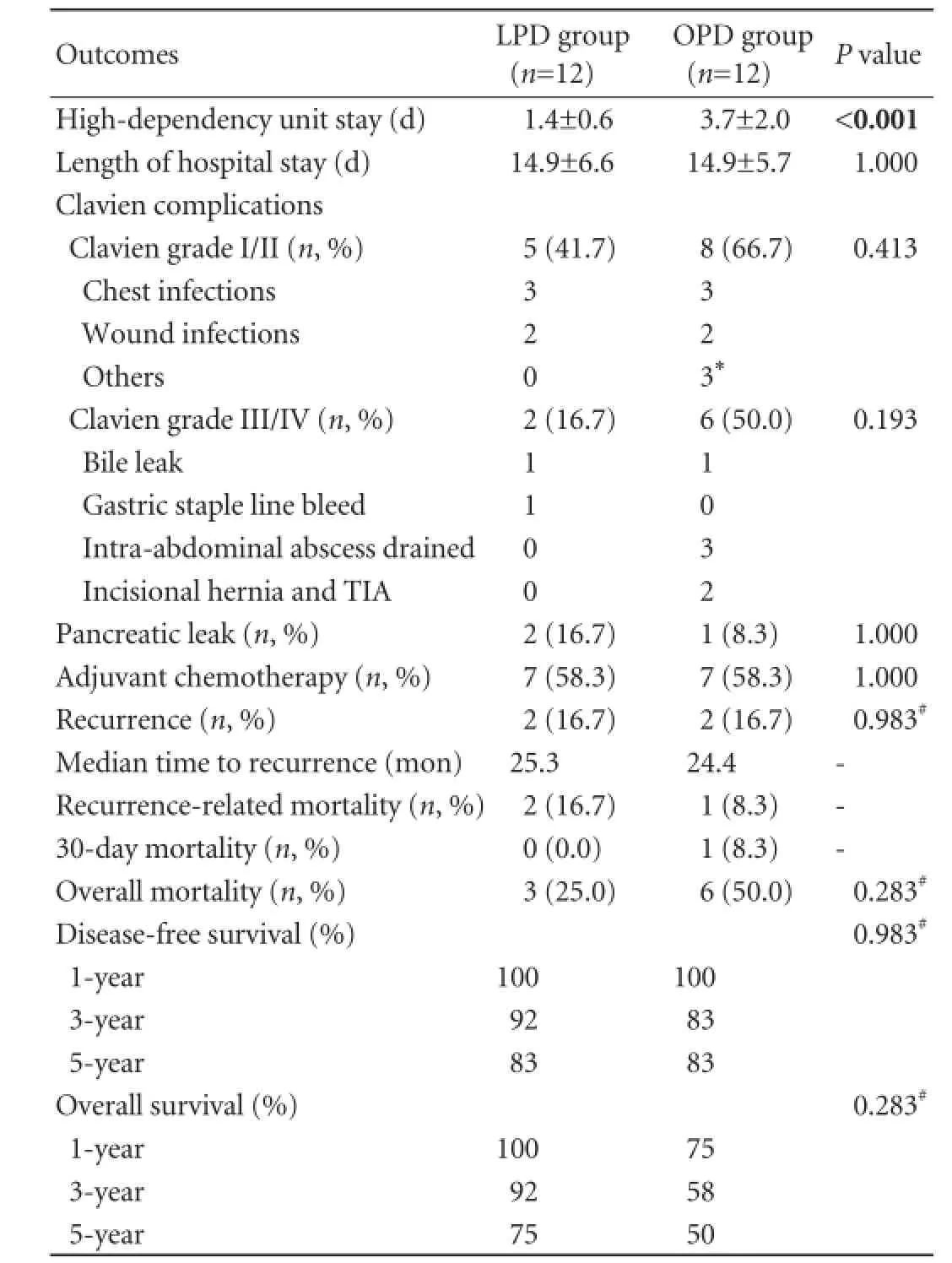
Table 2. Operative and clinical outcomes of LPD and OPD groups
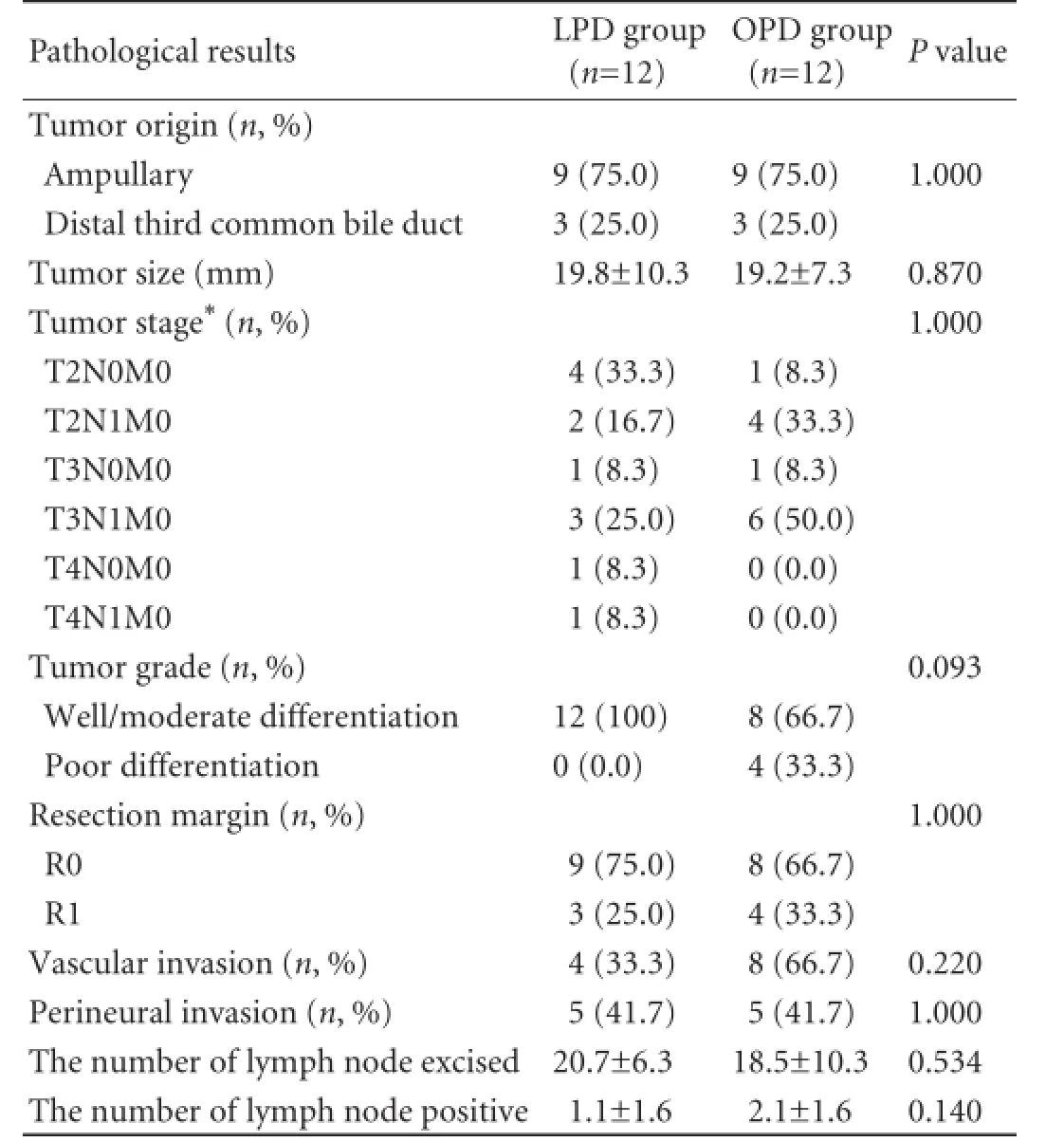
Table 3. Pathology data of LPD and OPD groups
There was one death within 30 days in the OPD group secondary to severe sepsis and none in the LPD group. On Kaplan-Meier survival analysis, the 1-, 3- and 5-year overall survival for LPD vs OPD was 100%, 92% and 75% vs 75%, 58% and 50%, respectively (log-rank,P=0.283). The 1-, 3- and 5-year disease-free survival was 100%, 92% and 83% for LPD and 100%, 83% and 83% for OPD, respectively (log-rank,P=0.983) (Figs. 1 and 2).
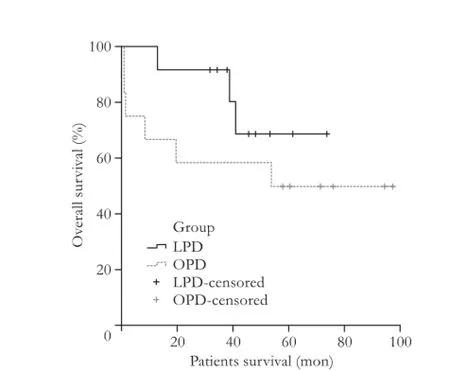
Fig. 1.Kaplan-Meier curve for patients overall survival of LPD and OPD groups (log-rank,P=0.283).
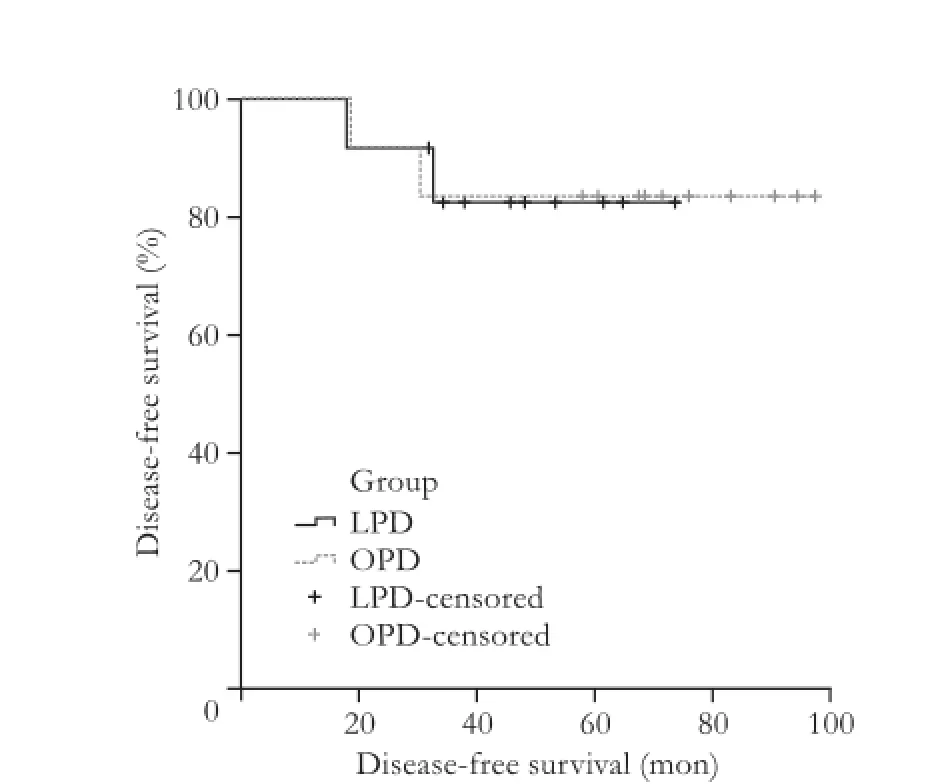
Fig. 2.Kaplan-Meier curve for disease-free survival of LPD and OPD groups (log-rank,P=0.983).
Discussion
Minimally invasive surgery has been expanding rapidly, and in most surgical specialties it is accepted as an acceptable alternative to traditional open surgery. Greater experience in pancreatic surgery and advancements in surgical techniques have reduced surgical morbidity and mortality following complex pancreatic resections. Over the last decade or so, LPD has proven its benefits in terms of reduced intraoperative blood loss, reduced high-dependency unit stay, faster recovery, shorter hospital stay and overall better quality of life.[14-17]Nonetheless, there is still reluctance in accepting minimally invasive techniques, mainly due to the learning curve associated with the procedure and uncertainties regarding oncological outcome.[30]
The present study reported the feasibility of LPD and also its oncological safety. Margin involvement is one of the important prognostic factors following pancreatic resection and R1 rates varied widely in the literature between 2% and over 75% for all cancers (pancreatic, ampullary and distal common bile duct).[31,32]The R1 rate was 25.0% (3 out of 12 patients) following LPD, that was comparable to 33.3% (4 out of 12 patients) of the case-matched OPD cohort. R1 rates we reported previously using the LEEPP were 25%-27% for ampullary and 46%-72% for distal common bile duct cancers. In the current study of LPD vs OPD, the R1 rates were 11.1% vs 22.2% for ampullary and 66.7% vs 66.7% for distal common bile duct cancers cancers respectively, which are similar to our previously published results.[28,29]
In a systematic review of 285 published cases of LPD, Gumbs et al[5]reported a much lower R1 rate of 0.4% for those with malignant disease (174 out of 235 cases had margin data, 74%). R1 rates for LPD were much higher in the present study, as firstly, we evaluated the margins using the validated LEEPP that allowed accurate assessment of tumor margins.[28]Secondly, data reported from the review by Gumbs et al[5]were inadequate for margin involvement, as most studies that were analyzed included both benign and malignant resections in their series.
The role of extended lymphadenectomy during PD is controversial. A number of prospective and retrospective cohort studies reported that the total number of lymph nodes resected and the involvement of the nodes or, more specifically, the lymph node ratio as the most important prognostic factor for survival following PD for ampullary tumors.[33-37]The mean number of lymph nodes resected was similar in the LPD group compared with that in the OPD group (20.7 vs 18.5). The mean number of lymph node positivity was again not differentin the LPD group (1.1) from that in the OPD group (2.1). Gumbs et al[5]reported a mean number of nodes retrieved in LPD series of 7 to 36 nodes with a weighted mean of 15 nodes (data available for 188 of 235 patients, 80%). The lymph node harvest in the current series was in accordance with the literature.
Prognostic factors that influence long-term outcome following PD were margin status, the number of nodes harvested, lymph node metastasis, tumor size, grade of tumor differentiation and vascular involvement.[38,39]The mean tumor size was similar in both groups (LPD vs OPD group; 19.8 vs 19.2 mm). Three patients in the LPD group had a tumor larger than 20 mm in size: 30, 36 and 40 mm, whereas two in the OPD group had tumor larger than 20 mm: 30 and 38 mm. It may be inferred from this comparison that appropriate patient selection in terms of tumor less than 20 mm may be oncologically safe for LPD resection, although lack of randomization limited such conclusion. There was no difference in our matched cohorts in terms of tumor stage, differentiation and extent of invasion.
Only three previous studies compared data between LPD and OPD.[27,40,41]To our knowledge, this is the first study to report a case-matched comparison of LPD vs OPD for malignant tumors. Also, this is the first matched study between LPD and OPD in a UK cohort. Zureikat et al[27]matched 14 LPDs with similar number of OPDs and demonstrated 100% R0 resection, mean lymph node yield of 18.5 nodes and no difference in clinical and oncological outcomes in comparison to the open group. Of note, 12 out of 14 cases in the LPD group were malignant. Cho et al[40]compared LPD vs OPD in a case-matched series of 15 patients in each group. The matching was done for patient's age, gender, ASA score and BMI grade. The 15 patients in the laparoscopic group included benign and low-grade malignant tumors, whereas all OPD patients had malignant tumors. This study showed no difference in margin status (negative in all 30 patients) between the two groups and similar the number of lymph nodes retrieved (LPD vs OPD, 18.5 vs 20.0, respectively). The study did not report on tumor size, recurrence rate or recurrence-related mortality. In this study, only patients with benign and low-grade malignant periampullary tumors were selected for LPD. In our study, we included ampullary and distal common bile duct tumors, irrespective of tumor staging. Recently, a Japanese study[41]compared 20 LPD with 31 OPD patients for periampullary disease. Again, the LPD group contained a mixture of both benign and malignant tumors, and oncological outcomes in terms of resection margin status, lymph nodes extracted or recurrence were not reported.
In keeping with oncological comparison of open with minimally invasive PDs, there were three studies which have performed case-matched comparison of RAPD with OPD.[42-44]Zhou et al[42]reported the first case-matched comparison between RAPD and OPD in a series of 16 patients in each group. Although the groups were comparable in the rates of R0 resection (RAPD vs OPD; 100% vs 87.5%, P=0.05), the study indeed increased the enthusiasm for minimally invasive PDs using robotics. In the largest comparative study between RAPD (n=44) and OPD (n=39), Buchs et al[43]showed a higher R0 resection rate for RAPD in comparison to OPD (90.9% vs 81.5%), that was not statistically significant. The study reported a higher harvest of lymph nodes in the RAPD group (mean 16.8) in comparison with that in the OPD group (mean 11), which was statistically significant. Recently, Chalikonda et al[44]compared 30 RAPDs with matched OPDs. The mean number of lymph nodes excised in RAPD vs OPD was 13.2 vs 11.7 respectively, which was not statistically significant (P=0.25). This was lower than the recommended lymph node harvest for a standard PD.[45]There were four margin positives in the OPD group, compared with none in the RAPD group, which was statistically significant.[44]Results from the robotic comparison of PD with OPD favored minimally invasive resections of pancreatic malignancies and highlighted the oncological safety of such resections.
Morbidity following LPD was reported in the range of 26%-40% from centers performing 25 or more procedures.[5]Our LPD morbidity rate was 58.3%, of whom majority were Clavien grade I and II, which were comparable to published data.[5,46]As regards longterm outcome, there has been significant improvement in 5-year survival after PD for malignancy, with recent studies from US and Europe reporting approximately 25% after R0 resection.[47-49]Results in our cohort were similar with all R0 resection patients being tumor free at death or alive at a median of 48 months follow-up. Two patients in each group had recurrence, both of whom had R1 resections. Although the current study is limited by its retrospective nature, non-randomized selection and small study population, an attempt was made to case-match the LPD with the OPD patients, so as to make a valuable comparison. The OPD patients were operated by different surgeons, which did add bias to comparing the outcomes with a single surgeon LPD procedure. We did not perform LPDs for pancreatic head tumors, as there are still oncological uncertainties, in particular the risk of positive resection margins with the total laparoscopic approach, with these pathologies.[26]
In summary, this case-matched comparison of LPD vs OPD showed that oncological outcomes of LPD are comparable to those of OPD, thereby providing evidence for the expanding role of and interest in LPD for the management of ampullary and distal common bile duct tumors. As with open surgery, patient selection is of utmost importance for successful clinical and oncological outcomes with LPD. The authors recommended that LPD can offer similar oncological benefits with careful patient selection, which would include ampullary and distal common bile duct tumors and a tumor size less than 20 mm. However, larger prospective studies or randomized controlled trials are needed between LPD and OPD, so as to evaluate the benefit of minimally invasive techniques regarding oncological outcome in carefully selected patients.
Contributors:MKV conceptualized the case-matched design and is the surgeon who performed all the laparoscopic procedures. HAR performed the case-matched analysis and wrote the manuscript. VCS and CA are the consultant histopathologists and involved in the pathological examination of the specimens. AA and SAM provided their surgical input in the open procedures. MKV made the final corrections to the manuscript. All authors corrected the manuscript. MKV is the guarantor.
Funding:None.
Ethical approval:This study was approved by the Local National Health Service.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Kim SC, Kim YH, Park KM, Lee YJ. Pancreatic cancer surgery: the state of the art. Curr Drug Targets 2012;13:764-771.
2 Kooby DA, Chu CK. Laparoscopic management of pancreatic malignancies. Surg Clin North Am 2010;90:427-446.
3 Kondo S, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Nagino M, et al. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepatobiliary Pancreat Surg 2008;15:41-54.
4 Ammori BJ, Baghdadi S. Minimally invasive pancreatic surgery: the new frontier? Curr Gastroenterol Rep 2006;8: 132-142.
5 Gumbs AA, Rodriguez Rivera AM, Milone L, Hoffman JP. Laparoscopic pancreatoduodenectomy: a review of 285 published cases. Ann Surg Oncol 2011;18:1335-1341.
6 Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-1892.
7 Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, Hsieh MC, et al. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg 2012;16:1303-1310.
8 Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg 2009;250: 842-848.
9 Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev 2008:CD003432.
10 Al-Taan OS, Stephenson JA, Briggs C, Pollard C, Metcalfe MS, Dennison AR. Laparoscopic pancreatic surgery: a review of present results and future prospects. HPB (Oxford) 2010;12:239-243.
11 Ammori BJ, Ayiomamitis GD. Laparoscopic pancreaticoduodenectomy and distal pancreatectomy: a UK experience and a systematic review of the literature. Surg Endosc 2011;25: 2084-2099.
12 Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-410.
13 Menon KV, Hayden JD, Prasad KR, Verbeke CS. Total laparoscopic pancreaticoduodenectomy and reconstruction for a cholangiocarcinoma of the bile duct. J Laparoendosc Adv Surg Tech A 2007;17:775-780.
14 Palanivelu C, Rajan PS, Rangarajan M, Vaithiswaran V, Senthilnathan P, Parthasarathi R, et al. Evolution in techniques of laparoscopic pancreaticoduodenectomy: a decade long experience from a tertiary center. J Hepatobiliary Pancreat Surg 2009;16:731-740.
15 Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc 2006;20:1045-1050.
16 Gumbs AA, Gayet B. The laparoscopic duodenopancreatectomy: the posterior approach. Surg Endosc 2008;22:539-540.
17 Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg 2010;145:19-23.
18 Whipple AO. Present-day surgery of the pancreas. N Engl J Med 1942;226:515-526.
19 Chu CK, Kooby DA. Laparoscopic surgery for pancreatic tumors. Surg Oncol Clin N Am 2010;19:311-333.
20 Zeh HJ 3rd, Bartlett DL, Moser AJ. Robotic-assisted major pancreatic resection. Adv Surg 2011;45:323-340.
21 Zeh HJ, Zureikat AH, Secrest A, Dauoudi M, Bartlett D, Moser AJ. Outcomes after robot-assisted pancreaticoduodenectomy for periampullary lesions. Ann Surg Oncol 2012;19:864-870.
22 Nguyen KT, Zureikat AH, Chalikonda S, Bartlett DL, Moser AJ, Zeh HJ. Technical aspects of robotic-assisted pancreaticoduodenectomy (RAPD). J Gastrointest Surg 2011;15:870-875.
23 Are C, Dhir M, Ravipati L. History of pancreaticoduodenectomy: early misconceptions, initial milestones and the pioneers. HPB (Oxford) 2011;13:377-384.
24 Nakeeb A. Laparoscopic pancreatic resections. Adv Surg 2009;43:91-102.
25 Briggs CD, Mann CD, Irving GR, Neal CP, Peterson M, Cameron IC, et al. Systematic review of minimally invasive pancreatic resection. J Gastrointest Surg 2009;13:1129-1137.
26 Matsuoka L, Parekh D. The minimally invasive approach to surgical management of pancreatic diseases. Gastroenterol Clin North Am 2012;41:77-101.
27 Zureikat AH, Breaux JA, Steel JL, Hughes SJ. Can laparoscopic pancreaticoduodenectomy be safely implemented? J Gastrointest Surg 2011;15:1151-1157.
28 Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006;93:1232-1237.
29 Menon KV, Gomez D, Smith AM, Anthoney A, Verbeke CS. Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP). HPB (Oxford) 2009;11:18-24.
30 Zhang T, Du X, Zhao Y. Laparoscopic surgery for pancreatic lesions: current status and future. Front Med 2011;5:277-282.
31 Fatima J, Schnelldorfer T, Barton J, Wood CM, Wiste HJ, Smyrk TC, et al. Pancreatoduodenectomy for ductal adenocarcinoma: implications of positive margin on survival. Arch Surg 2010;145:167-172.
32 La Torre M, Nigri G, Ferrari L, Cosenza G, Ravaioli M, Ramacciato G. Hospital volume, margin status, and longterm survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg 2012;78:225-229.
33 Hsu HP, Shan YS, Hsieh YH, Yang TM, Lin PW. Predictors of recurrence after pancreaticoduodenectomy in ampullary cancer: comparison between non-, early and late recurrence. J Formos Med Assoc 2007;106:432-443.
34 Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007;141:610-618.
35 Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008;15:165-174.
36 Falconi M, Crippa S, Domínguez I, Barugola G, Capelli P, Marcucci S, et al. Prognostic relevance of lymph node ratio and number of resected nodes after curative resection of ampulla of Vater carcinoma. Ann Surg Oncol 2008;15:3178-3186.
37 Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg 2009;13:1337-1344.
38 Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, et al. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer 2008;8:170.
39 Qiao QL, Zhao YG, Ye ML, Yang YM, Zhao JX, Huang YT, et al. Carcinoma of the ampulla of Vater: factors influencing long-term survival of 127 patients with resection. World J Surg 2007;31:137-146.
40 Cho A, Yamamoto H, Nagata M, Takiguchi N, Shimada H, Kainuma O, et al. Comparison of laparoscopy-assisted and open pylorus-preserving pancreaticoduodenectomy for periampullary disease. Am J Surg 2009;198:445-449.
41 Kuroki T, Adachi T, Okamoto T, Kanematsu T. A non-randomized comparative study of laparoscopy-assisted pancreaticoduodenectomy and open pancreaticoduodenectomy. Hepatogastroenterology 2012;59:570-573.
42 Zhou NX, Chen JZ, Liu Q, Zhang X, Wang Z, Ren S, et al. Outcomes of pancreatoduodenectomy with robotic surgery versus open surgery. Int J Med Robot 2011;7:131-137.
43 Buchs NC, Addeo P, Bianco FM, Ayloo S, Benedetti E, Giulianotti PC. Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg 2011;35:2739-2746.
44 Chalikonda S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc 2012;26:2397-2402.
45 Yeo CJ, Cameron JL, Sohn TA, Coleman J, Sauter PK, Hruban RH, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg 1999;229: 613-624.
46 Casadei R, Ricci C, Pezzilli R, Calculli L, Rega D, D'Ambra M, et al. Usefulness of the Clavien-Dindo classification after pancreaticoduodenectomy. ANZ J Surg 2011;81:747-748.
47 Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg 2003;27:324-329.
48 Schmidt CM, Powell ES, Yiannoutsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg 2004;139:718-727.
49 Kazanjian KK, Hines OJ, Duffy JP, Yoon DY, Cortina G, Reber HA. Improved survival following pancreaticoduodenectomy to treat adenocarcinoma of the pancreas: the influence of operative blood loss. Arch Surg 2008;143:1166-1171.
Received June 25, 2013
Accepted after revision November 8, 2013
Author Affiliations: Department of HPB and Transplant Surgery (Hakeem AR, Aldouri A, Smith AM and Menon KV), and Department of Histopathology (Cairns A), St James's University Hospital NHS Trust, Leeds Teaching Hospitals, Beckett street, Leeds, LS9 7TF, United Kingdom; Division of Pathology, Department of Laboratory Medicine, Karolinska Institutet, Stockholm, Sweden (Verbeke CS)
Krishna V Menon, MS, FRCS, Department of HPB and Transplant Surgery, St James's University Hospital, Beckett Street, Leeds, LS9 7TF, United Kingdom (Tel: +44-113-206-4887; Email: krishna. menon@nhs.net)
The study abstract was presented as a poster at theInternational Hepato-Pancreato-Biliary Association(IHPBA) meeting at Paris, 1-5 July 2012 and AUGIS (Association of Upper GI surgeons of Great Britain and Ireland) Digestive Disorders Federation (DDF) at Liverpool, 17-20 June 2012.
? 2014, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60048-5
Published online March 27, 2014.
 Hepatobiliary & Pancreatic Diseases International2014年4期
Hepatobiliary & Pancreatic Diseases International2014年4期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Effects of melatonin on the oxidative damage and pancreatic antioxidant defenses in ceruleininduced acute pancreatitis in rats
- Pancreaticoduodenectomy and pancreaticoduodenectomy combined with superior mesentericportal vein resection for elderly cancer patients
- Effect of external beam radiotherapy on patency of uncovered metallic stents in patients with inoperable bile duct cancer
- Prostacyclin decreases splanchnic vascular contractility in cirrhotic rats
- Liver transplantation using organs from deceased organ donors: a single organ transplant center experience
- Ultrasonic integrated backscatter in assessing liver steatosis before and after liver transplantation
