Prostacyclin decreases splanchnic vascular contractility in cirrhotic rats
De-Jun Liu, Wei Chen, Yan-Miao Huo, Wei Liu, Jun-Feng Zhang, Rong Hua and Yong-Wei Sun
Shanghai, China
Prostacyclin decreases splanchnic vascular contractility in cirrhotic rats
De-Jun Liu, Wei Chen, Yan-Miao Huo, Wei Liu, Jun-Feng Zhang, Rong Hua and Yong-Wei Sun
Shanghai, China
BACKGROUND:Prostacyclin has been shown to increase portal hypertension, but the mechanism is unclear. This study aimed to investigate whether the overproduction of prostacyclin (PGI2) in cirrhosis participates in the splanchnic vascular hyporesponsiveness to vasoconstrictors in cirrhotic rats.
METHODS:Cirrhotic model was created by subcutaneous injection of 60% carbon tetrachloride (CCl4) corn oil solution combined with intermittent drinking of 5% alcohol, and agematched rats served as controls. The isolated third-generation mesenteric arterioles were used to examine the contractile response to norepinephrine. The changes in vascular diameter were observed under a microscope imaging device. The plasma concentration of 6-ketone-prostaglandin F1α(6-keto-PGF1α, a stable metabolite of PGI2) was tested via enzyme immunoassays and the expression of cyclooxygenase (COX) in mesenteric arteries was detected by Western blotting.
RESULTS:In parallel with the increase of plasma 6-keto-PGF1α, the contractile response of arterioles from cirrhotic rats to norepinephrine was significantly impaired compared with that from controls. Inhibition of PGI2or protein kinase A with indomethacin or Rp-adenosine 3', 5'-cyclic monophosphothioate (Rp-cAMPS) partially reversed the vascular hypo-contractile response to norepinephrine in arterioles from cirrhotic rats. Indomethacin significantly decreased the plasma 6-keto-PGF1α. Furthermore, indomethacin significantly attenuated the effect of Rp-cAMPS on arterioles from cirrhotic rats. COX-1 expression was up-regulated in mesenteric arteries from cirrhotic rats, whereas COX-2 was not detectable in the mesenteric arteries from both cirrhotic and control rats.
CONCLUSION:Enhanced COX-1 expression in cirrhotic rats resulted in elevated PGI2production which partially contributedto the splanchnic vascular hyporesponsiveness to a vasoconstrictor via the protein kinase A pathway.
(Hepatobiliary Pancreat Dis Int 2014;13:416-422)
portal hypertension;
prostacyclin;
indomethacin;
protein kinase A;
vascular hyporesponsiveness
Introduction
Portal hypertension (PHT) is the culprit of many complications in cirrhosis such us upper gastrointestinal bleeding, hepatic encephalopathy, etc. Both intrahepatic resistance and splanchnic blood flow are increased in cirrhotic PHT. Increased intrahepatic vascular resistance is the initiating factor, whereas splanchnic blood flow is a secondary phenomenon that maintains and aggravates the portal venous pressure.[1,2]The increase of splanchnic blood flow is primarily caused by splanchnic vasodilation and therefore, the pathogenesis of splanchnic vasodilation in PHT should be elucidated. Extensive research in PHT animals and patients has revealed that the vasodilation is induced either by increased vasodilators or by the decreased vascular response to vasoconstrictors, termed "vascular hypocontractility".[3-6]Prostacyclin (PGI2), an endothelial vasoactive factor, is elevated in both animal models and patients with PHT.[7-9]PGI2increases splanchnic blood flow,[10,11]but up to now, it is still not fully understood whether and how the increased PGI2contributes to splanchnic vasodilation in PHT. PGI2is produced in endothelial cells. Both cyclooxygenase (COX) enzymes, COX-1 and COX-2, convert arachidonic acid into the PGI2precursor prostaglandin H2, which is subsequently synthesized into PGI2. PGI2is unstable with a very short half-time of <2 minutes and rapidly converted into the inactive hydration product 6-ketoneprostaglandin F1α(6-keto-PGF1α), which is typically tested as an indicator of PGI2. PGI2mediates itscellular activity via binding to G-protein-coupled receptors, leading to the activation of adenyl cyclase which produces the second messenger cyclic adenosine monophosphate (cAMP), the latter activates the cAMP-dependent protein kinase A (PKA). PKA subsequently induces vascular hypocontractility to vasoconstrictors by prevention of Ca2+mobilization into the cytoplasm and by phosphorylation of myosin light chain kinase.[12-15]Wu et al[15]demonstrated that Rp-adenosine 3', 5'-cyclic monophosphothioate (Rp-cAMPS), a PKA inhibitor, restored mesenteric vascular responses to norepinephrine (NE) in portal vein ligation induced PHT rats. The present study was to examine whether the increased PGI2in cirrhotic rats contributes to the splanchnic vascular hyporesponsiveness to NE via the PKA pathway.
Methods
Experimental model
Male Sprague-Dawley rats, weighing 250±50 g, were used for all experiments. Cirrhosis was induced by subcutaneous injection of 60% carbon tetrachloride (CCl4) corn oil solution (0.4 mL/100 g) twice a week for sixteen weeks. Five percent of alcohol was given intermittently in drinking water according to body weight and mental conditions. The rats which developed liver nodules by the end of the induction period were used as a cirrhotic group, while age-matched, corn oil treated rats served as controls.
One week after the induction, fourteen rats in the model group and controls received daily subcutaneous injections of indomethacin (0.3 mg/100 g) or its vehicle (saline) for five days. One hour after the last injection, the animals were subjected to sample collections.
All experiments were approved by the Animal Experiment Committee of Shanghai Jiaotong University School of Medicine.
Preparation ofin vitroarterioles
The arterioles were prepared as described.[16,17]Briefly, under intramuscular ketamine anesthesia, an abdominal midline incision was made and the small intestine with attached mesentery was excised and placed on a Petri dish containing cold (0-4 ℃) physiological salt solution (pH 7.4) containing 0.02 mmol/L EDTA, 145.0 mmol/L NaCl, 5.0 mmol/L KCl, 2.0 mmol/L CaCl2, 1.0 mmol/L MgSO4, 1.0 mmol/L NaH2PO4, 5.0 mmol/L glucose, 2.0 mmol/L pyruvate and 3.0 mmol/L MOPS. The mesentery was spread and pinned to the silastic bottom of a dish. With the use of a dissecting microscope (Motic) and micro scissors (World Precision Instruments), 1-2 mm in length segments of third-generation arterioles were isolated from the mesentery and transferred to the vessel chamber, containing glass micropipettes and physiological salt solution at room temperature. Two ends of the vessel were mounted and secured with a suture on the pipettes. The vessel was warmed slowly to 37 ℃ and the intravascular pressure was increased slowly to 80 mmHg.
Experimental procedure
When the preparation was completed, the vessel chamber was transferred to the stage of a video microscope (Motic) and the arteriole was allowed to equilibrate for 1-2 hours. During this time, vessels with favorable vasoactivity would contract and the diameter therefore was reduced and only those contracted arterioles were used in further experiments. When the arterioles reached a steady-state fixed diameter, they were exposed to increasing concentrations of NE (10-7-10-5mol/L) in a cumulative manner and each NE concentration was kept in the vessel for 5-10 minutes. To determine the involvement of PKA, another set of arterioles were preincubated with 50 μmol/L Rp-cAMPS for 30 minutes before vasoconstrictor response to NE was monitored. Vessel sizes were amplified by the microscope and the images were projected in a computer with an installed diameter-measuring software (Xuzhou Medical College).
Assay of 6-keto-PGF1α
Before rats were killed, 2 mL blood were collected from the inferior vena cava and placed in an iced tube. The sample was centrifuged at 3500 rpm for 10 minutes at 4 ℃ immediately, and frozen at -80 ℃ until assay. After all the samples have been collected, 6-keto-PGF1αas the stable metabolite of PGI2was measured by an enzyme immunoassay kit (Cayman) according to the manufacture's protocol.
Western blotting
During the preparation of arterioles, when the small intestine with attached mesentery was excised, the superior mesenteric artery with its branches was isolated from the mesentery by using a dissecting microscope and micro scissors. The superior mesenteric artery and branches were then washed in cold saline and frozen at -80 ℃ until assay. Western blotting was performed to detect the expression of COX-1 and COX-2 after all samples have been collected. Briefly, mesenteric arteries were homogenized in RIPA lysis buffer, the homogenates were centrifuged and the supernatants were collected. Appropriate samples were loaded in 10% sodium dodecylsulfate-polyacrylamide gels and after electrophoresis, the proteins were transferred topolyvinylidene fluoride membranes. Immunostaining of COX-1 and COX-2 was achieved using specific rabbit monoclonal anti-COX-1 (Abcam) and rabbit polyclonal anti-COX-2 (Abcam) antibodies and reacted with peroxidaseconjugated anti-rabbit antibody (Jackson) and enhanced chemiluminescence luminescence kit (Thermo Pierce).
Drugs
NE, Rp-cAMPS, and indomethacin, which were dissolved in saline containing 5% NaHCO3were purchased from Sigma.
Statistical analysis
The data were presented as mean±SD. Log concentrationresponse curves were fitted for the respective mean values of contraction by non-linear regression analysis (Software Prism, GraphPad). EC50 values (the molar concentration producing half maximum effect) were calculated from the curves. Statistical analysis was performed using unpaired Student'sttest. APvalue ≤0.05 was considered statistically significant.
Results
NE induced contractions
Stimulation of isolated third-generation mesenteric arterioles with NE caused a concentration-dependent contraction. The concentration-response curves to NE were similar in both rat groups, but cirrhosis induced an evident dextral shift of the concentration-response curves compared with saline treated control rats, and indomethacin partially reduced these changes (Fig. 1), while the EC50 values calculated from these curves changed in a parallel manner (Fig. 2). Thus, third-generation mesenteric arterioles from cirrhotic rats exhibited a depressed response to NE stimulation and this hyporesponsiveness could be partially improved by indomethacin.
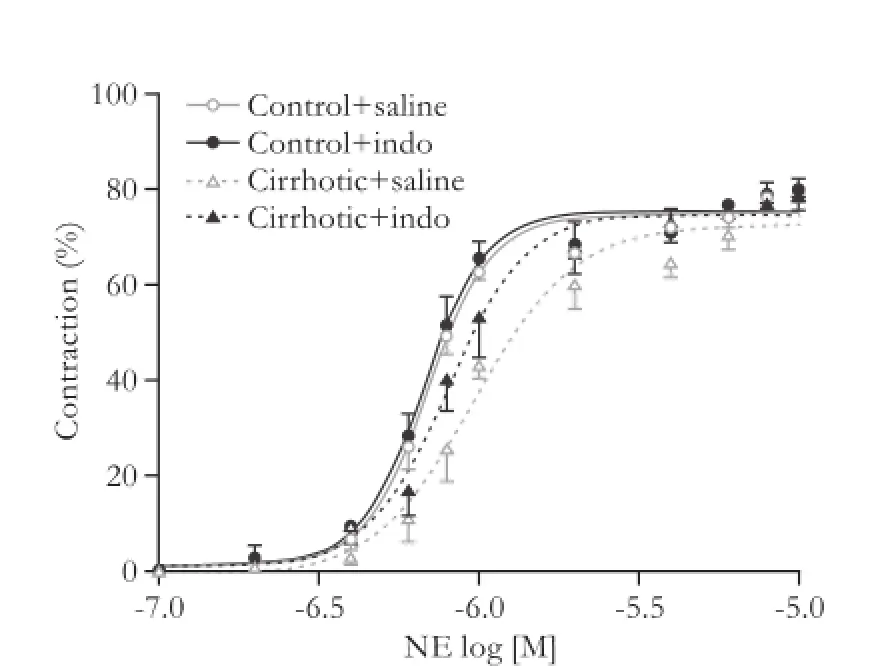
Fig. 1.Cumulative concentration-response curves to NE (10-7-10-5mol/L) of isolated third-generation mesenteric arterioles from the control (n=7) and cirrhotic rats (n=7) without treatment (saline) or with indomethacin treatment. Results were expressed as a percentage of contraction of the initial arteriolar diameter.

Fig. 2.EC50 values calculated from the fitted concentrationresponse curves of the control (n=7) and cirrhotic rats (n=7) without treatment (saline) or with indomethacin treatment. *:P<0.05, versus controls; #:P<0.05, versus saline.
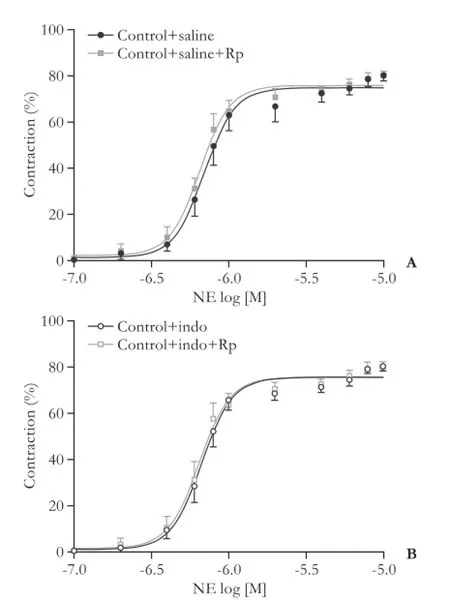
Fig. 3.Effect of Rp-cAMPS on the cumulative concentrationresponse curves to NE (10-7-10-5mol/L) in isolated third-generation mesenteric arterioles from the control rats (n=7) without treatment (saline) (A) or with indomethacin treatment (B). Results were expressed as a percentage of contraction of the initial arteriolar diameter.
In another set of experiments, third-generation mesenteric arterioles from all groups were pre-incubated with 50 μmol/L Rp-cAMPS for 30 minutes before the vasoconstriction response to NE was analyzed. The inhibition of PKA with Rp-cAMPS did not significantlymodify the vasoconstriction response to NE in arterioles from neither saline nor indomethacin treated control rats (Figs. 3 and 4). In contrast, in the arterioles from saline treated cirrhotic rats, Rp-cAMPS partially improved the vasoconstriction response to NE, but its effect was not visible in arterioles in indomethacin treated cirrhotic rats (Figs. 5 and 6).
6-keto-PGF1αproduction
Plasma concentrations of 6-keto-PGF1α, the stable metabolite of PGI2, were significantly elevated in saline treated cirrhotic rats compared with saline treated controls. Five-day consecutive treatment with indomethacin decreased the plasma 6-keto-PGF1αlevels in both control and cirrhotic rats compared with those treated with saline. The difference between indomethacin treated PHT rats and controls was not significant (Fig. 7).

Fig. 5.Effect of Rp-cAMPS on cumulative concentration-response curves to NE (10-7-10-5mol/L) in isolated third-generation mesenteric arterioles from the cirrhotic rats (n=7) without treatment (saline) (A) or with indomethacin treatment (B). The results were expressed as a percentage of contraction of the initial arteriolar diameter.
Western blotting assay of COX-1 and COX-2
The COX-1 protein was expressed in mesenteric arteries from saline treated control and cirrhotic rats and this expression was increased in cirrhotic rats (Fig. 8). COX-2 protein expression could not be detected in either of these vessels (data not shown).
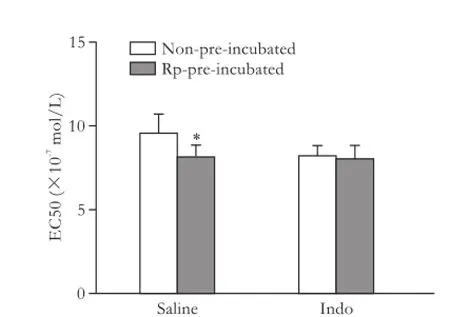
Fig. 6.Effect of Rp-cAMPS on EC50 values calculated from the fitted concentration-response curves of the cirrhotic rats (n=7) without treatment (saline) or with indomethacin treatment. *:P<0.05, versus non-pre-incubated.

Fig. 7.6-keto-PGF1αconcentrations in the control (n=7) and cirrhotic rats (n=7) in the absence (saline) or in the presence of indomethacin. *:P<0.05, versus controls; #:P<0.05, versus saline.
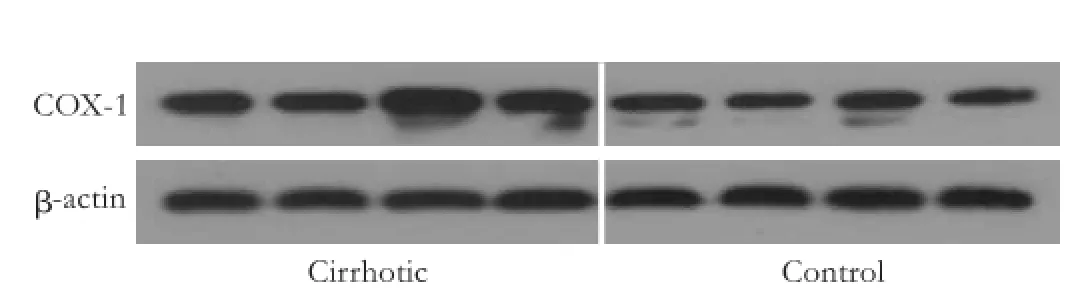
Fig. 8.COX-1 protein expressions in the mesenteric arteries from the saline treated control and cirrhotic rats.
Discussion
Vasodilation induced by impaired responses to vasoconstrictors despite elevated plasma levels of these vasoconstrictors is a key feature in PHT. In consistence with many other studies,[18-24]this study also demonstrated vascular hyporesponsiveness to NE in isolated splanchnic arterioles from cirrhotic rats. However, the underlying mechanisms are still not fully understood. Our results showed an evident increase of PGI2in cirrhotic rats which could be reversed by indomethacin. In parallel, we demonstrated that the arteriolar hyporesponsiveness was improved in indomethacin treated cirrhotic rats. However, in noncirrhotic control animals, although indomethacin reduced the production of PGI2, it did not change the vascular response to NE. Thus, we concluded that the increased production of PGI2in cirrhotic rats contributes to the splanchnic vascular hyporeactivity and the basal PGI2in control rats does not play a big role in the regulation of vascular constriction.
What is the mechanism of PGI2inhibition of vascular contraction? We found that PKA inhibition significantly increased the mesenteric artery contractility in cirrhotic rats. Our data are partially in accordance with those from a previous research.[15]However, Rp-cAMPS did not further improve the contractile-responsiveness in arterioles from indomethacin treated cirrhotic rats. We therefore proposed that the PGI2inhibition of splanchnic vascular responsiveness to NE is via the PKA pathway. PKA might attenuate the vasoconstriction by preventing the Ca2+-calmodulin-mediated conversion of inactive myosin light chain kinase (MLCK) to active MLCK or by inhibiting the ras homolog gene A (RhoA) signaling, both of which are necessary for vasoconstriction.[15,25]
To further clarify the mechanism for the PGI2increase in cirrhosis, we examined the protein expression of COX-1 and COX-2 in mesenteric arteries and found that the expression of COX-1 was significantly increased in the cirrhotic rats compared to the controls. In contrast, COX-2 expression was not detectable in mesenteric arteries from both groups.
In the past several years, many studies[18,26,27]have investigated the underlying mechanisms for this hypocontractility in PHT. Liao et al[18]reported that there were no changes in affinity or numbers of α1-adrenoceptors in the mesenteric and tail arteries of portal vein ligation induced PHT rats. Neef et al[26]observed the elevated mRNA levels of α1-adrenoceptor subtypes b, angiotension II type 1 receptor (AT1-R), arginine vasopressin receptor type 1a and endothelia receptor type A and B in hepatic arteries from cirrhotic patients. However, Hennenberg et al[27]did not find the changes of AT1-R as well as G-protein αq/11, α12 and α13 in both aortas from bile duct ligation induced cirrhotic rats and hepatic arteries from the patients with alcohol cirrhosis. These equivocal data suggested that it is not the defect of vasoconstrictor receptors but the post-receptor changes that lead to this hypocontractility in PHT. The main signal transduction system for the vasoconstrictor mediated vasoconstriction are the phospholipase C (PLC)/inositol-4, 5-triphosphate (IP3)-Ca2+/calmodulin pathway and the RhoA/Rho associated coiled-coil forming protein kinase (RhoA/ROCK) pathway.[6]A substantial number of studies have been conducted to detect the alterations of the two pathways in both animal models and patients with PHT. The results, however, were also equivocal concerning the alterations of G-protein, PKA, protein kinase G (PKG), RhoA, ROCK, β-arrestion-2, G-protein-coupled receptor kinases (GRKs) on expression or activity level.[15,21,22,24,27]
Although PGI2plays an important role in PHT, we should notice that there are many other vasodilators involved in the pathophysiology of PHT such as nitric oxide (NO), which can activate guanylate cyclase with production of cGMP and relax vascular smooth muscle cells subsequently.[2]This means more research should be done to clarify the underlying mechanisms for this hypocontractility. The mechanistic clarification may lead to the new approaches to the management of the PHT patients.
In the previous studies[11,22,24]investigating the pathogenic mechanisms leading to vasodilation in PHT, the most commonly used vessels were aortas and superior mesenteric artery rings. It should be noted, however, that the main site of the changes in vascular resistance is the splanchnic vasculature and conduit vessels such as aorta and superior mesenteric artery do not contribute to the regulation of vascular resistance.[28]Moreover, the change in vascular resistance is mainly due to the micro vessels with diameters of ≤150 μm. Therefore, the third-generation arterioles used in our study are appropriate for the investigation of splanchnic vascular responses to vasoconstrictors. In addition, arterioles were studied in vitro, and therefore the influences of neurohumoral regulations were excluded. Finally, the arteriolar response to vasoconstrictor was examined directly by measuring the changes in diameter, and because the microscope amplified the changes, even minor change could be detected. This method is frequently used in the study of vascular activity,[16,17,29,30]but rarely in PHT vasodilation.
In summary, we demonstrated that mesenteric artery response to vasoconstrictors was impaired and thePGI2levels were elevated in cirrhotic rats. Plasma PGI2elevation was associated with the overexpression of the COX-1 protein, whereas COX-2 protein expression was not detected. Our results suggested that PGI2may play its role through the PKA pathway and indomethacin can partially improve the hypocontractility by inhibiting the production of PGI2.
Acknowledgements:The authors gratefully thank Drs. Sun D and Huang A (Department of Physiology, New York Medical College, New York, USA) for their technical supervision and instrument assistance.
Contributors:SYW proposed the study. LDJ performed research and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts. SYW is the guarantor.
Funding:This study was supported by a grant from the Shanghai Science and Technology Commission (10411965200).
Ethical approval:All experiments were approved by the Animal Experiment Committee of Shanghai Jiaotong University School of Medicine.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Laleman W, Landeghem L, Wilmer A, Fevery J, Nevens F. Portal hypertension: from pathophysiology to clinical practice. Liver Int 2005;25:1079-1090.
2 Gatta A, Bolognesi M, Merkel C. Vasoactive factors and hemodynamic mechanisms in the pathophysiology of portal hypertension in cirrhosis. Mol Aspects Med 2008;29:119-129.
3 Newby DE, Hayes PC. Hyperdynamic circulation in liver cirrhosis: not peripheral vasodilatation but 'splanchnic steal'. QJM 2002;95:827-830.
4 Colle I, Geerts AM, Van Steenkiste C, Van Vlierberghe H. Hemodynamic changes in splanchnic blood vessels in portal hypertension. Anat Rec (Hoboken) 2008;291:699-713.
5 Heinzow HS, Lenz P, K?hler M, Reinecke F, Ullerich H, Domschke W, et al. Clinical outcome and predictors of survival after TIPS insertion in patients with liver cirrhosis. World J Gastroenterol 2012;18:5211-5218.
6 Hennenberg M, Trebicka J, Sauerbruch T, Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut 2008;57:1300-1314.
7 Hamilton G, Rosza I, Hutton R, Chow FP, Dandona P, Hobbs KE. Portal vein prostacyclin activity in experimental portal hypertension in rats. Clin Sci (Lond) 1981;60:327-329.
8 Sitzmann JV, Li SS, Adkinson NF. Evidence for role of prostacyclin as a systemic hormone in portal hypertension. Surgery 1991;109:149-153.
9 Guarner C, Soriano G, Such J, Teixidó M, Ramis I, Bulbena O, et al. Systemic prostacyclin in cirrhotic patients. Relationship with portal hypertension and changes after intestinal decontamination. Gastroenterology 1992;102:303-309.
10 Fernández M, García-Pagán JC, Casadevall M, Mourelle MI, Piqué JM, Bosch J, et al. Acute and chronic cyclooxygenase blockage in portal-hypertensive rats: influence in nitric oxide biosynthesis. Gastroenterology 1996;110:1529-1535.
11 Hou MC, Cahill PA, Zhang S, Wang YN, Hendrickson RJ, Redmond EM, et al. Enhanced cyclooxygenase-1 expression within the superior mesenteric artery of portal hypertensive rats: role in the hyperdynamic circulation. Hepatology 1998; 27:20-27.
12 Fetalvero KM, Shyu M, Nomikos AP, Chiu YF, Wagner RJ, Powell RJ, et al. The prostacyclin receptor induces human vascular smooth muscle cell differentiation via the protein kinase A pathway. Am J Physiol Heart Circ Physiol 2006;290: H1337-1346.
13 Vane J, Corin RE. Prostacyclin: a vascular mediator. Eur J Vasc Endovasc Surg 2003;26:571-578.
14 Stitham J, Midgett C, Martin KA, Hwa J. Prostacyclin: an inflammatory paradox. Front Pharmacol 2011;2:24.
15 Wu ZY, Benoit JN. Altered vascular norepinephrine responses in portal hypertensive intestine: role of PKA and guanylate cyclase. Am J Physiol 1997;272:G831-837.
16 Sun D, Messina EJ, Kaley G, Koller A. Characteristics and origin of myogenic response in isolated mesenteric arterioles. Am J Physiol 1992;263:H1486-1491.
17 Sun D, Kaley G, Koller A. Characteristics and origin of myogenic response in isolated gracilis muscle arterioles. Am J Physiol 1994;266:H1177-1183.
18 Liao JF, Yu PC, Lin HC, Lee FY, Kuo JS, Yang MC. Study on the vascular reactivity and alpha 1-adrenoceptors of portal hypertensive rats. Br J Pharmacol 1994;111:439-444.
19 Potenza MA, Botrugno OA, De Salvia MA, Lerro G, Nacci C, Marasciulo FL, et al. Endothelial COX-1 and -2 differentially affect reactivity of MVB in portal hypertensive rats. Am J Physiol Gastrointest Liver Physiol 2002;283:G587-594.
20 Atucha NM, Shah V, García-Carde?a G, Sessa WE, Groszmann RJ. Role of endothelium in the abnormal response of mesenteric vessels in rats with portal hypertension and liver cirrhosis. Gastroenterology 1996;111:1627-1632.
21 Hennenberg M, Biecker E, Trebicka J, Jochem K, Zhou Q, Schmidt M, et al. Defective RhoA/Rho-kinase signaling contributes to vascular hypocontractility and vasodilation in cirrhotic rats. Gastroenterology 2006;130:838-854.
22 Hennenberg M, Trebicka J, Kohistani AZ, Heller J, Sauerbruch T. Vascular hyporesponsiveness to angiotensin II in rats with CCl(4)-induced liver cirrhosis. Eur J Clin Invest 2009;39:906-913.
23 Heller J, Schepke M, Gehnen N, Molderings GJ, Müller A, Erhard J, et al. Altered adrenergic responsiveness of endothelium-denuded hepatic arteries and portal veins in patients with cirrhosis. Gastroenterology 1999;116:387-393.
24 Schepke M, Heller J, Paschke S, Thomas J, Wolff M, Neef M, et al. Contractile hyporesponsiveness of hepatic arteries in humans with cirrhosis: evidence for a receptor-specific mechanism. Hepatology 2001;34:884-888.
25 Luykenaar KD, Welsh DG. Activators of the PKA and PKG pathways attenuate RhoA-mediated suppression of the KDR current in cerebral arteries. Am J Physiol Heart Circ Physiol 2007;292:H2654-2663.
26 Neef M, Biecker E, Heller J, Schepke M, Nischalke HD, Wolff M, et al. Portal hypertension is associated with increased mRNA levels of vasopressor G-protein-coupled receptors in human hepatic arteries. Eur J Clin Invest 2003;33:249-255.
27 Hennenberg M, Trebicka J, Biecker E, Schepke M, SauerbruchT, Heller J. Vascular dysfunction in human and rat cirrhosis: role of receptor-desensitizing and calcium-sensitizing proteins. Hepatology 2007;45:495-506.
28 Wiest R. Splanchnic and systemic vasodilation: the experimental models. J Clin Gastroenterol 2007;41:S272-287.
29 Sun D, Liu H, Yan C, Jacobson A, Ojaimi C, Huang A, et al. COX-2 contributes to the maintenance of flow-induced dilation in arterioles of eNOS-knockout mice. Am J Physiol Heart Circ Physiol 2006;291:H1429-1435.
30 Resch M, Wiest R, Moleda L, Fredersdorf S, Stoelcker B, Schroeder JA, et al. Alterations in mechanical properties of mesenteric resistance arteries in experimental portal hypertension. Am J Physiol Gastrointest Liver Physiol 2009; 297:G849-857.
Received July 8, 2013
Accepted after revision January 23, 2014
Author Affiliations: Department of Biliary-Pancreatic Surgery, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200127, China (Liu DJ, Chen W, Huo YM, Liu W, Zhang JF, Hua R and Sun YW)
Yong-Wei Sun, MD, Department of Biliary-Pancreatic Surgery, Renji Hospital, School of Medicine, Shanghai Jiaotong University, 1630 Dongfang Road, Shanghai 200127, China (Tel: +86-21-68383773; Email: syw0616@126.com)
? 2014, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60270-8
Published online June 23, 2014.
 Hepatobiliary & Pancreatic Diseases International2014年4期
Hepatobiliary & Pancreatic Diseases International2014年4期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Effects of melatonin on the oxidative damage and pancreatic antioxidant defenses in ceruleininduced acute pancreatitis in rats
- A matched-pair analysis of laparoscopic versus open pancreaticoduodenectomy: oncological outcomes using Leeds Pathology Protocol
- Pancreaticoduodenectomy and pancreaticoduodenectomy combined with superior mesentericportal vein resection for elderly cancer patients
- Effect of external beam radiotherapy on patency of uncovered metallic stents in patients with inoperable bile duct cancer
- Liver transplantation using organs from deceased organ donors: a single organ transplant center experience
- Ultrasonic integrated backscatter in assessing liver steatosis before and after liver transplantation
