Separation of Recombinant Geranylgeranyl Diphosphate Synthase of Deinococcus radiodurans from Expressed Strain Cell Homogenate by Immobilized Metal Affinity Chromatography on a Characterized Monolithic Cryogel Column*
SHEN Shaochuan (沈紹傳)**, WANG Liangyan (王梁燕)**, SUN Zongtao (孫宗濤), LI Mingfeng (李銘峰) LIU Chengzhi (劉程智) TIAN Bing (田兵) YUN Junxian (贠軍賢)and HUA Yuejin (華躍進)***
1Key Laboratory for Nuclear-Agricultural Sciences of Chinese Ministry of Agriculture and Zhejiang Province, Institute of Nuclear-Agricultural Sciences, Zhejiang University, Hangzhou 310029, China
2State Key Laboratory Breeding Base of Green Chemistry Synthesis Technology, College of Chemical Engineering and Materials Science, Zhejiang University of Technology, Hangzhou 310032, China
3Department of Virology and Biotechnology, Zhejiang Academy of Agricultural Sciences, Hangzhou 310021, China
Separation of Recombinant Geranylgeranyl Diphosphate Synthase of Deinococcus radiodurans from Expressed Strain Cell Homogenate by Immobilized Metal Affinity Chromatography on a Characterized Monolithic Cryogel Column*
SHEN Shaochuan (沈紹傳)1,2,**, WANG Liangyan (王梁燕)1,**, SUN Zongtao (孫宗濤)3, LI Mingfeng (李銘峰)1, LIU Chengzhi (劉程智)1, TIAN Bing (田兵)1, YUN Junxian (贠軍賢)2and HUA Yuejin (華躍進)1,***
1Key Laboratory for Nuclear-Agricultural Sciences of Chinese Ministry of Agriculture and Zhejiang Province, Institute of Nuclear-Agricultural Sciences, Zhejiang University, Hangzhou 310029, China
2State Key Laboratory Breeding Base of Green Chemistry Synthesis Technology, College of Chemical Engineering and Materials Science, Zhejiang University of Technology, Hangzhou 310032, China
3Department of Virology and Biotechnology, Zhejiang Academy of Agricultural Sciences, Hangzhou 310021, China
Geranylgeranyl diphosphate synthase (GGPPS) plays a key role in the biosynthesis of antioxidative carotenoid from the extremely radioresistant bacterium Deinococcus radiodurans. In this work, the recombinant GGPPS expressed in Escherichia coli by cloning and transforming the gene dr1395 of D. radiodurans was isolated rapidly by an immobilized metal affinity supermacroporous cryogel, i.e., Cu2+-iminodiacetic acid (IDA)-cryogel. The properties of the Cu2+-IDA-cryogel were characterized using capillary-based mathematical model and experimental measurements. The obtained protein samples were analyzed by the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The porosity of the present Cu2+-IDA-cryogel is 90.4% and the water permeability is 5.04×10?12m2. From the capillary-based model, this cryogel presents a slightly wide normal pore (capillary) size distribution with the mean diameter of 55.2 μm, the standard deviation of 28.0 μm and the half of skeleton wall thickness of 2.8 μm. The pore size distribute from about 10 to 141 μm and the effective tortuosity of these capillary pores increases from 2.60 to 9.05. The isolation of the GGPPS from cell homogenate can be achieved at the flow velocity of 3.40×10?4m·s?1by the Cu2+-IDA-cryogel bed. High-purity GGPPS (about 91.4%) is obtained according to the SDS-PAGE analysis of the elution samples, indicating that the present method is a promising, simple and effective approach to isolate GGPPS from cell homogenate of engineering strains.
chromatography, separation, protein, modeling, bacterium
1 INTRODUCTION
Deinococcus radiodurans is a red-pigment bacterium well-known for its extraordinary resistance to a range of damages caused by ionizing radiation, desiccation, UV radiation, oxidizing agents, and electrophilic mutagens [1, 2]. This interesting organism has excellent capacities of damage tolerance and DNA repair and possesses efficient protein and non-protein protective systems [3]. In recent years, the non-protein protective system in D. radiodurans has attracted much attention due to its potential applications in various areas such as food, cosmetic and pharmaceutical industries [4-6]. Carotenoids, as the non-protein protective compounds, contribute to the cell resistant mechanism of D. radiodurans. Deinoxanthin, the major carotenoid of D. radiodurans, demonstrates stronger antioxidative capacity than lycopene and β-carotene in vitro [7]. The biosynthesis of deinoxanthin in D. radiodurans is complex and the process could be controlled by several synthases including phytoene synthase (CrtB, DR0862), phytoene desaturase (CrtI, DR0861), 1′,2′-hydratase (CruF, DR0091), carotenoid 3′,4′-desaturase (CrtD, DR2250) [7-10], and a geranylgeranyl diphosphate synthase (GGPPS, CrtE) [5]. Among them, GGPPS is the key synthase for the biosynthesis of geranylgeranyl diphosphate (GGPP), which is the crucial precursor in the deinoxanthin biosynthetic pathway. Generally, GGPPS catalyzes the synthesis of GGPP from farnesyl pyrophosphate, a C15 compound, and isopentenyl pyrophosphate (IPP), a C5 compound.
Actually, the family of GGPPS for terpene biosynthesis is widely found in a variety of organisms from prokaryotes to human [11, 12]. By using homologue analysis, the gene dr1395 has been predicted to encode GGPPS in D. radiodurans [5, 13, 14]. Moreover, our results have confirmed its function by gene mutation and transformation into Escherichia coli (data not shown). GGPPS plays a key role in carotenoid biosynthesis and has potential applications in industry. The separation and purification from the strains of enzyme are the important steps for its applications.However, there is a lack of applicable technology on the rapid separation and identification of recombinant GGPPS from D. radiodurans.
Monolithic cryogels are a new class of chromatographic supports for separation of biomolecules [15-20]. This interesting adsorbent has interconnected super-macropores with pore size of several microns to several hundreds of microns, which permit the high throughput of crude feedstocks containing microbial cells or cell debris. Cryogels have been used in capturing and isolating a variety of target biomolecules or particles including enzymes, bacterial endotoxins, adenosine triphosphate, microbial cells, and even viruses from complex crude biological suspensions or fermentation feedstocks [21-25].
GGPPSs, from bovine brain, pumpkin fruit, Thermus thermophilus, Taxus baccata and Phycomyces blakesleanus in original or expressed recombinant forms, have been purified by processes including ammonium sulfate precipitation and combined chromatography [26-30]. In this work, the DNA sequence of the gene dr1395 from D. radiodurans is amplified, cloned and transformed into E. coli to express GGPPS. The recombinant target GGPPS is purified by using a supermacroporous monolith, so called immobilized metal affinity cryogel. Properties of the cryogel column as well as the chromatographic separation of the recombinant GGPPS from E. coli homogenate are investigated.
2 MA TERIALS AND METHODS
2.1 Chemicals
Allyl glycidyl ether (AGE, 99%), N,N′-methylenebis(acrylamide) (MBAAm, 99%) and iminodiacetic acid (IDA, 96%) were purchased from Sigma-Aldrich (Steinheim, Germany), and acrylamide (AAm, 99.9%) was from Biobasic (Toronto, Canada). Bovine serum albumin (BSA, 98%) and N,N,N′,N′-tetramethylethylenediamine (TEMED, 99%) were purchased from Amresco (OH, USA). Other chemicals used were analytical grade. All reagents were used as received.
2.2 Bacterial strains and growth conditions
D. radiodurans R1 (ATCC 13939) was grown at 30 °C in tryptone-glucose-yeast extract (TGY) medium (5 mg·ml?1Bacto tryptone, 1 mg·ml?1glucose, and 3 mg·ml?1Bacto yeast extract) on an orbital shaker or on TGY plates solidified with 15 mg·ml?1agar. E. coli (DH5α) used as host for clone vectors and E. coli (BL21, DE3) used as host for expression vector were cultured in Luria-Bertani (LB) medium (10 mg·ml?1Bacto tryptone, 10 mg·ml?1sodium chloride, and 5 mg·ml?1Bacto yeast extract) at 37 °C.
2.3 Construction of recombinant expr ession GGPPS of D. Radiodurans
The DNA sequence of dr1395 was amplified from D. radiodurans using the primers 95P1 (5′-CATATGCGTCCCGAACTGCTCGCCCGCGTGC-3′) and 95P2 (5′-GGATCCGGAGGAC GCCGCTCACTTCTCCCG-3′). The polymerase chain reaction (PCR) products were ligated to thymine-adenine (TA) cloning vector pMD18-T for sequencing. The positive PCR products were cloned into His-tagged expression vector pET28a, forming recombinant vector pET-95. E. coli transformant contained recombinant vector pET-95 was incubated at 37 °C. Isopropyl-β-D-thiogalactopyranoside (IPTG) was added to a final concentration of 0.5 mmol·L?1after the OD600value of the culture had reached about 0.5. The culture was grown for additional 4 h and collected for recombinant GGPPS extraction by centrifugation at 8000 g and 4 °C for 5 min, washed three times with 50 mmol·L?1phosphate buffer (PB) (pH 7.2), and quickly frozen for storage at?80 °C for further use.
2.4 Preparation of the Cu2+-IDA-cryogel
A metal-chelated cryogel, i.e. Cu2+-IDA-cryogel, was prepared by coupling of Cu2+on the polyacrylamide-based cryogel matrix produced via the cryo-copolymerization of AAm, MBAAm and AGE initiated by TEMED and ammonium persulfate (APS) according to references, but with the freezing temperature variation manner in a glass column (I.D. 16 mm) as reported previously [19, 20, 24, 25, 31]. The concentration of monomers was 7% and the amounts of TEMED and APS used were 0.5% and 1.2% of monomers, respectively. In the metal coupling, the solution volumes and concentrations of Na2CO3and IDA, as well as other preparation conditions such as the flow rate, the reaction time and temperature, were established following those used previously [32]. In the immobilization procedure, 45 ml of 0.5 mol·L?1CuSO4was passed through the cryogel for 2 h and washed by deionized water for further use. The length of the resulting cryogel was 59 mm.
2.5 Characterization of the Cu2+-IDA-cryogel
The water permeability of the cryogel was determined by measuring the flow rates at different pressure drops using different heights of water-columns [24, 25, 32, 33]. The axial dispersion behavior was evaluated from the residence time distribution (RTD) by the tracer pulse method at various flow rates. 150 μl of 1 mg·ml?1BSA in 100 mmol·L?1PB (pH 7.2) was applied as the tracers in each run. The cryogel porosity was determined by measuring the water content and the cryogel volume according to references [21, 34, 35]. The pore size distribution was evaluated by the method with the combination of the capillary- based mathematical models and the experimental breakthrough curves at different flow rates, as described in reference [36]. The breakthrough experiments were conducted by pumping 1 mg·ml?1BSA in 100 mmol·L?1PB (pH 7.2) through the cryogel bed column at different flow rates under the non-binding condition, i.e., the protein was not bound by the cryogel but passed freely through the column. The process was monitored at UV 280 nm.
According to the model by Yun et al. [36-38], the Cu2+-IDA-cryogel here is assumed to be made up of tortuose capillaries with different lengths, but a constant thickness of skeleton walls and a normal size distribution written as

The capillaries in a given group have the same diameter, length, tortuosity and wall thickness. The tortuosity of a given capillary τiis defined as

The porosity of the cryogel bed ? is determined by the total volume of capillaries and the bed volume:
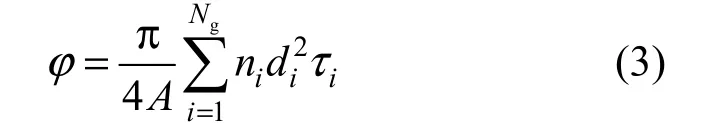
Since the cryogel bed volume is equal to the total volume of capillaries and their thin skeleton walls, we have

The water permeability of the cryogel is expressed as
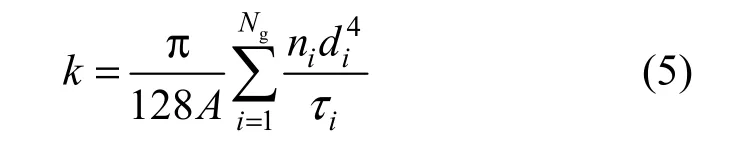
Under non-adsorption condition, the breakthrough of biomolecules such as BSA through the Cu2+-IDA-cryogel bed can be described by the differential mass balance equation [37-39]

The axial dispersion of protein within capillary i is given by [37]

The initial and the boundary conditions are expressed as


Details of the model and the numerical solution can be found in reference [37].
2.6 Isolation of GGPPS using cryogel column
3.2 g of thawed wet E. coli cell pellets containing recombinant GGPPS was re-suspended in 12.8 ml of 0.5 mol·L?1NaCl in 50 mmol·L?1PB (pH 7.2). The cells was disrupted with an ultrasonicator at 200 W output for a total of 5 min (5 s on and then 15 s off) on ice. The cell debris was removed by centrifugation (10000 r·min?1, 5 min), and the supernatants were subjected to the further chromatographic isolation. The chromatographic isolation of GGPPS from the E. coli homogenate was achieved using the Cu2+-IDA-cryogel at a constant flow velocity of 3.33×10?4m·s?1. The column was first equilibrated with 0.5 mol·L?1NaCl in PB (50 mmol·L?1, pH 7.2) and then 7.5 ml feedstock of E. coli homogenate in 0.5 mol·L?1NaCl in PB (50 mmol·L?1, pH 7.2) was loaded. The column was washed with 0.5 mol·L?1NaCl in PB (50 mmol·L?1, pH 7.2) and the elution was carried out using 0.1 mol·L?1imidazole in 0.5 mol·L?1NaCl in PB (50 mmol·L?1, pH 7.2) at the same liquid velocity. The process was monitored using a flow-through UV spectrometer at 280 nm as reported previously and the effluent was collected fractionally for further analysis [24, 25].
2.7 Analysis of protein purity
Protein samples from the stages of breakthrough, wash and elution were collected and analyzed by using the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The concentrations of the separating and the stacking gels were 14% and 4%, respectively. The electrophoresis was performed on the Mini Protean Tetra Cell device (Bio-Rad, USA) at a constant voltage of 120 V. The resulting gels were stained with 0.12% Coomassie Brilliant Blue R-250, destained and scanned using a digital gel imaging system (Tanon-3500, Tanon Science & Technology, China). The protein purity was estimated by the QuantityOne software (Version 4.2, Bio-Rad, USA) [40].
3 RESUL TS AND DISCUSSION
In order to set up effective separation strategy and suitable operation conditions to isolate GGPPS effectively from the E. coli homogenate and obtain highpurity target protein by using the Cu2+-IDA- cryogel, it needs to get an insight into the properties of the Cu2+-IDA-cryogel first. In this work, the properties of Cu2+-IDA-cryogel including cryogel porosity, permeability, pore size distribution, skeleton thickness and pore tortuosity are characterized. Furthermore, chromatographic isolation of GGPPS from the E. coli homogenate by using the Cu2+-IDA-cryogel is performed.
3.1 Properties of the Cu2+-IDA-cryogel
The cryogel porosity was determined by measuring the water content within a given sample volume. The obtained porosity of the present Cu2+-IDA-cryogel is 90.4%, which is close to those cryogels prepared under similar freezing conditions [23-25, 31-34]. The relationship of the pressure drop vs. flow rate was measured using different heights of water columns and the results are illustrated in Fig. 1. By fitting these experimental data with Darcy’s equation

the water permeability of the cryogel was determined as 5.04×10?12m2, which is also close to those values observed in other cryogels prepared under similar conditions [23-25, 31-34]. The values of the porosity and permeability indicate that the present cryogel has enough and high permeable space for homogenate and target enzyme to pass through and permit the achievement of chromatography at both low and high flow rates.
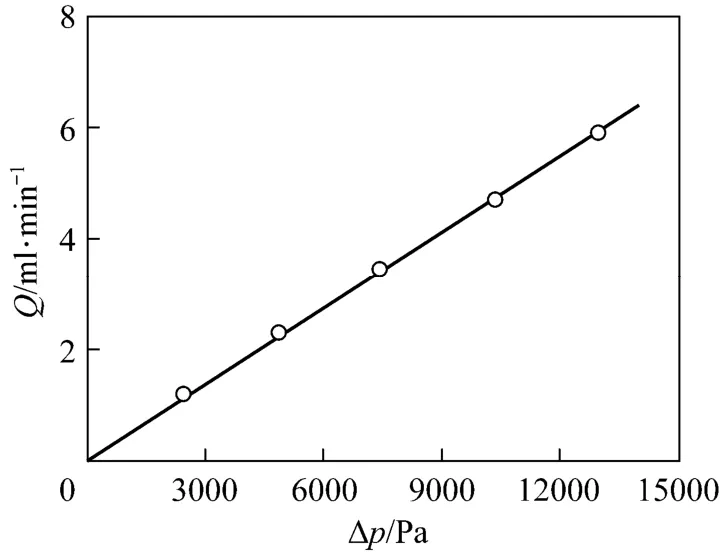
Figure 1 Fl ow rate vs. pr essure dr op in the Cu2+-IDA-cryogel bed○ experimental; fitted by Darcy’s equation
Parameters including the pore size distribution, skeleton thickness and pore tortuosity within the Cu2+-IDA-cryogel are crucial and significantly important to the chromatographic behavior of isolating GGPPS from the E. coli homogenate. However, due to the complexity of the cryogel microstructure, it is a challenging task to determine these parameters accurately by direct experimental measurements. Recently, capillary-based model, in which the super-macropores within cryogels are equivalent to bundles of capillaries, was suggested for describing the mass transfer and breakthrough behavior of proteins within cryogel beds [37, 38]. By fitting the model with the experimental data of porosity, permeability, cryogel length and diameter as well as the breakthrough data of given proteins at different flow rates, we can obtain an approximate estimation of these microstructure parameters. This method has been demonstrated to be effective in characterizing the microstructure properties of both polyacrylamide- and poly(hydroxyethyl methacrylate) (pHEMA)-based cryogels [37, 38]. In the present work, the method is employed to estimate the microstructure parameters of the Cu2+-IDA-cryogel, for which the experimental data of BSA breakthrough under nonbinding condition are used.
By using the experimental values of cryogel diameter, length, porosity, permeability and protein solution properties, the BSA breakthrough performance at different liquid flow velocities was evaluated by the model. The parameters of the present Cu2+-IDA-cryogel were then determined by comparing the model predictions of the BSA breakthrough profiles at different flow velocities with the corresponding experimental data. From the experimental breakthrough of BSA at the liquid flow velocity of 1.71×10?4m·s?1, the time for BSA tracers passing through the largest capillary within the cryogel bed was about 217 s, and this value was used to determine τdmax. Different values of the maximum and minimum pore diameters were tested and the parameter values of dmin=10 μm and dmax=160 μm give a good agreement between the model and experimental data. Fig. 2 shows the pore size distribution and the tortuosity of pores in the Cu2+-IDA-cryogel obtained by matching the model prediction with the experimental breakthrough curves as well as the water permeability, porosity and cryogel
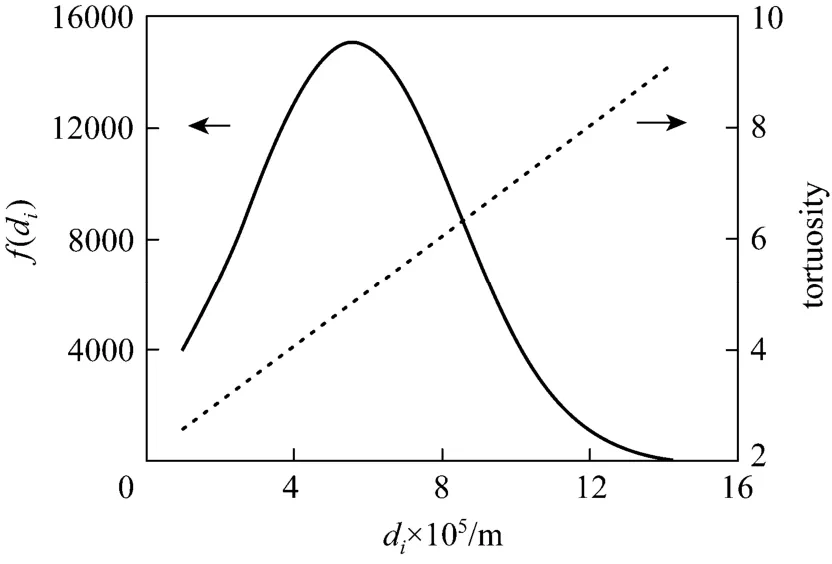
Figure 2 Diameter distribution () and tortuosity () of capillaries in the Cu2+-IDA-cryogel bed
bed size (volume) in this work. The cryogel presents a slightly wide normal pore (capillary) size distribution with the mean diameter of dm=55.2 μm, the standard deviation of σ=28.0 μm and the half of skeleton wall thickness ds=2.8 μm. The effective pore size varies from about 10 to 141 μm and the effective tortuosity of these capillary pores increases from 2.60 to 9.05. Fig. 3 shows the comparison between the calculated and experimental data of breakthrough for the determination of the microstructure parameters in the Cu2+-IDA-cryogel bed. The model fits the experimental breakthrough data well. The calculated porosity and permeability by the model are 91.7% and 4.99×10?12m2, respectively, which are very close to the experimental values.
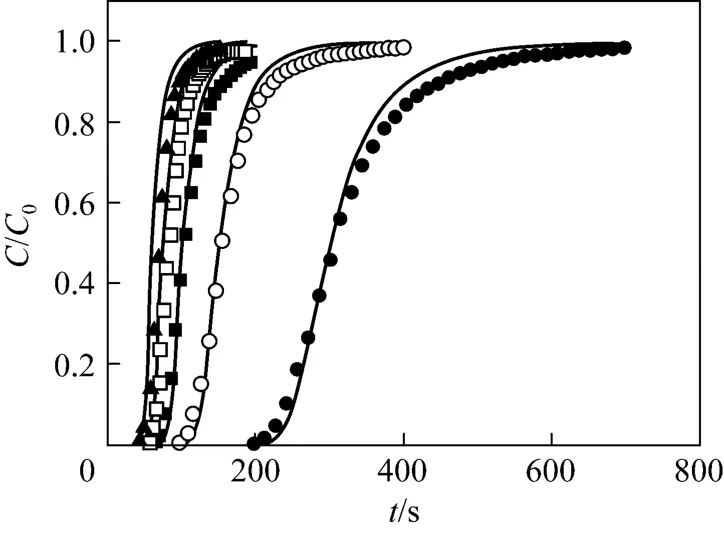
Figure 3 Comparison of the predicted and experimental breakthrough curves unde r non-adsorption c ondition at different liquid velocitiessolid lines—calculated data by the model; liquid velocity/m·s?1: ● 1.71×10?4; ○ 3.40×10?4; ■ 5.10×10?4;□ 6.88×10?4; ▲ 8.45×10?4
Generally, the target enzyme and the sizes of cell debris possibly contained in the centrifuged homogenate are several to hundreds of nanometers or several microns, which are much lower than those sizes of the pores within the present Cu2+-IDA-cryogel column. Thus, the cryogel is expected to be valid in the chromatographic isolation of GGPPS from the centrifuged E. coli homogenate.
The axial dispersion behavior is fundamental to the resolution and the spread of the elution peaks for the chromatography of the GGPPS by using the Cu2+-IDA-cryogel bed. In this work, the axial dispersion coefficients were determined from the residence time distribution (RTD) of BSA obtained at the flow velocities of 1.71×10?4, 3.40×10?4, 5.10×10?4, 6.88×10?4and 8.45×10?4m·s?1, as shown in Fig. 4. The calculated values of the overall dispersion coefficients at the same liquid velocities are in good agreement with those obtained by experimental measurements, demonstrating the availability of the model. The dispersion within the present Cu2+-IDA-cryogel is strong because the dispersion coefficients are in the range from 1.67×10?7to 1.27×10?6m2·s?1at the considered liquid flow velocities. Therefore, in order to get satisfied column efficiency, it is preferred to conduct the chromatography not at relatively high velocity for the present Cu2+-IDA-cryogel.
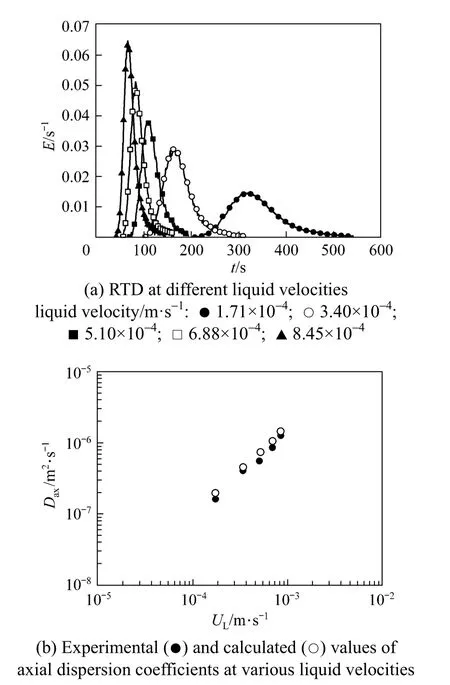
Figure 4 Axial dispersion behavior in the Cu2+-IDA-cryogel bed
3.2 Separation of GGPPS
The separation of GGPPS from the E. coli homogenate was carried out at the flow velocity of 3.40×10?4m·s?1and the chromatographic performance is displayed in Fig. 5, where the ordinate indicates the signals of UV280 translated and amplified linearly by the A/D converter in the chromatography process. There is a very limited amount of cell debris in the centrifuged E. coli homogenate. A total of 7.5 ml of the E. coli homogenate was loaded directly on the Cu2+-IDA-cryogel column. The elution was achieved successfully by using 0.1 mol·L?1imidazole and 0.5 mol·L?1NaCl in PB (50 mmol·L?1, pH 7.2). No peaks but only the plateau induced by the changing of elution liquid are observed for further increasing the concentration of imidazole (0.5 mol·L?1in this work).
Figure 6 shows the SDS-PAGE analysis on the samples of the homogenate and those from the breakthrough, wash and elution steps. The loaded feedstock in lane B is a complex containing lots of different proteins. In the breakthrough (lane C) and wash (lane D) stages, the band of GGPPS is observed, indicatingthat the present loaded volume is enough and some portion of the target enzyme are not bound and passes through the cyogel bed. In the elution stage, nearly the single band of GGPPS is observed as seen in lanes E, F and G. The average purity of these eluted samples is estimated by the Quantity One software to be about 91.4%. Therefore, it may be a promising, simple and effective approach to isolate the GGPPS from the E. coli homogenate by using the Cu2+-IDA-cryogel.
The separation of this recombinant enzyme could also be achieved directly from the crude cell homogenate by using the Cu2+-IDA-cryogel, as the supermacropores are large enough for the cell debris to pass thorough the cryogel without blockage as demonstrated by other researcher in different feedstocks [18-23], though the one-step separation directly from the crude cell homogenate is not exploited in this work.

Figure 5 Chromatographic behavior of GGPPS from the clarified E. coli homogenate by the Cu2+-IDA-cryogel bed[washed with 50 mmol·L?1PB (pH 7.2), eluted with 0.1 mol·L?1imidazole and 0.5 mol·L?1NaCl in PB (50 mmol·L?1, pH 7.2) (elution 1) followed by 0.5 mol·L?1imidazole and 0.5 mol·L?1NaCl in PB (50 mmol·L?1, pH 7.2) (elution 2);liquid velocity 3.40×10?4m·s?1in the chromatography process; C-G: fractions analyzed by SDS-PAGE as shown in Fig. 6]

Figure 6 Image of the SDS-PAGElanes A—standard protein markers; B—fermentation homogenate; C—breakthrough fraction; D—wash fraction; E, F, G—eluted peak fractions in elution; separating and stacking gels, 14% and 4%
4 CONCLU SIONS
GGPPS expressed in E. coli by cloning and transforming the gene dr1395 from D. radiodurans can be isolated rapidly by the super-macroporous Cu2+-IDA-cryogel. The fundamental properties of this immobilized metal affinity cryogel can be characterized by combining the capillary-based mathematical model and the experimental measurements. The results have shown that the present cryogel has high porosity and permeability, thin skeleton wall, lagersized tortuous super-macropores and a slightly wide normal pore size distribution, which is suitable for the rapid separation of GGPPS from the E. coli homogenate. The experimental results have also demonstrated that high-purity GGPPS (about 91.4%) can be obtained by the Cu2+-IDA-cryogel bed. This separation technology has potential application in industry for preparation of carotenoid biosynthesis from gene engineering strains.
NOMENCLATURE
A cross-area of the cryogel bed (=πdc2/4), m2
CDi(xD, tDi) dimensionless bulk-phase concentration of biomolecules in capillary i [=Ci( x, t)/C0]
Ci(x,t)bulk-phase concentration, mol·m?3
C0inlet concentration, mol·m?3
DABmolecular diffusion coefficient of biomolecules
Daxaxial dispersion coefficient of liquid
dcdiameter of the cryogel bed, μm
didiameter of capillary i, μm
dmmean diameter of capillaries in the cryogel column, μm
dmaxmaximum pore diameter, μm
dminminimum pore diameter, μm
dshalf of the skeleton thickness, μm
E residence time distribution
f(di) normal size distribution
k fluid permeability of the cryogel bed, m2
L length of the cryogel bed, m
Lilength of capillary i, m
Ngtotal number of capillary groups with the same diameter
ninumber of capillaries in group i
Peiaxial Peclet number (=τiLUi/Daxi)
Δp pressure drop, Pa
Q total flow rate in the cryogel bed, m3·s?1
t time, s
tDidimensionless time (=tUi/τiL)
tdmaxtime for the tracer passing through the largest capillary
within the cryogel bed, s
Uivelocity in capillary i (=ULdi2/32kτi), m·s?1
ULliquid flow velocity in the cryogel bed (=Q/ A), m·s?1
x distance, m
xDdimensionless distance from the inlet along the capillary length (=x/τiL)
μ water viscosity, Pa·s
σ standard deviation, μm
τdmaxtortuosity of the capillary with diameter dmaxτdmintortuosity of the capillary with diameter dminτitortuosity of capillary i (=Li/L )
φ porosity of the cryogel bed
ψ parameter used in the axial dispersion equation
REFERENCES
1 Minton, K.W., “DNA repair in the extremely radioresistant bacterium Deinococcus radiodurans”, Mol. Microbiol.,13, 9-15 (1994).
2 Cox, M.M., Battista, J.R., “Deinococcus radiodurans—the consummate survivor”, Nat. Rev. Microbiol., 3, 882-892 (2005).
3 Battista, J.R., Earl, A.M., Park, M.J., “Why is Deinococcus radiodurans so resistant to ionizing radiation?”, Trends Microbiol., 7, 362-365 (1999).
4 Lemee, L., Peuchant, E., Clerc, M., Brunner, M., Pfander, H.,“Deinoxanthin: A new carotenoid isolated from Deinococcus radiodurans”, Tetrahedron, 53, 919-926 (1997).
5 Tian, B., Hua, Y.J., “Carotenoid biosynthesis in extremophilic Deinococcus-Thermus bacteria”, Trends Microbiol., 18, 512-520 (2010).
6 Slade, D., Radman, M., “Oxidative stress resistance in Deinococcus radiodurans”, Microbiol. Mol. Biol. R., 75, 133-191 (2011).
7 Tian, B., Xu, Z.J., Sun, Z.T., Lin, J., Hua, Y.J., “Evaluation of the antioxidant effects of carotenoids from Deinococcus radiodurans through targeted mutagenesis, chemiluminescence, and DNA damage analyses”, BBA—Gen. Subjects, 1770, 902-911 (2007).
8 Xu, Z.J., Tian, B., Sun, Z.T., Lin, J., Hua, Y.J., “Identification and functional analysis of a phytoene desaturase gene from the extremely radioresistant bacterium Deinococcus radiodurans”, Microbiol. —SGM, 153, 1642-1652 (2007).
9 Tian, B., Sun, Z.T., Xu, Z.J., Shen, S.C., Wang, H., Hua, Y.J., “Carotenoid 3′,4′-desaturase is involved in carotenoid biosynthesis in the radioresistant bacterium Deinococcus radiodurans”, Microbiol. —SGM, 154, 3697-3706 (2008).
10 Sun, Z.T., Shen, S.C., Wang, C., Wang, H., Hu, Y.P., Jiao, J.D., Ma, T.T., Tian, B., Hua, Y.J., “A novel carotenoid 1,2-hydratase (CruF) from two species of the non-photosynthetic bacterium Deinococcus”, Microbiol. —SGM, 155, 2775-2783 (2009).
11 Math, S.K., Hearst, J.E., Poulter, C.D., “The crtE gene in Erwinia herbicola encodes geranylgeranyl diphosphate synthase”, P. Natl. Acad. Sci. USA, 89, 6761-6764 (1992).
12 Miyagi, Y., Matsumura, Y., Sagami, H., “Human geranylgeranyl diphosphate synthase is an octamer in solution”, J. Biochem., 142, 377-381 (2007).
13 Makarova, K.S., Aravind, L., Wolf, Y.I., Tatusov, R.L., Minton, K.W., Koonin, E.V., Daly, M.J., “Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics”, Microbiol. Mol. Biol. R., 65, 44-79 (2001).
14 Ghosal, D., Omelchenko, M.V., Gaidamakova, E.K., Matrosova, V.Y., Vasilenko, A., Venkateswaran, A., Zhai, M., Kostandarithes, H., Brim, H., Makarova, K., “How radiation kills cells: survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress”, FEMS Microbiol. Rev., 29, 361-375 (2005).
15 Lozinsky, V.I., Plieva, F.M., Galaev, I.Y., Mattiasson, B., “The potential of polymeric cryogels in bioseparation”, Bioseparation, 10, 163-188 (2002).
16 Lozinsky, V.I., Galaev, I.Y., Plieva, F.M., Savina, I.N., Jungvid, H., Mattiasson, B., “Polymeric cryogels as promising materials of biotechnological interest”, Trends Biotechnol., 21, 445-451 (2003).
17 Plieva, F.M., Galaev, I.Y., Mattiasson, B., “Macroporous gels prepared at subzero temperatures as novel materials for chromatography of particulate-containing fluids and cell culture applications”, J. Sep. Sci., 30, 1657-1671 (2007).
18 Lozinsky, V.I., “Polymeric cryogels as a new family of macroporous and super-macroporous materials for biotechnological purposes”, Russ. Chem. Bull., 57, 1015-1032 (2008).
19 Plieva, F.M., Kirsebom, H., Mattiasson, B., “Preparation of macroporous cryostructurated gel monoliths, their characterization and main applications”, J. Sep. Sci., 34, 2164-2172 (2011).
20 Kirsebom, H., Galaev, I.Y., Mattiasson, B., “Stimuli-responsive polymers in the 21st century: Elaborated architecture to achieve high sensitivity, fast response, and robust behavior”, J. Polym. Sci. Pol. Phys., 49, 173-178 (2011).
21 Arvidsson, P., Plieva, F.M., Savina, I.N., Lozinsky, V.I., Fexby, S., Bulow, L., Galaev, I.Y., Mattiasson, B., “Chromatography of microbial cells using continuous super-macroporous affinity and ion-exchange columns”, J. Chromatogr. A, 977, 27-38 (2002).
22 Plieva, F.M., Galaev, I.Y., Noppe, W., Mattiasson, B., “Cryogel applications in microbiology”, Trends Microbiol., 16, 543-551 (2008).
23 Yun, J.X., Shen, S.C., Chen, F., Yao, K.J., “One-step isolation of adenosine triphosphate from crude fermentation broth of Saccharomyces cerevisiae by anion-exchange chromatography using supermacroporous cryogel”, J. Chromatogr. B, 860, 57-62 (2007).
24 Wang, L.H., Shen, S.C., Yun, J.X., Yao, K.J., Yao, S.J., “Chromatographic separation of cytidine triphosphate from fermentation broth of yeast using anion-exchange cryogel”, J. Sep. Sci., 31, 689-695 (2008).
25 Chen, Y., Shen, S.C., Yun, J.X., Yao, K.J., “Isolation of ATP from a yeast fermentation broth using a cryogel column at high flow velocities”, J. Sep. Sci., 31, 3879-3883 (2008).
26 Sagami, H., Morita, Y., Ogura, K., “Purification and properties of geranylgeranyl-diphosphate synthase from bovine brain”, J. Biol. Chem., 269, 20561-20566 (1994).
27 Ogura, K., Shinka, T., Seto, S., “The purification and properties of geranylgeranyl pyrophosphate synthetase from pumpkin fruit”, J. Biochem., 72, 1101-1108 (1972).
28 Nishio, K., Nodake, Y., Hamada, K., Suto, K., Nakagawa, N., Kuramitsu, S., Miura, K., “Expression, purification, crystallization and preliminary X-ray studies of geranylgeranyl diphosphate synthase from Thermus thermophilus HB8”, Acta Crystallogr. D, 60, 178-180 (2004).
29 Laskaris, G., van der Heijden, R., Verpoorte, R., “Purification and partial characterisation of geranylgeranyl diphosphate synthase, from Taxus baccata cell cultures. An enzyme that regulates taxane biosynthesis”, Plant Sci., 153, 97-105 (2000).
30 Brinkhaus, F.L., Rilling, H.C., “Purification of geranylgeranyl diphosphate synthase from Phycomyces blakesleanus”, Arch. Biochem. Biophys., 266, 607-612 (1988).
31 Yao, K.J., Shen, S.C., Yun, J.X., Wang, L.H., He, X.J., Yu, X.M.,“Preparation of polyacrylamide-based super-macroporous monolithic cryogel beds under freezing-temperature variation conditions”, Chem. Eng. Sci., 61, 6701-6708 (2006).
32 Wang, L.H., Shen, S.C., He, X.J., Yun, J.X., Yao, K.J., Yao, S.J.,“Adsorption and elution behaviors of bovine serum albumin in metal-chelated affinity cryogel beds”, Biochem. Eng. J., 42, 237-242 (2008).
33 He, X.J., Yao, K.J., Shen, S.C., Yun, J.X., “Freezing characteristics of acrylamide-based aqueous solution used for the preparation of super-macroporous cryogels via cryo-copolymerization”, Chem. Eng. Sci., 62, 1334-1342 (2007).
34 Yao, K.J., Yun, J.X., Shen, S.C., Wang, L.H., He, X.J., Yu, X.M.,“Characterization of a novel continuous super-macroporous monolithic cryogel embedded with nanoparticles for protein chromatography”, J. Chromatogr. A, 1109, 103-110 (2006).
35 Plieva, F.M., Savina, I.N., Deraz, S., Andersson, J., Galaev, I.Y., Mattiasson, B., “Characterization of super-macroporous monolithic polyacrylamide based matrices designed for chromatography of bioparticles”, J. Chromatogr. B, 807, 129-137 (2004).
36 Yun, J.X., Lin, D.Q., Yao, S.J., “Predictive modeling of protein adsorption along the bed height by taking into account the axial nonuniform liquid dispersion and particle classification in expanded beds”, J. Chromatogr. A, 1095, 16-26 (2005).
37 Yun, J.X., Jespersen, G.R., Kirsebom, H., Gustavsson, P.E., Mattiasson, B., Galaev, I.Y., “An improved capillary model for describing the microstructure characteristics, fluid hydrodynamics and breakthrough performance of proteins in cryogel beds”, J. Chromatogr. A, 1218, 5487-5497 (2011).
38 Yun, J.X., Kirsebom, H., Galaev, I.Y., Mattiasson, B., “Modeling of protein breakthrough performance in cryogel columns by taking into account the overall axial dispersion”, J. Sep. Sci., 32, 2601-2607 (2009).
39 Persson, P., Baybak, O., Plieva, F., Galaev, I.Y., Mattiasson, B., Nilsson, B., Axelsson, A., “Characterization of a continuous supermacroporous monolithic matrix for chromatographic separation of large bioparticles”, Biotechnol. Bioeng., 88, 224-236 (2004).
40 Yan, L.D., Shen, S.C., Yun, J.X., Yao, K.J., “Isolation of lysozyme from chicken egg white using polyacrylamide-based cation-exchange cryogel”, Chin. J. Chem. Eng., 19, 876-880 (2011).
2012-04-05, accepted 2012-12-30.
* Supported by the National Natural Science Foundation of China (30830006, 20876145, 21036005), the International Science & Technology Cooperation Program from the Ministry of Science and Technology of China (1017), the Special Fund for Agroscientific Research in the Public Interest (201103007), the Fundamental Research Funds for the Central Universities and the Natural Science Foundation of Zhejiang Province (Y4080326, Y407366).
** These authors contributed equally to this work.
*** To whom correspondence should be addressed. E-mail: yjhua@zju.edu.cn
 Chinese Journal of Chemical Engineering2013年6期
Chinese Journal of Chemical Engineering2013年6期
- Chinese Journal of Chemical Engineering的其它文章
- Adsorption and Desorption Behavior of Tannic Acid in Aqueous Solution on Polyaniline Adsorbent*
- Synthesis of 2-Methyl-4-methoxyaniline from o-Nitrotoluene Using Pt/C and Acidic Ionic Liquid as Catalyst System*
- Supercritical Fluid Extraction of a Novel Template from Mesoporous Zirconia and the Effect on Porous Structure*
- Preparation of Mesoporous Carbons from Acrylonitrile-methyl Methacrylate Copolymer/Silica Nanocomposites Synthesized by in-situ Emulsion Polymerization*
- Effect of Hydrogen Reduction of Silver Ions on the Performance and Structure of New Solid Polymer Electrolyte PEI/Pebax2533/AgBF4Composite Membranes*
- Comparison on Thermal Conductivity and Permeability of Granular and Consolidated Activated Carbon for Refrigeration*
