有機染料D-SS和D-ST用于染料敏化太陽能電池光敏劑的比較
詹衛(wèi)伸 潘 石 王 喬 李 宏 張 毅
(大連理工大學(xué)物理與光電工程學(xué)院,近場光學(xué)與納米技術(shù)研究所,遼寧大連116024)
有機染料D-SS和D-ST用于染料敏化太陽能電池光敏劑的比較
詹衛(wèi)伸 潘 石*王 喬 李 宏 張 毅
(大連理工大學(xué)物理與光電工程學(xué)院,近場光學(xué)與納米技術(shù)研究所,遼寧大連116024)
為了揭示D-SS和D-ST分子敏化的染料敏化太陽能電池(DSSCs)的物理機制,采用密度泛函理論(DFT)、含時密度泛函理論(TDDFT)和自然鍵軌道(NBO)分析,模擬計算染料D-SS和D-ST分子的結(jié)構(gòu)、紫外-可見吸收光譜和能級結(jié)構(gòu).D-SS的紫外-可見吸收光譜相比于D-ST的有明顯的紅移,而且D-SS分子的摩爾吸光系數(shù)也高于D-ST分子的.D-SS分子本應(yīng)該比D-ST分子擁有更高的俘獲太陽輻射光子的能力,但由于D-SS分子的最高占據(jù)分子軌道(HOMO)能級位置比氧化還原電解質(zhì)(I-/I-3)的氧化還原能級高,處于光激發(fā)態(tài)的D-SS分子向TiO2電極注入電子而被氧化后,不能順利地從電解質(zhì)中得到電子而還原,使得D-SS分子俘獲光子的能力不能充分發(fā)揮,從而嚴(yán)重地降低了由其敏化的DSSCs的光電性能和光電能量轉(zhuǎn)換效率.揭示了D-SS敏化的DSSCs的光電性能,特別是光電能量轉(zhuǎn)換效率比D-ST敏化的DSSCs的低的原因.染料敏化劑分子的HOMO能級的位置對于DSSCs來說也是很重要的,用于DSSCs的有機敏化劑分子的HOMO能級的位置必須低于氧化還原電解質(zhì)的氧化還原能級.
密度泛函理論;含時密度泛函理論;染料敏化太陽能電池;分子模擬;電子結(jié)構(gòu);吸收光譜;能級結(jié)構(gòu)
1 Introduction
Since Gr?tzel et al.1-4reported the dye-sensitized solar cells (DSSCs)based on Ru complex in 1991,more attention has been paid to DSSCs due to its comparatively low cost and high efficiency.5,6However,DSSCs sensitized by free-metal organic dye has attracted more interest from researchers because of its much lower cost.7-14The dye molecules applied to DSSCs should have a structure of“donor-conjugate π bridge-acceptor(D-π-A)”,8,15-20in which the“electron acceptor”must contain the“anchoring group”.By the“anchoring group”,dye molecules can be adsorbed on the surface of TiO2nanocrystalline.9,20-24At present,the“anchoring group”is usually chosen as carboxyl (-COOH).Most D-π-A dyes take dialkylamines or diphenylamine moieties as electron donor,while carboxylic acid,cyanoacrylic acid,or rhodanine-3-acetic acid moieties as electron acceptor which also acts as an anchoring group.Carboxy groups can hang on the surface of TiO2,providing a strong restriction to dyes and a good electron-channel.Photoabsorption characteristic of D-π-A dyes is connected with intramolecular charge transfer(ICT),the excitation from electron donor to electron acceptor moiety,which results in efficient electron transfer from dyes excitation state via electron acceptor moiety(carboxy groups)to TiO2conduction band(CB)edge.Charge transfer or separation between electron donors and acceptors in the excitation state can facilitate the electron injection from dyes to TiO2CB,thus it can separate the cationic charge from surface and prevent the photoelectron(the injected electron)from compounding with oxidized dyes effectively.20,25The conjugate π bridge of sensitizer molecule for DSSCs is usually planar, which is benefit for ICT of electrons transition from the electron-donating group to the electron-accepting group14,22,26,27.In the electronically excited state,an anomalous 90°twist will take place in the donor moiety of dye molecules,which will promote the intramolecular charge transfer28.Hydrogen bonds between dye molecules and solvent molecules will facilitate the intramolecular charge transfer in dye molecules.29,30
Absorption spectra for dye are supposed to match well with solar radiation spectrum in order to effectively capture solar radiation photons.20,24,31To obtain effective injection electrons from excited dye to the conduction band of the TiO2electrode, the lowest unoccupied molecular orbital(LUMO)energy level of dye molecule must rank above the conduction band energy level of the TiO2.Not every excited dye molecule can inject electrons to TiO2electrode,because there are many other processes making the dye molecule de-excitation,which has a strong impact on the electron injection to the TiO2electrode. While energy level of the LUMO is higher,the driving forces of the electron injection from dye molecule to TiO2electrode will become stronger,which results in the higher transfer efficiency of DSSCs.32In order to make the oxidized(lose electrons)dye molecule efficiently recover(gain electrons)from I-/redox couple in the electrolyte,HOMO energy level of dye molecule has to be lower than the redox potential of.20,31To gain higher light-harvesting efficiency,dye molecule must possess higher molar absorption coefficient in the wide area of solar spectra.20
In 2006,Yang et al.33designed and synthesized two D-π-A polyene dyes D-SS and D-ST,and applied them to sensitized DSSCs.In the polar solution of tetrahydrofuran(THF),the maximum absorption peak of dye D-SS is located at 513 nm (molar absorption coefficient:ε=3.84×104L·mol-1·cm-1); while that of dye D-ST is located at 488 nm(ε=5.01×104L· mol-1·cm-1).Compared with D-ST,D-SS has one more methenyl chain on the bridge base,due to the extension of bridge base,which naturally gives rise to the red-shift of absorption spectra.23,27,34The incident photo-to-current conversion efficiency(IPCE)value of dye D-ST sensitized DSSCs exceeds 70% within 470-580 nm,and reaches a maximum value of 82%at 515 nm;this value of dye D-SS sensitized DSSCs is relatively low,and reaches a maximum value of 66%at 540 nm.The photovoltaic properties(short-circuit current density:JSC=10.64 mA,open-circuit voitage:VOC=0.52 V,fill factor:FF=0.70)of dye D-SS sensitized DSSCs are lower than those(JSC=15.23 mA,VOC=0.56 V,FF=0.73)of dye D-ST.Especially,the photovoltaic energy conversion efficiency(η=3.87%)of dye D-SS sensitized DSSCs is much lower than that(η=6.23%)of dye D-ST.33By comparison of the experimental results for dyes D-SS and D-ST,we found that,the absorption spectrum of dye D-SS exhibited red-shift larger than that of dye D-ST.However,the photovoltaic properties of D-SS sensitized DSSCs,especially photovoltaic energy conversion efficiency,are lower than those of dye D-ST.
The density functional theory(DFT)/time-dependent DFT (TDDFT)calculations has recently been used to investigate the absorption properties of molecules successfully.35-41The TDDFT method has been confirmed as a very useful and reliable tool to study the excited states of large molecules.42-46Compared with the experiment,the calculation of the TDDFT shows a considerable red-shift,especially the red-shift in the solution is more visible than the one in vacuum.The difference between the experiment and calculation of the TDDFT may come from the calculation method.The energy gaps calculated by the DFT are always smaller than those of the factual one,especially for the bigger conjugated system,which causes low calculated excited energy and significant red-shift in the calculated absorption spectra compared with the factual one.24,47,48It shows a difficulty in quantitative comparison of the calculation and the experiment.Though there is difference,the calculation of TDDFT can still describe the spectral features of the dye molecules,because the line shape and the relative intensity of spectra correspond with the experiment qualitatively.40,49In this article,we use a DFT/TDDFT method to investigate the molecular structures,absorption spectra,energy levels of D-SS and D-ST,trying to explain why the photovoltaic energy conversion efficiency of dye D-SS sensitized DSSCs is lower than that of D-ST.
2 Computational methods
DFT and TDDFT calculations were performed using the Gaussian 03 software package.50The ground-state geometries of dyes D-SS and D-ST were fully optimized in vacuum without any symmetry constraints at the B3LYP51-57level of theory with the 6-31G(d)basis set.The contribution of single excited state configurations to each electronic transition and the simulated absorption spectra of the dyes D-SS and D-ST were calculated. The electronic absorption spectra require calculation of the allowed excitations and oscillator strengths.These calculations were carried out using TDDFT with the same basis set and exchange-correlation functional in vacuum and solution.The TDB3LYP calculation containing the solvation effect in THF solution was performed on the geometries optimized in vacuum. The conductor polarizable continuum model(CPCM)19,58was conducted employing parameters and iterative computation methods as suggested by Klamt59,60to contain the solvation effect.Natural bond orbital(NBO)analysis was performed in ordertoanalyzethechargepopulationsof dyes D-SS andD-ST.48,61
3 Results and discussion
3.1 Molecular structures
Fig.1 shows the molecular structures of the dyes D-SS and D-ST.Fig.2 shows the optimized geometrical structures and the frontier molecular orbitals of the dyes D-SS and D-ST.
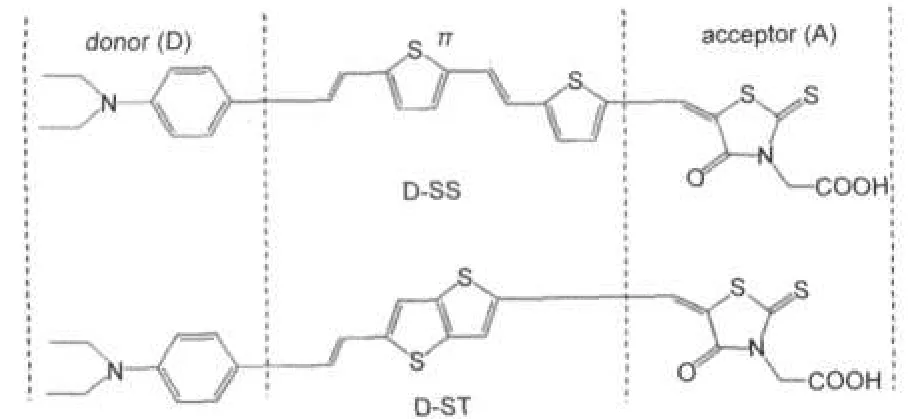
Fig.1 Molecular structures of dyes D-SS and D-ST
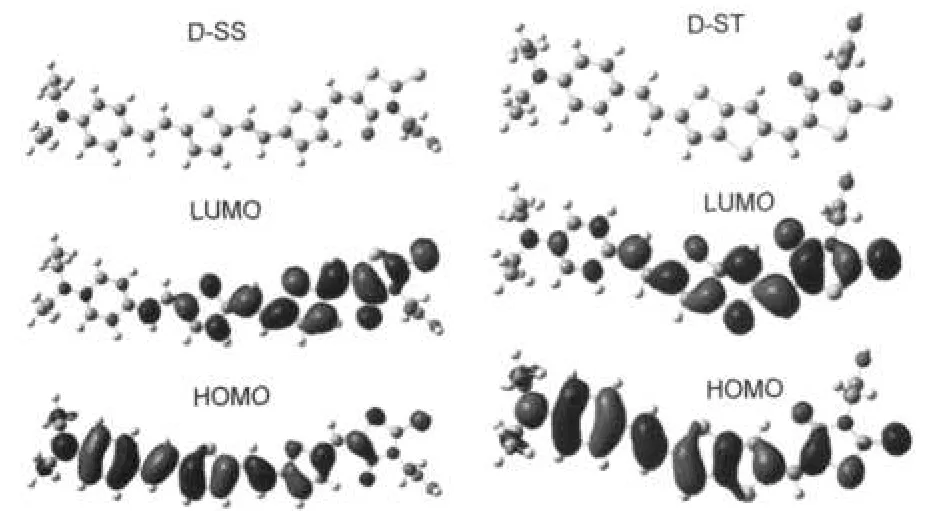
Fig.2 Optimized geometrical structures and frontier molecular orbitals of dyes D-SS and D-STHOMO:the highest occupied molecular orbital; LUMO:the lowest unoccupied molecular orbital
By analysis of NBO,we find that the charge populations of donor group,conjugated bridge,and acceptor group of D-SS are 0.065e,0.104e,and-0.166e,respectively;for D-ST,they are 0.074e,0.094e,and-0.171e,respectively.All of these molecules have similar D-π-A structures.The conjugate bridges of the dyes D-SS and D-ST are plane,which are conductive to intra molecular charge transfer for electrons from the electron-donating group to the electron-accepting group.14,22,26,27The electronic structures of HOMOs and LUMOs of the dyes D-SS and D-ST are alike.The HOMO is π orbital,while LUMO is single state of π*orbital.HOMOs have ground state characteristics, while LUMOs have excited state characteristics.In the ground state,the electrons are mainly distributed in the electron donor and conjugated bridge.In the excited state,the electrons are distributed in the thiophenes and cyanoacrylic acid groups,however,mostly in the anchoring groups(carboxyl:-COOH).Under illumination,through intramolecular charge transfer,electrons move from the HOMO to the LUMO,and eventually reach the anchoring groups.The molecular structures of the dyes D-SS and D-ST are very beneficial for solar cells.
3.2 Electronic absorption spectra
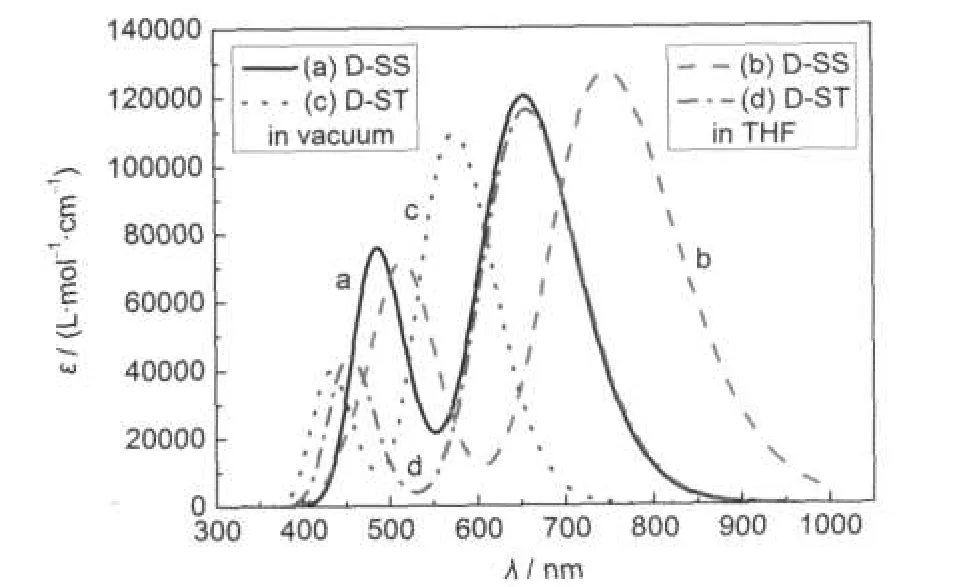
Fig.3 UV-Vis absorption spectra of the dyes D-SS and D-ST in vacuum and THF solution
Fig.3 shows the calculated UV-Vis absorption spectra for the dyes D-SS and D-ST in vacuum and THF solution.Whenever in vacuum or THF solution,the dyes D-SS and D-ST have two clear absorption bands within the UV-Vis region.The central wavelength(also maximum peak absorption wavelength λmax) of the first absorption band in the absorption spectrum of dye D-SS molecules in vacuum is approximately 655 nm(molar absorption coefficient approximately 1.2×105L·mol-1·cm-1),and the central wavelength of the second absorption band is approximately 485 nm(molar absorption coefficient approximately 0.76×105L·mol-1·cm-1);the central wavelength(λmax)of the first absorption band in the absorption spectrum in THF solution is approximately 750 nm(molar absorption coefficient approximately 1.27×105L·mol-1·cm-1),and the central wavelength of the second absorption band is approximately 514 nm (molar absorption coefficient approximately 0.72×105L·mol-1· cm-1).The central wavelength of the first absorption band in the absorption spectrum of dye D-ST molecules in vacuum is approximately 575 nm(molar absorption coefficient approximately 1.1×105L·mol-1·cm-1),and the central wavelength of the second absorption band is approximately 430 nm(molar absorption coefficient approximately 0.4×105L·mol-1·cm-1);the central wavelength of the first absorption band in the absorption spectrum in THF solution is approximately 658 nm(molar absorption coefficient approximately 1.16×105L·mol-1·cm-1), and the central wavelength of the second absorption band is approximately 452 nm(molar absorption coefficient approximately 0.44×105L·mol-1·cm-1).
In comparison with that in vacuum,the maximum peak absorption wavelength of dye D-SS molecules in THF solution has an approximately 95 nm red-shift;similarly,in comparison with the situation in vacuum,the maximum peak absorption wavelength of dye D-ST molecules in THF solution has an approximately 83 nm red-shift.This is due to the impact of solvent effect.58,62-66
In vacuum,in comparison with the case of dye D-ST molecules,the maximum peak absorption wavelength of dye D-SS molecules has an approximately 80 nm red-shift;while in THF solution,it turns out approximetely 92 nm red-shift.This is mainly because the conjugate π bridge of dye D-SS molecules is longer than that of dye D-ST molecules.
Table 1 shows details of the excitation energy,peak wavelength,and vibration strength of molecular absorption bands and the major components of electronic transitions.In fact, there is a very weak absorption band besides the two significant absorption bands shown in absorption spectra.Whether in vacuum or polar solution,the strongest absorption band(also the absorption band with a maximum red-shift)of the three absorption bands of molecules within UV-Vis region mainly consists of the transitions from the HOMO at the initial state to the LUMO at the final state.Other absorption bands mainly consist of the transitions HOMO→LUMO+1,HOMO-1→LUMO, HOMO-2→LUMO,and HOMO-3→LUMO.All the electronic transitions caused by these absorptions are π→π*transition, ICT,and the absorption spectra are electron spectrum.In these transitions,initial states are mainly related to electron donor groups,while the final states are mainly related to electron acceptor groups in the molecular orbital.This shows that absorption is a photoinduced electron transfer process,so the excitations generate charge separated states.
From the simulated absorption spectra,whether in vacuum or polar solution of THF,in comparison with the case of dye D-ST,the UV-Vis absorption spectra of dye D-SS have significant red-shift,and their molar absorption coefficients are close, even the molar absorption coefficient of dye D-SS is higher than that of dye D-ST.Therefore,the solar photon harvesting ability of dye D-SS molecules is stronger than that of dye D-ST molecules.However,the experimental results are that the photovoltaic properties of dye D-ST sensitized DSSCs are better than that of dye D-SS sensitized DSSCs.Especially,the photovoltaic energy conversion efficiency of dye D-ST sensitized DSSCs is nearly two folds higher than that of dye D-SS sensitized DSSCs.While such experimental results can not be explained in absorption spectrum,so we turned to the energy analysis.
3.3 Energy level diagram
Fig.4 gives the D-SS and D-ST molecular orbital energy level structures in vacuum and in THF solution,which are simulated using DFT-B3LYP/6-31G(d).Whether in vacuum or THF solution,the LUMO energy level positions of D-SS are lower than that of D-ST,and the HOMO energy level positions of DSS are higher than those of D-ST.This leads to the fact that the energy gaps ΔEH→Lbetween the LUMO and HOMO of D-SS, ΔEH-1→Lbetween the LUMO and HOMO-1,ΔEH→L+1between the LUMO+1 and HOMO,and ΔEH-1→L+1between the LUMO+ 1 and HOMO-1 are smaller than those of D-ST,especially theenergy gap of D-SS(ΔEH→L=2.014 eV(1.823 eV in THF solution))is smaller than that of D-ST(ΔEH→L=2.259 eV(2.096 eV in THF solution)).This is the main cause of the red-shift of the UV-Vis absorption spectrum of D-SS(electron spectrum)in comparison with that of D-ST.
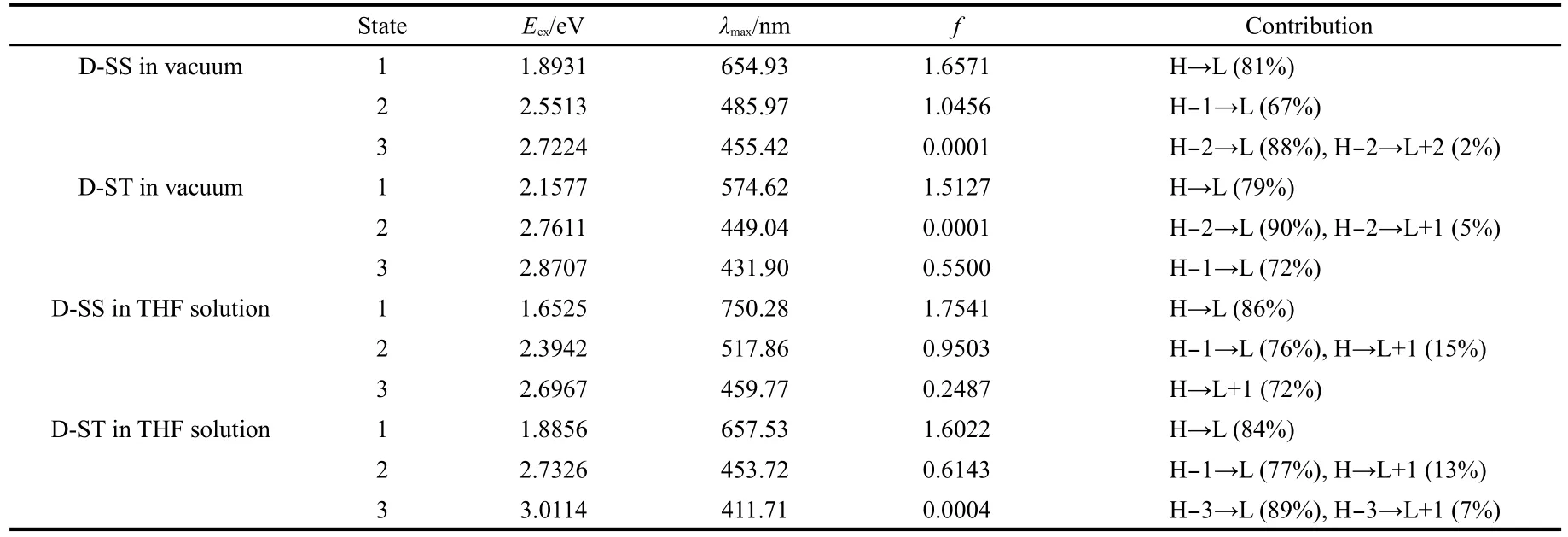
Table 1 Vertical excitation energies,wavelengths,oscillator strengths,and two highest electronic transition configurations for D-SS and D-ST in vacuum and THF solution
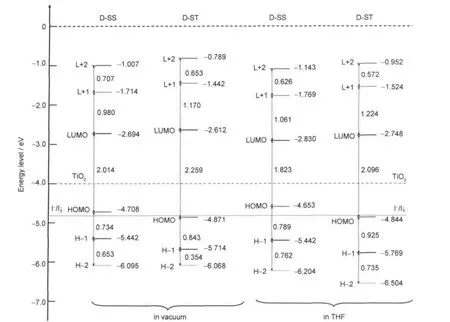
Fig.4 The frontier molecular orbital energy levels of D-SS and D-ST
Both the LUMO energy level(ELposition)of-2.694 eV in vacuum(-2.830 eV in THF solution)for dye D-SS molecules and the LUMO energy level of-2.612 eV in vacuum(-2.748 eV in THF solution)for D-ST molecules are much higher than the energy level(approximately-4.0 eV)of the conduction band of TiO2electrode.Therefore,the dyes D-SS and D-ST molecules at optical excited state can successfully inject electron towards TiO2electrode.
Whether in vacuum or polar solution,the LUMO energy levels of dye D-ST are slightly higher than those of dye D-SS. Therefore,the driving force for the electron injection of D-ST molecules towards the conduction band edge of TiO2electrode at excitation state is slightly greater than that of D-SS molecules.Thus,the photovoltaic properties of dye D-ST sensitized DSSCs should be slightly better than those of dye D-SS,and the photovoltaic energy conversion efficiency should be higher.However,at the same time,we notice that,the LUMO energy level of dye D-ST is not much higher than that of dye D-SS, which proves nothing about the experimental fact that the photovoltaic properties and photovoltaic energy conversion efficiency of dye D-ST sensitized DSSCs are much higher than that of dye D-SS.
We also notice that,the HOMO energy level(EHposition) of-4.871 eV in vacuum(-4.844 eV in THF solution)for dye D-ST molecules is lower than the redox energy level(-4.8 eV) of redox electrolyte(I-/I-3).The D-ST molecules that lose electron(electron is injected to TiO2electrode)and are oxidized still can be recovered by obtaining electron from electrolyte,in order to be excited through further photon absorption.However,the HOMO energy level(-4.708 eV in vacuum;-4.653 eV in THF solution)of dye D-SS molecules is higher than the redox energy level of redox electrolyte,so the D-SS molecules that lose electron(electron is injected to TiO2electrode)can not be successfully recovered by obtaining electron from electrolyte.Compared with dye D-ST molecules,although dye D-SS molecules have stronger solar photon harvesting ability but the HOMO energy level is higher than the redox energy level of redox electrolyte,dye D-SS molecules can not be successfully recovered by themselves(only can be de-excited through other slow process),which seriously hinders their further solar photon absorption,disables the capability to harvest photons and finally causes the decrease in the photovoltaic properties of dye D-SS sensitized DSSCs.That is to say,D-SS molecules are incapable of normally absorbing photon in DSSCs,or their photon absorption ability can not perform completely.Therefore, although its photon harvesting ability is theoretically strong, but such strong photon harvesting ability has not been explored experimentally,which causes its experimental molar absorption coefficient much lower than its theoretical molar absorp-tion coefficient.
4 Conclusions
The UV-Vis absorption spectrum of dye D-SS molecules has a significant red-shift in comparison with that of dye D-ST molecules,and the molar absorption coefficient of dye D-SS molecules is also higher than that of dye D-ST molecules.Dye D-SS molecules should have had higher solar radiation photon harvesting ability in comparison with dye D-ST molecules,but the HOMO energy level position of dye D-SS molecules is
higher than the redox energy level of redox electrolytetherefore,the D-SS molecules at optically excited state are incapable of being successfully recovered by obtaining electron from electrolyte after being oxidized by injecting electron towards TiO2electrode,which disables full play to be given to the photon harvesting ability.Thereby it seriously decreases the photovoltaic properties and photovoltaic energy conversion efficiency of dye D-SS sensitized DSSCs.Such organic dye molecules whose HOMO energy level is higher than the redox energy level of redox electrolyte are not suitable to be used as the sensitizer of DSSCs.In the process of designing and synthetizing the dyes for DSSCs,theoretical calculations should be carried out firstly to distinguish if the HOMO energy level of dyes can match the redox of redox electrolyte.The position of the HOMO energy level of dye molecules is also very important for DSSCs.When being applied to DSSCs,it must be lower than the redox energy level of redox electrolyte.
(1) O?Regan,B.;Gr?tzel,M.Nature 1991,353,737.
(2) Gr?tzel,M.J.Photochem.Photobiol.C 2003,4,145.
(3) Gr?tzel,M.J.Photochem.Photobiol.A 2004,164,3.
(4) Nazeeruddin,M.K.;Klein,C.;Liska,P.;Gr?tzel,M.Coord. Chem.Rev.2005,249,1460.
(5) Gr?tzel,M.Inorg.Chem.2005,44,6841.
(6) Peter,L.M.Phys.Chem.Chem.Phys.2007,9,2630.
(7)Wang,Z.S.;Cui,Y.;Dan-oh,Y.;Kasada,C.;Shinpo,A.;Hara, K.J.Phys.Chem.C 2007,111,7224.
(8) Chen,R.;Yang,X.;Tian,H.;Sun,L.C.J.Photochem. Photobiol.A-Chem.2007,189,295.
(9)Tian,H.;Yang,X.;Chen,R.;Pan,Y.;Li,L.;Hagfeldt,A.;Sun, L.C.Chem.Commun.2007,No.36,3741.
(10)Kim,S.;Kim,D.;Choi,H.;Kang,M.S.;Song,K.;Kang,S.O.; Ko,J.Chem.Commun.2008,No.40,4951.
(11) Ito,S.;Miura,H.;Uchida,S.;Takata,M.;Sumioka,K.;Liska, P.;Comte,P.;Péchy,P.;Gr?tzel,M.Chem.Commun.2008, No.41,5194.
(12) Li,C.;Yum,J.H.;Moon,S.J.;Herrmann,A.;Eickemeyer,F.; Pschirer,N.G.;Erk,P.;Sch?neboom,J.;Müllen,K.;Gr?tzel, M.;Nazeeruddin,M.K.ChemSusChem 2008,1,615.
(13) Jin,Y.;Hua,J.;Wu,W.;Ma,X.;Meng,F.Synth.Met.2008, 158,64.
(14) Burke,A.;Ito,S.;Snaith,H.;Bach,U.;Kwiatkowski,J.; Gr?tzel,M.Nano Lett.2008,8,977.
(15) Hagberg,D.P.;Marinado,T.;Karlsson,K.M.;Nonomura,K.; Qin,P.;Boschloo,G.;Brinck,T.;Hagfeldt,A.;Sun,L.C. J.Org.Chem.2007,72,9550.
(16) Qin,P.;Yang,X.;Chen,R.;Sun,L.C.;Marinado,T.; Edvinsson,T.;Boschloo,G.;Hagfeldt,A.J.Phys.Chem.C 2007,111,1853.
(17) Boschloo,G.;Marinado,T.;Nonomura,K.;Edvinsson,T.; Agrios,A.G.;Hagberg,D.P.;Sun,L.C.;Quintana,M.; Karthikeyan,C.S.;Thelakkat,M.;Hagfeldt,A.Thin Solid Films 2008,516,7214.
(18)Yen,Y.S.;Hsu,Y.C.;Lin,J.T.;Chang,C.W.;Hsu,C.P.;Yin, D.J.J.Phys.Chem.C 2008,112,12557.
(19)Balanay,M.P.;Kim,D.H.Phys.Chem.Chem.Phys.2008,10, 5121.
(20)Ooyama,Y.;Harima,Y.Eur.J.Org.Chem.2009,No.18,2903.
(21) Rochford,J.;Chu,D.;Hagfeldt,A.;Galoppini,E.J.Am.Chem. Soc.2007,129,4655.
(22) Chen,R.;Yang,X.;Tian,H.;Wang,X.;Hagfeldt,A.;Sun,L.C. Chem.Mater.2007,19,4007.
(23) Li,G.;Jiang,K.J.;Li,Y.F.;Li,S.L.;Yang,L.M.J.Phys. Chem.C 2008,112,11591.
(24) Marinado,T.;Hagberg,D.P.;Hedlund,M.;Edvinsson,T.; Johansson,E.M.J.;Boschloo,G.;Rensmo,H.;Brinck,T.;Sun, L.C.;Hagfeldty,A.Phys.Chem.Chem.Phys.2009,11,133.
(25) Chen,Z.;Li,F.;Huang,C.H.Curr.Org.Chem.2007,11,1241.
(26) Tsai,M.S.;Hsu,Y.C.;Lin,J.T.;Chen,H.C.;Hsu,C.P. J.Phys.Chem.C 2007,111,18785.
(27) Choi,H.;Lee,J.K.;Song,K.H.;Song,K.;Kang,S.O.;Ko,J. Tetrahedron 2007,63,1553.
(28) Zhao,G.J.;Chen,R.K.;Sun,M.T.;Liu,J.Y.;Li,G.Y.;Gao, Y.L.;Han,K.L.;Yang,X.C.;Sun,L.C.Chem.Eur.J.2008, 14,6935.
(29)Zhao,G.J.;Liu,J.Y.;Zhou,L.C.;Han,K.L.J.Phys.Chem.B 2007,111,8940.
(30) Zhao,G.J.;Han,K.L.Biophys.J.2008,94,38.
(31) Kurashige,Y.;Nakajima,T.;Kurashige,S.;Hirao,K.; Nishikitani,Y.J.Phys.Chem.A 2007,111,5544.
(32) Zhang,X.;Zhang,J.J.;Xia,Y.Y.J.Photochem.Photobiol. A-Chem.2008,194,167.
(33) Li,S.L.;Jiang,K.J.;Shao,K.F.;Yang,L.M.Chem.Commun. 2006,No.26,2792.
(34) Sayama,K.Tsukagoshi,S.;Mori,T.;Hara,K.;Ohga,Y.; Shinpo,A.;Abe,Y.;Suga,S.;Arakawa,H.Sol.Energy Mater. Sol.Cells 2003,80,47.
(35) DeAngelis,F.;Fantacci,S.;Selloni,A.;Nazeeruddin,M.K. Chem.Phys.Lett.2005,415,115.
(36) Xu,Y.;Chen,W.K.;Cao,M.J.;Liu,S.H.;Li,J.Q.; Philippopoulos,A.I.;Falaras,P.Chem.Phys.2006,330,204.
(37) Sun,J.;Song,J.;Zhao,Y.;Liang,W.Z.J.Chem.Phys.2007, 127,234107.
(38) Wang,Y.L.;Wu,G.S.Acta Phys.-Chim.Sin.2008,24,552. [王溢磊,吳國是.物理化學(xué)學(xué)報,2008,24,552.]
(39) Li,H.X.;Pan,S.J.;Wang,X.F.;Xiao,T.Chin.J.Chem.Phys. 2008,21,263.
(40)Zhang,C.R.;Wu,Y.Z.;Chen,Y.H.;Chen,H.S.Acta Phys.-Chim.Sin.2009,25,53.[張材榮,吳有智,陳玉紅,陳宏善.物理化學(xué)學(xué)報,2009,25,53.]
(41)Zhan,W.S.;Pan,S.;Li,Y.Z.;Chen,M.D.Acta Phys.-Chim. Sin.2009,25,2087. [詹衛(wèi)伸,潘 石,李源作,陳茂篤.物理化學(xué)學(xué)報,2009,25,2087.]
(42)Sobolewski,A.L.;Domcke,W.J.Phys.Chem.A 1999,103, 4494.
(43)Sobolewski,A.L.;Domcke,W.J.Phys.Chem.A 2004,108, 10917.
(44)Sobolewski,A.L.;Domcke,W.;H?ttig,C.J.Phys.Chem.A 2006,110,6301.
(45) Zhao,G.J.;Han,K.L.J.Phys.Chem.A 2007,111,2469.
(46) Zhao,G.J.;Han,K.L.J.Phys.Chem.A 2007,111,9218.
(47)Wang,Y.L.;Wu,G.S.Acta Phys.-Chim.Sin.2007,23,1831. [王溢磊,吳國是.物理化學(xué)學(xué)報,2007,23,1831.]
(48) Zhang,C.R.;Liu,Z.J.;Chen,Y.H.;Chen,H.S.;Wu,Y.Z.; Yuan,L.H.J.Mol.Struct.-Theochem 2009,899,86.
(49)Zhan,W.S.;Pan,S.;Li,Y.Z.;Chen,M.D.Acta Phys.-Chim. Sin.2010,26,1408. [詹衛(wèi)伸,潘 石,李源作,陳茂篤.物理化學(xué)學(xué)報,2010,26,1408.]
(50) Frisch,M.J.;Trucks,G.W.;Schlegel,H.B.;et al.Gaussian 03, Revision C.02;Gaussian Inc.:Pittsburgh,PA,2003.
(51) Becke,A.D.J.Chem.Phys.1993,98,1372.
(52) Becke,A.D.J.Chem.Phys.1993,98,5648.
(53) Stephens,P.J.;Devlin,F.J.;Chabalowski,C.F.;Frisch,M.J. J.Phys.Chem.1994,98,11623.
(54) Bene,J.E.D.;Person,W.B.;Szczepaniak,K.J.Phys.Chem. 1995,99,10705.
(55) Hertwig,R.H.;Koch,W.Chem.Phys.Lett.1997,268,345.
(56) Tozer,D.J.;Handy,N.C.J.Chem.Phys.1998,109,10180.
(57)Yanai,T.;Tew,D.P.;Handy,N.C.Chem.Phys.Lett.2004,393, 51.
(58) Barone,V.;Cossi,M.J.Phys.Chem.A 1998,102,1995.
(59) Klamt,A.J.Phys.Chem.1995,99,2224.
(60) Klamt,A.J.Phys.Chem.1996,100,3349.
(61) Reed,A.E.;Weinstock,R.B.;Weinhold,F.J.Chem.Phys. 1985,83,735.
(62)Cossi,M.;Barone,V.;Cammi,R.;Tomasi,J.Chem.Phys.Lett. 1996,255,327.
(63) Foresman,J.B.;Keith,T.A.;Wiberg,K.B.;Snoonian,J.; Frisch,M.J.J.Phys.Chem.1996,100,16098.
(64) Cossi,M.;Barone,V.;Mennucci,B.;Tomasi,J.Chem.Phys. Lett.1998,286,253.
(65) Klamt,A.;Jonas,V.;Bürger,T.;Lohrenz,J.C.W.J.Phys. Chem.A 1998,102,5074.
(66) Cossi,M.;Rega,N.;Scalmani,G.;Barone,V.J.Comput.Chem. 2003,24,669.
June 10,2011;Revised:November 7,2011;Published on Web:November 9,2011.
Comparison of D-SS and D-ST Dyes as Photo Sensitizers in Dye-Sensitized Solar Cells
ZHAN Wei-Shen PAN Shi*WANG Qiao LI Hong ZHANG Yi
(Institute of Near-Field Optics and Nanotechnology,School of Physics and Optoelectronic Technology, Dalian University of Technology,Dalian 116024,Liaoning Province,P.R.China)
The molecular structures,UV-Vis absorption spectra,and energy level structures of the dyes D-SS and D-ST were simulated using density functional theory,time-dependent density functional theory (TDDFT),and natural bond orbital analysis,which provided the physical mechanisms of dye-sensitized solar cells(DSSCs)containing D-ST and D-SS.The UV-Vis absorption spectrum of D-SS showed a significant red shift compared with that of D-ST and the molar absorption coefficient of D-SS was higher than that of D-ST.D-SS molecules should have a higher solar radiation photon-harvesting ability than D-ST molecules,but the energy level of the highest occupied molecular orbital(HOMO)of D-SS was higher than the redox energy level of the electrolyteAs a result,an optically excited D-SS molecule cannot be successfully recovered by accepting an electron from the electrolyte after being oxidized by injecting an electron towards the TiO2electrode.This limits the photon harvesting ability of D-SS molecules,and thereby significantly decreases the photovoltaic properties and energy conversion efficiency of DSSCs containing D-SS.This allows the photovoltaic properties of DSSCs containing D-SS to be understood, especially why its photovoltaic energy conversion efficiency is lower than that of DSSCs containing D-ST. The position of the HOMO energy level of dye-sensitized molecules is very important for the operation of DSSCs,and that of the organic sensitizer molecules used in DSSCs must be lower than the redox energy level of the electrolyte.
Density functional theory;Time-dependent density functional theory;Dye-sensitized solar cells;Molecular simulation;Electronic structure;Absorption spectrum; Energy level
10.3866/PKU.WHXB20122878
*Corresponding author.Email:span@dlut.edu.cn;Tel:+86-411-84707863;Fax:+86-411-84706061
O641

