濕化學(xué)法合成LiNi1/3Mn1/3Co1/3O2及其表征
張曉雨 江衛(wèi)軍 朱曉沛 其 魯,*
(1北京大學(xué)化學(xué)與分子工程學(xué)院應(yīng)用化學(xué)系,北京 100871;2中信國安盟固利電源技術(shù)有限公司,北京 102200)
Nowadays,lithium-ion batteries(LIB)have been widely used in portable power sources.Most LIB use LiCoO2as cathode material because of its simple synthesis,low irreversible capacity loss,and good cycling performance[1-2].However,due to high cost of LiCoO2,much effort has been made to develop other cheaper cathode materials and use them in price-sensitive and large-scale applications.Recently,a layer-structured compound LiNi1/3Mn1/3Co1/3O2(simplified as LNMCO),first introduced by Ohzuku′s group in 2001[3],has been considered as an attractive candidate of next-generation cathode material to replace LiCoO2due to its lower cost,more stable structure,larger capacity,and better thermal stability at charged state[4-6].The valence states of nickel,manganese,and cobalt ions in LNMCO powder are+2, +4,and+3,respectively,confirmed by X-ray photoelectron specctroscopy(XPS)study[7].For LNMCO,each transition metal plays its important role.The divalent nickel ions and trivalent cobalt ions are electroactive and the electrochemical reaction of lithium extraction/insertion takes place by oxidation/reduction of Ni2+/Ni4+and Co3+/Co4+ions depending on different cut-off voltages,while Mn4+remains inactive but maintains the structural stability[8].
However,cation mixing still remains a big problem for this material,since the ionic radius of Ni2+(0.069 nm)is close to that of Li+(0.076 nm)[9].A partial occupation of Ni2+by Li+and Ni2+generates a disorder in the structure and this could restrict the motion of Li+ions within the layers of the oxide,which leads to the tendency for the capacity to fade during long cycling[10]. Therefore,one aim of this work is to decrease the cation mixing byadjusting different acid to metal ion molar ratios(R)during the synthesis through wet-chemical method.
Moreover,It has been shown that magnetic experiment is quite a powerful tool to check the quality of samples and structural properties at nanoscopic scale,especially in the case of cathode materials for LIB[11-14]and it is possible to correlate the magnetic and structural properties of LNMCO[15-16].In particular, we have shown that the Ni2+ion in substitution for Li+on the 3b lattice site,Ni2+(3b)generates a ferromagnetic interaction with the Mn4+ions nearest neighbours on 3a sites,and this Ni2+(3b)-Mn4+(3a)ferromagnetic interaction is responsible for the formation of a ferromagnetic cluster centred on the Ni2+(3b)defect[17].The magnetic moment resulting from these ferromagnetic clusters can be detected from the magnetic measurements,from which the concentration of the Ni2+(3b)defects can be derived and compared with the concentration deduced from Rietveld refinement of XRD spectra.This correlation between magnetic and structural properties makes possible the determination of the concentration of Ni2+(3b)defects with very good accuracy[18].We then use the same procedure in this work.
In addition,although wet-chemical method has been used to synthesize LNMCO,using different chelating agents,e.g.,citric acid and oxalic acid[19-23],and succinic acid is also one of the widely used chelating agents for the synthesis of oxides,the synthesis of LNMCO assisted by succinic acid and the effect of its content on magnetic properties,especially on the relationship between structural and magnetic properties of LNMCO,has not been reported to the best of our knowledge.Therefore,varying the succinic acid to metal ion molar ratio and this effect on the structural,morphological,electrochemical,particularly on the magnetic properties of LNMCO,are studied detailedly in this paper.
1 Experimental
1.1 Sample synthesis
The synthesis occurred from metal acetates via inorganic polymerization reactions in the solution[24-26].This wet-chemical route was assisted by succinic acid(AR,Sigma-Aldrich product) as a polymeric agent,using appropriate molar ratios of lithium, nickel,manganese,and cobalt acetate as starting materials to synthesize LNMCO compound.Stoichiometric amounts of acetate hydrates ofLi(CH3COO)·2H2O(AR),Ni(CH3COO)2·4H2O(AR), Mn(CH3COO)2·4H2O(AR),and Co(CH3COO)2·4H2O(AR), Sigma-Aldrich products,were dissolved in distilled water and mixed homogenously with an aqueous solution of succinic acid, varying the molar ratio(R)of acid to metal-ion.The resulting solution was mixed by magnetic stirring at 80℃for 6 h to obtain a clear viscous gel.The gel was dried in an oven at 120℃for 12 h.The as-prepared precursor was pre-calcined at 450℃for 4 h to convert the metal carboxylates to oxides.After cooling down to room temperature,the obtained powder was grounded in an agate mortar and then sintered at 900℃in air for 15 h without pelleting to get the final LNMCO.
1.2 Apparatus
Thermogravity(TG)experiment was performed using a Q50 (TA,USA)instrument analyzer to monitor the mass loss/gain and heat treatment processes under a flow of dry air with a heating rate of 10℃·min-1.Measurements were carried out in the temperature range of 25℃≤T≤750℃.
For structural analysis of LNMCO,samples were characterized with X-ray diffraction(XRD)on a Philips(Netherlands)X′Pert PRO MRD(PW3050)diffractometer equipped with a Cu anticathode(Cu Kαradiation,λ=0.154056 nm)at room temperature.XRD patterns were collected under Bragg-Brentano geometry at 2θ with a step of 0.02°in the range of 10°-80°and were refined by the Rietveld method using the GSAS/EXPGUI package[27].For morphology analysis of LNMCO,a scanning electron microscope(SEM)study of the samples was performed using a JEOL(Japan)JSM-5600LV electron microscope.
The magnetic measurements (susceptibility and magnetization)were performed with a fully automated superconducting quantum interface device(SQUID)magnetometer(Quantum Design MPMS XL,USA)in the temperature range of 4-300 K. Powders were placed into small plastic vial,placed in a holder and finally inserted into the helium cryostat of the SQUID apparatus.The temperature dependence of the susceptibility data was recorded during heating of the sample using two modes:zero field cooling(ZFC)and field cooling(FC),to determine the mag-netic behavior.The procedure is based on performing two consecutive magnetization measurements:in ZFC the sample is first cooled down in the absence of magnetic field,then a magnetic field H=10 kOe is applied,and the ZFC magnetic susceptibility M(H)/H is measured where M is the magnetization measured upon heating.In the FC experiments,the same magnetic field is applied first at room temperature;the FC susceptibility is measured upon cooling.No difference,i.e.,no magnetic irreversibility effect has been detected between ZFC and FC measurements in anyofthe samples.Magnetic curves M(H)have been measured in an applied magnetic field in a range of 0-30 kOe.
Charge-discharge tests were performed on coin type cell (CR2032).Composite positive electrode was prepared by thoroughly mixing the active material(90%(w,mass fraction))with carbon black(2%(w)),acetylene black(2%(w)),polyvinylidene fluoride(6%(w))in N-methyl-pyrrolidinone and spread onto aluminium foils then dried at 120℃for 24 h in vacuum.Cells were then assembled in an argon-filled glove box(Braun,Germany) using foils of Li metal as counter electrode and Celgard 2400 as separator.The electrolyte was 1.0 mol·L-1LiPF6in a mixture of ethylene carbonate(EC)and diethyl carbonate(DEC)(1:1,volume ratio).The cells were galvanostatically cycled at 0.2C (1C=160 mA·g-1)between 3.0 V and 4.3 V(versus Li/Li+)on a Land CT2001A battery tester(Wuhan Jinnuo Electronics Co., Ltd.,China)at room temperature.
2 Results and discussion
2.1 TG analysis of LNMCO precursor
When acetate of an electropositive transition metal dissolves in succinic acid,there is a finite release of the acetate anion into solution.The basic species,acetate anion,can allow the dissolution of metal acetates.The formation of a chelation complex is important to prevent the segregation or precipitation of metal ions.The acetate ligand,succinic acid,has oxygen atoms and hydrogen atoms which can participate in hydrogen bonding.As a result,metal acetates are trapped in a glassy state by an extended network of hydrogen bonds.At the same time,succinic acid binds to the metal acetates and replaces the water of hydration in the complex to give acid-acetate species[28].Succinic acid also acts as fuel and provides local heat for the formation of compound during the decomposition process because of its selfigniting property,accelerating the decomposition of acetate ions. Therefore,varying the acid to metal ion molar ratio(R)can affect the decomposition/formation reaction.As an example,Fig.1 shows the TG analysis curve of the precursor of LNMCO synthesized via R=0.5.Several mass loss stages are observed in this TG curve.The first mass loss stage occurs at ca 223℃,which would be attributed to departure of residual water.After the departure of the remaining water molecule at ca 330℃,the anhydrous metal acetate can be decomposed into both metal oxide and gases such as carbon dioxide by further thermal treatment in air[29-30].It was reported that chelating agent(carboxylic-based acid)provoked decomposition during the synthesis of oxide powders and the gel precursor burned because the decomposed acetate ions acted as an oxidizer[24-26],so the mass loss in the third step is observed around 376℃which corresponds to the decomposition of succinic acid and acetate ions xerogel.After 450℃, there is little mass loss,so in this work,we choose 450℃as the heating temperature for pre-calcination.The reaction pathway can thus be given as follows:
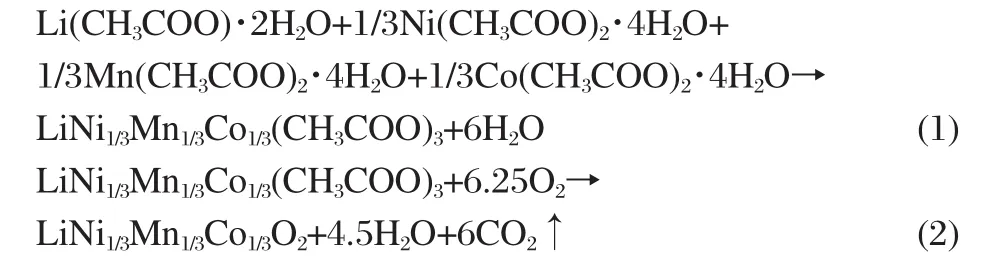
2.2 Structural and morphological analysis of LNMCO
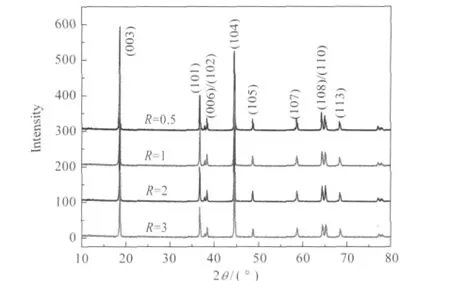
Fig.2 XRD patterns of LNMCO powders synthesized with different succinic acid to metal ion molar ratios(R)
Fig.2 shows the XRD patterns of LNMCO materials synthesized by wet-chemistry with calcination at 900℃in air for 15 h via different R values.From now on,all the experiments in this work will be focussed on the optimization of the parameter R. The Bragg lines are well indexed in the R3m space group with the hexagonal sitting.No impurity phases are detected and the powders are well crystallized in the α-NaFeO2type structure.As seen in Fig.2,the(006)/(102)and(108)/(110)doublets are well separated,which indicates a good hexagonal ordering of LNMCO[31].The narrow diffraction peaks of the pattern indicate a high crystallinity of the LNMCO powder and suggest a homogeneous distribution of the cations within the structure.The lattice parameters were obtained by analysis of the XRD data and the results were summarized in Table 1.The lattice parameter,a,is related to average metal-metal intraslab distance,the lattice parameter,c,is related to the average metal-metal inter-slab distance;and the trigonal distortion,c/a,is related to the hexagonal structure disorder.For layered compounds,higher value of c/a is desirable for better hexagonal structure[7].The trigonal distortion c/a is the largest for LNMCO R=1,which is used as a criterion of optimized material.In addition,the integrated intensity ratio of(003)to(104)peak,I003/I104,has been considered as an indicator of the degree of cation mixing[32].The higher the ratio is, the lower the cation mixing is.It is also believed that the lower value of(I006+I102)/I101is another indicator of better hexagonal ordering[33-34].The lowest value of(I006+I102)/I101,together with the highest value of I103/I104is obtained for R=1,which suggests that this is the optimized value of this synthesis parameter.As a result,varying R value has a direct effect on the structural properties of LNMCO,since the self-igniting property of succinic acid can influence the oxygen partial pressure during synthesis.The sudden variation in the oxygen partial pressure,especially during the burning of the succinic acid,has an impact on LNMCO formation.When R value is low,it cannot provide enough local heat for the formation of precursor.However,when R value is too high,the local temperature increases too much in a short period of time and the partial pressure of oxygen decreases due to the increased CO2,which results in the insufficient oxidation of ions and ferromagnetic defects that can aggravate the cation mixing during the formation of LNMCO.
The Rietveld refinement has been made with the constraints that the lithium ion and the nickel-manganese-cobalt ions occupy the 3b(0,0,0)and 3a(0,0,1/2)Wyckoff sites,respectively, while the oxygen anionsoccupythe 6c(0,0,zoxy)position(zoxywill be refined).In addition,we assume that each cationic site is fully occupied and the number of cations equals to that of anions,sothe overall charge neutrality is maintained.Furthermore,we assumed the existence of a small amount of nickel ions in the lithium sites,since the smaller difference in size between the Ni2+(0.069 nm)and the Li+ions(0.076 nm)in contrast with other cations(r(Mn4+)=0.053 nm,r(Co3+)=0.054 nm in an octahedral environment[9]).Therefore,we defined the formula as[Li1-yNiy]3b[LiyNi1/3-yMn1/3Co1/3]3aO2for Rietveld refinement:the occupancy parameter y of the Ni2+ions at the 3b sites was refined and constrained to be equal to that of Li+ions at the 3a sites,with the total nickel occupancy ratio constrained to 1/3.The results of the Rietveld refinement of the XRD patterns are also summarized in Table 1 as a function of R.Since the radius of Ni2+is smaller than that of Li+,Ni2+ions on 3b sites leads to an increase of the parameter a.Moreover,the presence of the Ni2+ions in lithium plane leads to the stronger electrostatic attraction between oxygen and Li+/Ni2+ions in the LiO2inter-slab plane,hence the decrease of the LiO2inter-slab space,I(LiO2),the correlated increase of the thickness S(MO2)of the MO2slabs,and the increase of c.
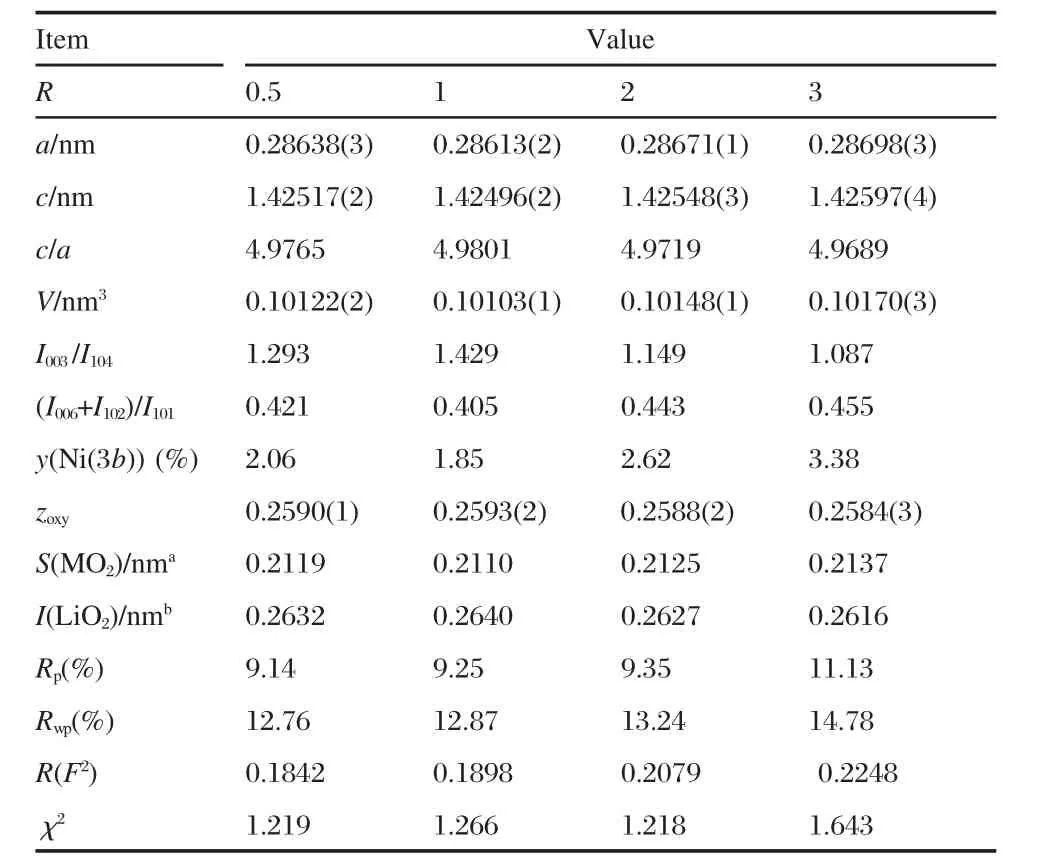
Table 1 Structural data of the LiNi1/3Mn1/3Co1/3O2samples synthesized with different R values
As seen from Table 1,we find a,c,S(MO2)are minimum and I(LiO2)is maximum for R=1,which confirms that this is the optimized value for this parameter.These correlated variations of a,c,S(MO2)and I(LiO2)are then additional proofs that R=1 is the optimized value for succinate route.Indeed,the amount of Li+/Ni2+cation mixing is the lowest in this case(y=1.85%),which is better than the results in Refs.[21,35].The Rietveld fit of the XRD pattern for this sample is shown in Fig.3.
The morphologies of powders were investigated by SEM.Fig. 4(a-d)shows the SEM images of LNMCO compounds synthesized via succinic acid route with different R values.The SEM pictures also show that the primary particles are stuck into agglomerates.The sample synthesized via the optimal value R=1 (Fig.4b),with minimum cation mixing and optimum structural integrity,seems to have a more uniform size distribution of particlesthan the others,which can facilitate the diffusion oflithiumion,so higher specific capacity and better capacity retention can be expected.
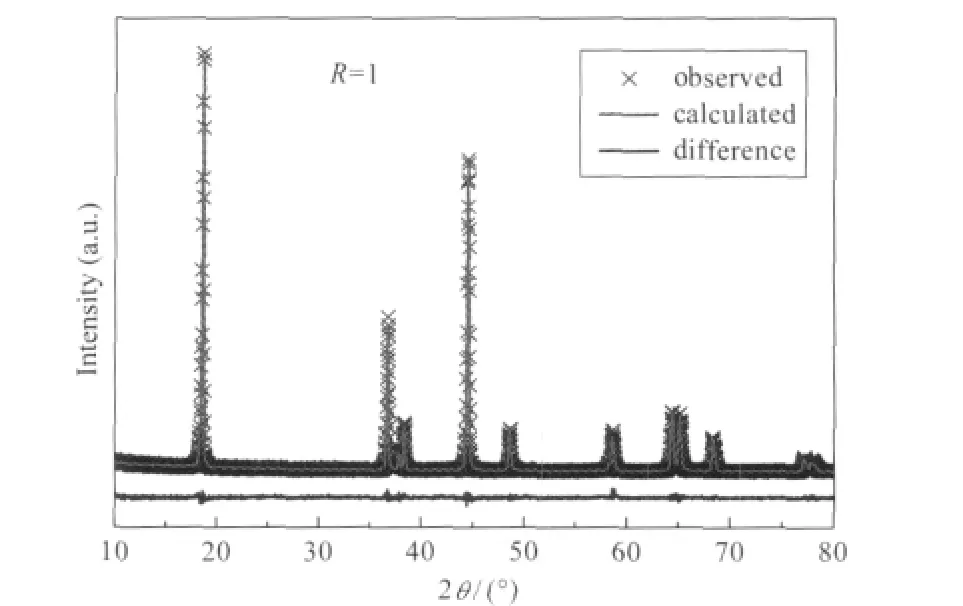
Fig.3 Rietveld refinement patterns of LNMCO synthesized at R=1 via wet-chemical methodThe cross marks show observed X-ray diffraction intensities and the solid line (in red on the web version)represents calculated intensities.The curve at the bottom(in blue on the web version)is the difference between the calculated and observed intensities on the same scale.
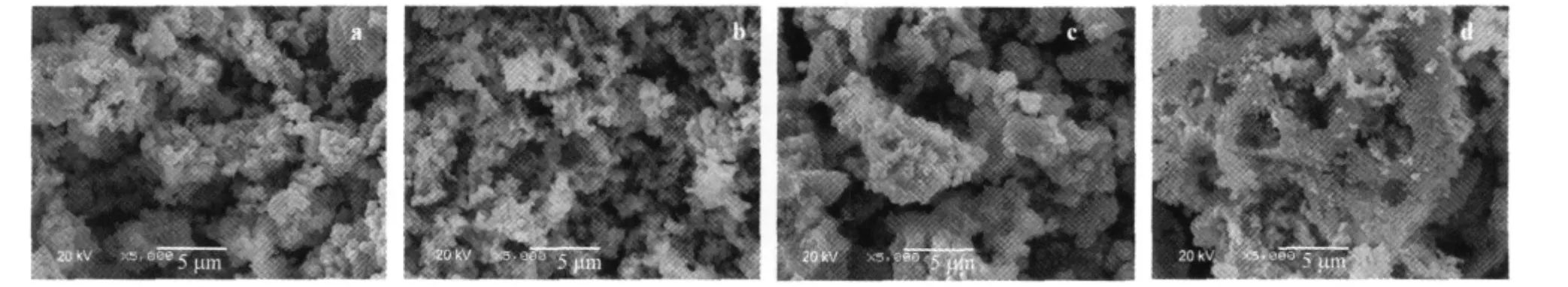
Fig.4 SEM images of LNMCO synthesized with different R valuesR:(a)0.5,(b)1,(c)2,(d)3
2.3 Magnetic properties of LNMCO
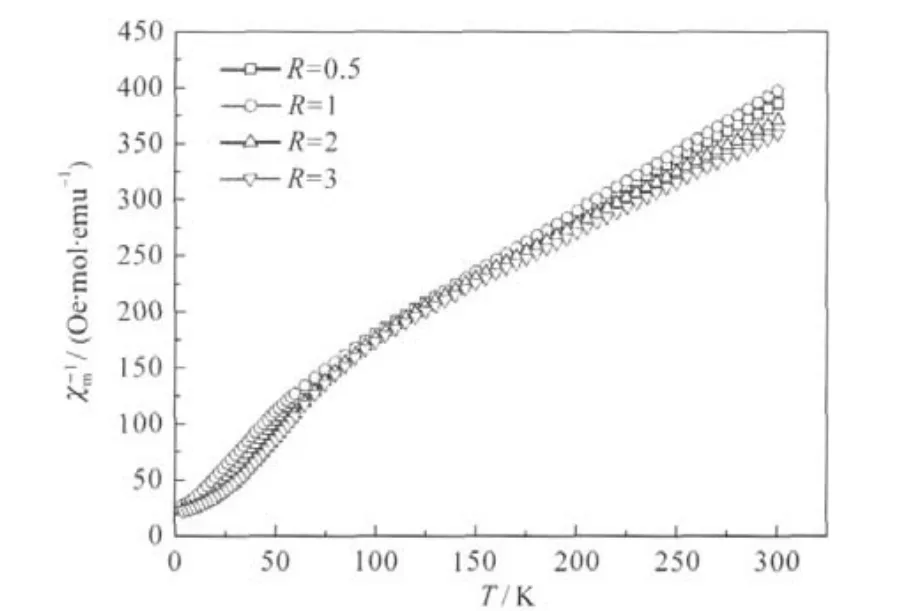
Fig.5 Plots of the reciprocal magnetic susceptibility χ-1mfor samples synthesized with different R valuesData were collected with a magnetic field H=10 kOe.
The temperature dependence of the reciprocal magnetic susceptibility,=H/M is presented in Fig.5 for all the samples. The magnetization curves are reported in Fig.6(a-d)for samples withthe“optimized”valueR=1,andtheotherthreesamples.Above 150 K,the curve-T shows a paramagnetic(PM)behavior and the magnetization is linear in field for all the samples,so thatis meaningful,i.e.,H/M=?H/?M.The quasi-linear variations ofwith T at the susceptibility can be described by a Curie-Weiss lawwith Θpthe Curie-Weiss temperature,and Cpthe Curie constant related to the effective magnetic moment μeffbythe relationwith kBthe Boltzmann constant and N the number of metal ions in one mole of product.The values of the two fitting parameters Θpand μeffobtained for the different acid to metal ion ratios R are reported in Table 2.Θpis negative in all the compounds and this is an intrinsic property due to the fact that intrinsic magnetic interactions are mainly the intra-layer superexchange interactions mediated via oxygen at 90°bonding angle,and they are dominantly antiferromagnetic(AFM)[14].Taking into account that the magnetic moments carried by Ni2+and Mn4+are 2.83μBand 3.87μB,respectively in this material while Co3+is diamagnetic[36-38],the theoreti-cal value of μeffin absence of Ni2+(3b)defects would be:
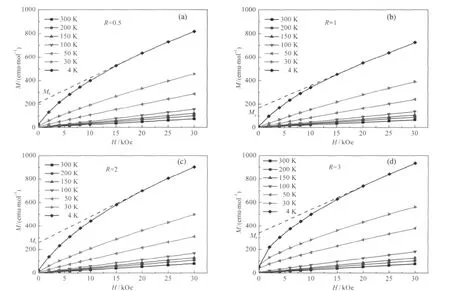
Fig.6 Isothermal plots of the magnetization M(H)for the LNMCO sample synthesized with different R values
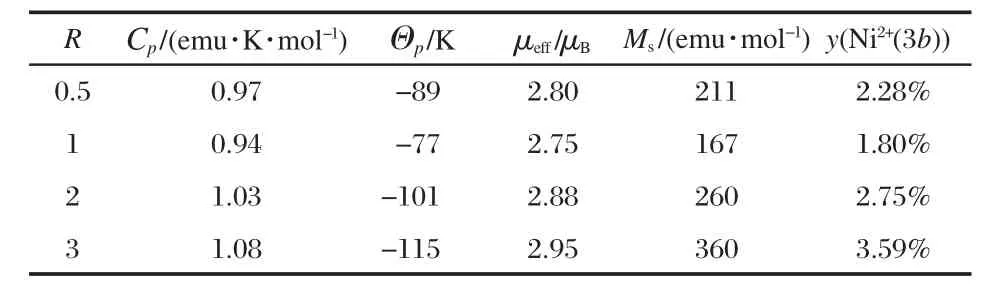
Table 2 Magnetic properties of LNMCO synthesized with different R values
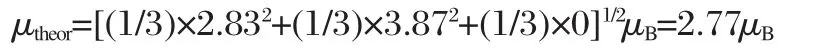
The experimental value of μefffor the R=1 sample is close to this theoretical value.For the other samples,however,μeffis found to be larger.To understand this effect,we note that the magnetization curves are no longer linear below 80 K.Note in this case, H/M≠?H/?M,so that χ-1min Fig.6(a-d)does not have any physical meaning at these low temperatures.Nevertheless,we have reported the data in Fig.6(a-d)since it will be useful to the discussion.For the moment,we just note that these deviations from linearity are related to the onset of a remanent magnetization at low temperature,evidenced in the magnetization curves of the R=3 in Fig.6(d).Following the prior works[14-16],we attribute this feature to the Ni2+(3b)defects.The substitution of Li by Ni on a 3b site generates a 180°interlayer Mn4+(3a)-O-Ni2+(3b)superexchange interactions which is ferromagnetic(FM)after the Goodenough rules[17].This interaction is strong enough to generate a ferromagnetic spin freezing of the Mn4+(3a)-O-Ni2+(3b)pair that is responsible for the onset of remanent magnetization at low temperature.This interaction is actually strong enough to induce a ferromagnetic spin-freezing of such pairs at low temperature.We can then estimate the amount of Ni2+(3a)defects as the ratio of the magnetic moment at saturation(Ms)of the ferromagnetic component of the magnetization,to the moment at saturation that the sample would have if all the Mn and Ni ions were saturated ferromagnetically,namely(1/3)g·μB·(SMn+SNi)=(5/3)μB= 1.67μBper chemical formula,since the Mn4+and Ni2+carry a spin SMn=3/2 and SNi=1.The gyromagnetic factor g is 2,since the orbital momentum is quenched by crystal field effects,and the factor 1/3 comes from the fact that there is only 1/3 Ni-Mn pair per chemical formula.Mshas been estimated from the linear extrapolation to H→0 of the isothermal magnetization curve M(H) at T=4.2 K taken in the range 20 kOe<H<30 kOe.The lowest temperature and this range of the highest field available in the experiments have been chosen to be sure that the ferromagnetic component is saturated.Despite this precaution,it should be noticed that the curvature of the magnetic curves below 20 kOe does not guarantee that the full saturation of the ferromagnetic component has been achieved even at higher field,and even at 4.2 K.Nevertheless,the result for the estimation of the rate of substitution y deduced from this magnetic analysis reported in Table 2,is in remarkable agreement with the result deduced from Rietveld refinement for all the samples investigated,which validates the analysis.For instance,in the case of the sample with R=1,we find Ms=167 emu·mol-1(Fig.6(b)),which amounts to a magnetic moment per formula 0.03μB.So the concentration of Ni2+at 3b sites can be calculated as 0.03μB/1.67μB=1.80%, which is the lowest value among all the samples and agrees well with the results obtained from Rietveld refinement.In the paramagnetic regime,the ferromagnetic Mn4+(3a)-O-Ni2+(3b)pairing enhances the effective magnetic moment of the material.This is the reason for the increasing large value of μeffupon increasing departure of the parameter R from its optimized value R=1,i.e., upon increasing y.
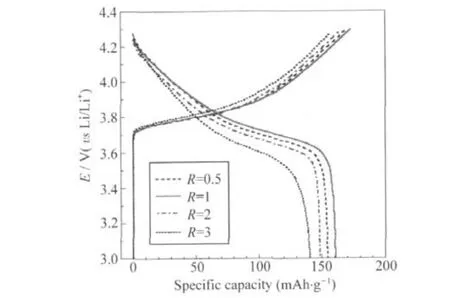
Fig.7 Initial charge-discharge profiles of LNMCO samples synthesized with different R values
2.4 Electrochemical properties of LNMCO
Fig.7 shows the initial charge-discharge curves of LNMCO cathode synthesized with different R values at 32 mA·g-1(0.2C) rate between 3.0 V and 4.3 V versus Li/Li+at room temperature. As shown in Fig.7,the prepared LNMCO materials display smooth charge-discharge curves without any plateaus,which indicates no spinel-related phases forming during cycling.As seen from Table 3,the initial discharge capacity of LNMCO samples are 155 mAh·g-1(R=0.5),161 mAh·g-1(R=1),149 mAh·g-1(R=2),and 141 mAh·g-1(R=3),respectively.The coulombic efficiencies are 90.6%(R=0.5),93.1%(R=1),90.3%(R=2),and 89.2%(R=3).
Fig.8 shows the differential capacity(?Q/?E)vs cell potential (E)of the Li//LNMCO(R=1)coin cell calculated from data presented in Fig.7.Upon charging the cell displays a major oxidation peak at 3.79 V,while the reduction peak occurs at 3.68 V upon discharging.As reported many times in the literature,the oxidation peak at ca 3.8 V with the corresponding reduction peak at 3.7 V is typical for the Ni2+/Ni4+redox reaction in the Li-Ni-Mn-Co oxide lattice[5,14].However,the asymmetry toward the high potential side is due to the partial redox contribution from Co3+to Co4+that corresponds to the second electron transfer.
The cycling performances of LNMCO samples are illustratedin Fig.9.The optimized sample(R=1)achieves higher initial discharge capacity(161 mAh·g-1)and its capacityretention is 91.3% after 50 cycles,which correspond well with the results from structural and magnetic analyses before.Higher structural integrity and less cation mixing result in better electrochemical performance,which can be compared with that of LNMCO synthesized by other wet-chemical methods[39-40],when using the same cut-off voltage(3.0-4.3 V)and current density(32 mA·g-1) for electrochemical characterization.
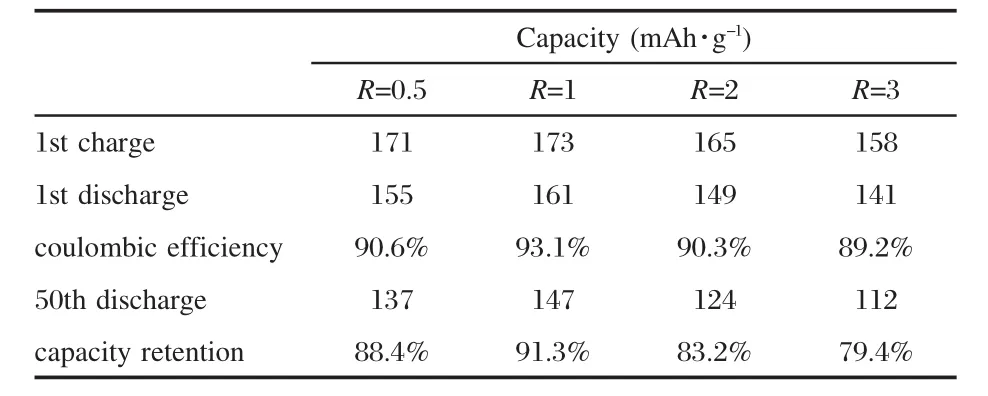
Table 3 Electrochemical properties of LNMCO synthesized with different R values
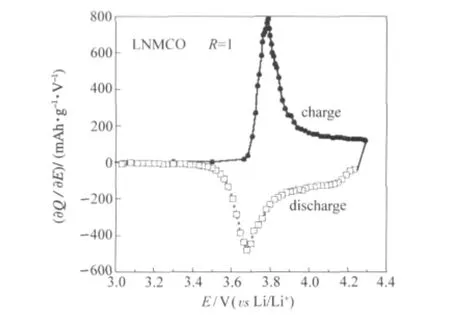
Fig.8 Differential capacity(?Q/?E)vs cell potential(E)of the Li//LNMCO(R=1)coin cell
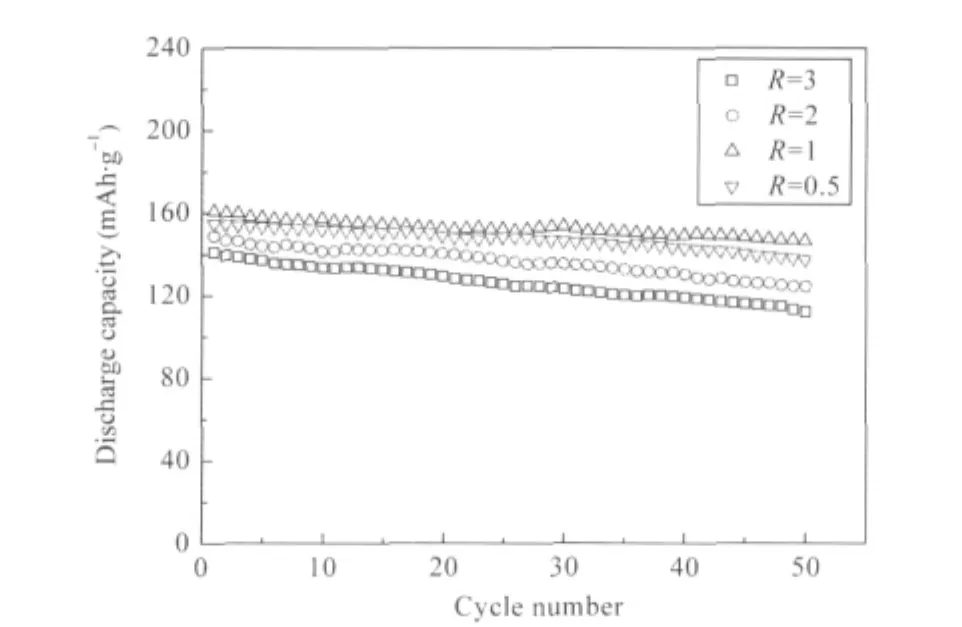
Fig.9 Cycling performance of LNMCO samples synthesized with different R values
3 Conclusions
The layered LNMCO was successfully synthesized via wetchemical route assisted by succinic acid with different R values. XRD,SQUID,and charge-discharge characterizations show that the structural,magnetic,and electrochemical profiles are sensitive to the synthetic conditions,i.e.,the R value that governs the cation mixing.The best performance of the LNMCO electrode has been obtained from an acid to metal ion ratio R=1 sintered at 900℃for 15 h.The amount of cation mixing estimated from the magnetization curve for R=1 is 1.80%,which agrees well with the Rietveld refinement result(1.85%).The LNMCO sample with R=1 shows the best reversibility with a coulombic efficiency of 93.1%for the first cycle.Its initial discharge capacity is 161 mAh·g-1and its capacity retention is 91.3%after 50 cycles in the cut-off voltage of 3.0-4.3 V.
1 Nagamura,T.;Tazawa,K.Prog.Batteries Sol.Cells,1990,9:209
2 Reimers,J.N.;Dahn,J.R.J.Electrochem.Soc.,1992,139:209
3 Ohzuku,T.;Makimura,Y.Chem.Lett.,2001,30:642
4 Yabuuchi,N.;Ohzuku,T.J.Power Sources,2003,119:171
5 Belharouak,I.;Sun,Y.K.;Liu,J.;Amine,K.J.Power Sources, 2003,123:247
6 Wang,J.;Qi,Y.J.;Li,Y.W.;Qi,L.Acta Phys.-Chim.Sin.,2007, 23(suppl.):46 [王 劍,祁毓俊,李永偉,其 魯.物理化學(xué)學(xué)報(bào),2007,23(增刊):46]
7 Shaju,K.M.;Rao,G.V.S.;Chowdari,B.V.R.Electrochim.Acta, 2002,48:145
8 Yoon,W.S.;Grey,C.P.;Balasubramanian,M.;Yang,X.Q.; Fischer,D.A.;McBreen,J.Electrochem.Solid-State Lett.,2004, 7:A53
9 Shannon,R.D.Crystallogr.Acta A,1976,32:751
10 Shaju,K.M.;Bruce,P.G.J.Power Sources,2007,174:1201
11 Julien,C.M.;Ait-Salah,A.;Mauger,A.;Gendron,F.Ionics,2006, 12:21
12 Amdouni,N.;Gendron,F.;Mauger,A.;Zarrouk,H.;Julien,C.M. Mater.Sci.Eng.B,2006,129:64
13 Zaghib,K.;Ravet,N.;Gauthier,M.;Gendron,F.;Mauger,A.; Goodenough,J.B.;Julien,C.M.J.Power Sources,2006,163:560
14 Abdel-Ghany,A.;Zaghib,K.;Gendron,F.;Mauger,A.;Julien,C. M.Electrochim.Acta,2007,52:4092
15 Abdel-Ghany,A.;Mauger,A.;Gendron,F.;Zaghib,K.;Julien,C. M.ECS Trans.,2007,3:137
16 Zhang,X.Y.;Mauger,A.;Gendron,F.;Qi,L.;Groult,H.; Perrigaud,L.;Julien,C.M.ECS Trans.,2009,16:11
17 Goodenough,J.B.Phys.Rev.,1960,117:1442
18 Zhang,X.Y.;Jiang,W.J.;Mauger,A.;Qi,L.;Gendron,F.;Julien, C.M.J.Power Sources,2010,195:1292
19 Zhang,W.;Liu,H.X.;Hu,C.;Zhu,X.J.;Li,Y.X.Rare Metals, 2008,27:158
20 Guo,H.J.;Liang,R.F.;Li,X.H.;Zhang,X.M.;Wang,Z.X.; Peng,W.J.;Wang,C.Trans.Nonferrous Met.Soc.China,2007, 17:1307
21 Li,X.;Wei,Y.J.;Ehrenberg,H.;Du,F.;Wang,C.Z.;Chen,G. Solid State Ionics,2008,178:1969
22 Liu,J.J.;Qiu,W.H.;Yu,L.Y.;Zhang,G.H.;Zhao,H.L.;Li,T. J.Power Sources,2007,174:701
23 He,Y.S.;Pei,L.;Liao,X.Z.;Ma,Z.F.J.Fluorine Chem.,2007, 128:139
24 Julien,C.M.;El-Farh,L.;Rangan,S.;Massot,M.J.Sol-Gel Sci. Technol.,1999,15:63
25 Julien,C.M.;Michael,M.S.;Ziolkiewicz,S.Int.J.Inorg.Mater., 1999,1:29
26 Julien,C.M.;Letranchant,C.;Rangan,S.;Lemal,M.;Ziolkiewicz, S.;Castro-Garcia,S.;El-Farh,L.;Benkaddour M.Mater.Sci.Eng. B,2000,76:145
27 Toby,B.H.J.Appl.Cryst.,2001,34:210
28 Wu,H.M.;Rao,C.V.;Rambabu,B.Mater.Chem.Phys.,2009, 116:532
29 Lee,B.W.J.Power Sources,2002,109:220
30 Caballero,A.;Cruz,M.;Hernán,L.;Melero,M.;Morales,J.; Castellón,E.R.J.Power Sources,2005,150:192
31 Rougier,A.;Gravereau,P.;Delmas,C.J.Electrochem.Soc.,1996, 143:1168
32 Ohzuku,T.;Ueda,A.;Nagayama,M.J.Electrochem.Soc.,1993, 140:1862
33 Dahn,J.R.;von Sacken,U.;Michal,C.A.Solid State Ionics, 1990,44:87
34 Reimers,J.N.;Rossen,E.;Jones,C.D.;Dahn,J.R.Solid State Ionics,1993,61:335
35 Shin,Y.J.;Choi,W.J.;Hong,Y.S.;Yoon,S.;Ryu,K.S.;Chang, S.H.Solid State Ionics,2006,177:515
36 Chernova,N.A.;Ma,M.M.;Xiao,J.;Whittingham,M.S.;Breger, J.;Grey,C.P.Chem.Mater.,2007,19:4682
37 Ma,M.M.;Chernova,N.A.;Toby,B.H.;Zavalij,P.Y.; Whittingham,M.S.J.Power Sources,2007,165:517
38 Xiao,J.;Chernova,N.A.;Whittingham,M.S.Chem.Mater., 2008,20:7454
39 Park,S.H.;Yoon,C.S.;Kang,S.G.;Kim,H.S.;Moon,S.I.;Sun, Y.K.Electrochim.Acta,2004,49:557
40 Liang,Y.G.;Han,X.Y.;Zhou,X.W.;Sun,J.T.;Zhou,Y.H. Electrochem.Commun.,2007,9:965
- 物理化學(xué)學(xué)報(bào)的其它文章
- PbmTen(m+n≤6)團(tuán)簇的結(jié)構(gòu)與穩(wěn)定性
- Ni含量對Co1-xNixB催化水解放氫性能的影響
- Synergistic Interactions in Mixed Micelles of Cationic 14-s-14 Gemini with Conventional Surfactants:Spacer and Counterion Effects
- 咪唑基離子液體的物理化學(xué)性質(zhì)估算及預(yù)測
- 化學(xué)氣相沉積法合成Fe/CMK-5及其對溶菌素的吸附性能
- 三種Au(111)催化水煤氣變換反應(yīng)機(jī)理的比較

