A Comparative Study of the Performance of Symmetric and Asymmetric Mixed-conducting Membranes*
CHANG Xianfeng (常先鋒), ZHANG Chun (張春), HE Yanjun (何艷君), DONG Xueliang (董學(xué)良), JIN Wanqin (金萬(wàn)勤) and XU Nanping (徐南平)
?
A Comparative Study of the Performance of Symmetric and Asymmetric Mixed-conducting Membranes*
CHANG Xianfeng (常先鋒), ZHANG Chun (張春), HE Yanjun (何艷君), DONG Xueliang (董學(xué)良), JIN Wanqin (金萬(wàn)勤)**and XU Nanping (徐南平)
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing University of Technology, Nanjing 210009, China
According to the configuration, mixed-conducting membranes are classified as symmetric membranes and asymmetric membranes consisting of a thin dense layer and a porous support. In this study, these two kinds of SrCo0.4Fe0.5Zr0.1O3-oxide-based membranes were systematically compared in terms of oxygen permeability and chemical stability, and their differences were elucidated by means of the theoretical calculation. For the oxygen permeability, the asymmetric membrane was greater than the symmetric membrane due to the significant decrease of bulk diffusion resistance in the thin dense layer of the asymmetric membrane. In regard to the chemical stability, the increase of oxygen partial pressure on the asymmetric membrane surface at CH4side produced the stable time of over 1032 h in partial oxidation of methane at 1123 K, while the symmetric membrane was only of 528 h. This studydemonstrated that the asymmetric membrane was a promising geometrical configuration for the practical application.
comparison,mixed-conducting oxide, symmetric membrane, asymmetric membrane, oxygen permeability, chemical stability
1 INTRODUCTION
Mixed ionic and electronic conducting (MIEC) oxides have both the oxygen ionic conductivity and the electronic conductivity at the temperature typically higher than 700°C [1]. Because of this excellent property, the membranes based on MIEC oxides have drawn considerable attention in many applications including the separation of oxygen-containing gas [2], partial oxidation of methane (POM) to syngas [3-6] and thermal decomposition of CO2[7].
For the commercial utilization of these MIEC membranes, the high oxygen permeability and especially good chemical stability are necessary. One route is to develop new membrane materials with high oxygen permeability and chemical stability. Sammells. [8] developed a brownmillerite-derived membrane material and the prepared membrane was continuously operated for over one year in the reactor for POM to syngas, but they did not reveal the specific composition of materials. Another promising route is believed to develop asymmetric membranes consisting of a thin dense layer and a porous support [9, 10]. In previous studies [3, 9-11], the membranes used were mainly composed of a single thick dense layer, which was called as symmetric membranes. However, there are some considerable challenges to prepare an asymmetric membrane [12, 13]. Fortunately, a great progress has been made after several years of endeavor [14-18]. For example, in our previous work [14], we proposed a co-sintering route and successfully prepared a La0.6Sr0.4Co0.2Fe0.8O3-δasymmetric membrane.
After the challenges in preparing asymmetric membranes were overcome successfully, the asymmetric membranes were gradually used in the practical application to investigate if they could effectively solve the problems of symmetric membranes in the oxygen permeability and chemical stability [14-18]. From the viewpoint of the comparison of their oxygen permeability, the asymmetric membranes were preliminarily demonstrated to be better than the symmetric membranes [14-18]. However, a clear illustration was not given for their differences, and the oxygen permeation flux through asymmetric membranes was generally lower than the expected values under the assumption of the oxygen permeation flux in direct proportion to the reciprocal of the thickness of dense layer [14, 15, 17]. The understanding of these phenomena might be helpful to deepen the knowledge of oxygen permeation mechanism and further improve the membrane performance effectively. As for the chemical stability, to the best of our knowledge, few papers have reported for the asymmetric membranes and made a detailed comparison with the performance of the symmetric membranes.
Therefore, the objectives of this work were to systematically compare the oxygen permeability and chemical stability of symmetric and asymmetric membranes and make a clear elucidation of their differences. The co-sintering route [14] was used to prepare the asymmetric membranes and in order to avoid the thermal expansion mismatch and reaction between the support and the thin dense layer, the support and the thin dense layer consisted of the same composition. SrCo0.4Fe0.5Zr0.1O3-δ(SCFZ) perovskite-type oxide, one of MIEC oxides, was selected as the representative membrane material, which was found to be structurally stable with higher oxygen permeability in our previous study [19].
2 THEORY
2.1 Calculation of the overall oxygen permeation resistance through a membrane
The oxygen flux through a membrane can be related to the overall driving force for oxygen permeation (Δ, in unit of J·mol-1) and the overall permeation resistance (overall, in unit of J·s·mol-2) as
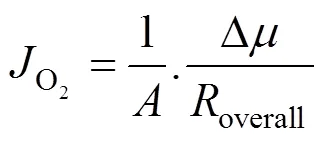
where(cm2) is the membrane area. Δis equal to the difference between the chemical potentials at the upstream and downstream sides of membrane.
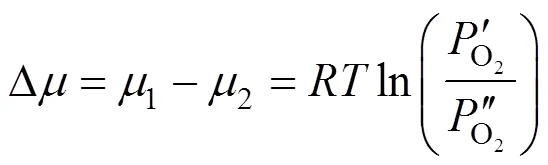
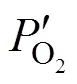
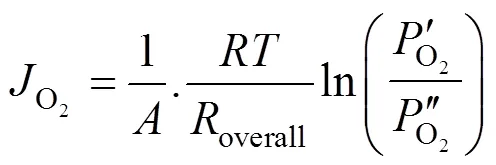
Transforming Eq. (3) yieldsoverallas

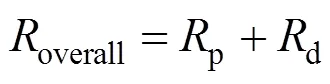
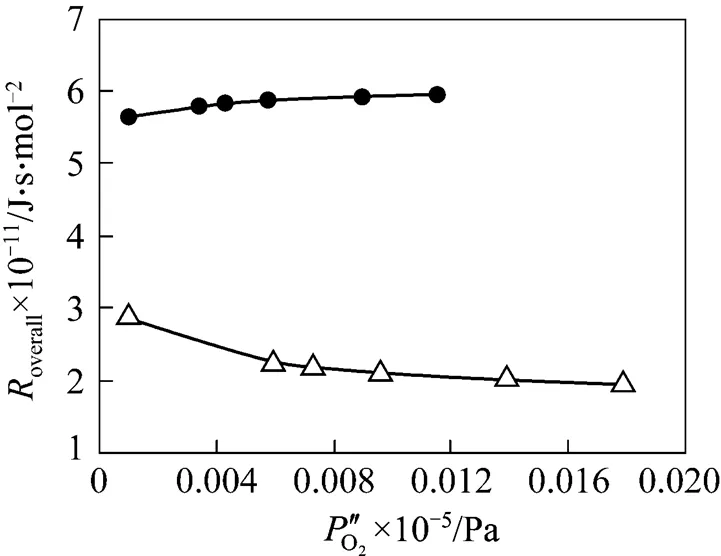
2.2 Proportion of transport resistance of support in the overall permeation resistance through an asymmetric membrane
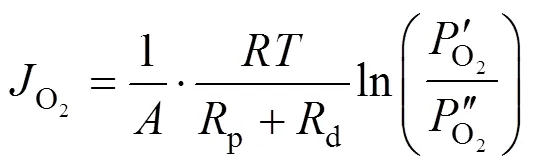
Taking the reciprocal of Eq. (5) gives Eq. (6).
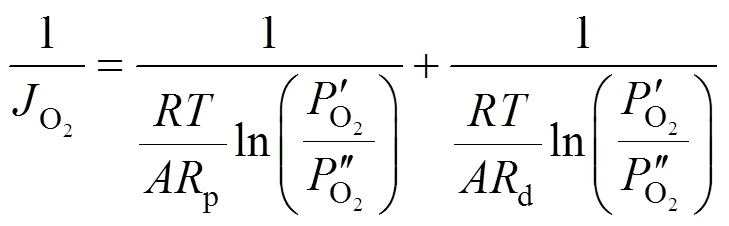
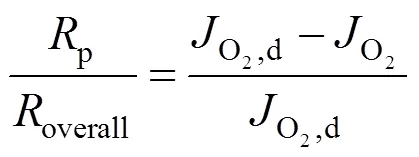
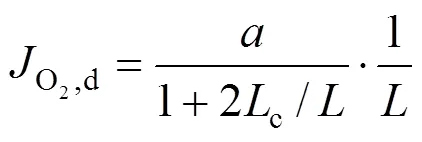

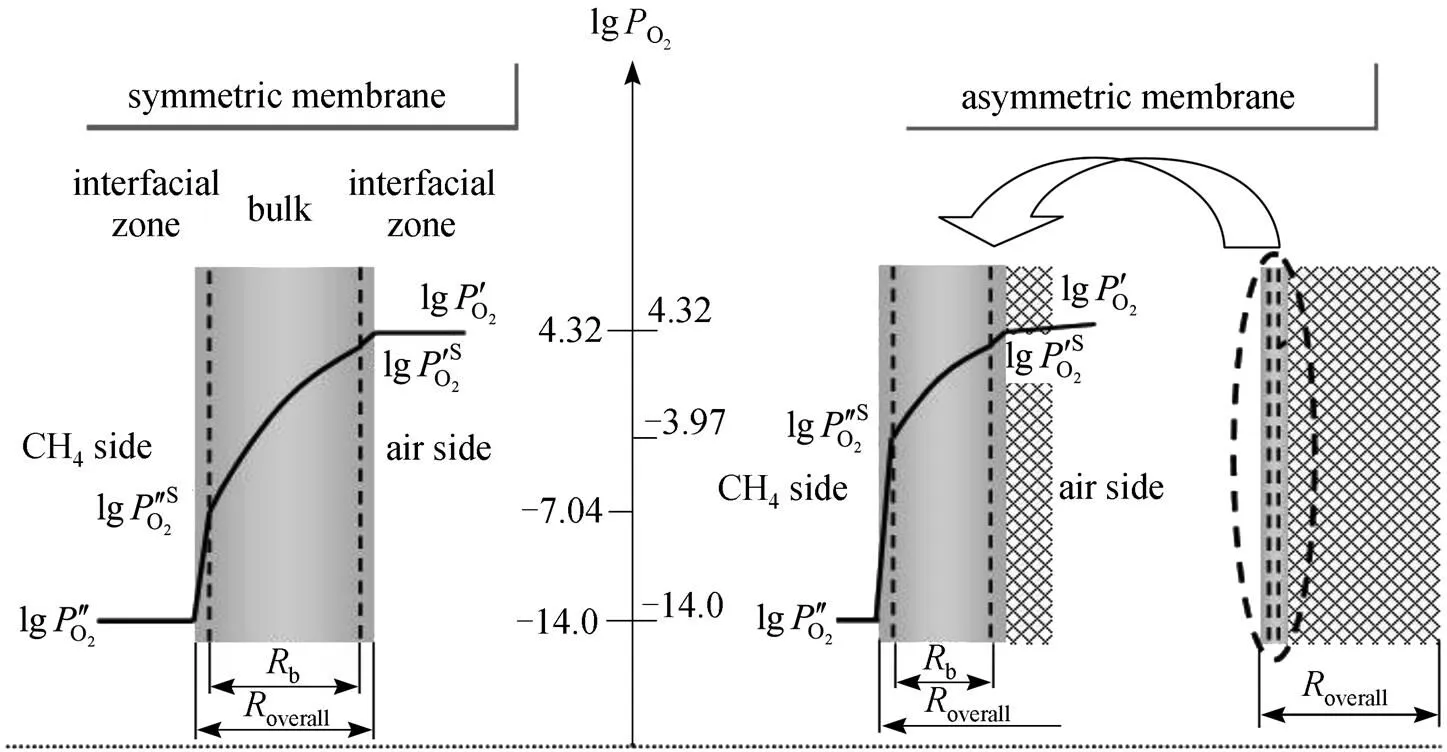
2.3 Estimation of the oxygen partial pressure on the membrane surface at CH4 side
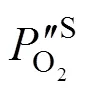
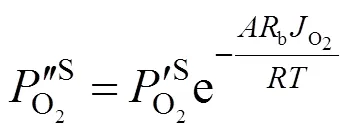
3 Experimental
3.1 Synthesis of SCFZ powders and preparation of membranes
SCFZ powders were synthesized by the conventional solid-state reaction method, which was similar to that in our previous study [19].
The asymmetric SCFZ membranes were prepared by the dry-pressing technique [15, 20]. Appropriate amounts of SCFZ powders were first poured into a stainless module. The surface of the SCFZ powders was leveled off at an intermediate pressure of 60 MPa. Then, the mixture of SCFZ powders and an inorganic porous former was added into the die to act as the support of asymmetric membranes. Pressing at a pressure of 240 MPa was followed to form the green SCFZ asymmetric membranes. Finally, green asymmetric SCFZ membranes were sintered at 1473 K for 2 h. For comparison, a dense symmetric SCFZ membrane and a porous SCFZ support were also fabricated under the identical condition with the asymmetric membranes.
3.2 Characterization
The crystal phase of membrane was determined by X-ray diffraction (XRD) with Cu Karadiation (Bruker, model D8 Advance, Germany). The diffraction patterns were collected at room temperature by step scanning at an increment of 0.05° in the range of 20°≤2≤80°. The morphology of membrane was examined by scanning electron microscopy (SEM) (FEI, model QUANTA-200, Netherlands) and the average pore size of support was evaluated by nitrogen permeability at room temperature. The tightness of membranes was checked by the N2gas-tight test at room temperature. A sintered membrane was first placed into a stainless module. After sealing the module, N2was introduced to one side of membrane. The other side of membrane was connected with a bubble flower. If the membrane was not dense, N2would permeate into the other side of membrane, and then the permeation of N2through the membrane could be detected by the bubble flower.
3.3 Oxygen permeation and membrane reaction measurement
The measurement of oxygen permeation and POM to syngas of membranes was conducted using an apparatus reported in our previous study [21]. Air was introduced into the upstream side (the porous support side of asymmetric membrane) of membrane at a rate of 100 ml(STP) per minute. He or a mixture of CH4and He was fed to the downstream side (the thin dense layer side of asymmetric membrane) of membrane. Both the upstream side and the downstream side of membrane were maintained at the atmospheric pressure. The effluent streams were analyzed by two on-line gas chromatographs (Shimadzu, model GC-8A, Japan) with about 1 cm3sample loop. A 2 m 5A molecular sieve column was used for the separation of H2, O2, N2, CH4and CO, and a 1 m TDX-01 column was for the separation of CO2and hydrocarbons. Fig. 1 illustrates the arrangement for comparison of performance of the symmetric and asymmetric membranes. Their oxygen permeabilities were compared and He was used as the sweeping gas in the permeation side in Fig. 1 (a). Fig. 1 (b) investigated their chemical stability, where CH4[2.9 ml·min-1(STP)] diluted by He [17.9 ml·min-1(STP)] was introduced into the reaction side of membrane. The CH4conversion and CO selectivity for the POM to syngas were defined as follows, respectively.
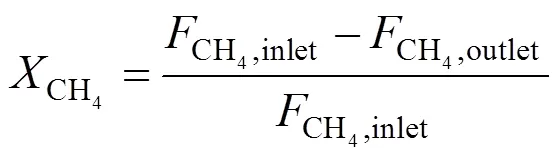
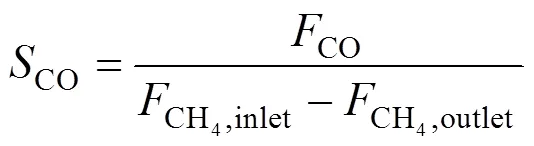
whereF(mol·s-1) is the flow rate of species. The oxygen permeation flux through membranes was calculated by
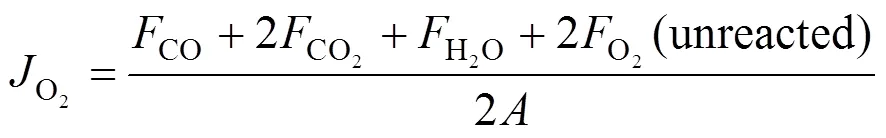
The blue-green 17.5% NiO/g-Al2O3catalyst used in the membrane reaction was preheated in 1︰1 mixture of H2︰He for 3 h at 700°C. In all membrane reaction experiments, 100 mg of the preheated 17.5% NiO/g-Al2O3catalysts were packed on the surface of membrane.
Figure 1 Schematic diagram for comparison of performance of symmetric and asymmetric membranes: (a) oxygen permeability; (b) partial oxidation of methane
4 RESULTS AND DISCUSSION
4.1 Membrane formation
The SCFZ symmetric and asymmetric membranes were prepared under the same conditions by means of the dry-pressing method [15, 20]. The morphology of prepared SCFZ asymmetric membranes was similar to that of La0.6Sr0.4Co0.2Fe0.8O3-δasymmetric membrane in our previous work [14]. The thin dense layer had a thickness of about 200 μm. Based on the nitrogen permeability of the sintered support at room temperature and the reports of Lin. [22], the support was estimated to be an average pore size of 1.18 μm and its nitrogen permeation was mainly controlled by the viscous flow, indicating that the porous support had a low gas transport resistance. Meanwhile, the XRD characterization confirmed that both the symmetric membrane and the asymmetric membrane exhibited a good perovskite-type structure.
The tightness of membranes was further checked by the N2gas-tight test at room temperature. When up to 0.2 MPa (absolute pressure) of N2was applied into one side of membrane, the permeation of N2was still not detected at the other side of membrane, which was at 0.1 MPa. However, in our oxygen permeation and membrane reaction experiments, the two sides of membrane were both maintained at the atmospheric pressure (0.1 MPa). The pressure difference in the oxygen permeation and membrane reaction experiments was less than that in the N2gas-tight test, indicating that the membranes prepared in our work was tight enough for the oxygen permeation and membrane reaction experiments.
4.2 Oxygen permeability

Figure 2 The temperature dependence of oxygen permeation fluxes of symmetric and asymmetric membranes at the oxygen partial pressure gradient of 2.1×104/1×102Pa△?asymmetric membrane;●?symmetric membrane
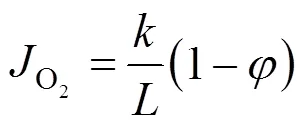
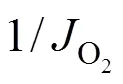
It could also be found from Fig. 2 that the apparent activation energy for oxygen permeation was decreased from 50.2 kJ·mol-1for the symmetric membrane to 30.8 kJ·mol-1for the asymmetric membrane, indicating that the asymmetric membrane had a greater capability for the oxygen permeation than the symmetric membrane. Similar phenomenon was observed by Teraoka. [12] and Ikeguchi[15].

Figure 3 The membrane thickness dependence of the oxygen permeation flux of symmetric membrane at 1123 K and the oxygen partial pressure gradient of 2.1×104/1×102Pa
4.3 Chemical stability
Figure 4 gives the performance of the POM to syngas of asymmetric SCFZ membranes (200 μm-thick thin dense layer and 1.3 mm-thick support) as a function of the temperature. Both the CH4conversion and the oxygen permeation flux through the membrane increase with an increase in the temperature. At 1123 K, the CH4conversion is 85.23% and the oxygen permeation flux is 4.20×10-6mol·cm-2·s-1. However, in the membrane reaction of symmetric membrane (1.5 mm thick), the CH4conversion and the oxygen permeation flux at 1123 K and the above conditions are only 66.47% and 3.14×10-6mol·cm-2·s-1, respectively. This was due to the greater bulk diffusion resistance in the symmetric membrane, which slowed down the supply of oxygen for the CH4reaction and then decreased the CH4conversion.

Figure 4 Plot of reaction performance of asymmetric membranestemperature
In order to examine the chemical stability of symmetric and asymmetric membranes at the elevated temperature and the reducing atmosphere, the long-term stability of membranes was performed in the POM reaction at 1123 K, as shown in Fig. 5. For the asymmetric membrane, the CH4conversion and the oxygen permeation flux are steadily kept at around 85.23% and 4.20×10-6mol·cm-2·s-1 in the first 696 h, respectively. After 696 h, the CH4conversion and the oxygen permeation flux show a remarkable increase. The CH4conversion increases from 85.23% to 92.21%, and the oxygen permeation flux is from 4.20×10-6to 4.63×10-6mol·cm-2·s-1. This was attributed to the formation of porous layer on the membrane surface at the CH4side, as shown in Fig. 6. The asymmetric SCFZ membrane prepared in our work was made of an oxide. At the CH4side, the membrane was surrounded by the reducing gases of CH4, H2and CO. These reducing gases were to destruct the membrane structure and break the membrane surface into some small grains to form the porous layer. It can be seen from Fig. 6 that although the perovskite-type characteristic peaks of membrane material on the membrane surface are remarkably weakened after being etched by the reducing gases, the membrane surface is still mainly composed of the perovskite-type oxide. Thus, this porous layer was acting as the surface modification of the membrane surface and increasing the area for the oxygen surface exchange on the membrane surface. The increase in the area for the oxygen surface exchange accelerated the oxygen surface exchange rate, and then enhanced the oxygen permeation flux through the membrane in the last 336 h. The increasing oxygen permeation flux through the membrane supplied the more amount of oxygen for the CH4reaction and led to an increase in the CH4conversion. This similar phenomenon was found by Tong. [24] and Wu[25]. When the POM reaction lasted for 1032 h, the experiment was voluntarily stopped. In the whole course, the CO selectivity was kept at about 95% and the H2/CO ratio was slightly lower than 2, which was caused by the instance that more amount of O2was permeated through the membrane from the air side and then consumed some of the product H2. Meanwhile, EDS analysis confirmed that no carbon was deposited on the catalyst after use in the POM reaction.
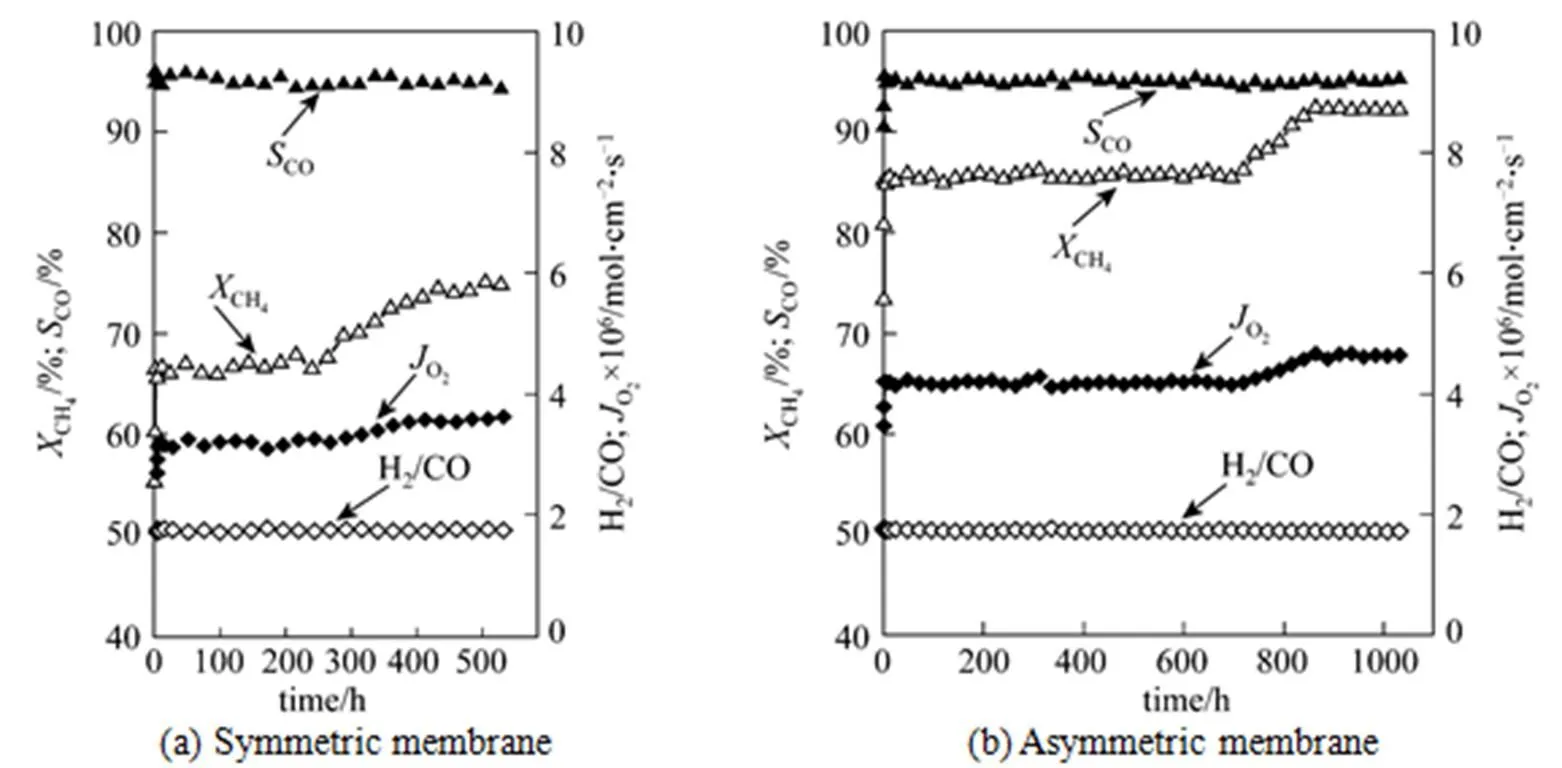
Figure 5 Long-term stability of membranes for the partial oxidation of methane to syngas at 1123 K
Figure 6 SEM images and XRD patterns of surface of asymmetric SrCo0.4Fe0.5Zr0.1O3-δmembranes
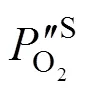

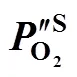
5 CONCLUSIONS
A comparison was systematically carried out between SrCo0.4Fe0.5Zr0.1O3-δsymmetric and asymmetric membranes in terms of oxygen permeability and chemical stability, and their differences were elucidated by means of the theoretical calculation. The main conclusions were as follows.
(1) For the oxygen permeability, the asymmetric membrane exhibited about two times that of the symmetric membrane in the range of 1073-1223 K under Air/He atmospheres, which was due to the significant decrease of bulk diffusion resistance in the thin dense layer of the asymmetric membrane.
(2) For the chemical stability, a remarkable extension of operating lifetime in POM was observed in the asymmetric membrane compared with the symmetric membrane because of the increase in the oxygen partial pressure on the membrane surface at CH4side. In the asymmetric membrane-based reactor, the steady time was over 1032 h at 1123 K, while there was only 528 h in the symmetric membrane-based reactor.
This study demonstrated that the asymmetric membrane-based reactor was a promising structure for the practical application.
NOMENCLATURE
membrane area, cm2
Fflow rate of species, mol·s-1
thickness of dense layer, cm
bbulk diffusion resistance in the dense layer, J·s·mol-2
doxygen permeation resistance in the dense layer, J·s·mol-2
overalloverall resistance for oxygen permeation, J·s·mol-2
poxygen transport resistance in the support, J·s·mol-2
sresistance of oxygen exchange process in the dense layer, J·s·mol-2
COCO selectivity
temperature, K
Superscript
S membrane surface
1 Bouwmeester, H.J.M., Burggraaf, A.J., Fundamentals of Inorganic Membrane Science Technology, Elsevier Science B.V., Amsterdam (1996).
2 Schiestel, T., Kilgus, M., Peter, S., Caspary, K.J., Wang, H., Caro, J., “Hollow fibre perovskite membranes for oxygen separation”,.., 258, 1-4 (2005).
3 Balachandran, U., Dusek, J.T., Mieville, R.L., Poeppel, R.B., Kleefisch, M.S., Pei, S., Kobylinski, T.P., Udovich, C.A., Bose, A.C., “Dense ceramic membranes for partial oxidation of methane to syngas”,..:., 133, 19-29 (1995).
4 Tsai, C.Y., Dixon, A.G., Moser, W.R., Ma, Y.H., “Dense perovskite membrane reactors for the partial oxidation of methane to syngas”,., 43, 2741-2750 (1997).
5 Jin, W., Gu, X., Li, S., Huang, P., Xu, N., Shi, J., “Experimental and simulation study on a catalyst packed tubular dense membrane reactor for partial oxidation of methane to syngas”,..., 55, 2617-2625 (2000).
6 Balachandran, U., Ma, B., “Mixed-conducting dense ceramic membranes for air separation and natural gas conversion”,.., 10, 617-624 (2006).
7 Jin, W., Zhang, C., Zhang, P., Fan, Y., Xu, N., “Thermal decomposition of carbon dioxide coupled with POM in a membrane reactor”,., 52, 2545-2550 (2006).
8 Sammells, A.F., Schwartz, M., Mackay, R.A., Barton, T.F., Peterson, D.R., “Catalytic membrane reactors for spontaneous synthesis gas production”,., 56, 325-328 (2000).
9 Bouwmeester, H.J.M., “Dense ceramic membranes for methane conversion”,., 82, 141-150 (2003).
10 Thursfield, A., Metcalfe, I.S., “The use of dense mixed ionic and electronic conducting membranes for chemical production”,..., 14, 2475-2485 (2004).
11 Pei, S., Kleefisch, M.S., Kobylinski, T.P., Faber, K., Udovich, C.A., Zhang-Mccoy, V., Dabrowski, B., Balachandran, U., Mieville, R.L., Poeppel, R.B., “Failure mechanisms of ceramic membrane reactors in partial oxidation of membrane to synthesis gas”,.., 30, 201-212 (1995).
12 Teraoka, Y., Fukuda, T., Miura, N., Yamazoe, N., “Development of oxygen semipermeable membrane using mixed conductive perovskite-type oxides (Part 2)”,......, 97, 523-529 (1989).
13 Liu, Y., Tan, X., Li, K., “Mixed conducting ceramics for catalytic membrane processing”,.., 48, 145-198 (2006).
14 Jin, W., Li, S., Huang, P., Xu, N., Shi, J., “Preparation of an asymmetric perovskite-type membrane and its oxygen permeability”,.., 185, 237-243 (2001).
15 Ikeguchi, M., Uchida, Y., Sekine, Y., Kikuchi, E., Matsukata, M., “Solid state synthesis of SrFeCo0.5Oasymmetric membranes for oxygen separation”,...., 38, 502-508 (2005).
16 Yin, X., Hong, L., Liu, Z.L., “Oxygen permeation through the LSCO-80/CeO2asymmetric tubular membrane reactor”,.., 268, 2-12 (2006).
17 Kovalevsky, A.V., Kharton, V.V., Maxim, F., Shaula, A.L., Frade, J.R., “Processing and characterization of La0.5Sr0.5FeO3-supported Sr1-xFe(Al)O3-SrAl2O4composite membranes”,.., 278, 162-172 (2006).
18 Büchler, O., Serra, J.M., Meulenberg, W.A., Sebold, D., Buchkremer, H.P., “Preparation and properties of thin La1-xSrCo1-yFeyO3-δperovskite membranes supported on tailored ceramic substrates”,, 178, 91-99 (2007).
19 Yang, L., Tan, L., Gu, X., Jin, W., Zhang, L., Xu, N., “A new series of Sr(Co, Fe, Zr)O3-perovskite-type oxides for oxygen permeation”,...., 42, 2299-2305 (2003).
20 Chang, X., Zhang, C., Jin, W., Xu, N., “Match of thermal performances between the membrane and the support for supported dense mixed-conducting membranes”,.., 285, 232-238 (2006).
21 Li, S., Jin, W., Huang, P., Xu, N., Shi, J., Hu, M., Payzant, E., Ma, Y., “Perovskite-related ZrO2-doped SrCo0.4Fe0.6O3-membrane for oxygen permeation”,., 45, 276-284 (1999).
22 Lin, Y.S., Burggraaf, A.J., “Experimental studies on pore size change of porous ceramic membranes after modification”,.., 79, 65-82 (1993).
23 Chang, X., Zhang, C., Wu, Z., Jin, W., Xu, N., “Contribution of the surface reactions to the overall oxygen permeation of the mixed conducting membranes”,...., 45, 2824-2829 (2006).
24 Tong, J., Yang, W., Cai, R., Zhu, B., Lin, L., “Novel and ideal zirconium-based dense membrane reactors for partial oxidation of methane to syngas”,.., 78, 129-137 (2002).
25 Wu, Z., Jin, W., Xu, N., “Oxygen permeability and stability of Al2O3-doped SrCo0.8Fe0.2O3-δmixed conducting oxides”,.., 279, 320-327 (2006).
26 Hendriksen, P.V., Larsen, P.H., Mogensen, M., Poulsen, F.W., Wiik, K., “Prospects and problems of dense oxygen permeable membranes”,., 56, 283-295 (2000).
2008-06-13,
2009-04-16.
the National Basic Research Program of China (2009CB623406), the National Natural Science Foundation of China (20636020), the National High Technology Research and Development Program of China (2006AA030204) and the Specialized Research Fund for the Doctoral Program of Higher Education of China (20060291003).
** To whom correspondence should be addressed. E-mail: wqjin@njut.edu.cn
 Chinese Journal of Chemical Engineering2009年4期
Chinese Journal of Chemical Engineering2009年4期
- Chinese Journal of Chemical Engineering的其它文章
- Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide*
- Phase Equilibrium of Isobutanol in Supercritical CO2
- Conversion of Methane by Steam Reforming Using Dielectric-barrier Discharge*
- Permeability and Selectivity of Sulfur Dioxide and Carbon Dioxide in Supported Ionic Liquid Membranes*
- Hydroxyapatite Coatings on Titanium Prepared by Electrodeposition in a Modified Simulated Body Fluid*
- Model Study on a Submerged Catalysis/Membrane Filtration System for Phenol Hydroxylation Catalyzed by TS-1*
