Bioresorbable magnesium-based alloys containing strontium doped nanohydroxyapatite promotes bone healing in critical sized bone defect in rat femur shaft
Shzi Shikh ,Irfn Qyoom ,R.Srvesh ,Ashok Kumr,b,d,e,?
a Department of Biological Sciences and Bioengineering,Indian Institute of Technology Kanpur,Kanpur 208016,India
b Centre for Environmental Science and Engineering,Indian Institute of Technology Kanpur,Kanpur 208016,India
c Department of Materials Science and Engineering,Indian Institute of Technology Kanpur,Kanpur 208016,India
d Center for Nanosciences,Indian Institute of Technology Kanpur,Kanpur 208016,India
e The Mehta Family Centre for Engineering in Medicine,Indian Institute of Technology Kanpur,Kanpur 208016,India
Abstract Magnesium-based biomaterials have been in extensive research for orthopedic applications for decades due to their optimal mechanical features and osteopromotive nature;nevertheless,rapid degradation restricts their clinical applicability.In this study,pristine magnesium was purifie (P-Mg) using a melt self-purificatio approach and reinforced using indigenously synthesized nanohydroxyapatite (HAP,0.6 wt.%)and strontium substituted nanohydroxyapatite (SrHAP,0.6 wt.%) using a low-cost stir assisted squeeze casting method to control their degradation rate.Using electron back-scattered diffraction (EBSD) and X-ray diffraction (XRD) examinations,all casted materials were carefully evaluated for microstructure and phase analysis.Mechanical characteristics, in vitro degradation,and in vitro biocompatibility with murine pre-osteoblasts were also tested on the fabricated alloys.For in vivo examination of bone formation,osteointegration,and degradation rate,the magnesium-based alloys were fabricated as small cylindrical pins with a diameter of 2.7 mm and a height of 2 mm.The pins were implanted in a critical-sized defect in a rat femur shaft (2.7 mm diameter and 2 mm depth) for 8 weeks and evaluated by micro-CT and histological evaluation for bone growth and osteointegration.When compared to P-Mg and P-MgHAP,micro-CT and histological analyses revealed that the P-MgSrHAP group had the highest bone formation towards the periphery of the implant and hence maximum osteointegration.When the removed pins from the bone defect were analyzed using GIXRD,they displayed hydroxyapatite peaks that were consistent with bio-integration.For P-Mg,P-MgHAP,and P-MgSrHAP 8 weeks after implantation,in vivo degradation rates derived from micro-CT were around 0.6 mm/year,0.5 mm/year,and 0.1 mm/year,respectively.Finally,P-MgSrHAP possesses the requisite degradation rate as well as sufficien mechanical and biological properties,indicating that it has the potential to be used in the development/fabrication of biodegradable bioactive orthopaedic implants.
Keywords: Magnesium purification Reinforcement;Degradation rate;Nanocement;Bone regeneration.
1.Introduction
Bone fractures and defects in load-bearing bones require clinical interventions as mechanical support to stabilize the fracture site and augment bone healing.These clinical interventions are usually accomplished by using metallic implants to support the fractured bone in the form of bone fixtures consisting of titanium and its alloys,stainless steel,and Co-Cr alloys.However,their slow or non-biodegradable nature with the release of toxic ions and other limitations like stress shielding,recurrent infections result in implant failures [1,2].The implant failure is dealt clinically by revision/secondary surgeries involving removal of old implant and replacing it with new implants thus making patients susceptible to secondary fractures.To improve current clinical practices and with technological advancement,biodegradable implants are constantly being explored for clinical translations as permanent implants [3–5].Hence,at present,the extensive focus of research has been in the direction of developing biodegradable metallic implant materials with a controlled degradation rate and mechanical properties similar/closer to that of human bone [6].
Magnesium (Mg) and Mg-based alloys have attracted considerable attention as biodegradable metallic implant materials for orthopedic applications,as they possess advantages over conventional bio-metallic materials,ceramics,and biodegradable polymers.Mg has mechanical properties close to that of human bone (elastic modulus Mg ~45 GPa,bone~20–40 GPa) and it is lighter than metal implants (density~2 gm/cm3) currently available for biomedical applications[7].In addition,Mg and Mg-based alloys gradually degrade in the human body environment and are eventually replaced by newly grown bone tissue after implantation,which eliminates the need for further surgery to remove these implants from the human body when they are used as a bone support temporary implants such as screws,plates,nails,and pins,etc.Despite its suitable mechanical and biological properties,Mg and its alloys have not found wide space in the medical implant industries yet.This is due to their high solubility profil in the human body environment.Moreover,magnesium and its alloys often degrade in the human body environment before the bone defect healsi.e.,the rate of degradation is faster than the rate of tissue healing and loses mechanical strength before sufficien healing.
The high degradation rate of Mg is basically due to the presence of intrinsic impurity elements (Fe,Cu,Ni,and Co)which form secondary phases with the alpha-Mg phase and lead to micro-galvanic corrosion due to their high corrosion potential than magnesium [8–12].Hence fabrication of magnesium-based biomaterials with acceptable levels of impurities is very important to reduce the intrinsic corrosion.Till now none of the purifie magnesium was found to have a long-term less corrosion rate than that of commercially available ultra-high purity (UHP) magnesium,i.e.,0.25 mm/year which is determined under 3.5 wt.% NaCl solutionviamass loss measurements [13].However,UHP magnesium is expensive.Therefore,to decrease the cost many steps were considered for controlling the impurities of pure magnesium and its alloys such as filtration vacuum distillation,use of flu es,etc [14].The Fe content of AZ31 and other commercially available alloys have been reduced without using any flu esvialow-temperature melt treatment using melt self-purifying technology [15].Therefore,there is an unmet need to further develop a simple method for pristine magnesium purificatio to achieve desired purity and subsequent degradability.
Also,it has been shown that alloying Mg can retard the biodegradation process.In reality,from the medical point of view,there are not many elements suitable for magnesium alloying.There is ample information available in the literature concerning the alloying of Mg to reduce its corrosion resistance for various industrial and medical applications.Moreover,all alloying elements form secondary phases with magnesium and lead to corrosion [16,17].Therefore,alloying with materials not forming any secondary phase with pure magnesium phase may be suitable for controlling corrosion rate [18].An army of studies has demonstrated that alloying magnesium with hydroxyapatite (2–15 wt.%) shows comparably reducedin vitrocorrosion rate,however,limitedin vivostudy available based on such alloys [19–21].Hydroxyapatite is a mineral component of bone comprising 65–70% bone mass and is also known to possess low solubility in a human physiological environment.However,to the best of the author’s knowledge,the inclusion of bone-like hydroxyapatite into magnesium melt in smaller quantities (<1 wt.%)has not been examined yet [22].Besides,none of the studies used strontium substituted hydroxyapatite as an alloying element in pure magnesium.Recentin vitrostudy based on hydroxyapatite reinforced magnesium tin alloy have concluded that the inclusion of tin into magnesium hydroxyapatite composite improves the mechanical and corrosion properties[23].
Considering all aforementioned concerns,two major steps have been taken in the present study;one is to provide desired purity magnesium and a preparation method for the purification Further,reinforcement of purifie magnesium with indigenously synthesized hydroxyapatite and strontium substituted hydroxyapatite nanoparticlesvialow-cost stir and squeeze casting method.The process developed in this study gives a method to design desired purifie magnesium and its alloys with a controlledin vivodegradation rate suitable for bone implant fabrication.The base material (magnesium) purificatio was performedviathe repeated casting method.Hence,the present study established here suggested that purifie magnesium-based bone-like apatitecontaining alloys could be a promising material for the fabrication of bioactive biodegradable magnesium-based bone fixtures
2.Materials and methods
2.1.Materials
Calcium nitrate,diammonium hydrogen phosphate,calcium sulfate dihydrate,and ammonia solution were purchased from SD Fine-Chem,India.Pure magnesium ingots (99.4% purity) were procured from SWAM EQUIP,Chennai,in India.Zoledronic acid was obtained from the Novartis,Switzerland.Minimum Essential Medium Eaglealpha modificatio (α-MEM) was purchased from Thermo Scientifi (Waltham,MA).3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT),trypsin?EDTA,and Masson’s trichrome staining kit were all sourced from Sigma Chemical Co.(St.Louis,MO).Fetal bovine serum(FBS)(US origin) was sourced from Gibco (Waltham,MA).All chemicals used in the present study were of analytical grade.Wistar rats (males) weighing between 250 and 300 g and age 2.5 months were obtained from the Indian Institute of Toxicology Research (IITR),Lucknow,India.
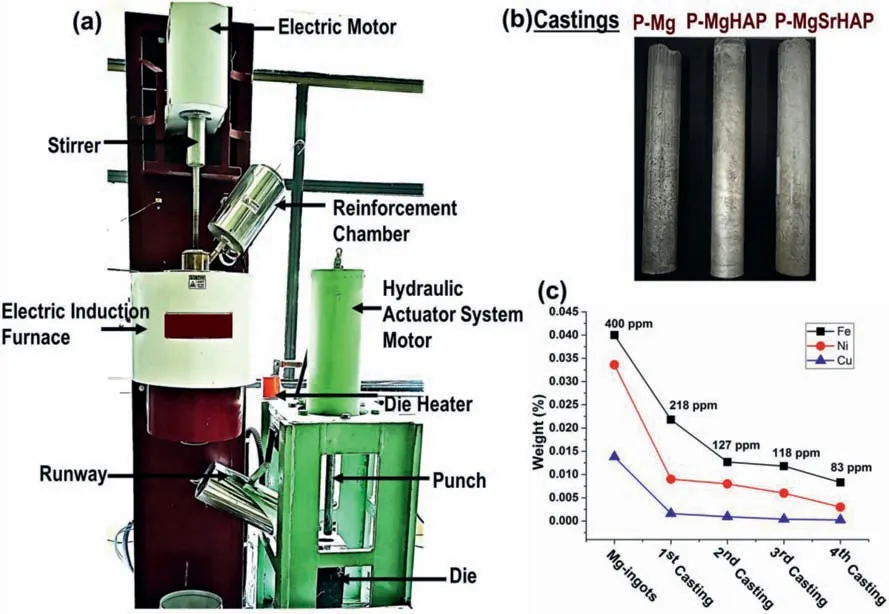
Fig.1.(a) Digital photographs of experimental Furnace with different parts labeled;(b) casted rods of purifie magnesium and its alloys and (c) graph shows the average impurities reduction via repeated casting method measured by optical emission spectroscopy.
2.2.Magnesium purification alloy fabrication,and their characterization
2.2.1.Magnesium purificatio
Magnesium ingots of purity 99.4% were purifie using the repeated casting method.Briefl,a stir casting furnace was heated to a temperature of 750 °C.After that,magnesium ingots were placed inside the crucible in an inert atmosphere as magnesium catches fir at a molten state in an air environment.Hence,highly purifie argon gas (99.9999% purity)was purged inside the crucible to prevent fir and oxidation of magnesium.Post complete smelting of magnesium ingots,melt temperature was lowered to 650 °C stirred for 10 min,and held for 15 min.After the completion of holding,the melt was poured into preheated die (250 °C) downward as shown in Fig.1.The aforementioned process of magnesium casting was repeated at least four times to achieve desired purity and named P-Mg.Post each casting a 0.5 mm thickness was milled and again casted with the same procedure described above four times.
2.2.2.Synthesis of nano sized hydroxyapatite,calcium sulfate α-hemihydrate (α-CSH),and zoledronic acid loaded bone cement
Hydroxyapatite nanoparticles were synthesized by wet chemical precipitation method described somewhere else[24–29].Briefl,calcium nitrate tetrahydrate (Ca(NO3)2·4H2O,0.96 M) an alkaline solution (pH 10.0) was maintained at 90?100 °C under constant stirring and mixed with aqueous solution of diammonium hydrogen orthophosphate((NH4)2HPO4,0.6 M) by drop casting.The pH of the system was constantly maintained at pH 10.0 by the addition of NH4OH solution.As a result,a white nHAP precipitate crystals were obtained.On completion of the drop casting and mixing of two source solutions (i.e.,calcium and phosphorous containing slurries),the precipitate formed were aged for at least 48 h at room temperature.Post complete maturation/ageing,the precipitate was filtere and washed thoroughly with Milli-Q water followed by drying at 120 °C.Dried powder of nHAP was subjected to sintering to enhance its crystallinity,and phase purity at 800 °C for 4 h holding.Similarly,strontium substituted nHAP was synthesized by additional substitution of strontium nitrate (5 mol%,Sr (NO3)2)slurry into calcium source slurry before drop casting of phosphorous source solution and followed by same procedure as described above for HAP synthesis.
An autoclave method was used to prepare calcium sulfateα-hemihydrate (CSH) (CaSO4·0.5H2O) from calcium sulfate dihydrate (CSD) (CaSO4·2H2O).Briefl,a dispersion media of CSD was prepared in aqueous solution of H2SO4(0.1 M)following one hour stirring and filtration Obtained crystals were then washed with milli-Q water for neutralization.The crystals were then dispersed in di-water at ratio CSD: water 1:2,respectively,and placed in autoclave system at 121–125 °C for 4 h.Post completion of autoclave treatment,CSH was filtere at 100 °C following washing in boiling water and drying at 95 °C in hot air oven over night.
Indigenously synthesized nHAP and CSH at ratio 40% and 60%,respectively,were mixed thoroughly to obtained biphasic nanocement (NC).A 600 μl of sterile water was added to each gram of NC which gives cement within 15 min of settling time.A bioactive molecule loadedi.e.,Zoledronic acid(ZA) containing nanocement was prepared by adding 10 μl of ZA to the liquid phase used for cementing and labelled as NC+ZA in the present study.
2.2.3.Magnesium alloying
For an alloy fabrication,indigenously synthesized HAP and SrHAP of size ranging between 50 and 60 nm were preheated to 300 °C for moisture removal before adding to the base material.Alloys were fabricated using a low-cost stirassisted squeeze casting method.P-Mg was used as base material.In the very firs step of alloy fabrication,P-Mg was placed inside crucible pre-heated at 750 °C temperature and left for melting under high purity argon (99.9999%) atmosphere.Post complete smelting of P-Mg,the melt was stirred at 350 rpm for melt confirmation After that preheated HAP was introduced into melt and stirred at 350 rpm for at least 10 min to ensure complete and homogenous mixing.Poststirring melt was held for 5–10 min for homogenization.After all the aforementioned steps finall,the mixture was cast into preheated die (250 °C) under a pressure of 30 tons for 20 s and named P-MgHAP.Similarly,P-Mg-based SrHAP containing alloy was fabricated and named as P-MgSrHAP.The concentrations of alloying elements were ranged between 0.6–2 wt%.In the present study the optimized concentrationi.e.,0.6 wt% of HAP and 0.6 wt% of SrHAP used for alloying P-Mg and processed further for the characterizations.
2.2.4.Characterization
Purity,density and porosity measurement.Purity and the chemical compositions of as-fabricated purifie magnesium and its alloys were investigated using optical emission spectroscopy (OES).
The density of as-fabricated purifie magnesium and its alloys via squeeze casting method was calculated using Archimedes’principle.For this,all the samples were weighed in air(Wa)and then samples were suspended in distilled water and weighed again(Ww).Experimental density was measured using the formula:
Where ?a is experimental density and ?w is the density of water.
For porosity measurements,the theoretical density of all samples was calculated using the rule of mixtures and denoted as ?t.With the help of experimental density and theoretical density,porosity (P) of the material was calculated using formulae:
Metallography.The metallography analysis of the casted rods was carried out by cutting ~3 mm thick slices using electrical-discharge machining (EDM).The samples were metallographically polished using 5 μm alumina dispersed in ethanol for several minutes until the oxidation marks formed due to EDM was removed.Further,polishing was carried out in 0.05 μm alumina dispersed in ethanol on Vibratory polisher (VibroMetTM2,Buehler) polished for 2 h.The sample was etched lightly agitating in 10% Nital (10 ml HNO3,90 ml C2H5OH) and immediately dried with a blast of hot air.Macro-photography of the samples was obtained using a Sony A7R III camera with a 50 mm macro lens.Orientation imaging microscopy (OIM) based on electron-back scattered diffraction (EBSD) was carried out with a NordlysNano detector Oxford instruments Nanotechnology Tools Ltd.,UK.A unique holder was fabricated for holding these large diameter samples during EBSD.Further,the EBSD scanning was done with an accelerating voltage of 20 kV with a probe current of ~13 nA.AZtec Crystal 1.1 (Oxford instruments Nanotechnology Tools Ltd.,UK) was for post OIM data analysis.The average grain size was evaluated based on equivalent circle diameter obtained from EBSD scanning,a minimum of three areas at the periphery and center of the samples were considered for plotting grain size distribution.
X-ray diffraction analysis.PAN analytical Empyrean instrument(2θ=20°–60°,step size:0.015°),with Cu-Kαradiation of 1.54,was used for X-ray diffraction measurement with an operating voltage and current 45 kV and 40 mA,respectively.
Mechanical properties.Cylindrical samples for compressive strength were prepared according to ASTM E9-89A using electrical-discharge machining (EDM) with length/diameter ratio 1.5,and ultimate compressive strength,and elongation percentage was obtained using Instron Universal testing machine (retrofi BiSS India Pvt.Ltd).The test was repeated at least 5 times and the average of the results presented.Tensile test was performed using Instron Universal testing machine(retrofi BiSS India Pvt.Ltd) at strain rate 0.005 s?1,at room temperature.A dog bone shape samples were prepared according to ASTM E8.
In vitrodegradation,hydrogen evolution test and corrosion layer characterization.P-Mg,MgHAP,and MgSrHAP were immersed in simulated body flui (SBF)(pH 7.4)with a solution to area ratio of 20 mL per cm2and incubated at 37°C for 30 days.SBF solution was changed alternatively to maintain pH 7.4.The degradation rate (DR) (mm/year) was evaluated post removing the corrosion products in chromic acid solution,using formula according to ASTM-G31-72:
Where W is the weight loss(mg)in time T(days),A is the initial surface area (cm2),D is the density (g/cm3) of casted materials,and T is the immersion time (days).
The morphology of the corrosion layer was observed using fiel emission electron microscopy (FE-SEM,Nova NanoSEM 450,operating voltage 15 kV) coupled with energy dispersive X-ray analysis (EDS).The phase analysis of the corrosion layer was performed using grazing angle X-ray diffraction (GIXRD) analysis.
2.3.Indirect cell culture
2.3.1.Cell culture
MC3T3-E1 murine pre-osteoblast cells were cultured inα-MEM media containing 10% fetal bovine serum (FBS) and 1% antibiotic (penicillin and streptomycin) known as complete media at 37 °C in a 5% CO2humidifie incubator.
Extract preparation.Prior to cell culture,all samples were sterilized in 70%ethanol under UV irradiation for 15 min and repeated for three times followed by washing in warm PBS for 15 min under UV irradiation thrice.Post complete sterilization,samples were incubated in completeα-MEM media with volume to surface ratio 1.3 ml/ cm2for 3 days at 37 °C in 5% CO2atmosphere.
Biocompatibility test.To investigate the biocompatibility and cytotoxic capacity of P-Mg,P-MgHAP,&P-MgSrHAP,MTT assay was performed at 24 and 72 h with 100,50,20 and 5% of extracts using murine pre-osteoblast MC3T3E1 cells.
2.4.Animal model
Anesthesia in animals was induced by 5% Isofluran inhalation which was further maintained at 2% during surgery.The right leg of rats was shaved and sterilized using povidone followed by blunt cut in the thigh muscles along the longitudinal axis of the femur to expose the femur shaft.A hole about 2.7 mm diameter and 2 mm depth was created in the central diaphysis of the femur using a drill burr operated by dental aerator under constant saline irrigation (Fig.6a).The defect was flushe with saline to remove the small bone pieces generated during the drilling.In all the groups which received implant (except empty control),the defect was firs fille with pre-set nanocement (NC) functionalized with zoledronic acid (ZA) (10 μg/animal) by impaction followed by implantation of Mg or its alloys in their respective groups(Fig.6b).The rationale for the use of ZA loaded NC was to enhance the osteointegration of the magnesium-based implants with the host bone as ZA loaded NC has been shown to promote bone regeneration in our earlier studies [24–29].The groups considered for animal experiments are presented in Table 1.

Table 1 Animal group details used for in vivo evaluation in rat femur defect.

Table 2 Weight (%age) of impurity contents along with reinforcing elements.
2.4.1.Blood testing
To evaluate the systemic inflammation blood from experimental rats were collected pre and post implantation by puncturing the retro-orbital sinus plexus at various time points (5,7,9,14,21,and 28 days).Approximately,500 μl of blood was drawn from each rat in a EDTA containing tube.Total leukocyte count(TLC)and differential leukocyte count(DLC)were outsourced and used as a parameter to evaluate the systemic inflammator effect of implantation of magnesiumbased implants.
2.4.2.In vivoradiography andex vivomicro-CT analysis
Thein vivoX-ray images of the animals were recorded at a 4-week time point post-surgery using a human X-ray machine with digital radiography to assess the changes at the implantation site.The excised femur samples were scanned using Skyscan 1172 (Bruker,Belgium)ex vivoscanner and the scanning parameters considered were,voxel size 10 μm,X-ray energy 50 keV,and exposure duration 800 ms.The images acquired after scanning were reconstructed using software (NRecon,Bruker,Belgium).Reconstructed images were realigned using Data Viewer (Bruker,Belgium) and the analysis was performed using CTAn (Bruker,Belgium) by selecting region of interest (ROI) in the periphery of the implant as concentric circle of dimension 0.3 mm diameter and height 2 mm (Fig.7d) to evaluate the CT-based morphometry (BV mm3).
Additionally,implant pins before and after implantation were also acquired for surface area and volume change analysis.In vivodegradation rate was evaluated using formulae:
WhereΔV is the reduced volume of implant pin,A is the implant surface area,and t is the implantation duration.
2.4.3.FE-SEM and XRD analysis of implants removed from defect site post 8 weeks
To investigate the interaction of implants with surroundings host tissues,the degradation layer and bone formation/mineralizationin vivoon the surface of implants post 8 weeks of implantation in rat bone defects,Filed-emission scanning electron microscopy (FE-SEM) and grazing angle X-ray diffraction (GIXRD) analysis were performed on excised implants.For FE-SEM,all samples were gold coated and Elemental analysis of the deposition/degradation layer on the surface of implant was performed using energy dispersive X-ray spectroscopy (EDS).
2.4.4.Histological analysis
Excised rat femurs post 8 weeks of implantation were fi ed in 10% neutral buffer formalin (NBF) at 4 °C for 48 h followed by washing in 70% ethanol for 72 h at 4 °C.The implanted metallic pins were removed before fixation Post fixatio and washing,samples were decalcifie in 10% Na-EDTA (pH=7.4) solution followed by paraffi embedding and sectioning at 10 μm thickness for hematoxylin and eosin(H&E),masson’s trichrome staining.
2.5.Statistical analysis
Statistical analysis was performed using standard deviation(SD) and presented as mean±SD,and one-way analysis of variance test with Bonferroni’s multiple comparison test to compare all the results,wherever required.
2.6.Animal ethics statement
All animal experiments were performed according to CPCSEA and Institute Animal Ethics Committee (IAEC) guidelines with reference no.IITK/IAEC/1093.All the animals were facilitated with enough food and water.
3.Results
3.1.Magnesium reinforcement and characterization
Magnesium was purifie via repeated casting method and achieved purity approximately 99.9%.The impurity contents post each casting are presented in the Fig.1c.Same magnesium material starting with raw magnesium ingots (99.4%,purity) was cast repeatedly four times and achieved Mg(99.9 wt.%,purity)with Fe content ~85 ppm(below the tolerance limit of secondary phase formation).Post each casting,the casted rod was milled 0.5 mm to remove the oxidized layer and casted again following the same procedure at least four times.Moreover,along with Fe,other impurity element contents were also decreased remarkably (Fig.1c).
Stochiometric hydroxyapatite (HAP)and nonstoichiometric strontium substituted hydroxyapatite (SrHAP,5 mol% of Sr) of size 50–60 nm approximately,were synthesizedviawet chemical precipitation method indigenously,as shown in the Fig.2a and b.These bone-like apatite were used as an alloying element in P-Mg.It had already been shown in previous studies that a high concentration of nHAP inclusion into magnesium leads to a faster degradation rate [30].Therefore,in this study,the optimized content of nHAP (0.666 wt.%) and nSrHAP (0.666 wt.%) were used for alloying P-Mg and were named as P-MgHAP and P-MgSrHAP,Table 2.The purifie magnesium-based HAP and SrHAP containing alloys were fabricated by stir assisted squeeze casting method as shown in Fig.1b.
The density and porosity of squeeze cast P-Mg and its alloys (P-MgHAP and P-MgSrHAP) were determined by Archimedes’ principle as presented in the Table 3.A significan increase in density was observed for all samples due to the squeeze casting process.Also,the porosity of all samples decreased due to the application of high-pressure during casting which removes entrapped air in the melt recrystallization/solidificatio process.

Table 3 Calculated densities and porosities of fabricated purifie magnesium and its based alloys.
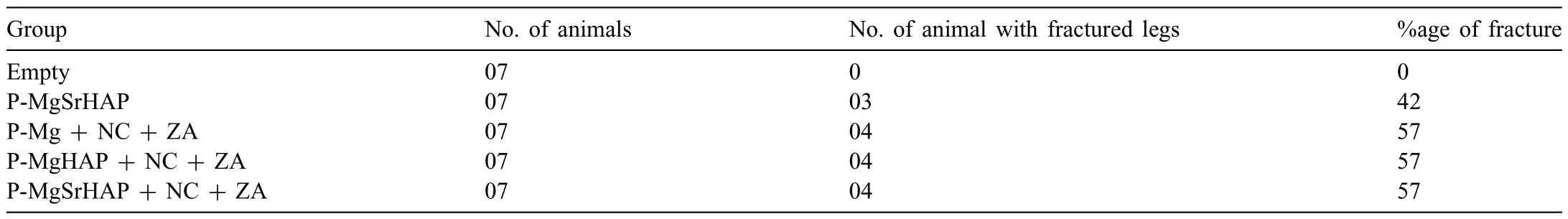
Table 4 Animals details (%age) undergone fracture in defect legs.
3.2.Metallography and phase identificatio
Macro images of pure Mg,P-MgHAP and P-MgSrHAP are shown in Fig.3a1,b1,and c1,respectively.Pure Mg has a coarser and columnar grains radiating outside,with the addition of HAP and SrHAP a decrease in grain size can be noticed (Fig.3b2 and c2).Previously it was reported that trace addition of Sr (0.01 wt.%) [31] and 5% HAP [32] in pure Mg resulted in a drastic decrease in grain size.In the present case (Fig.3c3) it is interesting to observe a combined effect of Sr and HAP resulted in better grain refinement The average grain size of as-casted P-Mg is ~271±151 μm,PMgHAP is 139±95 μm and P-MgSrHAP is 84±58 μm.Hence,in the present case it is interesting to observe a combined effect of Sr and HAP resulted in better grain refinement The probable reason for grain refinemen is nano particles of HAP and SrHAP that hinders the grain boundaries to grow and results in grain refinemen [33].Further,it is evident from IPF (inverse pole figure)-maps (Fig.3a–c) grains constitute deformation twins (highlighted by red lines).The twin has disorientation of 86.3±5° about 11ˉ20 direction,which corresponds to tensile twin in Mg which would have formed during the squeeze casting process.
Phase and crystal structure of all as casted samples were analyzed using X-ray diffraction analysis and the diffraction peaks were identifie using the international center for diffraction data database (ICDD).Fig.2c,presents the diffraction patterns of HAP,SrHAP,P-Mg,P-MgHAP,and P-MgSrHAP.Characteristic peaks of HAP were clearly seen in the XRD of HAP and SrHAP (PDF no: 01-086-1199).Peak shifting in the case of SrHAP was observed confirmin successful doping of Sr and formation of SrHAP [34].The diffraction pattern of P-Mg showed only peaks corresponding to pristine magnesium (PDF no: 00-004-0770) and no oxide peaks were observed confirmin the high purity of magnesium.Signifi cant intensity enhancement was observed at 2θvalue 32.86°,46.98°,and 49.64° in the diffraction pattern of P-MgHAP and P-MgSrHAP which corresponds to hydroxyapatite peaks.No additional peaks other than alpha Mg and nHAP were observed in the case of P-MgHAP and P-MgSrHAP,which suggests no new phase formation,indicating no chemical reaction occurred during reinforcement of P-Mg with HAP and SrHAP,respectively.
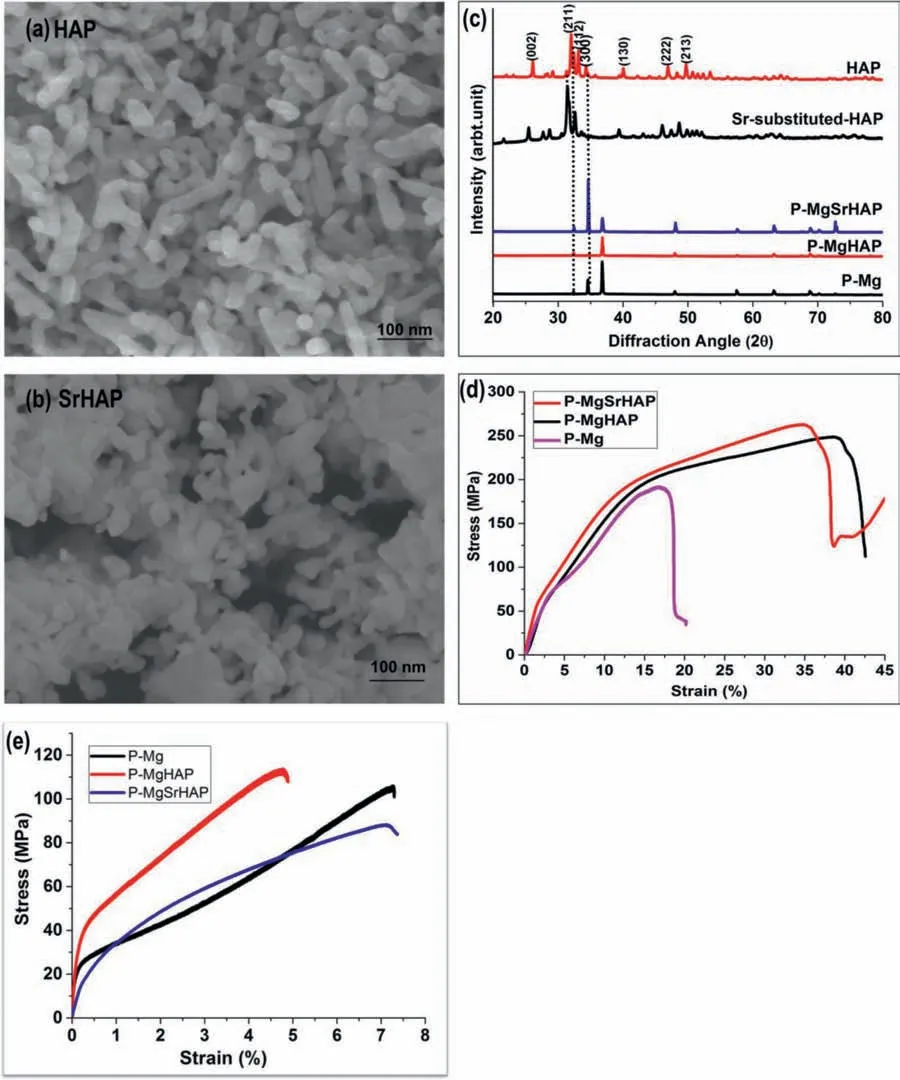
Fig.2.SEM micrographs of indigenously synthesized (a) nano hydroxyapatite (HAP) and (b) strontium substituted nano hydroxyapatite (SrHAP);(c) XRD diffractograms showing characteristic peaks for HAP,SrHAP,P-Mg,P-MgHAP and P-MgSrHAP,(d) &(e) compression and tensile stress-strain curve of P-Mg,P-MgHAP,and P-MgSrHAP,respectively.

Fig.3.Casting structure with orientation maps and grain size distribution by electron back-scattered diffraction (EBSD) of squeeze casted (a1,a2,a3) P-Mg,(b1,b2,b3) P-MgHAP,and (c1,c2,c3) P-MgSrHAP (For interpretation of the references to color in this figure the reader is referred to the web version of this article.).
3.3.Mechanical properties
The mechanical properties of magnesium and its alloys were determined in terms of stressvsstrain curve,the ultimate compressive strength (UCS),and elongation percentage(Fig.2d).It can be clearly seen that the addition of HAP and SrHAP to P-Mg leads to higher UCS,and elongation rate.The ultimate compressive modulus (UCM) significantl increased from 189 to 248 MPa and 262 MPa post alloying of P-Mg with HAP and SrHAP.The elongation percentage of P-MgHAP and P-MgSrHAP increased to almost double as compared to P-Mg.These results indicate that reinforcement of HAP and SrHAP into P-Mg positively improves the compressive behavior.The increased fracture strain indicates ductility and formability enhancement at room temperature.Fig.2e.depicts the tensile stress-strain curves of P-Mg,PMgHAP,and P-MgSrHAP.The tensile strength of P-Mg significantl enhanced post reinforcement with HAP as compared SrHAP [35].The tensile strength of P-Mg was found to decrease along with elongation post addition with SrHAP indicating increase in brittleness behavior.However,the compressive strength was found to increase post addition of HAP and Sr into P-Mg which again indicates brittleness.Hence,PMg turns to little brittle material post addition with HAP and SrHAP and these results are consistent with previous studies[32].
3.4. In vitro degradation and biocompatibility test
3.4.1.Degradation in simulated body flui and hydrogen evolution rate
Thein vitrodegradation rate of P-Mg,P-MgHAP,and PMgSrHAP was measured by mass loss measurements in simulated body flui at body temperature and pH 7.4,for different time points (Fig.4d).It was observed that the degradation in SBF for all the samples is less at initial time frames and started increasing after 2 weeks.A similar trend was observed for hydrogen evolution study (Fig.4e) wherein it was found that the rate of hydrogen gas evolution is less initially and increases after second week of incubation in SBF.
3.4.2.Scanning electron microscopy,energy dispersive X-ray spectroscopy,and grazing incidence X-ray diffraction analysis of degradation/corrosion layer
Surfaces of all samples immersed in SBF for 2 weeks were observed under FE-SEM coupled with EDS.It was observed that the surface got covered with mesh/honeycomb like structure (Fig.4a–c).EDS analysis showed high value of oxygen denoting magnesium hydroxide formation which was confirme by GIXRD data which presents peaks corresponding to magnesium hydroxide explicitly(Fig.4f).
3.5.Biocompatibility test
We observed that the effect of extracts on the cell proliferation and viability was dose dependent.At highest concentration (100%),all the extracts demonstrated cell death with time while as with decreasing concentration,we observed increase in the cell viability after 72 h (Fig.5a–c).Moreover,the pH of the extracts was also recorded and it was found that the pH of P-Mg,P-MgHAP and P-MgSrHAP is 10.0,8.5 and 8.5,respectively (Fig.5d).

Fig.5.Cell proliferation at 24 and 72 h by indirect cell culture using MTT assay for extracts derived from (a) P-Mg,(b) P-MgHAP,and (c) P-MgSrHAP extracts.(d) shows the pH of the different extracts derived from P-Mg,P-MgHAP and P-MgSrHAP.
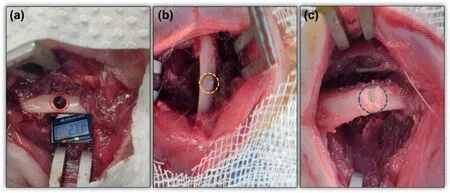
Fig.6.Representative images for (a) defect creation in rat femur shaft (diameter 2.7 mm×2 mm height) (red circle);(b) implantation of metal pin alone in the defect (yellow circle) and (c) implantation of metal pin after the defect was impacted with NC+ZA (blue circle) (For interpretation of the references to color in this figur legend,the reader is referred to the web version of this article.).
3.6.Blood cell counting
Systemic inflammator response post implantation was assessedviaTLC and DLC,and healthy animals were taken as control.At day 5 itself the blood counts were normal for all the groups indicating slow degradation and hence no critical systemic inflammator response observed for all the samples(Fig.S1).
3.7.Radiography post 4 weeks of implantation
The X-ray radiographs at 4 weeks indicated the presence of implants as intact,solid pins with little/slow degradation.In group 5 the bone was fractured at the defects site and also in the same group a gas bubble was observed in the soft tissues showing faster degradation (Fig.S2).
3.8.Micro CT analysis

Fig.7.(a) Representative micro-CT 2D images in different planes showing bone formation around the implant and implant integration in different groups;(b)representative 3D rendered images acquired from micro-CT scanning;(c) micro-CT based morphometry showing highest amount of bone mineralization (BV mm3) around the implant/defect in the ROI selected and (d) cylindrical ROI selected around the implant of size 300 μm diameter×2 mm height (brown ring) (For interpretation of the references to color in this figur legend,the reader is referred to the web version of this article.).
Micro CT based bone mineralization depicted enhanced bone formation around the implants with higher amount of mineralization seen in P-MgSrHAP (Fig.7c).The 2D and 3D rendered images acquired from micro-CT depicted presence of intact implant showing proper osteointegration with the host bone and enhanced trabecular bone deposition around the implants (Fig.7a and b).The morphometry-based bone mineralization (BV mm3) did not show any significan difference in the bone mineralization across the groups;however,a trend was observed where P-MgSrHAP showed higher amount of bone mineralization.The animals (in percentage)which showed fracture in each group at the end of 8 weeks is given in Table 4.
Thein vivodegradation (mm/year) was calculated from the images acquired from micro-CT based on the difference in volume of pre-implanted and post implanted pins(Fig.8d).Among all groups,P-Mg showed significantl higher rate ofin vivodegradation compared P-MgSrHAP.A very little dimension/structural change was observed in case of P-MgSrHAP as compared to P-Mg and P-MgHAP(Fig.8a–c).The material resorption here represents the resorption of nanocement and not the P-Mg.There seems to be the direct correlation between slow resorption of nanocement and fast degradation of P-Mg as the degradation products release in bulk amounts including magnesium ion completely disbalances the bone remodeling process eventually affecting the resorption of nanocement by osteoclasts.Moreover,the imbalance in bone remodeling due to burst release of magnesium ions also reduces the osteoblastic activities and thus decreases the overall bone formation thereby affecting the implant osteointegration.

Fig.8.Representative 3D model generated from micro-CT images of implant pins pre and post implantation for 8 weeks (a) P-Mg;(b) P-MgHAP and (c)P-MgSrHAP.(d) In vivo degradation rate (mm/year) of P-Mg,P-MgHAP and P-MgSrHAP calculated from images acquired from micro-CT scanning.
3.9.Field emission-scanning electron microscopy (FE-SEM)and grazing incidence X-ray diffraction (GIXRD) analysis of excised implants
All samples showed a white layer deposition containing Ca,O,P,and comparably small amount of magnesium as evident by EDS analysis (Fig.9a–c).The bone mineralization was confirme by Ca/P ratio which was matching with stochiometric ratio of natural bone (Ca/P=1.66) (Fig.9d–f).The characteristic peaks of HAP as observed by GI-XRD analysis of surfaces were detected on all implanted surfaces(Fig.9g).However,for metal implant with no cement incorporation,a dominant magnesium peak can be seen which denotes thin layer ofin vivoHAP formation on metal surface post 8 weeks of implantation.Comparably strong peaks of HAP were observed on surfaces implanted with the incorporation of NC+ZA.
3.10.Histology
Qualitative histological analysis of decalcifie samples after removing of the implanted pins was performed to evaluate the bone formation around the implant.H&E staining(Fig.10) demonstrated that in empty and metal alone groups there was no bone formation around and inside the defect site.However,in all other groups wherein the implantation of metallic pins was done along with NC+ZA,the new bone formation around the implant was higher with residual nanohydroxyapatite clearly visible.The new bone formation around the implants shows proper integration of pins with the host bone.The mature bone formation and collagen deposition around the implants was evaluated by masson’s trichrome staining (Fig.11).Collagen deposition (blue color) was observed in all the groups where NC+ZA was impacted in the defect site before implanting the pins thus showing neo-bone formation and proper integration with the host bone.
4.Discussion
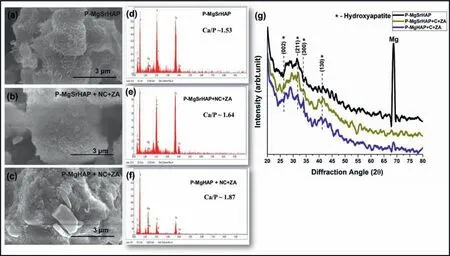
Fig.9.FE-SEM micrographs of excised (a) P-MgSrHAP;(b) P-MgSrHAP +NC +ZA and (c) P-MgHAP +NC+ZA implant pins.Elemental analysis by EDS of surfaces implant pins excised 8 weeks post implantation as (d) P-MgSrHAP;(e) P-MgSrHAP +NC +ZA and (f) P-MgHAP +NC+ZA with insets showing stochiometric Ca/P ratios.(g) GIXRD spectroscopy of implant pins harvested 8 weeks post implantation from the defects.
The degradation process of magnesium and its alloys are highly dependent on various factors including fabrication process,impurities,and alloying elements.It is well known that the corrosion rate of magnesium relay majorly on its purity because impurities such as iron (Fe),nickel (Ni),cobalt (Co),and copper (Co) severely deteriorates the corrosion resistance of magnesium and its alloys [34,36].Among all,Fe is majorly responsible for micro galvanic corrosion by forming secondary phase withα-Mg phase.It has also been [37,38] reported that the tolerance limit of Fe to be 170 ppm theoretically through the Mg-Fe phase diagram.It has been shown experimentally that the presence of Fe content from 150 ppm to 10 ppm in magnesium and concluded that the corrosion resistance increases with a decrease in Fe content [39].In the present study,magnesium was purifie using the repeated casting method by lowering the temperature and melt selfpurificatio method.In this method,the high-density particles such as Fe particles settle down in the crucible at 650 °C and lead to melt self-purification as the solid solubility of Fe in magnesium melt decreases at a temperature of 650 °C and thereby gets settled down easily.Moreover,by repeated casting method the chances/probability of settling down of Fe particles inside crucible increases and hence leads to more purificatio which has been observed in the present study Fig.1c.Therefore,the repeated casting method could be a simple and proper route for melt self-purificatio of pristine magnesium without adding any purificatio flu es or heavy metal elements [40].
The second step considered in this study was alloying of purifie magnesium with bone-like apatitei.e.,hydroxyapatite(HAP) and strontium substituted hydroxyapatite (SrHAP).A lower-level reinforcement has been done in this study which is 0.6666 wt.% of each,in purifie magnesium.Hydroxyapatite intrinsically shows low solubility in the human body environment [41].Additionally,HAP and SrHAP inclusion into P-Mg have not resulted in secondary phase at lower concentrations which was confirme from XRD phase analysis and hence controlledin vitrodegradation rate with adequate biocompatibility was observed.The mechanical properties were also enhanced significantl post addition of HAP and SrHAP into P-Mg.These improved mechanical properties are possibly due to the high heat capacity and stability of hydroxyapatite at higher temperaturesi.e.,above 750 °C.The fl w of compressive stress curves of all the samples presented a characteristic concave shape confirmin deformation twins’formation which was also observed in EBSD results.The grain refinemen of P-Mg was observed post reinforcement of HAP and SrHAP which may have contributed in the high mechanical strength[42].The probable reason for this is nano particles of HAP and SrHAP hinders the grain boundaries to grow and results in grain refinemen [43].

Fig.10.H&E staining of all groups post 8 weeks of implantation showing enhanced bone formation in groups where pins were implanted along with NC+ZA.The trabecular type bone plates are clearly visible in the periphery of the implant pin showing osteointegration (dotted red box).The hydroxyapatite residues are also visible (grey color) in group P-Mg+NC+ZA indicating incomplete material resorption and hence low bone formation as compared to groups P-MgHAP+NC+ZA and P-MgSrHAP+NC+ZA (For interpretation of the references to color in this figur legend,the reader is referred to the web version of this article.).

Fig.11.Masson’s trichrome staining showing maximum collagen deposition in the periphery(dotted red box)of implant pins in groups P-MgHAP+NC+ZA and P-MgSrHAP+NC+ZA as compared to P-MgSrHAP group indicating enhanced bone formation and osteointegration.Hydroxyapatite residues are also visible in P-Mg+NC+ZA group showing lesser material resorption and hence low bone formation as compared to P-MgHAP+NC+ZA and PMgSrHAP+NC+ZA (For interpretation of the references to color in this figur legend,the reader is referred to the web version of this article.).
The degradation process of magnesium and its based alloys involves electrochemical steps in aqueous solution such as;Mg+2H2O=Mg(OH)2+H2,which results in magnesium hydroxide formation along with hydrogen gas evolution.The faster degradation rate of magnesium and its alloys leads to more hydrogen bubbles release which forms a gas pocket around the defect site and stops the communication between the defect site and adjacent tissue which hampers the healing process [44].To overcome this,a slow and controlled degradation rate is required which has a dual role,one is to support the defect site mechanically,and the second is to help in healing.Magnesium hydroxide is highly soluble in a chlorine-containing environment with a limit above 30 mmol/L.The human physiological environment contains chlorine 150 mmol/L,which is beyond the limit,magnesium hydroxide gets converted into magnesium chloride which is highly soluble in water [45].Additionally,release of Mg2+ions cause alkalization of surrounding which further slowdown the degradation process [46].Besides this physiological environment also contains,phosphorous and calcium that triggers the surface to form calcium phosphate-based precipitation which inhibits further degradation of magnesium and its based alloys.Bothin vitroandin vivostudies confirme the presence of magnesium hydroxide,calcium,and phosphate on the sample surfaces which might have prevented the further degradation of all the samples and showed lower or controlled degradation rate.Additionally,P-MgSrHAP have presented low degradation rate as compared to P-Mg and PMgHAP.This could be due to substitution of strontium into HAP which promotes biological interactions and simultaneously reduces its degradation rate [47].Further,thein vivodegradation pattern was uniformly localized for all implanted materials as described by previous study [48],can be seen in Fig.8a–c.It has already been shown in previous study[49] thatin vitrocorrosion rate of magnesium and its alloys cannot be used to predict thein vivocorrosion rate.Various factors such asin vivolocal conditions,enzymatic activities,blood fl w rate,pH,site of implantation,protein attachment on implant surface,extensively effects the degradation rate of biological implants including magnesium implants.Hence,huge differencein vitroandin vivoconditions can be seen in the case of all the magnesium-based implants in the present study.
In thein vivostudy,we have used purifie magnesium(P-Mg) and its alloys (P-MgHAP and P-MgSrHAP) as hard tissue replacements in critical size bone defect,in which,PMgSrHAP is the only material implanted without cement incorporation.In nanocement and zoledronic acid (NC+ZA),NC is osteoconductive and ZA is an anticatabolic molecule that inhibits osteoclasts activity and thus promotes bone regeneration by reducing the amount of bone resorption[50].In group P-MgSrHAP,comparably less bone formation was observed than other implant pins implanted along with NC+ZA.In order to ensure that implant is not resorbed or loosened(aseptic loosening) before complete healing,we are hypothesizing that a bone fille like nanocement with ZA could be used to enhance bone formation around the implant and promote complete osteointegration.Moreover,the use of bone fille with antimicrobial agents will also prevent the failure of these implants due to septic loosening.It is well known that magnesium release helps in bone regeneration process biologically [51–54],and induced the bone regeneration around implants can be seen in the Figs.10 and 11,in H&E and masson’s trichrome staining.Early stages of bone regeneration include collagen production and osteoid formation[55],therefore,identifying collagen deposition around the implanted material implies bone regeneration initiation,which is visible in all groups Fig.11.This also denotes a slow degradation rate of all materials as a fast degradation rate does not promote collagen formation.No scar tissues observed around the defect site post implantation confirmin biosafety.
To confir ductility few prototypes such as nail,screw,plate,and a dental screw was fabricated/machined using PMgSrHAP (Fig.S3).
5.Conclusion
In conclusion,we have investigated the simple repeated casting approach for the purificatio of magnesium and achieved 99.9% purity.The purifie magnesium was successfully reinforced with indigenously synthesized hydroxyapatite and strontium substituted hydroxyapatite at low concentrations that resulted in grain refinemen and improved corrosion resistance,biocompatibility,and adequate mechanical properties.In vitroandin vivodata well collaborated with each other and presented uniform and controlled degradation rates.Histology tests confirme new bone formation along the side of implanted pins and no adverse effects were observed validatingin vivocompatibility.Hence,all the implanted materials showed a controlled degradation rate advising their applicability as temporary orthopedic implant fixtures
Declaration of Competing InterestAn Indian patent on “Process for the fabrication of biodegradable magnesium-based alloys for orthopaedic applications and thereof” has been file in the Indian Patent Offic and accorded with application number ‘202111017399’.
CRediT authorship contribution statementShazia Shaikh:Visualization,Methodology,Validation,Formal analysis,Software,Writing–original draft.Irfan Qayoom:Methodology,Formal analysis,Software,Writing–review &editing.R.Sarvesha:Formal analysis,Writing– review &editing.Ashok Kumar:Visualization,Methodology,Formal analysis,Writing– review &editing.
Acknowledgments
The authors would like to acknowledge the funding received from Ministry of Human Resource Development(MHRD),India and Indian Council of Medical Research(ICMR),India projects(IMPRINT-6714;UAY/MHRD_IITK_006),MHRD,India project(SPARC/2018–2019/P612/S),Science and Engineering Research Board (SERB),India project(IPA/2020/000026),Department of Science and Technology(DST),Govt.of India project (DST/NM/NT-2018/48),Department of Biotechnology (DBT),Govt.of India project(DBT/IN/SWEDEN/08/AK/2017–18),and Ortho Regenics Private Limited (ORPL).SS would like to acknowledge Prof.Dr.Sudhanshu Shekhar Singh (Assistant Professor,Department of Materials Science and Engineering,IIT Kanpur) for supporting in EBSD study,and Mr.Vikas Tiwari,Ms.Mamta Kanujia,and Mr.Surendra Mishra for their constant assistance during study.AK would like to acknowledge Rajeeva and Sangeeta Lahri Chair,IIT Kanpur,India.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2022.04.008.
 Journal of Magnesium and Alloys2023年1期
Journal of Magnesium and Alloys2023年1期
- Journal of Magnesium and Alloys的其它文章
- Formation of carbon and oxygen rich surface layer on high purity magnesium by atmospheric carbon dioxide plasma
- Local hardening and asymmetric twin growth by twin-twin interactions in a Mg alloy
- Brittle and ductile characteristics of intermetallic compounds in magnesium alloys: A large-scale screening guided by machine learning
- Experimental study on uniaxial ratchetting-fatigue interaction of extruded AZ31 magnesium alloy with different plastic deformation mechanisms
- Assessment of Mg(OH)2/TiO2 coating in the Mg-Ca-Zn alloy for improved corrosion resistance and antibacterial performance
- Simultaneous refinemen of α-Mg grains and β-Mg17Al12 in Mg-Al based alloys via heterogeneous nucleation on Al8Mn4Sm
