Simultaneous refinemen of α-Mg grains and β-Mg17Al12 in Mg-Al based alloys via heterogeneous nucleation on Al8Mn4Sm
Jun-Chen Chen ,Mei-Xuan Li ,Zhi-Yang Yu ,Zhao-Yuan Meng ,Cheng Wang ,Zhi-Zheng Yang ,Hui-Yuan Wanga,,?
a State Key Laboratory of Automotive Simulation and Control,Jilin University,Changchun 130012,PR China
b Key Laboratory of Automobile Materials of Ministry of Education &School of Materials Science and Engineering,Jilin University,Nanling Campus,No.5988 Renmin Street,Changchun 130025,PR China
cInternational Center of Future Science,Jilin University,Changchun 130012,PR China
Abstract Due to the significan differences in the formation temperature and crystal structure between the primary α-Mg and eutectic β-Mg17Al12,it is a great challenge to achieve simultaneous refinemen of the primary and eutectic phases in Mg-Al based alloys via heterogeneous nucleation.Surprisingly,we found that the α-Mg and β-Mg17Al12 in the AZ80 alloy can be simultaneously refine after 0.2 wt.% Sm addition,with the grain size decreasing from ~217±15 μm to ~170±10 μm and the β-Mg17Al12 morphology changing from a typical continuous network to a nod-like or spherical structure.The simultaneous refinemen mechanism is investigated through solidificatio simulation,transmission electron microscopy (TEM),and differential thermal analysis (DTA).In the AZ80-0.2Sm alloy,many Al8Mn4Sm particles can be observed near the center of the α-Mg grains or inside the β-Mg17Al12.Crystallographic calculations further reveal that the Al8Mn4Sm has good crystallographic matching with both the α-Mg and β-Mg17Al12,so it possesses the potency to serve as heterogeneous nucleation sites for both phases.The promoted heterogeneous nucleation on the Al8Mn4Sm decreases the undercooling required by the nucleation of the primary and eutectic phases,which enhances the heterogeneous nucleation rate,thus causing the simultaneous refinemen of the α-Mg and β-Mg17Al12.The orientation relationships between the Al8Mn4Sm and Mg/Mg17Al12 are identified which are and ,respectively.Furthermore,the refinemen of the β-Mg17Al12 accelerates its dissolution during the solution treatment,which is beneficia for cost saving in industrial applications.Other Al8Mn4RE compounds such as Al8Mn4Y might have the same positive effect on the simultaneous refinemen due to the similar physicochemical properties of rare earth elements.This work not only proves the possibility of simultaneously refinin the primary and eutectic phases in Mg-Al based alloys via heterogeneous nucleation,but also provides new insights into the development of refiner for cast Mg alloys.
Keywords: Magnesium alloys;Microstructure refinement Primary α-Mg;Eutectic β-Mg17Al12;Rare earth;Heterogeneous nucleation.
1.Introduction
Cast magnesium alloys possess significan economic advantages for the mass production of components in the automobile and 3C industries due to their shorter processing cycle and assembly costs [1–6].In particular,Mg-Al based alloys take a dominating position of consumption because they are readily cast and exhibit satisfactory strength [7–10].However,coarseα-Mg andβ-Mg17Al12are usually present in the as-cast Mg-Al series alloys and are extremely detrimental to the mechanical properties,which severely restricts the development and application of the alloys [11–13].Therefore,it is extremely important to simultaneously refin the primary dendriteα-Mg grains and eutecticβ-Mg17Al12phases.
Over the last two decades,lots of methods have been employed to refinα-Mg grains,such as rapid solidifica tion,ultrasonic stirring,superheating,and the addition of refiner [11,14–21].Among these methods,adding refiner to molten metals is a convenient and effective approach to refinin grains via the promotion of heterogeneous nucleation sites.Generally,the good crystallographic matching between the grain refiner and the metal matrix is a key factor for the refiner to be active nucleation sites,which decreases the critical undercooling of nucleation and enhances the heterogeneous nucleation rate.It is commonly acknowledged that Zr is a potent grain refine for Mg alloys,which is due to the similar crystal structure between Zr and Mg [22,23].However,the Zr cannot be used in the Mg alloys containing Al,Mn,Si,etc.,for the reason that Zr would form stable compounds with these alloy elements [22].In most of the Mg-Al based alloys,carbon-based particles are regarded as the main heterogeneous nuclei for theα-Mg grains during solidifica tion [22,24–29].For example,the Al4C3has long been selected as an effective nucleating substrate for theα-Mg grains[22,28,30,31].Unfortunately,most of the carbon-based refin ers can only refin theα-Mg,but have no effect on theβ-Mg17Al12refinement Moreover,refiner containing rare earth(RE) elements are also common choices for grain refinement Previous research [32,33] indicated that Al2RE compounds(such as Al2Gd,Al2Y,and Al2Sm) could act as effective refiner for cast Mg alloys due to their low crystallographic misfi with the Mg matrix [34,35].For example,the 2 wt.%Gd addition to the AZ31 alloy leads to the refineα-Mg grains due to the formation of Al2Gd particles [36].Li et al.[37] found that thein-situformed Al2Sm strongly refine the grains of the Mg-6Al-0.6Zn alloy.However,the satisfactory grain refinemen usually requires high content (>1 wt.%) RE addition to form sufficien nucleation sites,which raises the cost of the Mg-Al based alloys,thus restricting their industrial applications.
For AZ series alloys,when the Al content exceeds 6 wt.%,a large number of coarseβ-Mg17Al12phases could be formed[38,39].The crack is prone to propagate if the eutectic phases exhibit a continuous network or a coarse divorced morphology[12,13].Therefore,it is crucial to refin the eutectic phases and change theβ-Mg17Al12morphology.The common refin ing methods include rapid solidification mechanical deformation,alloying treatment,melt stirring method,and so on[19,40–43].Among these methods,alloying treatment is much facile and efficient Recently,Korgiopoulos and Pekguleryuz[39] summarized the studies [44–53] carried out in the last decade in terms of the modificatio of Mg17Al12by alloying.It seems that RE addition is still the favorable choice in regard to theβ-Mg17Al12refinemen [54].For instance,a morphology change of theβ-Mg17Al12from a coarse mesh structure to a fin short-rod morphology occurs in the AZ80 and AZ91 alloys after the addition of more than 1 wt.% Sm [55,56].However,to achieve a good refinin effect,it is necessary to add high content RE to consume the Al element in the liquid,which greatly increases the preparation cost.In addition,RE addition can also refin theβ-Mg17Al12via the promotion of heterogeneous nucleation.Unfortunately,the relevant studies are scarce,because very few compounds exhibit good crystallographic matching with theβ-Mg17Al12.One of the few recent breakthroughs is that the formation of Al4MgY by trace Y addition (0.2–1.5 ppm) could refin theβ-Mg17Al12in the Mg-6 wt.% Al alloy via a co-precipitation/nucleation effect [39].However,the investigation [39] only reveals the refinemen of theβ-Mg17Al12,but does not report the refine ment of theα-Mg.
Up till now,few reports are related to the simultaneous refinemen of the primaryα-Mg and eutecticβ-Mg17Al12via heterogeneous nucleation in Mg-Al based alloys because it is extremely demanding to discover a refine suitable for both phases.The refine should concurrently meet two fundamental requirements.Firstly,from the perspective of solidifica tion thermodynamics,the refine should have a high melting temperature and be formed prior to the primary and eutectic phases.Secondly,from the perspective of crystallography,the refine should possess favorable crystallographic matching with both theα-Mg andβ-Mg17Al12.Based on the forementioned requirements,the Al8Mn5commonly present in the Mg-Al based alloys [57] deserves more attention due to its high melting temperature.However,the Al8Mn5has no notable effect on the grain refinemen of the AZ series alloys[58,59].Meanwhile,the orientation relationship between the Al8Mn5and Mg17Al12is still unclear.
More recently,Pei et al.[60] reported that the Al8Mn4Ca formed in the AZ31 alloy on account of Ca segregation has good crystallographic matching with theα-Mg matrix,indicating that the Al8Mn4X (X,i.e.,alloy element) might act as the nucleation substrate.Therefore,we proposed a strategy to achieve the simultaneous refinemen of the primaryα-Mg and eutecticβ-Mg17Al12via promoting heterogeneous nucleation on the Al8Mn4X in Mg-Al based alloys.The alloy element should possess a large electronegativity difference with Al to ensure that the Al8Mn4X forms before theα-Mg andβ-Mg17Al12during solidification Hence,RE elements are very suitable.Among them,Sm as a light RE element has a relatively low cost.Surprisingly,the simultaneous refinemen is realized by adding trace Sm (0.2 wt.%)to the AZ80 alloy.The refinin mechanism has been illuminated by utilizing a set of techniques,including solidificatio simulation,crystallographic calculations,microstructure characterization,and thermodynamic analysis.This work might tackle the bottleneck problem of simultaneously refinin theα-Mg andβ-Mg17Al12and broaden the industrial applications of the Mg-Al based alloys.
2.Experimental procedure
The compositions of the studied alloys were determined by an optical spectrum analyzer (ARL 4460,Switzerland).The nominal and measured compositions are listed in Table 1.Note that Fe is the unavoidable impurity,and the AZ80-0.2Y alloy is used to verify whether other Al8Mn4RE compounds,such as Al8Mn4Y,could serve as the heterogeneous nucleation substrate for the primary and eutectic phases.The investi-gated alloys were fabricated by melting the mixture of pure Mg (99.85 wt.%),pure Al (99.90 wt.%),Mg-3.35Mn (wt.%),Mg-35Sm (wt.%),and Mg-23Y (wt.%) master alloys in an electric resistance furnace under the atmosphere of 99.5% CO2and 0.5% SF6at ~700 °C.After completely melted,the molten liquid was manually stirred for about 3 min to produce a homogeneous mixture and the impurities were removed effectively.After being purifie and homogenized for about 10 min at 680°C,the melt was poured into a steel mold preheated at 200°C through a bottom gating system.The outside dimension of the steel mold is 210×120×90 mm3and the inside dimension is 130×40×20 mm3.The details of the mold can be obtained from our previous work [61].The samples for observation with the size of 10×10×5 mm3were cut from the bottom of the ingot,where the mean solidificatio cooling rate was measured as~30 K/s.
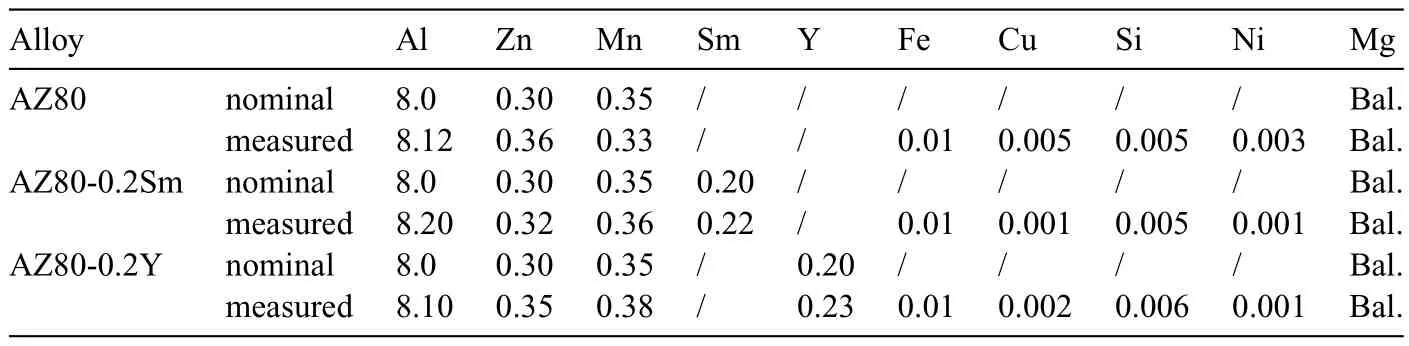
Table 1 The nominal and measured compositions of the investigated alloys (wt.%).

Table 2 The possible ORs and corresponding interatomic/interplanar misfit between Mg (α) and Al8Mn4Sm (γ).
The specimens were carefully ground,manually polished,and etched for about 10 s in an acetic picric solution (1.5 g picric acid,2 mL acetic acid,1 mL distilled water,and 20 mL ethyl alcohol).Afterwards,the metallographic samples were examined by using an optical microscope (OM,Carl Zeiss-Axio Imager A2m,Germany) and a scanning electron microscope (SEM,Sigma 500) equipped with an energy dispersive spectrometer (EDS) analyzer (INCA-X-Max,UK).Transmission electron microscopy (TEM) analysis was carried out to analyze the compositions and crystal structures of the phases using a JEM-2100 (JEOL,Japan) equipped with an EDS analyzer operated at the accelerating voltage of 200 kV.Thin foils for TEM observations were manually ground to about 80 μm in thickness and punched into disks with a diameter of 3 mm.Afterwards,ion milling was conducted on the disks at an accelerating voltage of 4 kV for ~1.5 h.Differential thermal analysis (DTA,SDT-Q600,TA Instruments Inc.USA) was conducted at a constant rate of 10 °C/min during the cooling process from 700 to 200 °C.Standard tensile samples were taken from the bottom of the ingot,and the tensile test was performed using an AGS-X-100kN electric universal testing machine (SHIMADZU,Suzhou,China).The results were obtained from at least fi e tensile samples under a strain rate of 1.0×10?3s?1at ambient temperature.The sizes of theα-Mg grains and Al8Mn4Sm compounds in the studied alloys were measured by the Nano Measure 1.2 software using the linear intercept method and derived from at least fiftee optical micrographs.The area fraction of theβ-Mg17Al12was determined by the ImageJ software and twenty images were used to ensure the accuracy.
3.Results
3.1.Solidificatio simulations
To predict the type and the formation sequence of the phases in the alloy with trace Sm addition,the equilibrium phase diagram of the AZ80-xSm alloy was calculated based on the Pandat software package.
When 0.2 wt.% Sm is added,according to the equilibrium solidificatio path marked by the red line in Fig.1a,the Al8Mn4Sm is formed prior to the primaryα-Mg and eutecticβ-Mg17Al12during the solidificatio process,which indicates that the Al8Mn4Sm is possible to act as heterogeneous nucleation sites for theα-Mg andβ-Mg17Al12.The existence of Al8Mn5,Al11Mn4,Al4Mn,and even Al3Sm may dilute the mass fraction of the Al8Mn4Sm.Therefore,non–equilibrium(Scheil) calculations were performed to predict the mass fraction of the Al8Mn4Sm.As shown in Fig.1b,c,the mass percentage of the Al8Mn4Sm and Al8Mn5is 0.779% and 0.032%,respectively,and the fraction of Al11Mn4,Al4Mn,and Al3Sm can be almost neglected.That is,under the Scheil solidifi cation condition,~96% of the intermetallic compounds containing Al and Mn elements are Al8Mn4Sm in the AZ80-0.2Sm alloy.Previous studies indicated that ~95% of the Al-Mn intermetallic compounds in the AZ80/AZ91 alloy are Al8Mn5after Scheil solidificatio [62–65].Therefore,it can be deduced that Al8Mn5is almost completely converted into Al8Mn4Sm after 0.2 wt.% Sm addition.
3.2.Crystallographic calculations
It is well known that the potency of the nucleation catalysts can be predicted based on the edge-to-edge matching model[66,67].According to the model,the densely packed matching directions and planes should be selected.Then the interatomic misfi along the directions and the d-value mismatch between the matching planes are calculated.If the interatomic spacing misfi along the matching directions are less than 10%,and the d-value mismatch between the matching planes is equal to or less than 6%,it is considered that an orientation relationship (OR) possibly exists between the two phases.

Fig.1.(a) Calculated Mg-8.0Al-0.3Zn-0.35Mn-xSm (AZ80-xSm) equilibrium phase diagram,x changes from 0 to 0.4 wt.%.(b) Phase fraction as a function of temperature for the AZ80-0.2Sm alloy under the Scheil solidifi cation condition.(c) The partial enlargement of (b).


Table 3 The possible ORs and corresponding interatomic/interplanar misfit between Mg17Al12 (β) and Al8Mn4Sm (γ).
3.3.As-cast microstructures
Fig.2a–f show the as-cast microstructures of the AZ80 and AZ80-0.2Sm alloys.As shown in the optical micrographs(Fig.2a,d),the AZ80 and AZ80-0.2Sm alloys exhibit a developed dendritic structure.Theα-Mg grain size drops from~217±15 μm to ~170±10 μm after 0.2 wt.% Sm addition,exhibiting apparentα-Mg grain refinement
Fig.2b,c,e,and f are the SEM images in secondary electron (SE) mode,showing the morphology and distribution of the intermetallic phases in the two alloys.According to the EDS results (Table 4) obtained from Fig.2c,the AZ80 alloy contains theα-Mg (phase 1),β-Mg17Al12(phase 2),and Al8Mn5(phase 3).Theβ-Mg17Al12exhibits a typical continuous network structure distributed along the grain boundaries,where several Al-Mn intermetallic compounds also exist.More than thirty Al-Mn compounds were chosen and identifie as Al8Mn5by EDS.Meanwhile,almost no Al11Mn4or Al4Mn is detected in the microstructure,which agrees well with the calculation results (Fig.1b,c) and the relevant studies [62].Note that trace Fe is detected in the Al8Mn5,which confirm the contribution of Mn to removing Fe impurities.As the atom radii of Mn and Fe are very close,the substitution of Mn by Fe does not cause significan variation in the lattice parameters [25].The Zn solutes are mainly enriched in theα-Mg andβ-Mg17Al12,consistent with the results in the previous reports [35,70].
For the AZ80-0.2Sm alloy,theβ-Mg17Al12exhibits the discontinuous nod-like or spherical morphology (phase 5).The added Sm mainly appears in the compounds (phase 6 and phase 7) identifie as Al8Mn4Sm according to the EDS(Table 4) and the following TEM results (Fig.4),and almost no Sm is detected in theα-Mg matrix.While a small quantity of Al8Mn5(phase 8) phases still exist,nearly 93% of the intermetallic compounds containing Al and Mn elements are Al8Mn4Sm in the AZ80-0.2Sm alloy (more than thirty Al-Mn/Al-Mn-Sm phases are involved in the statistics),which agrees very well with the simulation prediction in Section 3.1.In addition,the area fraction of theβ-Mg17Al12in the AZ80 and AZ80-0.2Sm alloys is measured as 5.60±0.05% and 5.41±0.04%,respectively,indicating no significan difference.Therefore,the 0.2 wt.% Sm addition promotes the simultaneous refinemen of the primaryα-Mg and eutecticβ-Mg17Al12.

Fig.2.Optical micrographs showing the α-Mg grains in the (a) AZ80 alloy and (d) AZ80-0.2Sm alloy.SEM images in secondary electron (SE) mode showing the intermetallic phases in the (b,c) AZ80 alloy and (e and f) the AZ80-0.2Sm alloy.
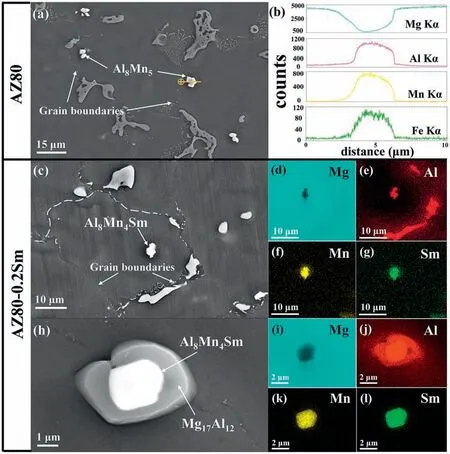
Fig.3.(a) SEM image in secondary electron (SE) mode of the AZ80 alloy and (b) the elemental line scanning spectra of the Al8Mn5 particle.(c) SEM image of the AZ80-0.2Sm alloy and (d–g) corresponding EDS elemental mappings for the Al8Mn4Sm in the α-Mg grain.(h) SEM image of the AZ80-0.2Sm alloy and (i–l) corresponding EDS elemental mappings for the Al8Mn4Sm inside the β-Mg17Al12.

Table 4 EDS results (at.%) for locations 1–8 in Fig.2c,f.

Fig.4.(a and b) BF STEM images,(c–j) corresponding EDS elemental mappings,and (k–n) selected area electron diffraction (SAED) patterns of the AZ80-0.2Sm alloy.
Fig.3 further illustrates the typical distribution of the Al8Mn5and Al8Mn4Sm compounds in the AZ80 and AZ80-0.2Sm alloys.As for the AZ80 alloy,the Al8Mn5particles are mainly located around the grain boundaries (Fig.3a,b).In contrast,in the AZ80-0.2Sm alloy,the Al8Mn4Sm compounds are located either near the center of theα-Mg grain or inside theβ-Mg17Al12(Fig.3c–l).Fig.4a–n show the TEM images of the AZ80-0.2Sm alloy.The Bright Field Scanning TEM(BF-STEM)images(Fig.4a,b),corresponding EDS mappings (Fig.4c–j),and selected area electron diffraction (SAED) patterns (Fig.4k–n) further confir that the Al-Mn-Sm phase in theα-Mg matrix or insideβ-Mg17Al12is Al8Mn4Sm (I4/mmm,tetragonal structure,a=0.8826 nm andc=0.494 nm).
3.4.DTA analysis
DTA analysis is performed on the two alloys and the results are shown in Fig.5.The formation temperature of theα-Mg andβ-Mg17Al12in the AZ80 alloy is 592.5 °C and 421.3 °C,respectively (Fig.5a).Interestingly,the 0.2 wt.%Sm addition increases the formation temperature of theα-Mg andβ-Mg17Al12to 593.8 °C and 425.6 °C,respectively(Fig.5b).
3.5.Accelerated dissolution of β-Mg17Al12 in solution treatment
To examine the effect of the Sm addition on the microstructure evolution during solution treatment,the AZ80 and AZ80-0.2Sm alloys were subjected to solution treatment at 415°C for different time(1,2,or 3 h).For the AZ80 alloy,the typical continuous network structure of theβ-Mg17Al12hardly changes during the early stage of the solution treatment (Fig.6a,b).After solution treatment for 3 h,the area fraction ofβ-Mg17Al12decreases from the initial ~5.60%to ~2.14% (Table 5),and a number ofβ-Mg17Al12phases can still be observed (Fig.6c).For the AZ80-0.2Sm alloy,with the prolongation of the solution time,the area fraction of nod-likeβ-Mg17Al12decreases distinctly from ~5.41% to~0.68%.After solution treatment for 3 h,few Mg17Al12can be inspected (Fig.6f) and minor Al8Mn4Sm/Al8Mn5compounds remain due to their high melting temperature.Note that theβ-Mg17Al12area fraction (~2.03%) of the AZ80-0.2Sm alloy after solution treatment for 1 h is similar to that(~2.14%) of the AZ80 alloy after solution treatment for 3 h.This indicates that the 0.2 wt.% Sm addition accelerates the dissolution of theβ-Mg17Al12during the solution treatment,which significantl shortens the heat treatment process in industrial applications.

Table 5 The area fraction of β-Mg17Al12 (%) in the AZ80 and AZ80-0.2Sm alloys subjected to solution treatment at 415 °C for different time.
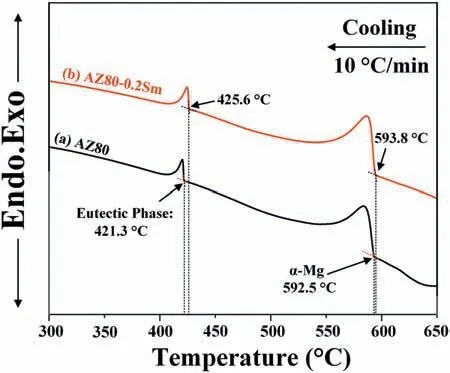
Fig.5.DTA profile of the (a) AZ80 and (b) AZ80-0.2Sm alloy.
The accelerated dissolution is mainly owing to theβ-Mg17Al12refinement The dissolution time of eutectic constituent phases has a strong relationship with the thickness or lamellar spacing of the phase in a power law [71],
whereτρrepresents the time needed for complete dissolution.αrepresents the coefficientmis the thickness or lamellar spacing of the eutectic phase.bis the exponent that is above 1.It implies that the fine eutectic phase (m) could result in less dissolution time (τρ).Consequently,the refineβ-Mg17Al12promotes its dissolution during the solution treatment.
3.5.Enhanced mechanical property
Fig.7 shows the typical stress-strain curves and Table 6 lists the tensile properties of the as-cast AZ80 and AZ80-0.2Sm alloys at ambient temperature.The ultimate tensile strength (UTS) and elongation for the AZ80 alloy are 202±2.5 MPa and 10.1±0.4%,respectively,while those for the AZ80-0.2Sm alloy are 225±3.2 MPa and 14.3±0.5%,respectively.Compared with the AZ80 alloy,the AZ80-0.2Sm alloy exhibits a remarkable improvement of tensile strength and ductility,which is mainly attributed to the simultaneous refinemen ofα-Mg grains andβ-Mg17Al12achieved by 0.2 wt.% Sm addition.Theβ-Mg17Al12refinemen is particularly beneficia for ductility because the crack propagates a shorter distance before stopping at the interface between the ductileα-Mg matrix and Mg17Al12.

Table 6 Tensile properties of the as-cast AZ80 and AZ80-0.2Sm alloys at ambient temperature.
4.Discussion
4.1.Mechanism of α-Mg grain refinemen
It is widely acknowledged that the two dominating factors affecting the grain refinemen are: (i) solutes that can rapidly generate sufficien constitutional supercooling(i.e.,solutes with a high-value growth restriction factor,Q) and (ii)heterogeneous nucleant particles with high nucleation potency[11,72–74].Correlatively,the grain size (dgs) can be define as [72]:
where the slopebis related to the potency of the nucleant particles and a steeper slope (larger b-value) corresponds to a lower potency;acorresponds to the number of particles that actually nucleate grains when theQvalue is infinite which is also known as the maximum number of activable nuclei.As for a binary alloy,Qcan be define as [73,75]:
wherekis the partition coefficien (Cs/Cl),mis the slope of the liquidus,andc0is the concentration of the solute in the alloy.
According to the parameters given in the literature [14,76],theQvalue of Sm forα-Mg is calculated to be about 0.25 K,indicating that the grain refinemen caused by the variation ofQvalue is very limited.Theavalue of the AZ80-0.2Sm alloy should be nearly the same as that of the AZ80 alloy,since the total mass fraction of Al8Mn4Sm and Al8Mn5intermetallic compounds in the AZ80-0.2Sm alloy is almost equal to that of Al8Mn5in the AZ80 alloy.It is observed that theα-Mg grain size of the AZ80-0.2Sm alloy is dramatically refine to ~170 ± 10 μm compared with that of the AZ80 alloy(~217±15 μm),which is mainly ascribed to the remarkable decrease inbvalue,indicating that the potency of the heterogeneous nucleant particles present in the AZ80-0.2Sm alloy has been greatly enhanced with the Sm addition.

Fig.6.SEM images in secondary electron (SE) mode for the (a-c) AZ80 and (d-f) AZ80-0.2Sm alloys subjected to solution treatment at 415 °C for different time (1,2,or 3 h).
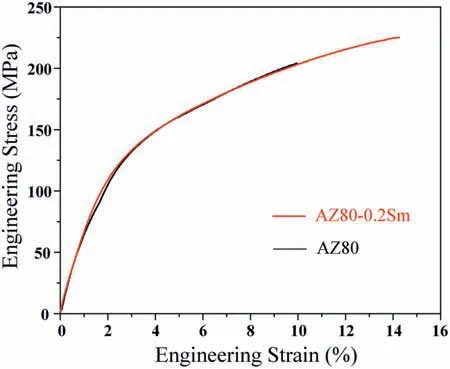
Fig.7.Typical stress-strain curves of the as-cast AZ80 and AZ80-0.2Sm alloys at ambient temperature.
It has been reported [34,35] that a small amount of Sm addition (less than 2 wt.%) could lead to the grain coarsening in the Mg-3Al and AZ31 alloys.The reason is that Sm combines with the Al-Fe-C-O particles to form Al-Fe-Sm-C-O particles which have a lower nucleation potency than the native Al-Fe-C-O nuclei in the Mg-Al alloys [34,35].Whereas,in the present work,a small amount of Sm addition does not lead to grain coarsening but significan grain refinement This result is because most of the Fe impurities are absorbed in the Al8Mn5/Al8Mn4Sm intermetallic compounds (Table 4),hence inhibiting the formation of Al-Fe-Sm-C-O particles.It is consistent with the result that no Al-Fe-C-O or Al-Fe-Sm-C-O particles were detected in the alloys (Fig.2).
Based on the calculation results in Table 2,the Al8Mn4Sm possesses enhanced potency to serve as an effective nucleation site for theα-Mg on account of its better crystallographic matching with Mg.Furthermore,in regard to the distribution of the intermetallic compounds,the Al8Mn5particles in the AZ80 alloy are mainly distributed around the grain boundaries(Fig.3a,b),indicating that they fail to act as the heterogeneous nucleation sites for theα-Mg grains.In contrast,in the AZ80-0.2Sm alloy,the Al8Mn4Sm is found near the center of theα-Mg grain (Fig.3c–g),showing its great potential to act as an effective nucleation substrate for the primaryα-Mg.
From the DTA results (Fig.5),the 0.2 wt.% Sm addition increases the formation temperature of theα-Mg by about 1.3°C.It means that theα-Mg in the AZ80-0.2Sm alloy could solidify at a lower undercooling,which also implies that theα-Mg nucleation needs less undercooling.The decreased undercooling required by theα-Mg nucleation is closely related to the formation of heterogeneous nuclei in the modifie melt.According to the classical nucleation theory [77],the critical undercooling of nucleation (ΔTn) is proportional to the interfacial energy between liquid and nucleation particles (γSL):
wheredpis the diameter of the spherical nucleation particle andSvis the fusion entropy of the nucleation per unit volume.Eq.(4) predicts that a lowerγSLresults in a decrease ofΔTnwhendpandSvbasically remain constant,so the decreased critical undercooling ofα-Mg nucleation is owing to the lowerγSLvalue between the Al8Mn4Sm andα-Mg.It is thermodynamically believed that the interfacial energy between an effective nucleation substrate and the primary phase is lower than that between the primary phase and the metal melt [77,78].Consequently,the Al8Mn4Sm particles in the melt could act as effective nucleation sites for theα-Mg nucleation during solidification which gives rise to an increasing number of nuclei,thus refinin theα-Mg grains in the AZ80-0.2Sm alloy.
4.2.Mechanism of β-Mg17Al12 refinemen
According to the previous studies[44–53],theβ-Mg17Al12refinemen is mainly by adding other elements to form Alcontaining intermetallic phases.In the AZ80-0.2Sm alloy,the area fraction of theβ-Mg17Al12shows almost no decrease.Furthermore,the Al8Mn4Sm compounds are found inside theβ-Mg17Al12(Figs.3 and 4).These results imply that theβ-Mg17Al12refinemen is probably attributed to the heterogeneous nucleation on the Al8Mn4Sm.
According to the DTA results,the formation of theβ-Mg17Al12starts at a higher temperature (425.6 °C) in the AZ80-0.2Sm alloy,compared with that (421.3 °C) in the AZ80 alloy (Fig.5).The higher formation temperature implies a lower undercooling (ΔTn) required by the nucleation of theβ-Mg17Al12,which further proves that the Al8Mn4Sm could act as the effective heterogeneous nucleation site for theβ-Mg17Al12,hence promotingβ-Mg17Al12refinement
It is noted that with the 0.2 wt.% Sm addition,the refinin effect of theβ-Mg17Al12is more significan than that of theα-Mg grains.The distinct difference may be closely related todap,i.e.,the diameter of the active nucleation particles.Eq.(4) reveals that heterogeneous nucleation occurs more readily on larger particles than on smaller ones.This means that the larger particles possess higher nucleation activity,which could result in fine grains.However,the trend is not unlimited because grains that nucleated on larger particles tend to grow faster owing to lower curvature undercooling.Therefore,in a specifie alloy system,an optimum size of the active nucleation particles definitel exists to generate the fines grains.For instance,Qiu and Zhang [79] proposed that the most effective size of active Al2Y nucleation particles for the Mg-10 wt.% Y cast alloy is 6–6.5 μm.In this work,the mean diameter of the Al8Mn4Sm particles is nearly 3.2 μm,which is probably closer to the optimum size of active nucleation particles for theβ-Mg17Al12.As a result,the nucleation activity of Al8Mn4Sm particles for theβ-Mg17Al12is higher than that for theα-Mg grains,thus leading to a more signifi cant reduction inΔTnof theβ-Mg17Al12and more prominentβ-Mg17Al12refinement
4.3.The orientation relationships (ORs) between Al8Mn4Sm and α-Mg/Mg17Al12
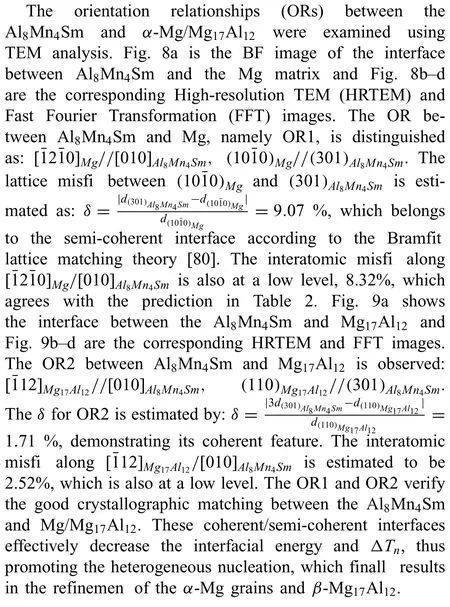
4.4.Prospects for the effect of Al8Mn4RE on simultaneous refinemen
Apart from the Al8Mn4Sm,other Al8Mn4RE (RE,i.e.,Ce,Nd,Y,and Gd) compounds also exhibit the potential to act as heterogeneous nucleation sites for theα-Mg andβ-Mg17Al12.Firstly,the Al8Mn4RE compounds with high melting temperatures are formed prior to theα-Mg andβ-Mg17Al12.Secondly,crystallographic calculations (Table 7 and 8) based on the obtained OR results (Figs.8 and 9) show that the interatomic/interplanar misfit between the Al8Mn4RE and Mg are nearly the same with the counterparts between the Al8Mn4Sm and Mg (Table 7).Meanwhile,the interatomic/interplanar misfit between the Al8Mn4RE and Mg17Al12are also close to those between the Al8Mn4Sm and Mg17Al12(Table 8),which is basically attributed to the same crystal structure and similar lattice parameters of the Al8Mn4RE compounds.The above results indicate that these Al8Mn4RE compounds all possess sufficientl good crystallographic matching with both theα-Mg matrix andβ-Mg17Al12.Furthermore,Fig.10a–j show that the Al8Mn4Y particle is observed near the center of theα-Mg grain or inside theβ-Mg17Al12,serving as the heterogeneous nucleation substrates.Therefore,owing to the similar physicochemical properties of RE elements,it is reasonable to expect that other Al8Mn4RE (RE,i.e.,Ce,Nd,Y,and Gd)compounds could also act as effective heterogeneous nucleation sites to promote the simultaneous refinemen of theα-Mg andβ-Mg17Al12.Our results may provide more options for low-cost and highly efficien microstructure refiner employed in the cast Mg alloys,particularly in the Mg-Al based alloys.
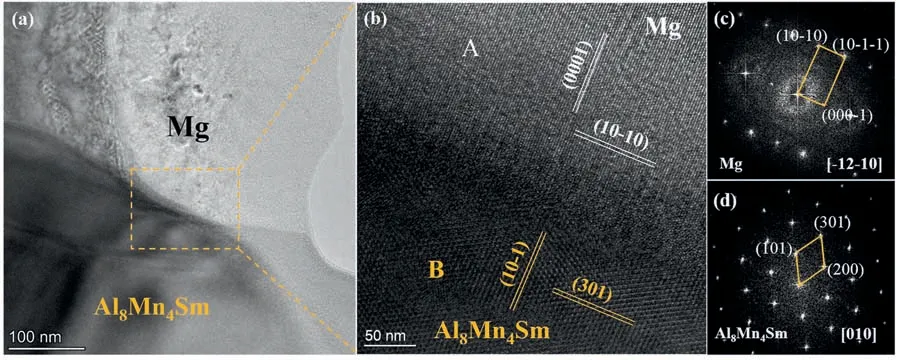
Fig.8.The interface between Al8Mn4Sm and the Mg matrix: (a) BF image of the interface,(b) HRTEM image of the marked area in (a),(c,d) corresponding FFT images obtained from areas marked with “A” and “B” in (b).
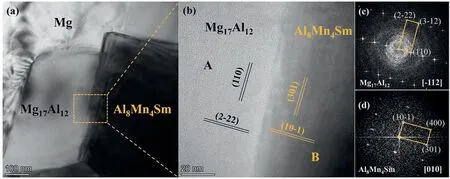
Fig.9.The interface between Al8Mn4Sm and Mg17Al12: (a) BF image of the interface,(b) HRTEM image of the marked area of (a),(c,d) corresponding FFT images obtained from areas marked with “A” and “B” in (b).

Table 7 The interatomic/interplanar misfit between Al8Mn4RE (γ) and Mg (α).
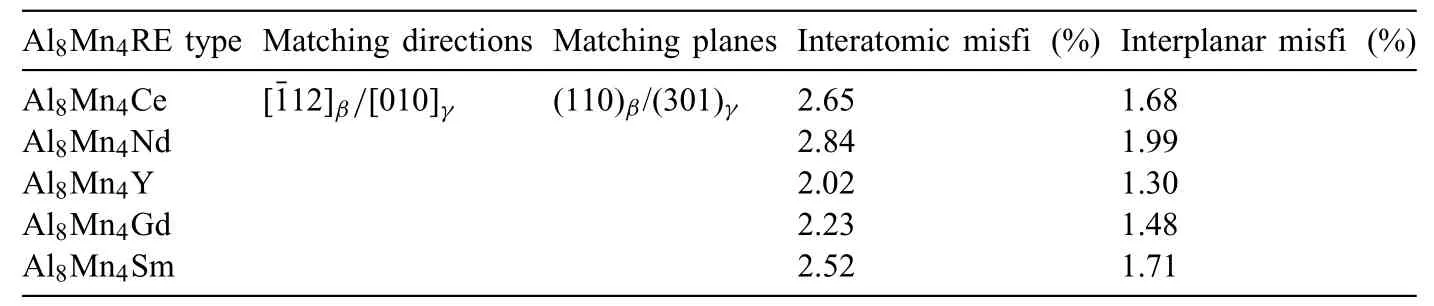
Table 8 The interatomic/interplanar misfit between Al8Mn4RE (γ) and Mg17Al12 (β).
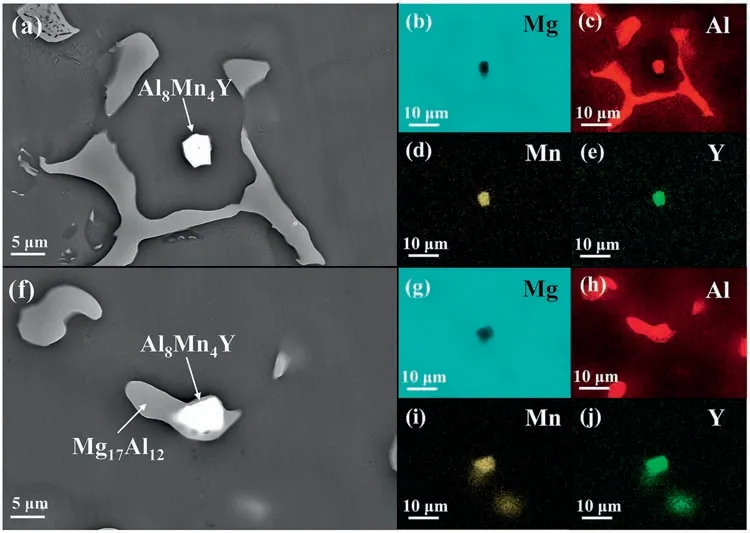
Fig.10.(a) SEM image in secondary electron (SE) mode and (b–e) corresponding EDS elemental mappings for the AZ80-0.2Y alloy showing the Al8Mn4Y as the heterogeneous nucleation site for the α-Mg.(f) SEM image and (g–j) corresponding EDS elemental mappings for the AZ80-0.2Y alloy showing the Al8Mn4Y as the heterogeneous nucleation site for the β-Mg17Al12.
5.Conclusion
In summary,a strategy was proposed to simultaneously refin the primaryα-Mg and eutecticβ-Mg17Al12by promoting heterogeneous nucleation on Al8Mn4Sm in Mg-Al based alloys.With 0.2 wt.% Sm addition to the AZ80 alloy,theα-Mg grain size decreases from ~217±15 μm to ~170±10 μm,and concurrently,theβ-Mg17Al12morphology changes from a typical continuous network to a nod-like or spherical structure.The main conclusions are summarized as below:
(1) The 0.2 wt.% Sm addition leads to almost all the Al8Mn5transforming into Al8Mn4Sm.Unlike the Al8Mn5,the Al8Mn4Sm with better crystallographic matching with theα-Mg matrix andβ-Mg17Al12could be a potent nucleation agent.This view is confirme by the result that most of the Al8Mn5particles are distributed around the grain boundaries,while the Al8Mn4Sm particles can be observed near the center of theα-Mg grains or inside theβ-Mg17Al12.
(2) The 0.2 wt.% Sm addition decreases the critical undercooling (ΔTn) required by theα-Mg/β-Mg17Al12nucleation.It indicates that the Al8Mn4Sm compound is an effective nucleation substrate for both theα-Mg andβ-Mg17Al12,which gives rise to an increasing number of nuclei,thus refinin theα-Mg andβ-Mg17Al12simultaneously.
(4) The refinemen of theβ-Mg17Al12accelerates its dissolution during the solution treatment,which greatly shortens the heat treatment process in industrial production,thereby significantl reducing energy consumption and manufacture costs.
(5) Apart from the Al8Mn4Sm,other Al8Mn4RE compounds such as Al8Mn4Y have the same positive effect on the simultaneous refinemen of the primaryα-Mg and eutecticβ-Mg17Al12,which broadens the option range of microstructure refiner and boosts the widespread industrial applications for cast Mg-Al based alloys.
Declaration of Competing InterestThe authors declare that they have no known competing financia interests or personal relationships that could have appeared to influenc the work reported in this paper.
Acknowledgements
Financial supports from The National Natural Science Foundation of China (Nos.52104376,U19A2084,52074132,and 52004100) and China Postdoctoral Science Foundation (2021T140250 and 2021M701376).Partial financia support came from The Science and Technology Development Program of Jilin Province (Nos.20200401025GX and 20200201002JC),and Program for JLU Science and Technology Innovative Research Team (JLUSTIRT,2017TD-09).
 Journal of Magnesium and Alloys2023年1期
Journal of Magnesium and Alloys2023年1期
- Journal of Magnesium and Alloys的其它文章
- Formation of carbon and oxygen rich surface layer on high purity magnesium by atmospheric carbon dioxide plasma
- Local hardening and asymmetric twin growth by twin-twin interactions in a Mg alloy
- Brittle and ductile characteristics of intermetallic compounds in magnesium alloys: A large-scale screening guided by machine learning
- Experimental study on uniaxial ratchetting-fatigue interaction of extruded AZ31 magnesium alloy with different plastic deformation mechanisms
- Assessment of Mg(OH)2/TiO2 coating in the Mg-Ca-Zn alloy for improved corrosion resistance and antibacterial performance
- Unveiling the influenc of dendrite characteristics on the slip/twinning activity and the strain hardening capacity of Mg-Sn-Li-Zn cast alloys
