Potential use of a dried saliva spot(DSS)in therapeutic drug monitoring and disease diagnosis
Yu Hn ,Xi-Ling Li ,Minghui Zhng ,Jing Wng ,Su Zeng ,Jun Zhe Min ,*
a Key Laboratory of Natural Medicines of the Changbai Mountain,Ministry of Education,Department of Pharmaceutical Analysis,College of Pharmacy,Yanbian University,Yanji,Jilin,133002,China
b Institute of Drug Metabolism and Pharmaceutical Analysis,Zhejiang Province Key Laboratory of Anti-Cancer Drug Research,College of Pharmaceutical Sciences,Zhejiang University,Hangzhou,310058,China
Keywords:Human saliva Dried saliva spot Therapeutic drug monitoring Disease diagnosis
ABSTRACT In recent years,scientific researchers have increasingly become interested in noninvasive sampling methods for therapeutic drug monitoring and disease diagnosis.As a result,dried saliva spot(DSS),which is a sampling technique for collecting dried saliva samples,has been widely used as an alternative matrix to serum for the detection of target molecules.Coupling the DSS method with a highly sensitive detection instrument improves the efficiency of the preparation and analysis of biological samples.Furthermore,dried blood spots,dried plasma spots,and dried matrix spots,which are similar to those of the DSS method,are discussed.Compared with alternative biological fluids used in dried spot methods,including serum,tears,urine,and plasma,saliva has the advantage of convenience in terms of sample collection from children or persons with disabilities.This review aims to provide integral strategies and guidelines for dried spot methods to analyze biological samples by illustrating several dried spot methods.Herein,we summarize recent advancements in DSS methods from June 2014 to March 2021 and discuss the advantages and disadvantages of the key aspects of this method,including sample preparation and method validation.Finally,we outline the challenges and prospects of such methods in practical applications.
1.Introduction
Generally,blood(including plasma,serum,or whole blood),urine,and saliva are the most common biological fluids used for the detection of metabolites,which is of great significance for therapeutic drug monitoring(TDM)and disease diagnosis[1].However,TDM and disease diagnosis using conventional biological fluids often require samples in large amounts,which are complicated and potentially unstable,and makes it difficult to store and transport the samples[2,3].In the past few decades,the increase in the use of dried spot sampling techniques has provided an avenue for technical development in various fields because of its advantages,such as less tissue damage,less sampling quantity,and convenience[4].Compared with traditional sampling techniques,dried blood spot(DBS),dried matrix spot(DMS),dried saliva spot(DSS),and alternative dried biological fluid spot technologies are increasingly used in the field of basic medical research.
DBS technology is an established technique and its use is being expanded.Based on the DBS technique,DMS and DSS have been rapidly developed over the last 10 years.The DMS method is used for the analysis of the non-blood matrix.This method often uses filter paper with a color indicator to enhance the visibility of transparent fluids spotted onto DMS cards.Saliva collection is noninvasive and painless.Therefore,saliva is superior to blood and other non-blood matrices(cerebrospinal fluid,synovial fluid,tears,urine,and plasma)as a diagnostic fluid[5-9].Moreover,the DSS method usually uses 3-100μL of saliva as the sampling volume,which is conducive to obtaining many samples rapidly.The DSS technique has made good progress in terms of TDM and disease diagnosis.
This review summarizes the research progress of the DSS technique from June 2014 to March 2021.The shortcomings of the DSS method in terms of sample preparation and methodological verification are the focus of this review,including the selection of appropriate filter paper types and filter paper cutting tools,internal standard addition,extraction conditions,analyte stability,and sensitivity of the instruments.In addition,DBS,dried urine spot(DUS),and DMS are also introduced and discussed.This review summarizes the currently used dried spot sampling techniques and provides novel methods and strategies for the collection,storage,and transportation of biological samples.In the future,this method can be used not only for the analysis of biological samples but also for other analytical sampling and analysis fields.
2.DSS technique
DSS is a well-known sampling technique for collecting saliva samples by spotting salivary specimens on filter paper.Saliva sample collection is inherently noninvasive and painless;furthermore,collection of saliva samples does not require specialized training,can be performed at home,has practically no risk of infection,is readily accepted by patients,and has a considerable advantage in terms of sample collection from special groups,including children or disabled persons or those with anxiety disorders[10].Saliva is produced from the parotid,sublingual,submandibular,and numerous minor salivary glands.Differences in the composition of saliva secreted by different salivary glands have been observed[6].However,saliva from different salivary glands is not widely used because of the high degree of training required for the specialist collecting the specimen.Whole saliva is easy to collect and is suitable for patients and volunteers participating in scientific research.The composition of saliva also depends on whether salivary secretion is basal or stimulated.Therefore,different salivary sampling styles can cause variations in salivary composition[9].For instance,passive drool and direct collection of saliva into a sampling tube are two ways of collecting unstimulated whole saliva.An obvious shortcoming of directly collecting saliva is that more bacteria may be present in such samples,which may affect the analysis of several compounds.Gentle mastication,using citric acid,gently chewing a cotton roll,and polystyrene foam swabs are usually used to collect stimulated whole saliva.These methods have their own limitations;for instance,citric acid affects testosterone analysis.Therefore,researchers should choose a sampling method that is most suitable for analyte quantification.For DSS sampling,the saliva drops from the lower lip are collected into the sampling tube.This is a suitable method of saliva collection because it is convenient,does not require additional sampling equipment,and is suitable for everyone.Brushing,ingesting food and liquids(except water),and chewing gum should be forbidden for at least 30 min before collection[8].
Different filter papers have different diameters and liquid absorption efficiencies.The amount of saliva required varies with the selected filter paper,but is approximately 3-100μL.After collecting the DSS samples,the salivary collection card is allowed to dry and is stored at room temperature.Because saliva and target analytes adhere to the filter paper,this feature enables the preservation and stability of the saliva sample to improve.Therefore,the advantage of DSS significantly reduces transportation costs.Subsequently,the DSS is extracted using a suitable solvent using a combination of vortex-assisted extraction(VAE)and ultrasound-assisted extraction(UAE).Subsequently,the extraction is concentrated and reconstituted using a mobile phase or extraction solvent.
To date,DSS has been widely used in diagnosis of several diseases[11-15],for the bio-detection of several drugs[16-24]and in metabolomics analysis[25,26].Seven years(June 2014-March 2021)of literature research on DSS as a sampling technology are summarized(Table 1).In particular,we focus on the establishment of the DSS methods,which consists of the sample preparation process and method validation.
2.1.Different types of filter paper used in the DSS method
Among the reported studies that employ DSS,four types of filter paper are used in DSS(Fig.1).In the case of the Whatman FTA?DMPK-C card(Fig.1A)used to collect saliva samples,the dried saliva spots are not easily visualized.Thus,color-indicating cards(Fig.1B)were developed to allow the analyst to visually verify the location of the dried sample spot[27].We recommend that if saliva samples are collected using filter paper with a color indicator,analyte recovery should be compared with that found using a noncolor-indicating filter paper because the color additive can result in ionization suppression.
FTA DMPK-A and FTA DMPK-B are two types of Whatman FTA?DMPK cards,which are usually used for DBS sample collection.FTA DMPK-A and FTA DMPK-B cards have chemical reagents capable of denaturing proteins,whereas the Whatman FTA DMPK-C card does not[28].In the DBS method,FTA DMPK cards were used to investigate the extraction efficiency and stability of plasma samples[29].However,the efficiency and stability of saliva extraction from filter paper using the DSS method have not been reported.Therefore,the development of the DSS method in the future should focus on the performance of the filter paper and verify whether the filter paper used is suitable for the target analyte.
Ordinary filter paper with a fixed diameter for the collection of saliva samples is used for the determination of lidocaine(Fig.1C)[18].Salivary spots are punched in the center,which are graphically defined locations for biological sample collection.However,analysts find it difficult to control the location of the applied saliva samples.Although saliva samples can be collected using this DSStype filter paper,the size of the samples itself may be outside the fixed diameter collection spots of the filter paper.An ordinary filter paper(Fig.1D)is used to detect uric acid in human saliva[26].First,this filter paper is cut into an appropriate size,thus preventing the saliva from spreading beyond the prescribed range.In contrast to DBS,DSS spotted on filter paper is not as obvious;thus,reliably knowing the location of the dried sample can improve the accuracy of the analysis.One approach to enhancing the visibility of dried saliva spots for DSS is using ultraviolet(UV)light[23].The advantage of using a UV lamp for visualization is that UV illumination does not affect the analyte recovery rate.
The Whatman?903 protein saver card,also known as the Whatman 903 card,is usually used in the DSS method(Fig.1E).As the name indicates,this filter paper is used to protect proteins from biological samples.Thus,the Whatman?903 protein saver card is not the same as the FTA Classic Card,which is impregnated with chemicals that lyse cells,denature proteins,and protect nucleic acids from nucleases,oxidative damage,and UV damage.This filter paper has been proven to detect nucleic acids in blood[30-32].Recently,Hsiao et al.[11]described a new method that used matrix-assisted laser desorption ionization-time of flight mass spectrometry(MS)for the diagnosis of oral squamous cell carcinoma.Krone et al.[13]described a robust method using polymerase chain reaction in conjunction with the Whatman?903 protein saver card for the detection ofStreptococcus pneumoniaecarriage in human saliva,which is stable in DSS stored with a desiccant for up to 1 month over a broad range of temperatures.This filter paper is therefore also suitable for analyzing nucleic acids in saliva.Furthermore,it has been used to detect drug metabolites in human saliva,including antiepileptic drugs,antipsychotic drugs,methadone,and 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine[16,17,22].However,the recovery of analytes is lower than that of biological metabolites[24,25].This could be due to either the high protein binding rate of some drugs or the absence of denaturation of the protein in saliva.Therefore,we recommend that the FTA Classic Card be used when analyzing t h e c o n c e n t r a t i o n o f d r u g s i n s a l i v a.T h i s f i l t e r p a p e r m a k e s t h e p r o t e i n m o r e e f f e c t i v e f o r d e n a t u r a t i o n a n d r e l e a s e s m o r e d r u g m e t a b o l i t e s.B e c a u s e t h e c h e m i c a l s o n t h e f i l t e r p a p e r s a f f e c t t h e t e s t r e s u l t s w h e n s u c h f i l t e r p a p e r s a r e u s e d f o r s a l i v a c o l l e c t i o n,t h e m a t r i x e f f e c t s h o u l d b e c o n s i d e r e d i n a s s a y s u s i n g t h e D S S m e t h o d.the concentration of drugs in saliva.This filter paper makes the protein more effective for denaturation and releases more drug metabolites.Because the chemicals on the filter papers affect the test results when such filter papers are used for saliva collection,the matrix effect should be considered in assays using the DSS method.

Refs.[11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26]Instrument Matrix-assisted laser desorption/ionization time-of-flight MS Ultra-performance liquid chromatographyelectrospray ionization-MS/MS Quantitative PCR Reverse transcriptase PCR PCR HPLC-diode array detector GC-MS/MS LC-MS/MS HPLC-photo-diode array LC/MS GC-MS GC-MS/MS UHPLC-MS/MS Thermo Fisher Scientific Q-Exactive Focus LC-MS/MS Long-term 8 days 14 days 1 month 1,2,and 3 weeks 1,2,and 3 weeks 4 or 8 days 3 days 60 days 2 weeks 2 months At least 2 weeks 9 days(45 °C)5 days(4 or 20 °C)HPLC-ultraviolet Short-term 7 days(37 °C)1 day 24 h(RT)24 h(RT)24 h,7 days,14 days(RT,4 °C,and-20 °C)3 h,24 h,and 3 days 24 h(RT)2 or 24 h(RT)1 and 7 days(RT)27 days(RT)24 h(20 °C)Freeze/thaw(CV,%)all AEDs<11<15 Stability Autosampler(CV,%)<13<15<13<11.1 Limit of detection 6.75 fg/mL 25 CFU/reaction 0.05μg/mL<10 for 0.03μg/mL 10-100 ng/mL 5 ng/mL 0.5μg/mL<8 Limit of quantitation 3.07 ng/mL 0.1μg/mL 10 ng/mL(HAL:5 ng/mL)40 ng/mL 10 ng/mL 2 ng/mL 10 ng/mL Recovery(%)80-100 99.5-104.6 22.5 fg/mL 63-97 100 110.71>70 45-74 86-91 89-115 90.52-102.42 Interday precision CV(%)4.3-6.04 1-14.3 6.24-14.89 84.35-Lower than 8.8 5.9-12.2 Intraday precision(%)CV 2.6-7.75 4.39-11.52 2.15-12.28 41-61 0-12.9 4.02-10.99 2.17-9.84 Lower than 8.8 5.7-13.0 1.08-4.30 1.06-3.78 Applications of the dried saliva spot(DSS)in therapeutic drug monitoring and disease diagnosis.Linearity regression(R2)and linear range 0.999;3.38-180.1 ng/mL At least 0.99;1.125-225 mg/mL At least 0.99;0.1-10 μg/mL ng/mL(HAL:5-100 ng/mL)At least 0.9993;40-500 ng/mL ng/mL>0.994;2-1000 ng/mL >0.9;0.5-1000 ng/mL At least 0.99;10-1000 ng/mL At least 0.99;2-300 μg/mL time Drying 30 min Cool air from a hairdryer 2 h(RT)40 min 1 h 1 h(36 °C)At least 0.99;10-400 A few minutes Overnight(RT)2.5 h Overnight At least 0.99;10-250 2 h 30 min Extraction time 10 min(95 °C)15 s 5 min 5 min 1 min 1 min 10 min 60 min 15 min 10 min Optimization of the extraction procedure Solvent volume 400μL 5 min 50μL 180μL 15 min 710μL/600μL 900μL 30 min 1 mL 2 mL 150μL 30 s 150μL 5 min 200μL 10 min 3 mL 1 mL 200μL 50μL 600μL 5 mL 200μL 5 min Extraction solvent 260 mL of 100 mM Tris-HCl(pH 8.5),100 mL of 20%(m/V)deoxycholate,and 40 mL of 50 mM tris(2-carboxyethyl)phosphinehydrochloride Acetonitrile 20 mM Tris-Cl,2 mM ethylene diamine tetraacetic acid Prepared viral lysis buffer containing carrier RNA and terile 1×phosphate-buffered saline/ethanol EasyMAG? lysis buffer Methanol acidified with formic acid(pH 5.5)Methanol(pH=5.0)with 25μL of IS solution(CPZ-d3 in 0.1μg/mL and promazine in 0.5μg/mL)Acetonitrile containing the IS pentycaine at a concentration of 20 nmol/L Methanol and IS Acetonitrile,ammonium acetate buffer 14 mM,and methanol(55:35:10,V/V/V)Ethylacetate Isopropanol and 20μL of IS 5 ng/mL in MeOH:water(20:80,V/V)100 mM ammonium acetate in water with 1.0% Triton-X-100/ethyl acetate Eethyl acetate:hexane(90:10,V/V)0.1 mL of IS solution(5 FU 2μg/mL)and ethyl acetate:isopropanol(85:15,V/V)Lithium carbonate solution Sample volume(μL)25 100 50 50 50 100 50 250 50 15 5 50 5 Analytical substances/diagnosed diseases Matrix metalloprotease 1/oral squamous cell carcinoma D,L-Lactic acid/diabetes 3 Pneumoniae DNA/Streptococcus pneumoniae Congenital cytomegalovirus AEDs:phenobarbital,phenytoin,carbamazepine,and carbamazepine-10,11-epoxide Antipsychotic drugs:CPZ,levomepromazine,cyamemazine,clozapine,HAL,and quetiapine Lidocaine Non-steroidal antiinflammatory drugs Benzoylecgonine,AMP,MDMA,cocaethylene,and COC AMP,methamph etamine,MDMA,Δ9-THC,COC,morphine,MTD,and clonazepam MTD and 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine BMS-927711 THC and its metabolites(THC,11-hydroxy-Δ9-THC,11-nor-9-carboxy-Δ9-THC,AM-2201,ABCHMINACA,and fentanyl)Uracil and Whatman S&S # 903 paper Measles virus/measles 100 dihydrouracil Uric acid/hyperuricemia 903 protein Sampling filter paper Whatman 903 paper Whatman 903 protein saver 903 protein FTA DMPK-C card saver cards 903 protein 903 protein Table 1 Whatman?saver card saver cards Fabric phase sorptive extraction membranes 903 Grade card Whatman?Whatman?Filter paper Whatman?paper Filter paper Whatman?saver cards Whatman FTA DMPK-C Whatman grade 31 ET and 3 MM chromatography paper Whatman 903 paper Ordinary filter paper CV:coefficient of variation;MS:mass spectrometry;PCR:polymerase chain reaction;RT:room temperature;HPLC:high performance liquid chromatography;AEDs:antiepileptic drugs;CPZ:chlorpromazine;HAL:haloperidol;GC:gas chromatography-mass spectrometer;IS:internal standard;LC:liquid chromatography;AMP:amphetamine;MDMA:3,4-methylenedioxymethamphetamine;COC:cocaine;THC:tetrahydrocannabinol;MTD:methadone.
Conventionally,as for dried spot sampling,biological fluids are dropped on filter paper made of pure cellulose.In recent years,soluble materials have been used for sampling and storing blood and saliva.Lodoen et al.[33]reported a soluble material composed of an alginate and chitosan foam for saliva sampling(Fig.1F).Soluble materials have two advantages over filter papers made of pure cellulose.First,the sample preparation time is significantly reduced,and second,all the analytes(100%)from the spot are transferred.This is possible because such filters are foams,which are inherently porous,swollen,and hydrophilic matrices that can instantly absorb biological fluids.However,there are drawbacks to this novel type of soluble material.As the extracted solution for analysis contains the dissolved biopolymer and other foam components,in addition to saliva components and analytes,an additional step of analyte extraction or biopolymer precipitation is necessary prior to analysis.Thus,compared to the use of conventional fiber filter paper,DSS testing with foam-based soluble material requires an additional dissolution step.Nevertheless,we believe that the findings from preliminary experiments with alginate and chitosan foams are highly promising.By switching from traditional saliva collection to dry storage of saliva on sheets of solid materials,any challenges associated with proprietary diluents can be avoided.A considerable effort is still needed to further investigate soluble biopolymers as sampling media for DSS.
According to the information presented above,filter papers used in the DSS method include filter papers used for spotting dried blood,ordinary filter paper,and filter paper of soluble materials.The Whatman?903 card,which can absorb saliva samples of 25-100μL,is widely used in this method.In this case,the large volume of saliva is a disadvantage with respect to the complete drying of the sample.If the amount of saliva dropped on the filter paper is very small,for example,3μL of saliva,a hairdryer can be used to dry it in order to speed up the drying time.This is an advantage for daily applications because the universal drying time is 1-2 h,and even overnight for some samples.Simultaneously,the impact on the temperature stability of the DSS and analytes should be considered.Therefore,in the future,the DSS method requires the development of a filter paper using newer materials that have a lower cost and use less volume of saliva.

Fig.1.Types of filter paper used in the dried saliva spot(DSS)method:(A)Whatman FTA?DMPK-C card;(B)color-indicating card;(C)filter paper for the determination of lidocaine;(D)filter paper for the determination of uric acid;(E)Whatman?903 protein saver card;and(F)solid-material filter paper made of an alginate and chitosan foam.(Reprint from Refs.[18,23,26,33]with permission.)
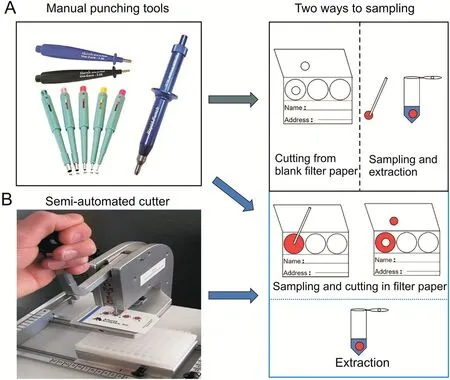
Fig.2.Different types of equipment for cutting filter paper for dried saliva spot(DSS)testing:(A)manual hole punch and(B)semi-automated cutter from TOMTEC.(Reprint from Ref.[34]with permission.)

Fig.3.Preparation and brief schematic of dried saliva spot(DSS)for determination content of uric acid(UA).LC-UV:liquid chromatography-ultraviolet;IS:internal standard.(Reprint from Ref.[26]with permission.)
2.2.Types of filter paper cutting equipment
It is commonly known that DSS is punched from the filter paper.Two different types of equipment are used for cutting the filter paper.Typically,manual punching tools such as the Harris Micro-Punch,Miltex?biopsy punch with plunger,and rapid-punch(Microscopy Products for Science and Industry)are used to punch out the spots(Fig.2A)[34].However,these have limitations in that they can only punch one hole at a time and the punching accuracy may be reduced when analyzing more dried spots.Alternatively,a device from TOMTEC(Fig.2B)was used by Johnson et al.[34]as an improved punching method for semi-automated dried whole blood spots.No statistical difference was observed between the results of the semi-automated methods when compared with the results of the DMS methods using manual punching and extraction.Therefore,neither type of equipment(manual punching or semi-automated)was found to affect the experimental outcome.A newly published DSS article uses a manual punch,the concept version of which is shown in Fig.3[26].The whole saliva is collected by asking the user to open the mouth and place the collection tube on the lips so that the saliva flows naturally into the collection tube.This method is simple and nonirritating,and can be operated by the patient.Salivary gland collection requires insertion of a collecting tube/special device into the salivary gland to obtain saliva.In terms of operating procedures,the latter is complicated in operation and slow in sampling,and an expert is required to perform the operation.Therefore,the first collection method is recommended.The capillary tube may be used to transfer saliva from the collection tube to the filter paper.Different filter papers have different diameters and liquid absorption efficiencies,and the amount of saliva varies with the selected filter paper,ranging from 3 to 100μL.After patient sampling and drying,the dried saliva spot is extracted using a Li2CO3solution by VAE.The extracted sample is concentrated and reconstituted using the same solution.This is a specific step that is applied to the detection of uric acid concentration in human saliva.To date,we believe that it is easier to use the entire spot containing the biological sample for analysis rather than to punch a hole in the spot.There are two reasons why it is better to cut the filter paper into spots and then add the saliva sample:1)for filter paper without color indication,dried saliva and other transparent biological fluids are not readily located;and 2)the concentration of the analyte in dried spots may be unevenly distributed by cutting the filter paper containing the sample.There is no doubt that either of these methods will increase the workload of analysts.Therefore,the filter paper can be cut into spots of fixed diameter and partitioned into a 96-well plate with filter paper placed in each well.After the user places the sample on the spot and dries it,this spot is mailed to the analysis laboratory.Analysts no longer need to use cutting tools,which not only reduces the workload but also ensures the accuracy of the analyte concentration.
2.3.DSS sample extraction methods
After the preparation of suitable filter paper and selecting the appropriate cutting tools,extraction methods should be evaluated.To verify the feasibility of the extraction method,a methodological investigation was conducted.For practical applications,the stability of the analyte on filter paper should also be considered.Therefore,in this section,we summarize the key strategies,including the addition of an internal standard(IS),optimization of the extraction procedure,and ensuring of analyte stability.
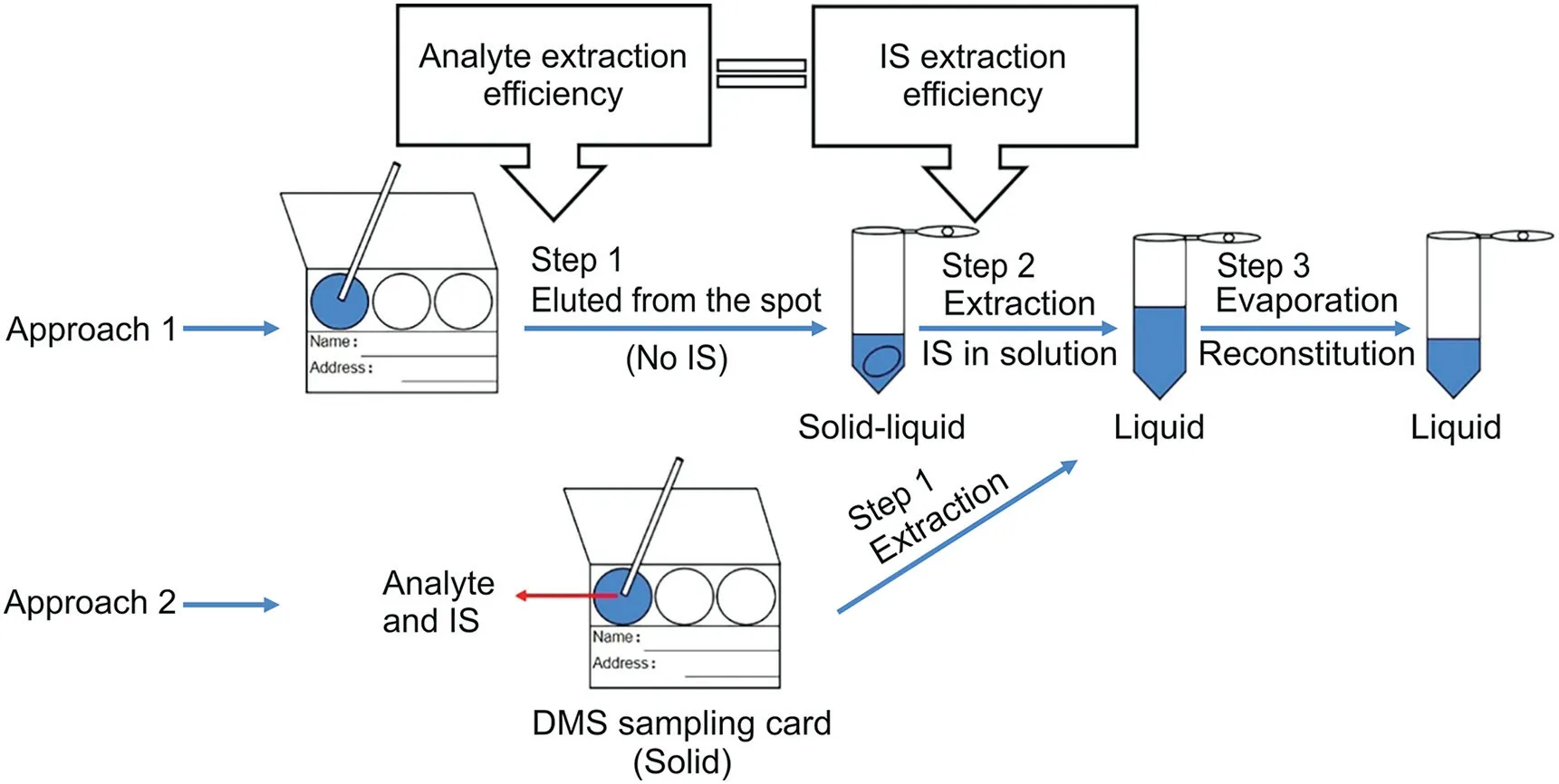
Fig.4.Processing steps of dried matrix spot(DMS)sample extraction.IS:internal standard.(Reprint from Refs.[23,35]with permission.)
2.3.1.Addition of an IS in DSS analysis
Table 1 shows published articles on the DSS methods[11-26],in which an IS was added to the extraction solvent instead of dropping it onto the filter paper.Zheng et al.[23]recommended the addition of IS to the extraction solution and for the DSS sample to be extracted after the analyte was eluted;however,Zimmer et al.[35]stated that IS should be added to the filter paper prior to the biological sample during the DMS sample preparation(Fig.4).It is important to ensure that the extraction efficiency of the analyte and IS are equal.Christianson et al.[27]and Manicke et al.[36]compared the average accuracy(%)and precision(coefficient of variation,%)of adding IS before or after applying the biological sample to the filter paper.Both studies found that adding IS to the filter papers before the biological sample improved the precision and accuracy of the method.Therefore,we believe that these improvements in accuracy and precision are attributed to one factor:IS compensates for analytes from the collection paper during the punching process.Therefore,for DSS,we propose not punching disks from the filter paper containing the saliva sample,but rather,using the entire,indicated circular area of the sample.This confers two advantages:1)when punching holes in the sample card,one need not be concerned about the uneven distribution of the saliva on the filter paper if the entire collection spot is used;and 2)IS does not compensate for the analyte because the entire saliva sample is used during extraction for DSS.In addition,it is also a useful approach to cutting out the filter paper before adding the saliva sample as the diameter of the filter paper spot can be adjusted according to the volume of the sample.This approach reduces the amount of the filter paper used and reduces the sample drying time.
2.3.2.Optimization of the extraction procedure
Optimization of extraction conditions in DSS should consider five factors:extraction solvent,solvent volume,extraction time,drying time,and evaluation of extraction time.Most extraction solvents are either methanol or acetonitrile because they effectively precipitate the protein in saliva(Table 1).Some researchers believe that freezing saliva samples can prevent the degradation of some molecules in saliva,and when necessary,this can also prevent bacterial growth.Saliva contains bacterial protease enzymes,which can degrade several salivary proteins;consequently,this can affect the investigation of the protein compound[37,38].If the extraction solvent uses either methanol or acetonitrile and the target analyte is not decomposed,we believe that it is not necessary to freeze the saliva sample,thereby preferring the DSS approach in which the user directly applies the saliva sample on the filter paper instead of freezing it.Generally,solid-phase extraction(SPE)or liquid-liquid extraction(LLE)requires greater amounts of extraction solvents.Therefore,fewer extraction solvents facilitate the sample preparation step.Many studies on the DSS method have discussed solvent volume.The extraction solvent used is usually at least 50μL,with a maximum volume of 5 mL.The volume of the extraction solvent used in the DSS method is lower,which is better than that for SPE and LLE.Regarding the extraction time,the extraction process is not completed instantly,but a specific amount of time is required for the extraction solvent to soak the entire spot.Of the published studies reviewed,we found that the shortest and longest extraction time was 15 s and 60 min,respectively(Table 1).The extraction time is also dependent on the type of the instrument and filter paper used.Therefore,the extraction time should ensure that the maximum amount of analyte can be recovered.In addition,DSS samples must be completely dried before storage or transport because moisture may harm the specimen by inducing bacterial growth or altering its elution.To evaluate the DSS methods,linear regression,intra-day and inter-day precision,recovery,limits of quantification,and limits of detection were determined.Surprisingly,many studies that were reviewed did not optimize the extraction conditions,including the type of extraction solvent,solvent volume,extraction time,and drying time(Table 1).Furthermore,we found only one published study that evaluated extraction time[26].Solvent volume may be a factor in cases where a single extraction does not completely recover the analyte.The recovery rate of some analytes was less than 80%(Table 1).One of the reasons for the low recovery rate is that analytes that have not been extracted may still be present in the filter paper.Therefore,evaluation of the extraction times should be considered in the DSS extraction procedure.
2.3.3.Stability of analytes in DSS
Analyte stability during the entire analytical procedure is a prerequisite for reliable quantification[39].Analyte stability is divided into autosampler stability,freeze/thaw-cycle stability,short-term stability,and long-term stability(Table 1).It is important to note that instability occurs not only in the sample matrix but also in the processed samples.Therefore,it is important to test analyte stability in samples prepared using an autosampler.It is essential to understand the short-term and long-term analyte stabilities in order to determine how long dried saliva can be consistently stored and under what conditions it must be transferred to the laboratory for analysis.
In the DSS method,some researchers thawed frozen saliva and applied it to filter paper as samples[16,17,22].Because of the presence of salivary amylase in saliva,freezing will cause it to precipitate or the analyte concentration will need to be tested again.Therefore,the freeze/thaw stability of the analytes should be evaluated over at least three freeze/thaw cycles at three concentrations in triplicate.However,this method of freezing saliva at the time of collection is not conducive to the on-site collection process for DSS because it adds an extra step for the patient at the time of sample collection,making it impractical for common adoption and standard use.
2.3.4.High sensitivity analytical instrumentation
Abdel-Rehim and Abdel-Rehim[18]and Numako et al.[12]reported the first instances of using the DSS sampling method for TDM and disease diagnosis in 2014 and 2015,respectively.Even after years of development,the correlation between detection sensitivity and sample volume remains challenging.Some studies detected analytes using high performance liquid chromatography(HPLC)coupled to either a photodiode array(PDA)or UV detector(Table 1).The limit of detection(LOD)for the DSS analysis using the PDA detector was 0.03μg/mL.However,the LOD was fg/mL level(6.75 fg/mL)using tandem mass spectrometry(MS/MS)detection.Moreover,we found(in this review)that the minimum biological sample volume using MS/MS as a detector was 3μL.The increased sensitivity and selectivity of these detectors has reduced the demand for bio-sample volumes in DSS methods.Compared to UV detection,MS/MS offers improved sensitivity and enables a wider linear detection range[40].It can also provide relative molecular mass and structural information has great advantages in both qualitative and quantitative aspects[41].However,the operation of an MS requires a skilled analyst with specific training and knowledge,and MS detectors are more expensive than UV detectors,both in acquisition and maintenance.
3.Other dried spot method strategies
In addition to the DSS method,DBS and DMS are also techniques where filter paper is used for the shipment of dried sample spots,including blood,plasma,serum,urine,and herbal medicines.These samples spotted on filter papers have been widely reported,which has stimulated the development of such methods.Those sampling methods have received enormous attention because of their simplicity,convenience,robustness,and reliability.
3.1.DBS and dry plasma spot(DPS)
In 1963,the DBS method was used to diagnose phenylketonuria disease in human neonate screening[42].Since then,this method of simplifying the collection and processing of samples has developed rapidly in the application of newborn screening and disease diagnosis.At present,the use of DBS has expanded to a wide range of applications,such as large epidemiological studies[43],human immunodeficiency virus[44-46],coronavirus disease 2019[47]and hepatitis C(HCV)[48]testing,and newborn genetic screening[49].Compared with conventional sampling techniques,DBS has several advantages:1)significantly less blood is required;2)collection and preparation costs are low;3)the method is simple and reliable to perform using peripheral blood from fingertip or heel pricks with little trauma and minimal discomfort,which is readily acceptable to the patient;4)remote sampling is permissible and can even be performed at home,making DBS applicable in remote areas,5)low transportation and sample storage costs,and 6)no pollution risk to the environment[50,51].However,the cost of developing new methods using DBS technology is high,and a DBS sample can be affected by a patient's hematocrit,which affects the diffusion area of the DBS,and thus,seriously affects the concentration measurement of target compounds[52].There is also a risk of poor sampling quality.For patients performing at-home sampling,it is challenging to provide reproducible and uniform high-quality spots.Furthermore,for anti-HCV screening,DBS technology is confronted with false-negative and false-positive results.
As mentioned above,DBS has some drawbacks,among which the results are biased because of the influence of erythrocytes in whole blood[53].Plasma does not contain red blood cells and is a more appropriate sample for analysis[54-60].Currently,this DPS sampling method has been widely used in TDM[61-63],disease diagnosis[64-66],monitoring antiretroviral treatment[67],and clinical pharmacokinetic studies[68].However,the collection of plasma requires specialized equipment and personnel,which contradicts the advantages of the dried spot method.Brahmadhi et al.[69]combined microwave-assisted extraction with a dried plasma spot,which greatly shortened the sample processing time.However,this method requires professional machines to obtain plasma.Marins et al.[70]used the cobas?Plasma Separation Card to collect whole blood and compared it with plasma samples.This study solved the problem of using specialized equipment in the preparation of plasma.However,the sample size of whole blood is larger than that required when using the DBS method,which increases the risk of infection in patients.Compared with a membrane of new material,a non-solvent-thermally induced phase separation(N-TIPS)and a book-type dried plasma spot card was developed by Gao et al.[71]and Ryona et al.[72],which require a smaller blood sample and can be applied in patients who cannot go out and deliver samples.
3.2.DMS
DMS sampling is an application of DBS technology used in nonblood matrices[73,74],in which a dye is added to the collection paper to indicate the location of the dried sample points.When a transparent or nearly transparent analyte,such as cerebrospinal fluid,saliva,tears,or synovial fluid,is dried on the collection paper,it is difficult to not only know the location of the visual spot,but also guarantee accurate removal and extraction of the sample spot for testing.Therefore,a color-indicating dye is applied to sample collection cards to help analysts visually confirm the location of the dried sample.This method ensures a reliable and robust collection of transparent fluid samples,thereby improving the accuracy and precision of testing.Compared with DBS,the addition of a color indicator in DMS to indicate the location of dried sample spots complicates the method and makes it more time consuming.In addition,a color-indicating dye may not be suitable for all analyte properties to be tested because the high dye concentration in the spot may affect the selectivity of the target analyte.Therefore,it can be a challenge to find a suitable dye for DMS sampling.
In addition to the most commonly used biological fluids for the detection of metabolites,such as blood,plasma,serum,and saliva,urine is a commonly analyzed sample.Compared with blood and plasma collection,urine collection is noninvasive and simple.Currently,urine is used as a biological fluid in the identification of illicit substances,therapeutic adherence monitoring,and detection of toxic compounds[75,76].Urine samples are dropped on filter paper for preservation,called a DUS.A suitable volume of the sample is then transferred to the filter paper.Usually,the volume of the sample spotted on the filter paper cannot exceed the maximum loading capacity of the filter paper.Subsequently,the obtained spots are dried for hours or overnight at room temperature or at the desired temperature.To extract the analytes,a whole DUS is punched out from the card and extracted with a certain amount of organic solvent using a combination of VAE and UAE.The solution is dried and re-dissolved in the mobile phase.The extraction solvent,solvent volume,extraction time,and drying time should be optimized.This method is similar to the DSS method,and the commonly used filter papers for DUS are the Whatman?903 protein saver card[77,78]and the Whatman FTA classic cards[60,79].DUS has developed rapidly in terms of application in disease diagnoses,such as the diagnosis of glutaric aciduria type 1[80],congenital cytomegalovirus infections in newborns[81],and human papillomavirus testing[82].The current examples of DUS in TDM applications are not comprehensive,and this is also the direction of future DUS method development.
In addition to biological samples,the dried spot method is also used to analyze herbal medicines,which are called dried spots of herbal medicines(DSHM)[83].A flowchart of this process is presented in Fig.5.Dried sample spots are loaded into an in-line filter holder.This device is placed in front of the chromatographic column so that the mobile phase can pass through the dried spot to achieve online sample extraction.Compared with the conventional DMS sampling,the analyte from the DSHM method is eluted online in an in-line filter holder.Although this approach appears to be more convenient,it is not suitable for analyzing many samples.
At present,the DSS and DUS techniques are also used in combination with paper-spray MS,which allows for direct ionization of the analyte from a biofluid spot on a piece of paper.For instance,Bills et al.[24]described a method to concentrate and preserve tetrahydrocannabinol(THC)and synthetic cannabinoids in urine and saliva on paper strips for analysis by paper-spray MS.The sample was passed through an oil spot on a strip of 40 mm paper,which is known as paper strip extraction.Usually,to evaluate the homogeneity of the paper strip,the dried paper is cut into a 5 mm strip and each 5 mm paper segment is analyzed.Normally,THC is unstable and difficult to detect using urine and saliva spots.Therefore,sesame seed oil was used as a means to preconcentrate the analytes.Combining these two techniques improved the LOD for THC to ng/mL levels in urine and saliva.It is an effective method for the future detection of compounds with unstable saliva or other bio-sample matrices.The application of these direct analysis techniques to DSS bioanalysis can significantly increase the throughput of these bioanalytical methods.Paper spray has been shown to have high sensitivity and a short analysis time,which makes it a potential option for disease diagnosis and TDM.Although not having an extraction process confers many advantages to this approach,differentiating isomers remains a problem for ambient ionization techniques.
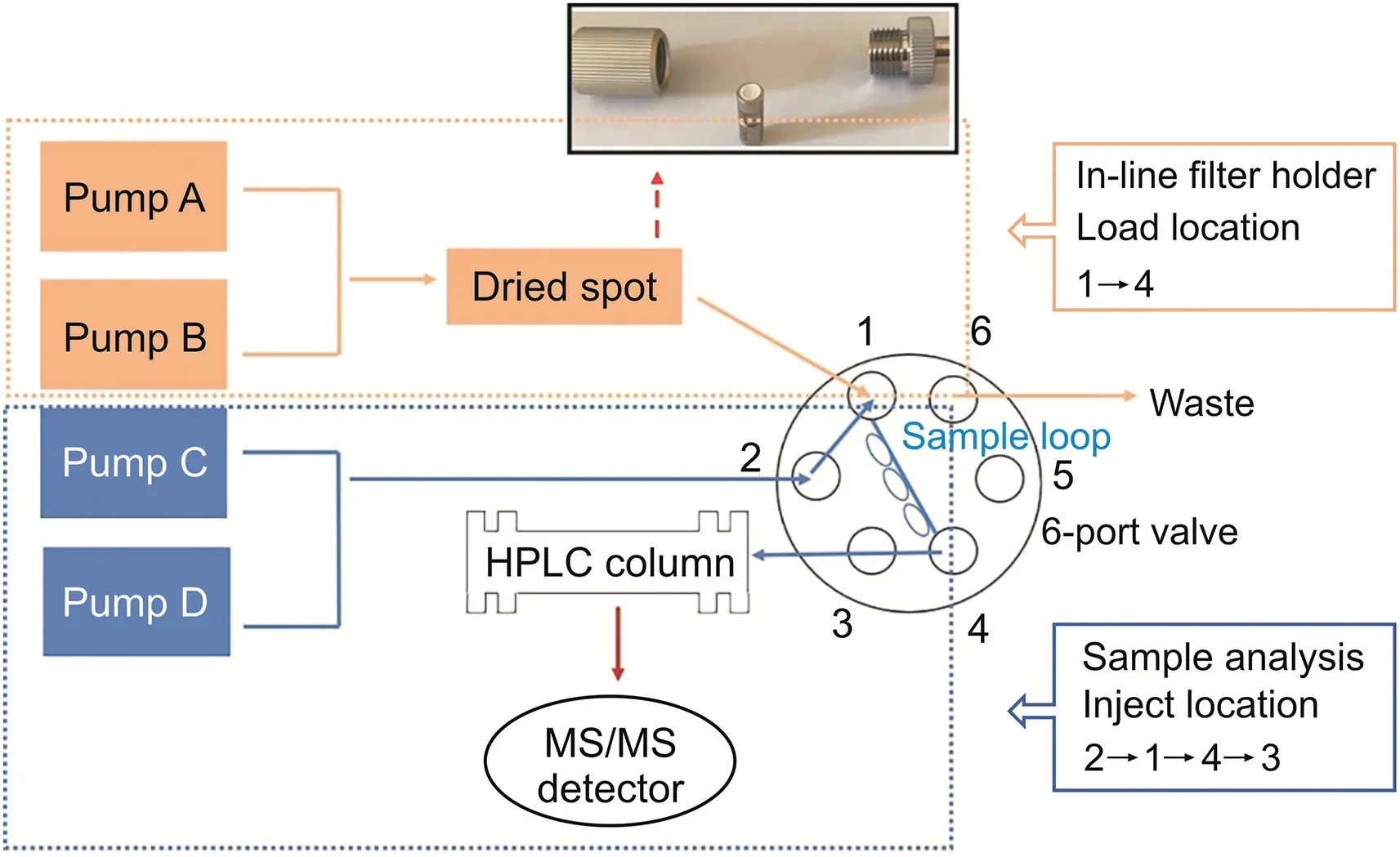
Fig.5.Schematic diagrams of the dried herbal medicines spot(DHMS)method.HPLC:high performance liquid chromatography.(Reprint from Ref.[83]with permission.)
4.Conclusions and future perspectives
Our reviewsummarizes the DSSmethodused for TDM and disease diagnosis in recent years and discusses the developmental deficiencies of this method in sample preparation and validation.It provides a comprehensive analysis process and recommendations for researchers using this method.This convenient,robust,and reliable sampling method has opened up a new development direction for medical testing.In addition,blood,plasma,urine,and herbal medicines have been used to detect samples in the methodsknown as DBS,DPS,DUS,and DSHM,respectively.The abovementioned methods are dried spot methods that are advantageous in terms of storage and transportation of biological and plant samples.
Currently,most commercial filter papers used for collecting alternative biological fluids are inadequate.With the wide application of these methods in various fields,the development of novel filter paper materials is inevitable.In daily applications,to shorten the analysis time,the direct analysis method replaces the traditional filter paper extraction procedure and determines a new development direction for fast and simple dried spot methods.To reduce invasiveness or facilitate drying,the volume of the determined sample is usually a microliter.Therefore,the sensitivity of either the DSS method or the dried spot of different biological fluids is a major challenge.Most experiments were conducted in a laboratory for sampling.In practical applications,the proficiency and accuracy of sample collection by patients or users will also be a challenge.With the rapid development of these methods,people will be able to understand their physical condition without leaving their home in the near future,which can become a reality.
CRediT author statement
Yu Han:Conceptualization,Formal analysis,Investigation,Methodology,Visualization,Writing-Original draft preparation;Xi-Ling Li:Methodology,Data curation,Formal analysis,Writing-Original draft preparation;Minghui Zhang:Investigation;Jing Wang:Investigation;Su Zeng:Supervision,Conceptualization,Visualization;Jun Zhe Min:Data curation,Writing-Reviewing and Editing,Funding acquisition.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study has been supported by the National Natural Science Foundation of China(Grant Nos.:82173782 and 32160234)and the Science and Technology Development Project,Education Department of Jilin Province of China(Grant No.:JJKH20191151KJ).
 Journal of Pharmaceutical Analysis2022年6期
Journal of Pharmaceutical Analysis2022年6期
- Journal of Pharmaceutical Analysis的其它文章
- Corrigendum to“The potential of miRNA-based therapeutics in severe acute respiratory syndrome coronavirus 2(SARS-CoV-2)infection:A review”[J.Pharma.Anal.11(2021)265-271]
- Development of a surface plasmon resonance biosensor for accurate and sensitive quantitation of small molecules in blood samples
- Peptide-RNA complexation-induced fluorescence“turn on”displacement assay for the recognition of small ligands targeting HIV-1 RNA
- Fluorescent aptasensor for detection of live foodborne pathogens based on multicolor perovskite-quantum-dot-encoded DNA probes and dual-stirring-bar-assisted signal amplification
- Tumor-targeting intravenous lipid emulsion of paclitaxel:Characteristics,stability,toxicity,and toxicokinetics
- Metabolomic and elemental profiling of blood serum in bladder cancer
