Tumor-targeting intravenous lipid emulsion of paclitaxel:Characteristics,stability,toxicity,and toxicokinetics
Jun Ye ,Lin Li ,Jiye Yin ,Hongliang Wang ,Renjie Li ,Yanfang Yang ,Yongbiao Guan ,*,Xuejun Xia ,Yuling Liu
a State Key Laboratory of Bioactive Substance and Function of Natural Medicines,Institute of Materia Medica,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing,100050,China
b Beijing Key Laboratory of Drug Delivery Technology and Novel Formulation,Institute of Materia Medica,Chinese Academy of Medical Sciences & Peking Union Medical College,Beijing,100050,China
c National Beijing Center for Drug Safety Evaluation and Research,Beijing Institute of Pharmacology and Toxicology,Academy of Military Medical Sciences,Beijing,100850,China
Keywords:Nanodrug delivery systems Lipid emulsion Paclitaxel Colloidal Stability Toxicokinetics
ABSTRACT Lipid nanoemulsions are promising nanodrug delivery carriers that can improve the efficacy and safety of paclitaxel(PTX).However,no intravenous lipid emulsion of PTX has been approved for clinical treatment,and systemic safety profiles have not yet been reported.Here we outline the development of a PTXloaded tumor-targeting intravenous lipid emulsion(PTX Emul)and describe its characteristics,colloidal stability,and systemic safety profiles in terms of acute toxicity,long-term toxicity,and toxicokinetics.We also compare PTX Emul with conventional PTX injection.Results showed that PTX Emul exhibited an ideal average particle size(approximately 160 nm)with narrow size distribution and robust colloidal stability under different conditions.Hypersensitivity reaction and hemolysis tests revealed that PTX Emul did not induce hypersensitivity reactions and had no hemolytic potential.In addition,where the alleviated systemic toxicity of PTX Emul may be attributed to the altered toxicokinetic characteristics in beagle dogs,including the decreased AUC and increased plasma clearance and volume of distribution,PTX Emul alleviated acute and long-term toxicity as evidenced by the enhanced the median lethal dose and approximate lethal dose,moderate body weight change,decreased bone marrow suppression and organ toxicity compared with those under PTX injection at the same dose.A fundamental understanding of the systemic safety profiles,high tumor-targeting efficiency,and superior antitumor activity in vivo of PTX Emul can provide powerful evidence of its therapeutic potential as a future treatment for breast cancer.
1.Introduction
Paclitaxel(PTX)is one of the most successful natural anticancer agents with a unique mechanism.It has been widely applied for the clinical treatment of ovarian,breast,and nonsmall cell lung cancers since its approval for marketing in 1992[1,2].Given the extremely low solubility of PTX in water(0.3μg/mL),the first commercially available PTX injection(Taxol?,Bristol-Myers Squibb,USA)was formulated in combination with a surfactant of polyoxyethylene castor oil(Cremophor EL?)and dehydrated ethanol.Although the introduction of surfactants enhances PTX solubility,serious acute hypersensitivity reactions associated with Cremophor EL?considerably limits the clinical use of PTX[3].Thus,efforts have been directed toward developing a novel Cremophor EL?-free PTX formulation with reduced toxicity and enhanced antitumor activity.
Nanodrug delivery systems(NDDS)present a promising approach to personalized medicine with enormous potential for enhancing water solubility/stability,pharmacokinetics,biodistribution and therapeutic efficacy,and mitigating side effects[4-7].Owing to these multiple advantages,many preclinical studies have been conducted on NDDS and have reported encouraging preliminary outcomes in the fields of disease treatment,imaging,and theranostics,especially in cancer diagnosis and treatment[5,8].Many sophisticated NDDS have been designed for the classic chemotherapy agent PTX to enhance its antitumor efficacy and reduce its toxicity and serious allergic reactions from traditional PTX injection.Several NDDS-based PTX nanoformulations have been approved for clinical use,such as Abraxane?(albumin-based nanoparticles,USA),Cynviloq?(polymeric micelles,South Korea),Paclical?(micelles,Russian Federation),and Lipusu?(liposomes,China)[6,9].These approved PTX nanoformulations have the advantages of enhanced antitumor efficacy or decreased toxicity compared with Taxol?[9].Over the past few decades,lipid emulsions have been reported as promising vehicles for poorly water-soluble agents owing to the high capacity for drug loading and ease of industrial production.Oil-in-water-based lipid emulsions commonly range from 100 to 500 nm and are characterized by their core-shell structure,in which the oil core provides a reservoir for encapsulating lipophilic drugs and a phospholipid monolayer shell for the stability of particles[10-14].Many lipid emulsion-based nutrition and therapeutic products have been used clinically for over 50 years,such as Intralipid?,Liple?,and Diprivan?,confirming the safety and potential of lipid emulsions[12].The development of PTX-loaded lipid emulsion has received extensive attention worldwide.However,the poor solubility of PTX in commonly used oil phases leads to low encapsulation efficiency,poor colloidal stability,and premature drug leakage.To overcome these challenges,researchers attempted to design advanced PTXloaded lipid emulsions,including lipophilic PTX prodrugs,using tocopherol as the oil phase,polyethylene glycol-mediated lipid nanoemulsion,tocopherol polyethylene glycol 1000 succinate(TPGS)-incorporating nanoemulsion,and cholesterol-rich nanoemulsion[15-19].Tocosol?,which is prepared with tocopherol as the oil phase for PTX solubilization and vitamin E TPGS as the primary emulsifier,is the first vitamin E-based emulsion of PTX approved for clinical trials.Despite its positive outcomes in early clinical trials,Tocosol failed to perform in a pivotal phase III clinical trial[16].To date,no intravenous lipid emulsion of PTX has been approved for clinical use.
We previously developed a PTX-loaded tumor-targeting intravenous lipid emulsion(PTX Emul)consisting of an oil core for PTXcholesterol complex solubilization surrounded by a monolayer of phospholipids[20-24].PTX Emul resembles the structure of native low-density lipoprotein(LDL),shows a nanoparticle size of 160 nm,and remarkably promotes the targeted delivery of PTX into triplenegative breast cancer cells through an LDL receptor-mediated active targeting pathway[22].In addition,it shows enhanced safety and superior antitumor activity compared with conventional PTX injection at the maximum tolerated dose against triplenegative breast cancer[21].PTX Emul has a unique advantage over commercially available lyophilized PTX nanoformulations because it can be directly administered via intravenous infusion in clinical use without reconstitution or dilution[24].Analysis of its in vitro and in vivo antitumor effects and underlying mechanism in triple-negative breast cancer cells confirmed that,in the clinical treatment of breast cancer[21,22,24],the LDL receptor-mediated tumor-targeting PTX Emul has a potential use.However,the systemic safety profiles including acute toxicity,long-term toxicity,and toxicokinetics of PTX Emul have not yet been reported.These are keys in evaluating the clinical application potential of this new therapeutic product,especially for cytotoxic chemotherapy agents.
This study aimed to evaluate the systemic safety profiles of PTX Emul prepared by high-pressure homogenization.Its characteristics such as average particle size and size distribution,zeta potential,and colloidal stability in vitro were also examined.Its systemic safety profiles,including hypersensitivity reaction,hemolysis test,acute toxicity,long-term toxicity,and toxicokinetics,were investigated.A fundamental understanding of the systemic safety profiles of PTX Emul may provide powerful evidence of its therapeutic potential as a future treatment for breast cancer.
2.Materials and methods
2.1.Materials
PTX was purchased from Guilin Huiang Biochemistry Pharmaceutical Co.,Ltd.(Guiling,China).PTX injection(trade name:Zisu?)was obtained from Beijing Union Pharmaceutical Factory(Beijing,China).Soybean oil and medium-chain triglycerides(MCT)were acquired from Liaoning Shinsun Pharmaceutical Co.,Ltd.(Tieling,China).Soybean lecithin(Lipoid S75)was bought from Lipoid GmbH(Ludwigshafen,Germany),and poloxamer 188 was obtained from BASF Corporation(Ludwigshafen,Germany).Glycerol(for injection)was purchased from Shantou Jiahe Biologic Technology Co.,Ltd.(Shantou,China).All other chemicals and reagents used in the experiments were of analytical or high-performance liquid chromatography grade.
2.2.Animals
Guinea pigs(5-6 weeks old)and Sprague-Dawley(SD)rats(6-8 weeks old)were supplied by Beijing Vital River Laboratory Animal Technology Co.,Ltd.(Beijing,China).Beagle dogs(6-8 months old)were provided by Beijing Marshall Biotechnology Co.,Ltd.(Beijing,China).Toxicity studies were performed at the National Beijing Center for Drug Safety Evaluation and Research(Beijing,China)in compliance with the Good Laboratory Practice Regulations and with the approval of the Association for Assessment and Accreditation of Laboratory Animal Care International(Approval Nos.:IACUC-2010-012,IACUC-2010-065,IACUC-2010-108,IACUC-2011-015,and IACUC-2015-067;Beijing,China).All animal procedures were conducted following the Guide for the Care and Use of Laboratory Animals[25].
2.3.Preparation and characteristics of PTX Emul
The PTX-cholesterol complex and PTX Emul were prepared by solvent evaporation and high-pressure homogenization,respectively[20].The PTX-cholesterol complex(100 mL)was prepared as follows:PTX(1.0 g)and cholesterol(0.45 g;1:1,molar ratio)were dissolved in acetone(250 mL)and stirred at 40°C for 1.5 h.A solid PTX-cholesterol complex was obtained after the acetone was removed under agitation and vacuum.PTX Emul(100 mL)was prepared as follows:first,the oil phase consisting of soybean oil(10.0 g),MCT(10.0 g),and vitamin E acetate(10.0 mg)was stirred at 60°C for uniform dispersion.The PTX-cholesterol complex(170.9 mg)was dissolved in the oil phase at 60°C.The aqueous phase was formed by dispersing soybean lecithin(1.2 g),poloxamer 188(2.0 g),and glycerol(2.25 g)in ultrapure water(73.0 mL)at 60°C and then slowly mixed with the oil phase at 60°C,followed by high-speed shear mixing(FA25;Fluko,Shanghai,China)at 19,000 r/min for 8 min.Thereafter,the coarse emulsion was homogenized using homogenization equipment(NS1001L 2 K;GEA Niro Soavi S.p.A,Parma,Italy)at 800 bar for eight cycles to obtain PTX Emul.The pH of the obtained PTX Emul was adjusted to 4.5 with 0.1 M hydrochloric acid.Following nitrogen gas purging,PTX Emul was aliquoted into quantities of 10 mL and sterilized by autoclaving at 115°C for 30 min.[3H]PTX Emul was prepared using the same procedure,except that[3H]PTX-cholesterol complex was dissolved in oil.
Particle size,polydispersity index(PDI),and zeta potential were measured using dynamic light scattering(NICOMP 380 ZLS,PSS,Santa Barbara,CA,USA).After negative staining,morphology was examined by transmission electron microscopy(TEM)(JEM-1200EX,JEOL,Tokyo,Japan).
The entrapment efficiency of PTX in PTX Emul was measured by minicolumn centrifugation[23].In brief,500μL of PTX Emul was transferred to the prepared Sephadex G-50 minicolumn(Sigma-Aldrich,St.Louis,MO,USA),which was then centrifuged at 2,000 r/min for 3 min.After centrifugation,the minicolumn was eluted with deionized water(1 mL)three times and with ethanol(2 mL)twice to elute PTX Emul and free PTX,respectively.The amount of PTX entrapped in the PTX Emul was measured by disrupting the eluted PTX Emul with ethanol.Entrapment efficiency(EE%)was calculated by the formula:EE%=centrappeddrug/ctotaldrug×100%,wherecrepresents the content of PTX.
2.4.In vitro colloidal stability of PTX Emul
The colloidal stability of PTX Emul was evaluated with static multiple light scattering(SMLS)on a Turbiscan Tower(Formulaction,Toulouse,France)[24,26,27].Approximately 4 mL of PTX Emul was added into a cylindrical glass cell placed in the Turbiscan Tower for real-time monitoring within 48 h at 4,25,and 40°C.Variations in the backscattering light intensity(ΔBS),mean light intensity,and Turbiscan stability index(TSI)calculated from backscattering light signals were used to assess the in vitro colloidal stability of PTX Emul[28],where backscattering light intensity curve with respect to the position was obtained by scanning.
2.5.Hypersensitivity reaction
Guinea pigs(5-6 weeks old)were randomly allocated into seven groups(n=6,3 males and 3 females)as follows:1)saline as negative control group,2)10%(m/V)bovine serum albumin as positive control group,3)PTX-free lipid emulsion group,4)PTX injection(1.5 mg/kg)group,5)PTX injection(3.0 mg/kg)group,6)PTX Emul(1.5 mg/kg)group,and 7)PTX Emul(3.0 mg/kg)group.All treatments were intraperitoneally administered on days 0,2,and 4.On the 10th day following the last immunization,the animals in each group were challenged with the two-fold intraperitoneal dose.The reactions to the challenge injection were monitored and scored for 3 h.
2.6.Hemolysis test in vitro
Hemolysis assay was performed using rabbit blood in vitro.Rabbit blood was collected,and fibrinogen was removed from the blood by quick stirring.The fibrinogen-free blood samples were subsequently mixed with saline(1:10,V/V).The supernatant was discarded after centrifugation at 1,500 r/min for 15 min.Sedimentary erythrocyte pellets were washed with saline until the supernatant became colorless.Finally,erythrocyte pellets were resuspended in saline as 2%(V/V)erythrocyte standard dispersion.
The 2% erythrocyte standard dispersion(2.5 mL)was added with predetermined volumes of PTX Emul,followed by saline to make a final volume of 5 mL.Distilled water and saline were used as positive and negative controls,respectively.After mixing,the samples were incubated at 37°C.The precipitation of erythrocyte pellets and the changes in the medium color were observed with the naked eye at 15 min,30 min,1 h,2 h,3 h,and 12 h.After incubation for 12 h,the erythrocyte pellets at the bottom of the tube were collected for each group,and the morphology of the erythrocytes in each group was observed under a microscope.
2.7.Acute toxicity
The acute toxicity of PTX injection and PTX Emul was assessed in SD rats and beagle dogs.SD rats were randomly allocated into eight groups(n=10;5 males and 5 females)and injected intravenously with either a single dose of PTX injection or PTX Emul at 12,24,36,and 48 mg/kg via the tail vein.General symptoms,body weight,and mortality after administration were monitored daily for 14 days.The median lethal dose(LD50)of PTX injection and PTX Emul was calculated based on the mortality of rats.
The acute toxicity of PTX Emul to beagle dogs was assayed using the approximate lethal dose(ALD)method.Four beagle dogs were randomly divided into four groups(n=1)and infused intravenously with a single dose of PTX Emul at 12,24,36,and 48 mg/kg.The dogs were monitored for toxic symptoms,body weight,and mortality daily for 14 days after administration.Beagle dogs were added to the acute toxicity assessment to primarily explore the dosage for long-term toxicity.
2.8.Long-term toxicity
Beagle dogs were randomly allocated into six groups(n=6;3 males and 3 females),namely,PTX-free emulsion group(vehicle group);PTX injection groups administered with doses of 8 or 12 mg/kg;and PTX Emul groups administered with doses of 12,18,or 27 mg/kg,followed by intravenous infusions every week for 4 weeks(for a total of five times).Four dogs in each group(2 males and 2 females)were sacrificed 24 h after the last dose was given.Complete necropsy examination,including gross and histological examinations by hematoxylin-eosin(HE)staining of all major organ tissues and organ weight measurements,was conducted on the four animals from each group.The remaining animals were observed during the 4-week recovery phase and examined daily for general symptoms,including behavior,mental status,outward signs,glandular secretion,fecal traits,skin mucosa,food consumption,and mortality.Body weight and temperature were measured weekly.Hematological examination,serum biochemistry analysis,and immunological tests were performed before the initial administration,after the last administration,and during the recovery phase.
2.9.Toxicokinetic study
Toxicokinetic study was carried out to determine the long-term toxicity in beagle dogs.Blood samples(approximately 2.0 mL)were collected from each dog by limb vein venipuncture at 0,0.5,1,4,6,10,and 24 h after the first and last administration(weeks 1 and 5 of dosing).Blood was transferred to heparinized anticoagulant tubes,and plasma was collected by centrifugation at 4,000 r/min for 10 min.Plasma samples were stored at-80°C until the liquid chromatography-tandem mass spectrometry(LC-MS/MS)analysis of PTX concentration.Primary pharmacokinetic parameters were calculated using DAS 2.0(BioGuider Medicinal Technology Co.,Ltd.,Shanghai,China).
2.10.LC-MS/MS analysis of PTX
The plasma concentration of PTX was measured by LC-MS/MS[29].In brief,100μL of plasma,50μL of internal standard(400 ng/mL docetaxel in acetonitrile),50μL of acetonitrile,and 500μL oftert-butyl methyl ether were mixed and centrifuged at 12,000 r/min for 10 min.The supernatant was then removed and dried under a stream of nitrogen.The residues were dissolved in 100μL of the mobile phase,vortexed for 30 s,and centrifuged at 12,000 r/min for 5 min.Afterward,2μL of the supernatant was injected into the LC-MS/MS system(6410 Triple Quad;Agilent Technologies,Santa Clara,CA,USA)for analysis.
Chromatographic separation was conducted using ZORBAX Eclipse XDB-C18column(4.6 mm×50 mm,1.8μm;Agilent Technologies,Santa Clara,CA,USA)under the following conditions:mobile phase,acetonitrile and 0.1% acetic acid(60:40,V/V);flow rate:0.4 mL/min;injection volume,2μL;and column temperature,35°C.The MS conditions were electrospray ion source,positive ionization,and multiple reaction monitoring mode.The ion pairs used for monitoring werem/z876.3→308.0 for PTX andm/z830.3→549.0 for the internal standard(docetaxel).The standard curve of PTX was linear over a concentration range of 5-2000 ng/mL:T=0.0073c+0.0156,r=0.9997,whereTrepresents the peak area ratio of PTX to the internal standard,andcrepresents PTX concentration.The method's specificity,precision,and recovery rate met the requirements of the methodology.
2.11.Statistical analysis
Statistical analysis was performed by Student'st-test for two groups or one-way analysis of variance for more than two groups using GraphPad Prism version 7.00 for Windows(GraphPad Software,La Jolla,CA,USA).Differences were considered statistically significant atPvalue<0.05.
3.Results
3.1.Characteristics of PTX Emul
3.1.1.Particle size,zeta potential,and morphology
The average particle size,PDI,zeta potential,and morphology of PTX Emul were characterized by dynamic light scattering and TEM.As shown in Fig.1,the average particle size and zeta potential were 164.6±0.2 nm and-33.8±0.4 mV,respectively.The regular spherical structure of PTX Emul was observed under TEM,and its size was consistent with that determined by dynamic light scattering.In addition,PTX Emul displayed extremely low PDI values(<0.1),suggesting a narrow size distribution.PTX was efficiently encapsulated in the developed lipid nanoemulsions with a high encapsulation effciiency of 94.87%±0.38%,which may be attributed to the enhanced lipophilicity of the PTX-cholesterol complex.
3.1.2.PTX Emul exhibited good colloidal stability
The colloidal stability of the nanodrug delivery system is a critical quality that is directly related to its clinical efficacy and safety[24,30,31].A fundamental understanding of the colloidal stability of PTX Emul can provide meaningful insights into its rational clinical use.The colloidal stability of PTX Emul was monitored under different conditions(4,25,and 40°C)in real-time for 48 h using the Turbiscan Tower,an advanced analytical instrument based on the SMLS technique that can detect early,imperceptible changes in nanodispersion before macroscale physical changes appear[24,26,27].The dispersion instability of nanoparticles,such as creaming,sedimentation,flocculation,and coalescence,occurs during monitoring and is reflected as a variation in theΔBS and mean light intensity.TSI values were determined using the changes inΔBS for further evaluation of stability.In general,high TSI values indicate less stability[28,32].
As shown in Fig.2,all the variations in theΔBS of PTX Emul were extremely steady(<3%)under the different test conditions(4,25,and 40°C).In addition,the mean light intensity demonstrated no instability at 4,25,or 40°C for 48 h.Similar stability results were observed for TSI,which showed extremely low values(<0.2).Comparison of stability results at different temperatures showed that the stability tended to decrease with the increase in temperature.TheΔBS of PTX Emul at 4,25,and 40°C was<0.8%,<1%,and<3%,respectively.Similarly,the TSI of PTX Emul at 4,25,and 40°C was<0.1%,nearly 0.1%,and<0.2%,respectively.The excellent colloidal stability of PTX Emul may be attributed to its nanoparticle size,narrow size distribution,and sufficient absolute zeta potential.
3.1.3.PTX Emul demonstrated no hemolytic potential
Following the 3 h observation,which indicated that no hemolysis occurred,the tube of the negative control revealed no red color though the red blood cells precipitated at the bottom.By contrast,when complete hemolysis was indicated,a red solution without red blood cells was found in the positive control group.Given that PTX Emul is a white emulsion,and although the red blood cells precipitated at the bottom of the test tube after 12 h,solution stratification is difficult to observe after it is mixed with the red blood cell suspension.For further verification,the red blood cells in the samples were examined under a microscope.As shown in Fig.3,the tubes of the negative control and PTX Emul contained a number of intact red blood cells,indicating no hemolysis.Meanwhile,no intact red blood cells were observed in the positive control.These results demonstrated that different concentrations of PTX Emul did not induce hemolysis or erythrocyte agglutination at 37°C.
3.2.PTX Emul did not induce hypersensitivity reactions
The intensity of hypersensitivity reactions was evaluated according to Tables S1 and S2.In the active anaphylaxis experiment,the positive control guinea pigs all showed serious symptoms of hypersensitivity:two out of six guinea pigs showed an extremely strong hypersensitivity(++++)reaction,two out of six guinea pigs had a strong hypersensitivity(+++)reaction,and two out of six guinea pigs had a positive(++)reaction.No anaphylactic symptoms were found in the negative control or PTX-free lipid emulsion groups.The guinea pigs treated with low-and high-dose PTX Emul(1.5 and 3.0 mg/kg,respectively)did not show any abnormal symptoms.No significant abnormalities were observed in the lowdose PTX injection(1.5 mg/kg)group.However,the high dose PTX injection(3.0 mg/kg)group exhibited varying degrees of allergic reactions:four out of six guinea pigs had positive anaphylaxis(++),and two out of six guinea pigs had weakly positive anaphylaxis(+).Table 1 shows the degree of reaction of each group.These results indicated that PTX Emul did not cause hypersensitivity reactions when administered intravenously.
3.3.Reduced acute toxicity with PTX Emul treatment
The acute toxicity of PTX injection and PTX Emul was evaluated in SD rats and beagle dogs.In the SD rats,PTX injection at all doses(12,24,36,and 48 mg/kg)resulted in lethal toxicity;thus,none survived the administration.None of the rats that had received 12 or 24 mg/kg PTX Emul exhibited abnormal activities or mortalities.When the administered dose increased,the animals showed additional symptoms.The rats in the 36 and 48 mg/kg PTX Emul groups showed symptoms of toxicity including hypoactivity,sparse body hair,and loose stools.The increase in dose was correlated with severe symptoms of toxicity.One female rat died after receiving a dose of 36 mg/kg PTX Emul on day 5.In the group treated with 48 mg/kg PTX Emul,two female rats died on days 7 and 8,and six out of ten rats survived.The body weights of all the rats were monitored throughout the experiment,and the animals in all groups gained weight after administration(Fig.4A).The LD50of PTX Emul administered by intravenous injection in female and male rats was above 36 and 48 mg/kg,respectively,and that of PTX injection was less than 12 mg/kg(Fig.4B).Compared with PTX injection,PTX Emul had significantly reduced acute toxicity and at least three-fold higher lethal dosage.
The beagle dogs,not exhibiting any indication of acute toxicity for the following 14 days,showed no gross behavioral abnormality after receiving 12 mg/kg PTX Emul.No distinct abnormalities were observed during administration after the dose was increased to 24 mg/kg.On day 3 after administration,the animals showed symptoms of lethargy and hypoactivity that disappeared after three days.In addition,the beagle dogs exhibited hair loss on day 10 after administration.When the dose was increased to 36 mg/kg,mortality was observed in two of the beagle dogs on days 4-5 after administration.Therefore,the ALD of intravenous PTX Emul in beagle dogs was determined as 24-36 mg/kg(Fig.4C).
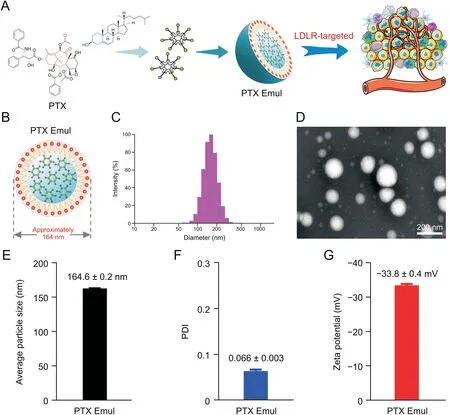
Fig.1.In vitro characterization of paclitaxel(PTX)-loaded tumor-targeting intravenous lipid emulsion(PTX Emul).(A)Schematic of the preparation of PTX Emul.(B)Schematic structure of PTX Emul.(C)Particle size distribution.(D)Transmission electron microscopy(TEM)image.(E)Average particle size.(F)Polydispersity index(PDI).(G)Zeta potential.Each value represents the mean±standard deviation(n=3).LDLR:low-density lipoprotein receptor.
3.4.Alleviated long-term toxicity with PTX Emul treatment
According to the above results,PTX Emul was superior to PTX injection in terms of hypersensitivity reaction and acute toxicity(LD50and ALD).The long-term toxicity of PTX Emul was evaluated in beagle dogs using PTX injection as a reference to determine i ts toxic response,target organ,and toxic reversibility.Based on the ALD(24-36 mg/kg)of intravenous PTX Emul in beagle dogs,the low,middle,and high doses of PTX Emul for long-term toxicity in the dogs were set as 12,18,and 27 mg/kg,respectively.The low and high doses of PTX injection were set as 8 and 12 mg/kg,respectively.The primary evaluation indices included general behavior,body weight,hematology,serum biochemistry analysis,organ coeffciients,and histopathologic examination were recorded at predetermined time points.
3.4.1.General behavior
After the repeat dosing of PTX injection or PTX Emul,the beagle dogs showed some toxicity symptoms such as vomiting,decreased activity,lethargy,loose stools,hair loss,and weight loss that occurred during the administration period.These symptoms worsened with the increase in dosage(Table S3).The toxicity of the PTX formulations was evaluated by comparing the toxicity symptoms and mortality.In terms of toxicity symptoms,the toxicity of PTX Emul was significantly lower than that of PTX injection at an equivalent dose(12 mg/kg).Even at a high dose(18 mg/kg),the toxicity of PTX Emul was less than that of PTX injection at 12 mg/kg.In terms of gastrointestinal reaction,one out of six dogs vomited during each administration of PTX injection(8 mg/kg).Incomplete digested feed was observed in the 8 mg/kg PTX injection group 3-5 days after each administration.When the PTX injection dose was increased to 12 mg/kg,five out of six dogs vomited during almost each administration.For the PTX Emul group given with the same dose(12 mg/kg),three out of six dogs vomited on the third day after the initial dose and no vomiting symptom was observed during thefollow-up administration.When the PTX Emul dose was increased to 18 mg/kg,three out of six dogs vomited after the first two doses.In terms of mortality,one animal died in the 12 mg/kg PTX injection group on day 4,and all the animals survived the experiment period in the 12 mg/kg PTX Emul group.When PTX Emul dose was increased to 18 and 27 mg/kg,one and four animals died after day10,respectively.These results showed that PTX Emul caused significantly fewer gastrointestinal reactions and deaths than PTX injection at the same dose with the animal body temperatures
fluctuating within the normal range(Fig.S1),and electrocardiogram showing no abnormalities in any group during the entire experiment(Table S4).
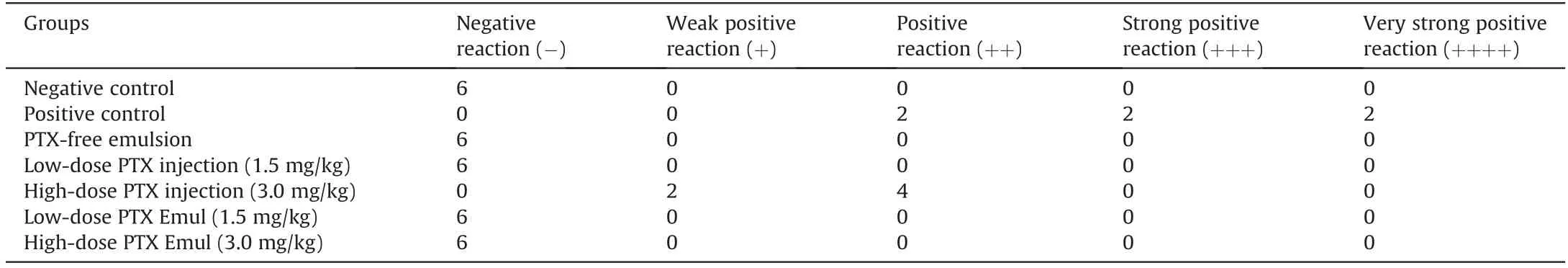
Table 1 Number of guinea pigs with allergic reactions and the degrees of allergic reactions to the active systemic anaphylaxis test after administration of paclitaxel(PTX)injection or PTX-loaded tumor-targeting intravenous lipid emulsion(PTX Emul)(n=6).
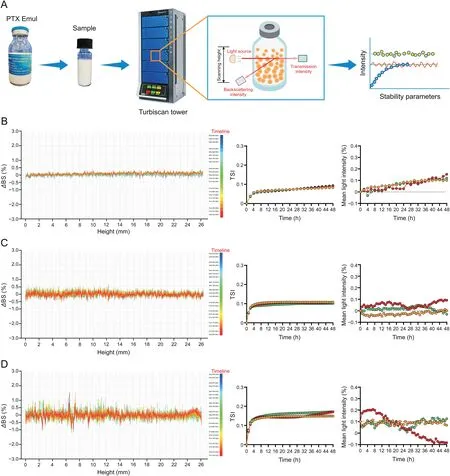
Fig.2.In vitro colloidal stability of paclitaxel(PTX)-loaded tumor-targeting intravenous lipid emulsion(PTX Emul)at different conditions.(A)Schematic of the measurement process.Variations of backscattering light intensity(ΔBS),turbiscan stability index(TSI),and mean light intensity of PTX Emul at(B)4,(C)25,and(D)40 °C for 48 h(n=3).
3.4.2.Changes in body weight
All beagle dogs were weighed within 8 weeks including the 4-week administration period and 4-week recovery phase.PTX-free lipid emulsion was used as the vehicle group.As shown in Fig.5,the dogs in the vehicle group showed a gradual increase in body weight throughout the experiment but no toxicity symptoms,indicating that the PTX-free lipid emulsion was not toxic.After PTX injection or PTX Emul was administered every week for 4 weeks,a dose-dependent reduction in average body weight was observed.At 8 mg/kg PTX injection,no clear toxicity was observed,and the body weight hardly changed throughout the study.However,a steep drop in body weight was observed when the dose of PTX injection was increased from 8 to 12 mg/kg,indicating that the treated dogs experienced severe toxicity.Compared with PTX injection,the encapsulation of PTX into lipid emulsion greatly decreased the systemic toxicity of PTX at an equivalent dose(12 mg/kg)as evidenced by the static body weight of the dogs in the PTX Emul group.Similar changes in body weight were found between the dogs treated with PTX Emul at 12 mg/kg and those treated with PTX injection at 8 mg/kg.These results demonstrated that PTX injection and PTX Emul caused systemic toxicity in terms of general behavior and body weight change due to PTX's toxicity.However,the effect of PTX injection on body weight change was more distinct than that of PTX Emul.
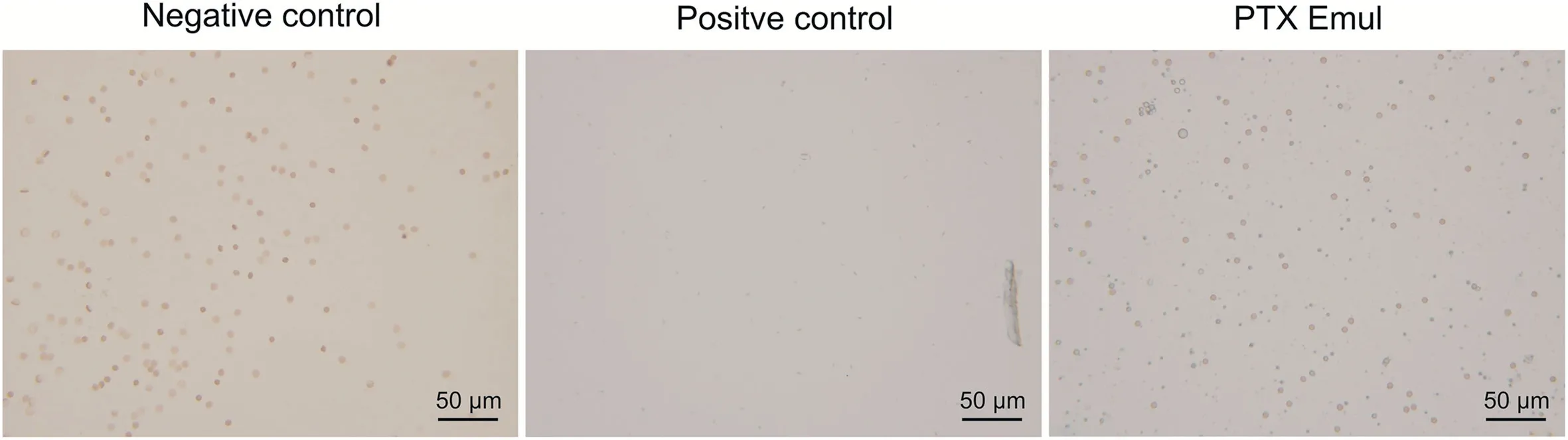
Fig.3.Morphology of red blood cells under an optical microscope.Paclitaxel(PTX)-loaded tumor-targeting intravenous lipid emulsion(PTX Emul)(0.5 mL)was added to the 2%erythrocyte standard dispersion(2.5 mL),followed by saline to make a final volume of 5 mL.Distilled water and saline were used as positive and negative controls,respectively.After mixing,samples were incubated at 37 °C for 12 h.
3.4.3.Hematology and serum biochemistry analysis
The parameters assessed in the hematology examination were white blood cell count(WBC),red blood cell count(RBC),mean corpuscular hemoglobin,mean corpuscular hemoglobin concentration(MCHC),hemoglobin(HB),hematocrit(HCT),mean corpuscular volume(MCV),platelet count(PLT),and reticulocyte count(Fig.S2).At the end of drug administration(fifth regimen),WBC,RBC,HB,HCT,and MCHC were significantly lower in all therapeutic groups than in the vehicle group.No statistically significant differences were observed among the therapeutic groups.PTX injection and PTX Emul did not affect MCV and PLT.After the recovery period,the hematological indices of the surviving animals returned to normal.These findings demonstrated that both PTX formulations had inhibitory effects on peripheral blood levels.
The results for serum biochemical parameters,including alanine aminotransferase(ALT),aspartate aminotransferase(AST),alkaline phosphatase(ALP),creatine phosphokinase(CK),blood urea nitrogen(BUN),creatinine,total protein(TP),albumin(Alb),and cholesterol(Chol),are presented in Fig.6.At the end of the drug administration,an increase in the levels of AST and CK and a decrease in the levels of TP and Alb were observed in the PTX injection and PTX Emul groups compared with levels of AST and CK and levels of TP and Alb in the vehicle group.The high dose of PTX injection increased BUN levels.Other parameters such as ALT,ALP,and Chol remained unchanged in the therapeutic groups relative to those of the vehicle group.Serum biochemistry parameters returned to the level observed in the vehicle group during the recovery period.These changes suggested that PTX injection and PTX Emul might cause reversible liver and heart damage.
3.4.4.Humoral immunological examination
At the end of administration(fifth regimen),the IgG of the animals treated with PTX injection(8 and 12 mg/kg)and high dose of PTX Emul(18 mg/kg)decreased significantly compared with that of the vehicle group(Fig.S3).No significant change in IgG levels was observed in the animals treated with the low dose of PTX Emul(12 mg/kg)and among the therapeutic groups during the recovery period.These results suggested that PTX injection was likely to decrease the level of IgG,and this effect was reversible.No significant changes in the other immunological indices including C3,C4,IgA,and IgM were observed during the experiment.
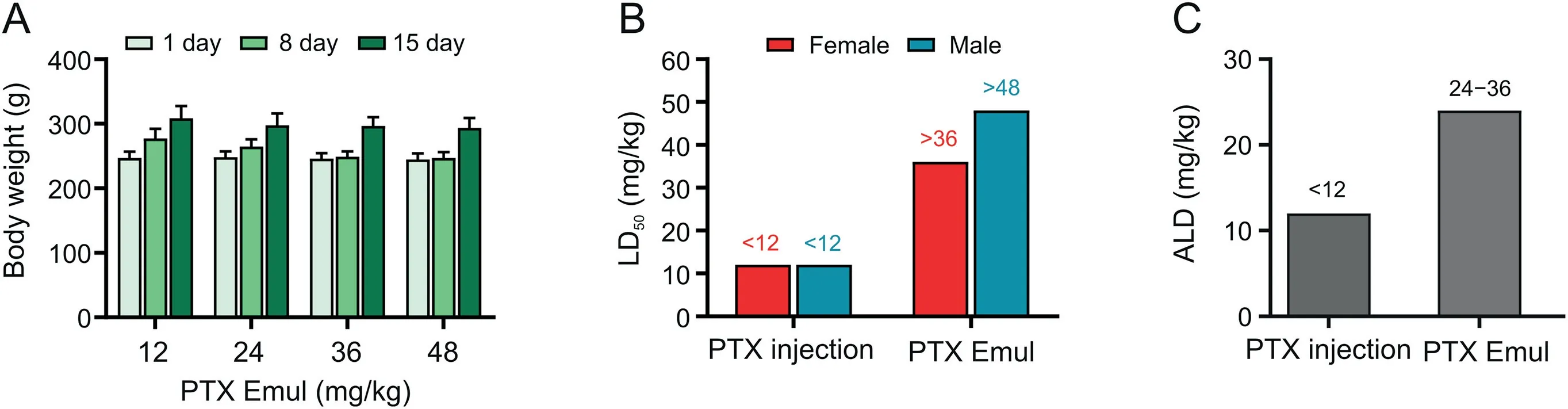
Fig.4.Evalution on the acute toxicity of paclitaxel(PTX)injection and PTX-loaded tumor-targeting intravenous lipid emulsion(PTX Emul)in Sprague-Dawley(SD)rats and beagle dogs.(A)Changes in the body weight of SD rats after treatment of PTX Emul(12,24,36,and 48 mg/kg).Each value represents the mean±standard error of mean(n=6 to 10).(B)The median lethal dose(LD50)of PTX injection and PTX Emul in SD rats.(C)Approximate lethal dose(ALD)of PTX injection and PTX Emul in beagle dogs.
3.4.5.Organ coefficients and histopathology examinations
Organ coefficients and histopathology examinations were performed to evaluate the organ damage caused by PTX injection or PTX Emul(Fig.S4).After the fifth regimen,the organ coefficient of the thoracic glands in all PTX groups decreased significantly compared with those in the vehicle group.This finding indicated organ atrophy,which was more severe in the PTX injection groups compared with that in the PTX Emul groups.No significant change in organ coefficientsofotherorgansincludingthebrain,heart,liver,spleen,lung,kidney,testes,epididymides,and adrenal gland was found between the vehicle and PTX groups.The organ coefficients of the ovaries and uteruses in all PTX groups showed a tendency to decrease.
Histological examination was performed by staining the sections with HE and observing them under a microscope.The results revealed that high doses of PTX Emul(27 mg/kg)induced thoracic gland atrophy and bone marrow suppression(Figs.S5 and S6).Similar results of bone marrow suppression were verified by bone marrow cell microscopy experiments:hypoplastic myelopoiesis was found in all the four animals with PTX injection(8 and 12 mg/kg)and in one out of four and three out of four animals with PTX Emul(12 and 18 mg/kg).These results indicated that the inhibitory effect of PTX Emul on bone marrow was weaker than that of PTX injection.The gonads of beagle dogs in all the PTX groups were damaged as evidenced by the atrophy in the testis and epididymis in male beagle dogs and the injury to ovaries in female beagle dogs.A dose-toxicity relationship was found between PTX treatment and organ damage.The animals in the PTX Emul group showed less evidence of toxicity than those in the PTX injection group of the same dose.For example,PTX Emul induced slight atrophy of the thoracic glands,spleens,and mesenteric lymph nodes,and PTX injection at the same dose of 12 mg/kg induced mild atrophy of the same organs.This finding indicated that PTX encapsulated in lipid emulsion may reduce the damage to the hematopoietic and immune systems.Organ atrophy slowly disappeared during the recovery period,and gonad damage also partially recovered,demonstrating that the PTX damage in beagle dogs was reversible.
The results of organ coefficients and histopathology examination demonstrated that thoracic glands,spleens,mesenteric lymph nodes,bone marrow,and gonads were the possible toxic target organs associated with PTX injection and PTX Emul.Compared with those induced by PTX injection,the bone marrow suppression and organ toxicity induced by PTX could be alleviated in the form of PTX Emul.
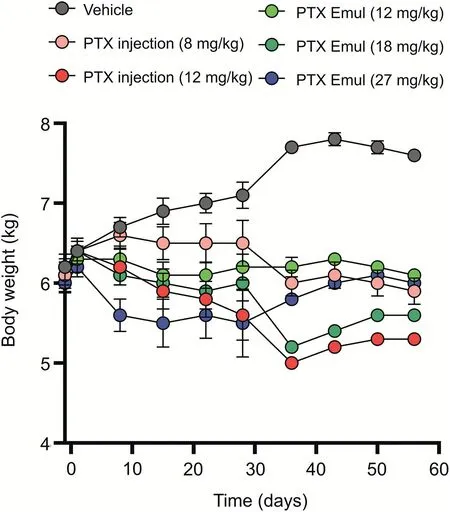
Fig.5.Changes in the body weight of beagle dogs after the repeat administration of vehicle,paclitaxel(PTX)injection(8 and 12 mg/kg),and PTX-loaded tumor-targeting intravenous lipid emulsion(PTX Emul)(12,18,and 27 mg/kg).Each value represents the mean±standard error of mean(n=5 to 6)in the first six measurements(day 28),except the PTX Emul(27 mg/kg)(n=1 to 2)in the last four measurements(days 36-56).
3.5.Altered toxicokinetic characteristics of PTX Emul in beagle dogs
The plasma concentration-time curves and the main pharmacokinetic parameters after the initial administration of PTX injection(8 and 12 mg/kg)and PTX Emul(12,18,and 27 mg/kg)are shown in Figs.7A and C.The PTX concentration of PTX Emul was significantly lower than that of PTX injection in the first 6 h at the same dose(12 mg/kg).The area under the concentration-time curve(AUC0-t)of PTX injection at doses of 8 and 12 mg/kg was 7917.72±1208.80 and 26576.69±3952.19 h·ng/mL,respectively.The AUC0-tof PTX Emul at doses of 12,18,and 27 mg/kg was 5761.04±1060.90,17869.79±12934.92,and 45542.38±12473.98 h·ng/mL,respectively.Consistent with the changes in the plasma concentration,the AUC0-tof PTX Emul was significantly lower than that of PTX injection(approximately 21.7%)at the same dose(12 mg/kg).PTX Emul exhibited higher plasma clearance(CLz)and volume of distribution(Vz)than PTX injection.With a 1.5-fold increase in dose,approximately 3.4-fold increase was observed in the AUC0-tof PTX injection.However,the AUC0-tof PTX Emul increased by 2.5-3.1 times when the dose was increased by 1.5 times.These results demonstrated that compared with PTX injection,PTX Emul was easily distributed into the peripheral tissues and had low exposure in blood,which was beneficial in reducing blood toxicity.
The mean plasma concentration-time curves and the main pharmacokinetic parameters after repeated administrations are shown in Figs.7B and D.The AUC0-tof the PTX injection group at doses of 8 and 12 mg/kg was 7872.94±1436.68 and 29060.31±10172.08 h·ng/mL,respectively.The AUC0-tof PTX Emul at doses of 12,18,and 27 mg/kg was 5083.24±1312.31,9460.98±4463.85,and 19014.53±2281.84 h·ng/mL,respectively.Similar to that in the first administration,the AUC0-tof PTX Emul was also greatly lower than that of PTX injection(approximately 17.5%)at the same dose(12 mg/kg).With a 1.5-fold increase in dose,there was an approximately 3.5-fold increase in AUC0-tof PTX injection.However,the AUC0-tof PTX Emul increased by 1.9-2.0 times when the dose was increased by a factor of 1.5.These results strongly suggested that PTX Emul was beneficial in reducing blood toxicity.
Compared with that after the initial administration,the AUC0-tof PTX injection after repeated doses showed no significant change.Meanwhile,the AUC0-tof PTX Emul decreased up to 88%,53%,and 42% of that of the initial administration.The clearance rate was accelerated after repeated administration.
4.Discussion
Lipid nanoemulsions have gained popularity over the past decade because of their exceptional properties,such as nano size,large surface area,high capacity for drug loading,robust stability,and targeted drug delivery.Their good biocompatibility and ease of industrialization have driven several lipid emulsion products to commercialization since the 1960s.Oil-in-water-based lipid emulsions generally have a core-shell structure,in which the oil core serves as a reservoir for encapsulating lipophilic drugs and a phospholipid monolayer shell for the stability of the particles[10-13].The oil phase plays a critical role in lipid emulsion formulations because it solubilizes lipophilic agents.Solubility in the oil phase is a key factor in determining the drug loading,encapsulation efficiency,stability,and drug release characteristics of lipid emulsions,all of which affect in vivo performance[12].Therefore,high solubility in the oil phase is a desirable criterion for maximum drug loading using a minimum amount of oil[33,34].Owing to its poor lipophilic property,PTX cannot be efficiently encapsulated in lipid emulsions.To date,no intravenous lipid emulsion of PTX has been approved for clinical treatment.
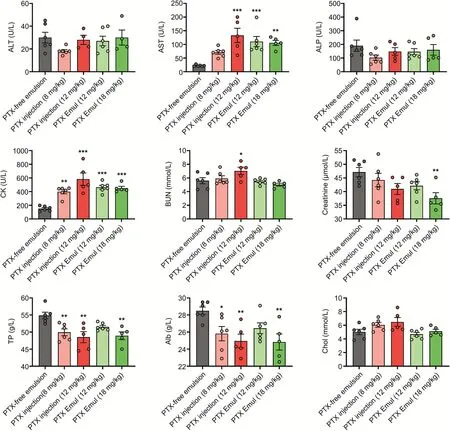
Fig.6.Serum biochemical parameters of beagle dogs after repeat administration of paclitaxel(PTX)injection and PTX-loaded tumor-targeting intravenous lipid emulsion(PTX Emul).The serum biochemistry analysis was performed after the last administration.Serum biochemical parameters included alanine aminotransferase(ALT),aspartate aminotransferase(AST),alkaline phosphatase(ALP),creatine phosphokinase(CK),blood urea nitrogen(BUN),creatinine,total protein(TP),albumin(Alb),and cholesterol(Chol).Each value represents the mean±standard error of mean(n=4 to 6).*P<0.05,**P<0.01,and***P<0.001 compared with the vehicle.
We previously developed a PTX-cholesterol complex connected by intermolecular interactions to enhance the lipophilicity of PTX.Considering its enhanced solubility in the oil phase,we developed a PTX Emul with ideal nano size,high loading capability,and high encapsulation efficiency based on the PTX-cholesterol complex[20-24].Compared with the conventional PTX injection,PTX Emul showed stronger and more rapid inhibitory activities in twodimensional and three-dimensional tumor models in vitro,higher specificity and efficiency in tumor accumulation,and better antitumor activity in vivo against breast tumors[21].The improved antitumor activity in vitro and in vivo is due to the enhanced permeability and retention effect mediated by the nanosize and the active tumor-targeting effect mediated by LDL receptors on breast cancer[22].Based on previous findings,PTX Emul,as reported here,exhibited great potential as an NDDS for PTX in a clinical setting as a targeted treatment of breast cancer[24].However,systemic safety evaluation,including acute and long-term toxicity and toxicokinetics,of PTX Emul has not been investigated,which is the key to determining the clinical potential of the new therapeutic product,especially for cytotoxic chemotherapy agents.To provide compelling evidence of its therapeutic potential,we systematically evaluated the safety profiles of PTX Emul in vitro and in vivo.
The colloidal stability of NDDS is a critical quality that affects its safety and efficacy during clinical use[24,30,31].Therefore,the colloidal stability of PTX Emul must be investigated before preclinical safety evaluations.In this study,the colloidal stability of PTX Emul was evaluated by SMLS,which provides a more objective and accurate assessment of colloidal stability than dynamic light scattering[24,26,27].The primary advantage of SMLS is the direct characterization of nanodispersions without dilution or perturbation of their initial forms[35-37].In vitro colloidal stability analysis revealed that PTX Emul exhibited excellent stability within 48 h at 4,25,and 40°C,which mimic refrigerated storage,room temperature,and high temperature,respectively.This robust colloidal stability may be attributed to the small average particle size(approximately 160 nm)and uniform size distribution(PDI<0.1).In addition,the sufficient absolute zeta potential(absolute value>30)is highly beneficial in enhancing colloidal stability by providing an electrostatic repelling force between the nanoparticles[38-40].Although an increase in temperature can speed up the Brownian motion of the nanoparticles and decrease the colloidal stability,the overall fluctuation is extremely small and remains within a limited range.Good stability can prevent the burst release of encapsulated agents and particle aggregation and reduce in vivo safety risks.
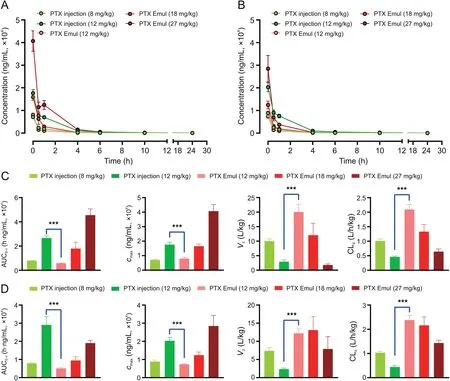
Fig.7.Plasma concentration-time curves and main pharmacokinetic parameters after the(A and C)initial administration and(B and D)repeated administrations of paclitaxel(PTX)injection and PTX-loaded tumor-targeting intravenous lipid emulsion(PTX Emul).Each value represents the mean±standard error of mean(n=5 to 6).For the repeated administrations of PTX Emul(27 mg/kg;n=2).***P<0.001.
The high incidence of hypersensitivity reactions is a major clinical problem that limits the clinical use of PTX injection.The high concentration of Cremophor EL?in PTX injection was confirmed to be the cause of the toxic effects and allergic reactions[41].Commercial Cremophor EL?-free nanoformulations of PTX,such as Abraxane?,greatly reduced allergic reactions in the clinic compared with PTX injection[42].PTX is encapsulated in lipid emulsion and not in direct contact with blood rapidly,which is also responsible for reducing allergic reactions.Hypersensitivity reaction experiments revealed that PTX injection exhibited varying degrees of allergic reactions and Cremophor EL?-free PTX Emul did not induce any hypersensitivity reactions at the same dose in guinea pigs possibly due to the free particles of Cremophor EL?,high encapsulation efficiency of PTX in PTX Emul(>90%),and sustained drug release profile[23].
In our previous study,the maximum tolerated dose(MTD)of PTX Emul was 2.25 times higher than that of PTX injection in nude mice,which is similar to the 2.24-fold increase for Abraxane?(albumin-based PTX nanoparticle)than that for PTX injection.This finding indicated that PTX Emul and Abraxane?were equally well tolerated and exhibited a superior safety profile in vivo compared with PTX injection[21].According to further comparative study on acute toxicity outcome between PTX injection and PTX Emul,the LD50of PTX Emul was about threefold higher than that of PTX injection in SD rats.The ALD of PTX Emul in beagle dogs was 24-36 mg/kg.In general,most chemotherapeutic drugs are administered at the MTD to maximize their clinical efficacy[43].Therefore,the enhanced MTD and LD50of PTX Emul could attenuate the toxicity of PTX and greatly amplify its antitumor efficacy.The reduced acute toxicity of PTX Emul may also benefit from the removal of Cremophor EL?and its high encapsulation efficiency.
The long-term toxicity and toxicokinetics of PTX Emul were determined and compared with those of PTX injection in beagle dogs to further explore the safety and underlying mechanism of less toxicity.In consideration of the repeated administrations of PTX in the clinic,long-term toxicity was evaluated through the intravenous infusion of PTX Emul to beagle dogs every week for 4 weeks.The PTX-free lipid emulsion did not induce any systemic toxicity,indicating the good biocompatibility of the lipid emulsion as an NDDS.Compared with PTX injection,PTX encapsulated into a lipid emulsion could alleviate the systemic toxicity of PTX at an equivalent dose(12 mg/kg),as evidenced by the symptoms of the general behavior and the change in body weight during the experiment.Although PTX injection and PTX Emul had similar toxic target organs including thoracic glands,spleens,mesenteric lymph nodes,bone marrow,and gonads,the degree of toxicity induced by PTX was less when it was in PTX Emul compared with that when it was in PTX injection.The alleviated systemic toxicity of PTX Emul may be attributed to the altered toxicokinetic characteristics of PTX Emul in beagle dogs.The AUC0-tof PTX Emul was significantly lower than that of PTX injection at the same dose(12 mg/kg)after the initial administration and repeated administration.In addition,high CLzandVzwere detected,demonstrating that PTX Emul was easily distributed into the peripheral tissues and had lower exposure in blood compared with PTX injection,which is beneficial in reducing systemic toxicity.The plasma pharmacokinetic characteristics of PTX Emul in beagle dogs were consistent with those in SD rats.Tissue distribution results of PTX Emul in SD rats demonstrated that PTX Emul showed a significant increase of concentration in the liver and spleen and a significant decrease of concentration in the heart and kidney compared with PTX injection[29].The high Vz laid the foundation for the tumor-targeting distribution of PTX Emul in vivo,which was confirmed in nude mice bearing MDA-MB-231 tumors in our previous study[21].Therefore,the high CLzandVzof PTX Emul may also be beneficial in improving the antitumor efficacy of PTX Emul by promoting tumor-targeting distribution in vivo.The excretion of PTX Emul in SD rats was determined by the[3H]PTX isotope tracer method.These results showed that the average recovery rate of total radioactivity was 91.48%,among which the average total excretion rate of urine samples was 11.30%,that of fecal samples was 78.83%,that of cage cleaning solution was 0.91%,and that of the residual amount of cadaveric and tissue was 0.27%of the dose,indicating that there was almost no drug residue in the tissue(Fig.S7).
The specific structure of PTX Emul,which consists of an oil core for PTX-cholesterol complex solubilization surrounded by a monolayer of a phospholipid,might be the cause of the high encapsulation efficiency,sustained drug release profile,and altered toxicokinetic characteristics of PTX Emul compared with those of PTX injection.Taken together,the properties of PTX Emul,including the small average particle size with a uniform size distribution,high encapsulation efficiency,excellent colloidal stability,and altered toxicokinetic characteristics,provide a solid basis for its in vivo safety profiles.However,one limitation of this study is the biological analysis,with the lack of innovation in the method or method application.
5.Conclusion
PTX Emul,an intravenous lipid emulsion based on the PTXcholesterol complex,was developed.Its characteristics,colloidal stability,and systemic safety profiles in terms of acute toxicity,long-term toxicity,and toxicokinetics were evaluated and compared with those of PTX injection.PTX Emul exhibited an ideal average particle size with uniform size distribution and excellent colloidal stability.Systemic safety evaluation demonstrated that PTX Emul did not induce hypersensitivity reactions,had no hemolytic potential,and alleviated acute and long-term toxicity by altering the toxicokinetic characteristics in vivo.This fundamental understanding of the systemic safety profiles,high tumor-targeting efficiency,and superior antitumor activity in vivo of PTX Emul provides powerful evidence for its application as a future treatment for breast cancer.
CRediT author statement
Jun Ye and Lin Li:Conceptualization,Methodology,Investigation,Formal analysis,Writing-Original draft preparation;Jiye Yin,Hongliang Wang,Renjie Li,and Yanfang Yang:Methodology,Investigation;Yongbiao Guan and Xuejun Xia:Supervision,Project administration;Yuling Liu:Supervision,Project administration,Funding acquisition,Writing-Reviewing and Editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was financially supported by the National Science and Technology Major Project of China(Grant No.:2018ZX09711001),Beijing Nova Program(Grant No.:Z211100002121127),Beijing Natural Science Foundation(Grant No.:L212059),Fundamental Research Funds for the Central Universities(Grant No.:3332021101),and CAMS Innovation Fund for Medical Sciences(CIFMS,Grant No.:2022-I2M-JB-011).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2022.08.002.
 Journal of Pharmaceutical Analysis2022年6期
Journal of Pharmaceutical Analysis2022年6期
- Journal of Pharmaceutical Analysis的其它文章
- Corrigendum to“The potential of miRNA-based therapeutics in severe acute respiratory syndrome coronavirus 2(SARS-CoV-2)infection:A review”[J.Pharma.Anal.11(2021)265-271]
- Development of a surface plasmon resonance biosensor for accurate and sensitive quantitation of small molecules in blood samples
- Peptide-RNA complexation-induced fluorescence“turn on”displacement assay for the recognition of small ligands targeting HIV-1 RNA
- Fluorescent aptasensor for detection of live foodborne pathogens based on multicolor perovskite-quantum-dot-encoded DNA probes and dual-stirring-bar-assisted signal amplification
- Metabolomic and elemental profiling of blood serum in bladder cancer
- A highly efficient protein corona-based proteomic analysis strategy for the discovery of pharmacodynamic biomarkers
