COVID-19 drug-induced liver injury: A recent update of the literature
Lekha Saha, Soumya Vij, Kajal Rawat
Abstract The severity of coronavirus disease 2019 (COVID-19) may be correlated with the risk of liver injury development. An increasing number of studies indicate that degrees of hepatotoxicity have been associated with using some medications in the management of COVID-19 patients. However, limited studies have systematically investigated the evidence of drug-induced liver injury (DILI) in COVID-19 patients. An increasing number of studies indicate that degrees of hepatotoxicity have been associated with using some of these medications in the management of COVID-19 patients. Significantly, it was relieved after the cessation of these agents. However, to our knowledge, no studies have systematically investigated the evidence of DILI in COVID-19 patients. In this review, we discussed the association between hepatotoxicity in COVID-19 patients and the drugs used in these patients and possible mechanisms of hepatotoxicity. The currently available evidence on the association of different therapeutic agents with hepatotoxicity in COVID-19 patient was systematically reviewed.
Key Words: Liver injury; COVID-19; Anti-COVID drugs; Mechanisms; Clinical evidences
INTRODUCTION
A novel coronavirus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was initially discovered in Wuhan, China in early December 2019. This discovery was followed by an outbreak that spread across the globe. SARS-CoV-2 is a new pathogen in the coronavirus family, and the condition it causes is called coronavirus disease 2019 (COVID-19), a severe acute respiratory condition[1]. As of August 28, 2022, over 598 million confirmed cases and over 6.4 million deaths had been reported globally by the World Health Organization[1]. Patients commonly present with symptoms of fever, dry cough, and fatigue; however, symptom severity varies. Severe symptoms include respiratory distress, high-grade fever, chest discomfort, and loss of appetite. The disease is usually self-limiting,with recovery in nearly 80% of patients with no significant interventions required. Up to 15% may need respiratory support, and 5% of patients need intensive care due to severe disease[2,3].
COVID-19 consists of a systemic disease that can present numerous complications. Complications from the severe illness include acute respiratory distress syndrome, septic shock, and thrombolytic and multiorgan failure affecting the liver, heart, and kidneys[4]. The virus can affect not only the lungs of the patient but also other tissues that express the angiotensin-converting enzyme (ACE)-2 receptor, such as the vascular endothelium, gastrointestinal tract, and squamous epithelium of the nasal, oral mucous,and pharynx. Therefore, COVID-19 is a systemic illness that may cause arrhythmia, thrombolytic episodes, prolongation of the QT interval, acute coronary syndrome, myocardial dysfunction,ketoacidosis, hepatocellular damage, kidney injury, neurological symptoms, hyperglycemia, sepsis, and,in more severe cases, multiorgan failure.
Patients with COVID-19 experience various degrees of liver function abnormalities. Numerous studies have demonstrated that liver damage is frequent in people with COVID-19 and may contribute to the severity of the disease[5-7]. Liver damage can be complicated and varied, requiring a thorough investigation and continuous monitoring. In the context of COVID-19, clinicians will have to determine whether the liver injury is related to underlying liver disease, drugs used to treat COVID-19, the direct effect of the virus, or a complicated disease course. Recent studies have suggested a number of potential mechanisms of probable liver injury in COVID-19 patients. However, the exact cause and specific means of COVID-associated liver injury need to be elucidated further. Drug-induced liver injury (DILI) should be closely monitored in COVID-19 patients, especially considering the widespread off-label usage of medications in preventive and therapeutic regimens.
Liver disorders in COVID-19 patients using several concomitant off-label drugs (potentially causing further liver damage) should be a warning sign for rapid identification and early intervention, thus preventing severe impairment in patients. The inherent liver damage associated with COVID-19 patients and the use of numerous concurrent off-label medications leading to increased severity of liver damage should be a warning indicator for early diagnosis and treatment, averting severe impairment in COVID-19 patients. This review summarizes current evidence related to hepatobiliary complications in COVID-19, provides an overview of the available evidence and critically elucidates the proposed mechanisms. We anticipate that this review will assist medical professionals in developing more effective methods for managing such patients.
COVID-19 AND ITS IMPACT ON THE LIVER
The effect of COVID-19 on liver functions can be observed by abnormalities in the levels of liver enzymes. There may be a decrease in the albumin levels as well as increasing bilirubin levels.Laboratory findings of liver injury include an increase in aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase, lactate dehydrogenase,hyperbilirubinemia, prolonged prothrombin time. and hypoalbuminemia (Table 1)[8-11].
Any alterations in liver function can be either due to parenchymal injury, cholestasis, or mixed type of etiology[12,13]. The incidence of a parenchymal, cholestatic, and mixed form of liver injury was observed to be 75.0%, 29.2%, and 43.4%, respectively, in patients with COVID-19 infections. The elevation in liver enzymes (AST and ALT) was found to be within five times the upper limit of normal.The rates of ALT and AST increases were observed to be in a range of 2.5%-50.0% and 2.6%-61.1%,respectively. It was observed that 56% elevation in AST and 28% in ALT was observed in those patients who had severe COVID-19 infection[14]. Elevation of gamma-glutamyl transferase may occur in up to 28% of patients, whereas ALT elevation was observed in nearly 23% of patients as are the AST levels.Increases in gamma-glutamyl transferase have been observed to increase mostly in severe forms of infection and could be possibly linked to drug toxicity[15]. AST level increases can be linked to hepatocyte injuries and mitochondrial damage by the virus. It was observed that an AST/ALT ratio > 1 helps predict the occurrence of severe pneumonia, need for intensive care, and risk of mortality.Elevations in ALP were rare but occurred in patients who had either multiorgan failure, liver failure or died due to COVID-19 infection[16].
Liver damage in patients with COVID-19 is currently limited to moderate to severe cases and the damage may be transient, with liver tests returning to normal without needing specific treatment[4,17].The acute or chronic liver failure occurrence is yet to be investigated. However, the severity of the condition, the likelihood that a patient would require admission to the intensive care unit or a lengthy hospital stay[18], and the risk of death, all increase with higher serum levels of AST/ALT and total bilirubin[19]. In patients with pre-existing conditions, the occurrence of liver injury may be aggravated or accelerated. Some of these conditions include fatty liver disease with co-existing metabolic disorders,reactivation of viral hepatitis, as well as other causes of chronic liver diseases.
Some liver biopsy and histopathology specimens that were analyzed from patients with COVID-19 showed moderate microvascular steatosis and mild lobular and portal vein activity. There was evidence of infiltration of lymphocytes, sinusoidal expansion of the central lobule, and patchy necrosis[4]. There were no signs of severe liver injury such as extracellular fibrosis, coagulation necrosis, or severe cholestasis in the biopsy specimens examined. The difficulty to perform autopsy and liver biopsies of COVID-19 patients due to the virus’ high transmissibility and the lack of guidelines for defending medical practitioners during the start of the pandemic lead to the deficit of histological information in the literature[17].
Several studies have reported that severe cases of COVID-19 were more likely to have severe liver injury than mild cases[20-22]. Male patients were more likely to have liver function injuries than females(P< 0.05)[23]. The association of multiorgan abnormalities has also been observed to correlate with the severity of the disease and the worsening of clinical outcomes. Liver involvement is not necessarily associated with a picture of multiorgan failure but may be seen in early disease stages. However, in these patients, there is no need for additional therapy for recovery of hepatic functions and is generally reversible. The frequency of liver damage in COVID-19 patients ranges from 14.8% to 53.0%[24].
POTENTIAL MECHANISMS OF LIVER INJURY IN PATIENTS WITH COVID-19
Direct effect of viral infection on the liver
The etiopathogenesis of liver damage related to direct COVID-19 infections has not been completely determined yet. However, it is hypothesized to be direct hepatocellular damage, as is observed by the elevation in the cytonecrosis enzymes levels (Figure 1). ACE2 is a key receptor of the SARS-CoV-2 virus[25]. The ACE2 receptor is expressed in the lungs and the bile duct cells and acts as a gateway for the entry of the virus. The cell-specific expression of the ACE2 receptor in healthy liver tissues was evaluated by Chaiet al[6] and found that ACE2 was expressed in 2.6% of hepatocytes and 59.7% of cholangiocytes, suggesting that liver injury may be caused by direct viral invasion. The hypothesis of direct viral damage can also be explained by liver biopsies that show signs of hepatocyte apoptosis,along with the presence of viral RNA in the tissue[26]. A recent study by Wuet al[27] found that half of the COVID-19-infected patients who had completely cleared respiratory tract infections had virus shedding in their fecal specimens up to 11 d after viral detection in the respiratory tract samples became negative. This suggests that there may be viral replication in extrapulmonary sites (digestive tract and liver).
Role of endotheliitis in hepatic injury
Another suggested mechanism of liver injury is secondary to the hypercoagulability leading to thrombosis of the portahepatic system. Occurrence of hypoxia as a result of pulmonary involvement may lead to a state of hypoxia in liver tissue followed by reperfusions, thereby causing an influx of reactive oxygen species and worsening the proinflammatory state[28]. The inflammation of the vascular endothelium caused by SARS-CoV-2 infection leads to microvascular dysfunction, which leads to a hypercoagulable state, tissue edema, and organ ischemia[29,30]. It was previously reported that a variety of viruses, such as dengue fever virus, human immunodeficiency virus (HIV), and Ebola virus,have been affecting the coagulation system[29]. The underlying mechanism of the hepatic injury associated with COVID-19 may be the liver ischemia-reperfusion injury, a pathophysiological process that frequently occurs following a rapid recovery of blood circulation to the liver tissue. When there is vascular endothelial damage, either directly by virus or through immune-mediated inflammation, the vessels have a vasoconstriction and procoagulant state (Figure 1). Studies in the literature demonstrated significantly higher plasma levels of fibrinogen and D-dimer in severe cases of COVID-19 than those in healthy controls[31-33].
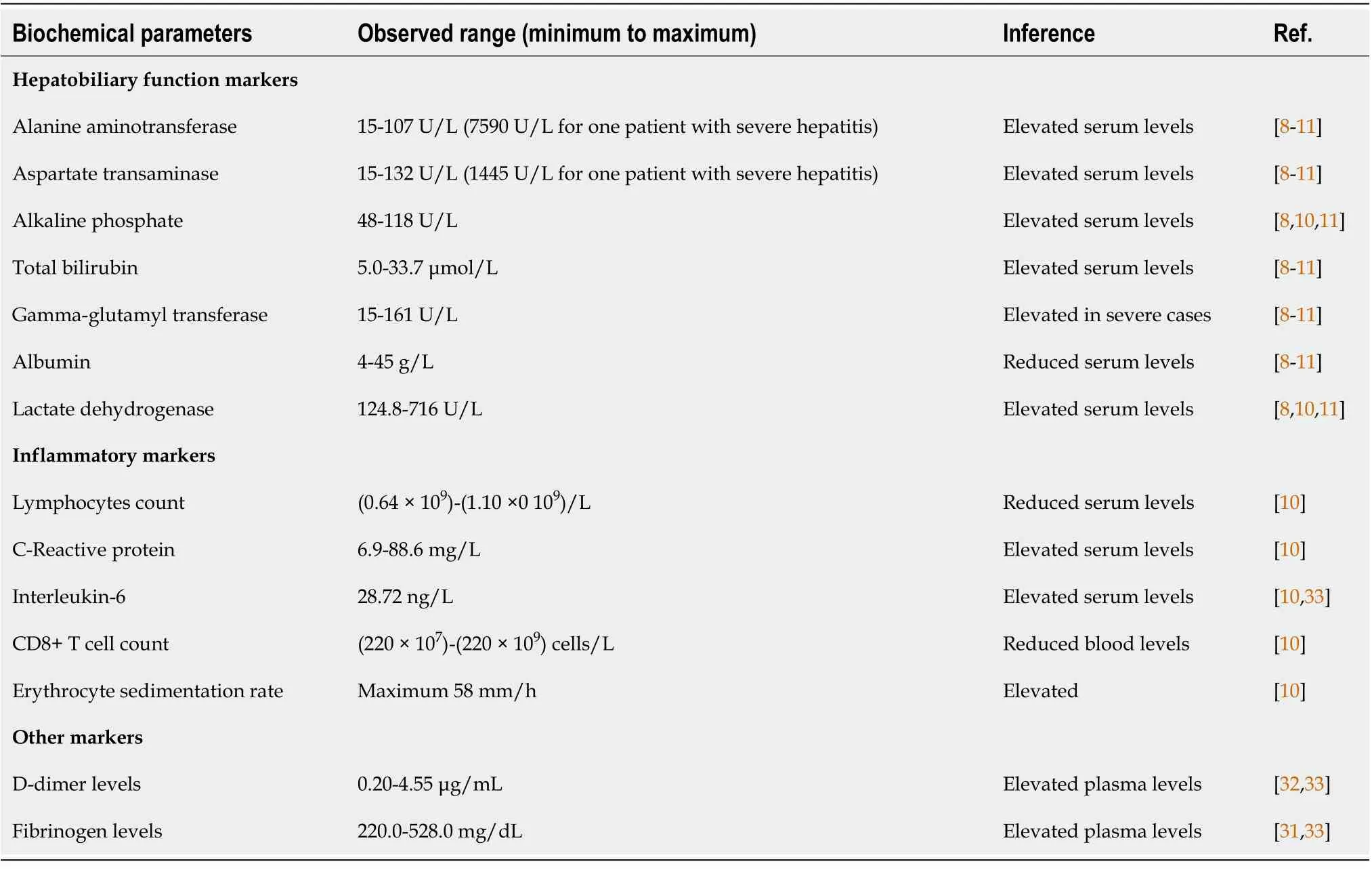
Table 1 Clinical evidence of coronavirus disease 2019-mediated impact on various biochemical indexes
Inflammatory storm in COVID-19-associated hepatic injury
The excessive immune response induced by COVID-19 infection causes an inflammatory cytokine storm that might also be one of the critical factors in hepatic injury (Figure 1)[34,35]. Lower lymphocyte counts(both helper T cells and suppressor T cells) and higher plasma levels of various inflammatory cytokines[monocyte chemoattractant protein 1, interleukin (IL)-2, IL-10, IL-7, GCSF, MIP1A, IP10, and tumor necrosis factor-alpha] were commonly observed in patients with COVID-19, especially in the critically ill patients[3,36]. Increases in IL-6 and IL-10 as well as a decrease in CD4+ T cells were found to be independent risk factors for severe liver damage in a cohort study of 192 patients[37]. Another study discovered an independent relationship between lymphopenia and C-reactive protein levels and hepatic damage[38].
Systemic inflammatory response syndrome in patients presenting with moderate to severe illness leads to uncontrolled immune-mediated inflammatory response secondary to the release of cytokines and other inflammatory markers such as interleukins and tumor necrosis factors. The state of cytokine storm leads to secondary liver injury by causing systemic inflammation, and the patients can often present with multiorgan failure[39].
The liver injury associated with COVID-19 was determined to lead to a poorer patient outcome. This could be due to the involvement of other organ systems as well. In severe and critical patients, there was a rise in the biomarkers of inflammation, myocardial injury, liver and kidney functions, and coagulation functions[20]. Patients with liver injury usually had a longer hospital stay, and in those with a preexisting liver disease, the risk of hospitalization and mortality was higher. Systematic reviews have shown that an elevation in liver biochemistry during the first visit was also found to be an indicator of the disease severity.
In a metanalysis of over 15000 patients with COVID-19, liver injury was reported in 23.1% patients in the early period and 24.4% incidence in the overall disease course. They found that 48.5% of the cases of liver injury were reported within the 1st2 wk of admission, and 26.7% patients with severe pneumonia had liver injury as well[12]. The incidence of liver injury in patients who have died from COVID-19 was found to be as high as 58%-78%[40,41].
The poor prognosis of patients developing liver injury is possibly related to patients older than 60 years, severe COVID-19 infection, and underlying metabolic diseases such as hypertension and diabetes. Therefore, it was concluded that for patients with other comorbidities, there is a greater need for in-depth monitoring and individualizing the treatment given[42].
COVID-19 DILI: An overview
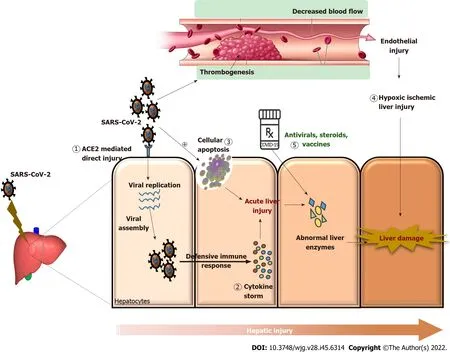
Figure 1 Molecular mechanisms of severe acute respiratory syndrome coronavirus 2 and coronavirus disease 2019 drug-induced liver injury. 1: Direct action of the virus, i.e., angiotensin-converting enzyme 2 receptor mediated action; 2: Immuno-inflammation caused by cytokine storm leading to liver damage; 3: Hepatocellular apoptosis induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection; 4: SARS-CoV-2-induced thrombosis leading to vascular endothelial damage and causing hypoxic-ischemic liver injury; 5: Several drugs and therapies used to counteract coronavirus disease 2019 including antivirals, immunomodulatory agents, and vaccines reportedly led to abnormal liver enzymes causing drug-induced liver injury. All these mechanisms aggravate liver injury by causing abnormal liver enzymes like increasing the levels of alanine aminotransferase, aspartate transaminase, alkaline phosphate, gammaglutamyl transferase, lactate dehydrogenase, and total bilirubin and reducing the albumin levels. ACE2: Angiotensin converting enzyme-2; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; COVID-19: Coronavirus disease 2019.
DILI is a rare diagnosis and can occurviamany drugs, leading to liver damage. DILI may be severe but rarely fatal; recovery after drug discontinuation generally occurs. DILI is defined by increased liver enzymes either due to hepatocellular necrosis, cholestasis, or both[43]. The hepatocellular injury pattern resembles acute viral hepatitis-induced injury, hallmarked by hepatocyte necrosis and inflammation.Agents that typically cause this injury pattern include isoniazid, nitrofurantoin, and methyldopa.Cholestatic injury resembles bile duct obstruction, hallmarked by bile stasis, portal inflammation, and proliferation or injury of bile ducts and ductules. Drugs that exhibit this type of injury include amoxicillin/clavulanate, ciprofloxacin, and sulfonylureas. Mixed type liver injury is most characteristic of DILI, with the liver biopsy showing necrosis and inflammation along with bile stasis[44].
DILI may be caused by either an intrinsic or a distinctive mechanism. The clinical pattern associated with DILI is usually varied and can mimic other forms of liver disease. The patient’s presentation can resemble acute hepatitis, acute liver failure, chronic hepatitis, biliary obstruction, or fatty liver disease.As the evidence of DILI concerning various drugs is often large and scattered across multiple disciplines, it becomes difficult for clinicians and researchers to search for evidence related to each drug.Therefore, the reference for the most established evidence related to a drug and its ability to cause liver injury isviadatabases such as LiverTox[44].
Intrinsic DILI is usually dose-dependent and predictable and occurs after a brief latency period. The most common DILI is idiosyncratic, and it is primarily unpredictable, with a variable latency period[45]. The severity of DILI may vary, and up to 10% of severe cases may need a liver transplant for the same. Mortality is three times higher in patients with pre-existing liver disease, along with a DILI.Monitoring is vital in a patient exhibiting signs of DILI. Any signs of jaundice, hyperbilirubinemia,hepatomegaly, or new onset right upper quadrant pain warrant further investigations and discontinuation of the suspected drugs[4]. Mostly, liver injury is reversible, with recovery after drug discontinuation. One possible treatment option is ursodeoxycholic acid, which protects elevated transaminase and serum total bilirubin levels[46].
The Roussel Uclaf Causality Assessment Method scale is a seven-domain scale, which considers the temporal association of liver injury, various risk factors such as age, alcohol intake, and concomitant hepatotoxic drugs, and occurrence of repeated liver damage on the administration of the suspect drug.It also helps in the exclusion of other causes of transaminase elevations, such as ischemia, myositis,cytokine storm, and previous chronic liver disease[4].
The societal pressures of COVID-19 infections, including hospital bed shortages and added stress to the health system, are further compounded by liver injury secondary to drug use. Although in mild to moderate cases, the risk of mortality remains low, it still leads to the added burden on healthcare systems due to prolongation of hospital stays and an increased risk of contracting a nosocomial infection[47].
The treatment of COVID-19 saw the use of many therapeutic options, from the repurposing of older drugs to new drug trials and the administration of new vaccines to prevent infection. Assessing the hepatotoxicity profile of drugs used in COVID-19 is complicated as many drugs have been used offlabel and with dosages not routinely used. Thereby, reports of DILI may be rarely encountered in routine practice with some drugs but may be more often when used in COVID-19[48].
LITERATURE REVIEW
The general information on SARS-CoV-2-associated hepatic injury was gathered by using a systematic search strategy. The eligible systematic reviews, meta-analysis, and review articles on COVID-19-induced and COVID-19 DILI were searched in four databases: EMBASE, Scopus, MEDLINE, and Web of Science. The literature search was performed through September 5, 2022 and terms used for search across all the databases included: ((COVID-19) AND (Drug-induced) AND (Hepatotoxicity)) OR((SARS-CoV-2) AND (Drug-induced) AND (Hepatotoxicity)) OR ((COVID-19) AND (Drug-induced)AND (Hepatic injury)) OR ((SARS-CoV-2) AND (Drug-induced) AND (Hepatic injury)) OR ((COVID-19) AND (Drug-induced) AND (Liver injury)) OR ((SARS-CoV-2) AND (Drug-induced) AND (Liver injury)). The search returned a total of 113 articles, which were further screened for duplicates. A total of 56 articles were included, all fitting the eligible criteria,i.e.,reviews and meta-analyses on COVID-19-induced and COVID-19 DILI.
Evidence of hepatotoxicity risk associated with COVID-19 therapy
One meta-analysis reported the incidence of drug toxicity with COVID-19 therapy as high as 25.4%[49].Some of the most commonly reported cases of hepatotoxicity have been associated with the prescription of antipyretics such as acetaminophen, anti-inflammatories such as tocilizumab, and antivirals such as favipravir, remdesivir, lopinavir/ritonavir (LPV/r), chloroquine, oseltamivir, and ribavirin (Table 2). In patients with pre-existing chronic liver disease or with severe infection, the risk of developing hepatotoxicity was reported to be higher[50]. The liver is the leading site for metabolism and elimination for many drugs. Some of the drugs that were repurposed for COVID-19 treatments such as nucleoside analogs and protease inhibitors are primarily metabolized in the liver[10] (Table 2).
ANTIVIRAL DRUGS AND LIVER INJURY
One of the antiviral drugs explored for therapy in COVID-19 included lopinavir-ritonavir, a combination widely prescribed in the treatment of HIV/AIDS. As a combination, it has been previously reported to be associated with moderate to severe elevations in serum aminotransferases. The mechanism of liver injury is probably due to metabolism through the CYP3A4 system[51]. Various studies reported liver function abnormalities when patients were treated with LPV/r[52]. The incidence of reported adverse events with the use of this combination was as high as 63.8% in one study, with the second most common adverse event being liver injury[53] (Table 2).
A pooled meta-analysis reported the incidence of DILI with LPV/r as 37.2%[49]. Once hepatotoxicity develops with LPV/r or ribavirin, restarting the drug is not recommended[54]. LPV/r prescription is associated with moderate to severe elevations in serum transaminase levels. Ritonavir, given as a boosting drug in this combination, acts as an enzyme inhibitor and may also lead to compounded hepatotoxicity of co-administered medicines[55] (Table 2).
Favipiravir is an inhibitor of RNA-dependent RNA polymerase enzyme. It has shown efficacy against Ebola, West Nile virus, yellow fever virus, and influenza. It acts by embedding in the viral RNA,causing mutations and leading to viral death[56]. It has been demonstrated to have activity against the SARS-CoV-2 virus by interfering with the viral replication by competing with the purine nucleosides.There have been some reports of elevation in the hepatic transaminases with favipiravir therapy, but it was generally mild and reversible[57] (Table 2).
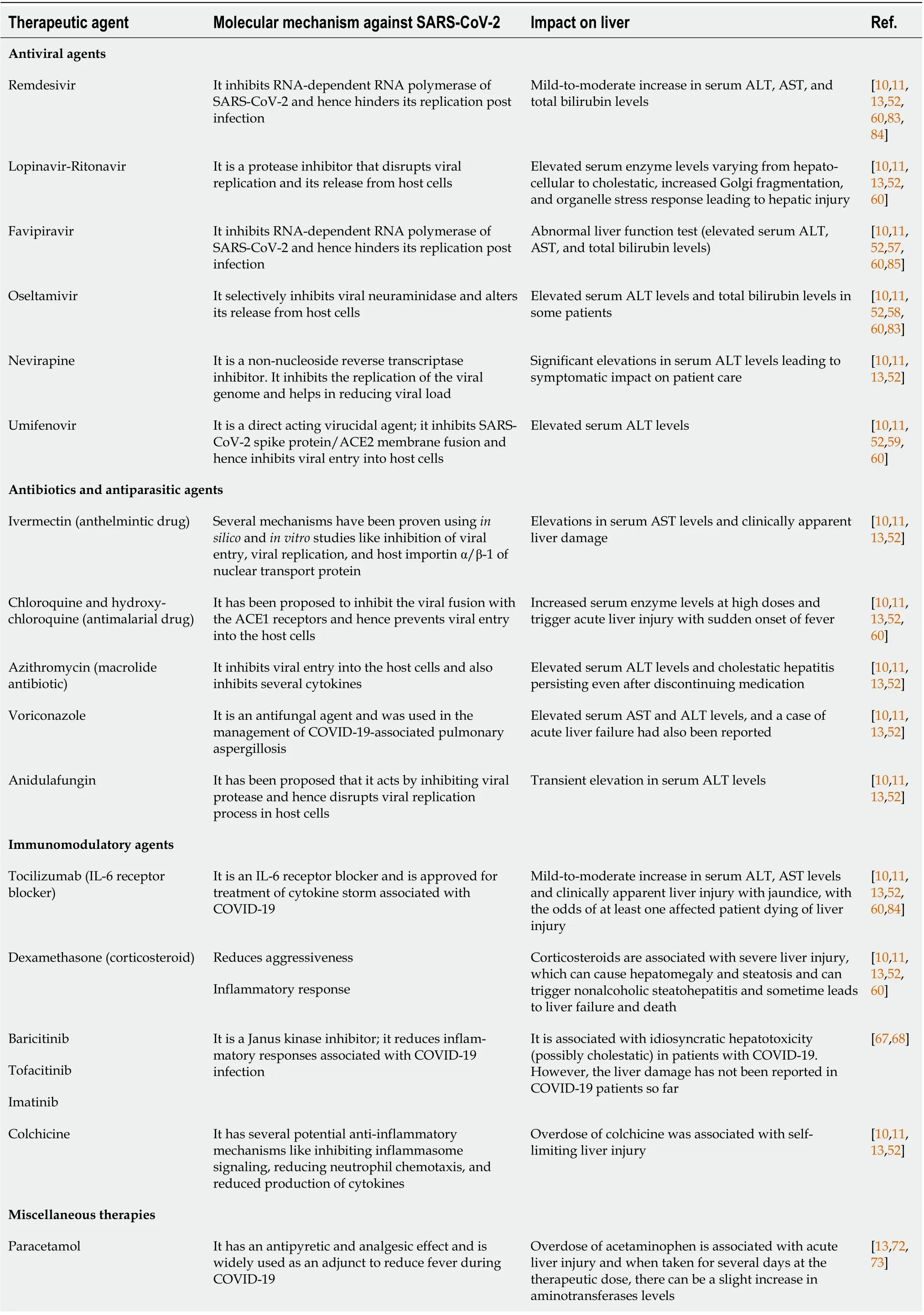
Table 2 Clinical evidence of coronavirus disease 2019 drug-induced liver injury
Enoxaparin It is low molecular weight heparin and was indicated in thromboembolic venous complications of COVID-19 Elevated serum AST levels and mild elevation in serum alkaline phosphate and total bilirubin levels[10,11,13,52]LMWH It is an anticoagulant used in COVID-19 to reduce the risk of thromboembolism LMWH are associated with acute hepatotoxicity, and elevations in the liver enzymes are usually observed after 5-8 d of initiation of therapy[74,85]SARS-CoV-2 vaccines (Pfizer-BioNTech, Moderna, and Oxford-AstraZeneca)mRNA and viral vector encoding SARS-CoV-2 spike protein were used for vaccinations in healthy subjects The clinical phenotype is mostly hepatocellular and showed features of immune-mediated hepatitis[77,78]ALT: Alanine aminotransferase; AST: Aspartate transaminase; ACE: Angiotensin converting enzyme; IL-6: Interleukin 6; LMWH: Low molecular weight heparin; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; COVID-19: Coronavirus disease 2019.
Oseltamivir has also been initially explored for therapy in patients with COVID-19. The efficacy of oseltamivir could not be established in various trials and is now no longer prescribed. Some case reports have reported a transient rise in liver enzymes associated with its use; however evidence in COVID-19 is lacking[58]. Umifenovir is used for influenza treatment and was prescribed initially in COVID-19 in China. It is metabolizedviaCYP3A4, and this mechanism may be associated with the occurrence of DILI. Its use in cirrhotic patients is cautioned due to risk of liver injury[59,60] (Table 2).
Remdesivir is a nucleotide analogue that was explored for the treatment of hepatitis C virus and Ebola. One case series reported 25% and 33% increases AST and ALT, respectively[61]. The risk of abnormalities in the AST and ALT levels were higher with the use of remdesivir as compared to any other therapy for COVID-19. Elevations were noted immediately after starting remdesivir, but the gradual improvement was noted afterwards[62]. Prior to starting remdesivir, a baseline liver function test should be obtained. If the transaminase level is increased more than 10 times, then the use of remdesivir should be discontinued[63]. However, hepatotoxicity is generally asymptomatic, fully reversible, and not associated with any jaundice[64] (Table 2).
IMMUNOMODULATORS AND LIVER INJURY
Hydroxychloroquine and chloroquine are immunomodulator drugs, mainly used in autoimmune disorders. During the early days of the pandemic, these drugs were widely explored for the treatment of COVID-19 infections due to their proposed role in decreasing the hyperinflammatory states associated with COVID-19. Hydroxychloroquine is metabolized in the liver, and its metabolites may also accumulate in the liver leading to hepatotoxicity, although rarely reported[44]. One case of a 10-fold increase in transaminases was reported with administration of hydroxychloroquine, which was reversible and recovery occurred after withdrawal of the drug[65] (Table 2).
Tocilizumab is a monoclonal antibody against the IL-6 receptor and is indicated for use in cytokine storm associated with severe disease. It has received Food and Drug Administration approval for use in COVID-19 infections. It has been demonstrated to have a transient elevation in the hepatic enzymes, but rare cases of severe liver injury have occurred[55]. Patients with a pre-existing hepatitis B viral infection have experienced reactivation of the virus after treatment with tocilizumab[66]. The recommendation suggests patients treated with tocilizumab should receive regular liver function test monitoring, and hepatitis B virus-DNA levels should be rechecked in patients with pre-existing infections after discharge from the hospital[39] (Table 2).
Baricitinib, tofacitinib, and imatinib belong to a class of Janus kinase inhibitors. Of these, baricitinib has been used the most for treating COVID-19 because of its effect on decreasing the inflammatory response to COVID-19 infection[67]. There is evidence of a nearly 1% incidence of liver enzyme and bilirubin increases with patients, but no data has been published so far for patients with COVID-19. In the randomized controlled trial conducted for baricitinib in COVID-19, there were no reports of liver damage[68] (Table 2).
Azithromycin was widely used in the beginning of the pandemic, as it had some demonstrated antiviral and anti-inflammatory activity[69]. Combined use of hydroxychloroquine and azithromycin was explored by a French study. However, the findings were disputed, and the study was withdrawn[70]. Azithromycin has a concern over causing cardiotoxicity, but there have been instances of hepatotoxicity reported with azithromycin use as well[44]. The risk of liver injury is higher in patients with pre-existing liver disease, and they are at a higher risk of developing more severe complications[71](Table 2).
MISCELLANEOUS THERAPIES AND LIVER INJURY
Paracetamol has both antipyretic and analgesic effects and is a well-known cause of dose-dependent DILI; it is usually difficult to manage and can be fatal to the patients. The underlying mechanism for toxicity is direct damage through a metabolite generated from liver metabolism[72]. Paracetamol is used as an adjunct therapy, along with many other medications, but the actual incidence of hepatotoxicity associated with acetaminophen is still undetermined[73] (Table 2).
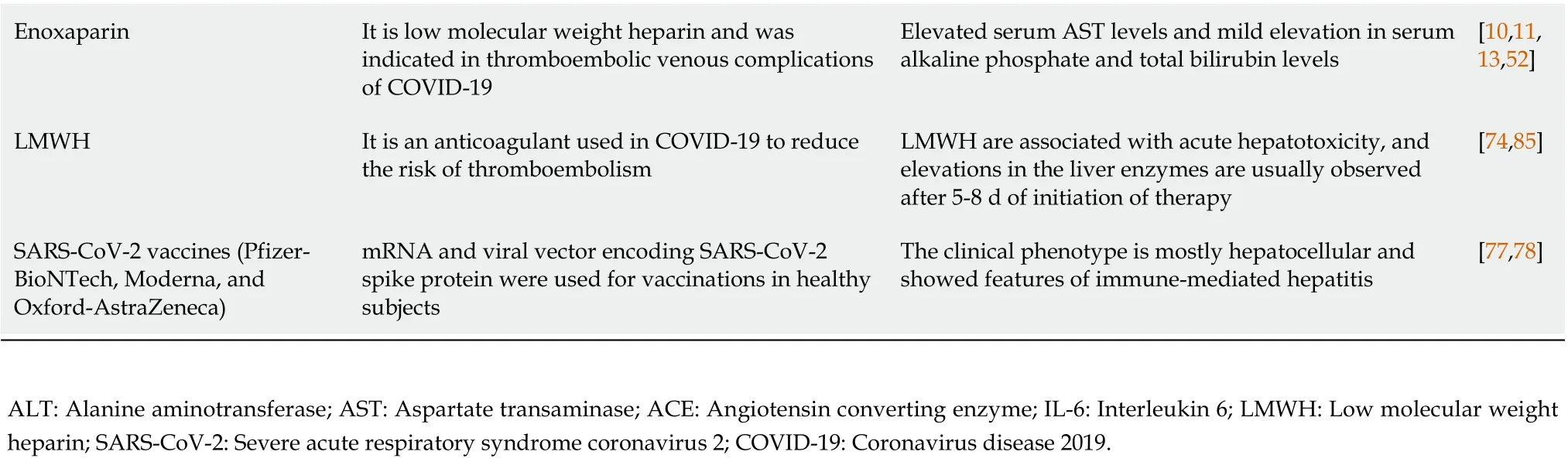
Low molecular weight heparin is one of the most commonly prescribed anticoagulants used in COVID-19 to reduce the risk of thromboembolism and its sequelae. Low molecular weight heparin is known to cause hepatotoxicity in the range of 4.3%-13.0%[74]. Liver enzyme elevation usually occurs 5-8 d after initiation of therapy and are reported to normalize within 2 wk of stopping the drugs[75](Table 2).
One of the cornerstones of treatments is the systemic administration of corticosteroids. Corticosteroids have been shown to reduce mortality in mechanically ventilated patients and in patients who require supplemental oxygen support at the time of admission. Although, corticosteroid use is associated with many adverse events, ranging from deranged glycemic control to thromboembolism,secondary infections and reactivation of hepatitis B infection[39]. The prolonged use of corticosteroids may be associated with hepatic steatosis. However, this effect is less likely to be seen with the relatively short duration of corticosteroid therapy in COVID-19 infections[76] (Table 2).
Vaccinations in healthy subjects for prophylaxis against COVID-19 had been reported to be associated with acute liver injury in some patients. A world-wide case series involving 87 patients administered with COVID-19 vaccines, including 59% of cases with the Pfizer-BioNTech (BNT162b2) vaccine, 23% of cases with the Oxford-AstraZeneca (ChAdOX1 nCoV-19) vaccine, and 18% of cases with the Moderna(mRNA-1273) vaccine, had reported clinical features of acute liver injury associated with SARS-CoV-2 vaccination[77,78]. However, the benefits of COVID-19 vaccines during the pandemic cannot be neglected because of the rare events of liver injury in the small proportion of the overall vaccinated population (Table 2).
DIAGNOSIS AND MANAGEMENT OF DILI
DILI is a diagnosis by exclusion. Patient workup includes thorough patient history, including a history of chronic liver disease, alcohol or drug abuse, and complete information on past and concomitant drug history[79]. A complete history of herbal and dietary supplement use should also be taken[80]. Patients developing liver injury require regular monitoring of ALT, AST, total and direct bilirubin, albumin,prothrombin time, and international normalized ratio[24].
According to the updated Roussel Uclaf Causality Assessment Method, the cutoffs for the diagnosis of DILI is as follows: ALT > 5 times the upper limit of normal and/or ALP > 2 times upper limit of normal. Based on the ratio of serum ALT to ALP values (R-value), DILI pattern may be hepatocellular (≥5), cholestatic (≤ 2), or mixed (> 2 and < 5)[43]; 50% of the patients with DILI exhibit a hepatocellular pattern[64].
Acute liver injury in patients with COVID-19 is usually mild and is transient, with recovery without requiring any specific therapy[79]. However, supportive therapy is often needed, such as albumin infusion or administration of fresh frozen plasma. In patients with pre-existing liver dysfunction and severe infection, the drugs prescribed should be carefully monitored, taking care that no more than two drugs with the potential to cause DILI should be administered at the same time. Also, regular monitoring of liver functions should be done for such patients. If patients develop DILI, early discontinuation should be initiated, or dosage should be altered if complete discontinuation is not possible[81].
There are no defined guidelines for managing DILI in patients with COVID-19. The most effective option available is to discontinue the offending agent. This leads to recovery in 90% of patients. In cases where required, therapy can be initiated with medications such as ursodeoxycholic acid[82]. Some other therapeutic options available include drugs such as polyene phosphatidylcholine, glycyrrhizic acid, and adenosylmethionine. Glycyrrhizic acid has been demonstrated to have a strong affinity for liver enzymes and has anti-inflammatory, anti-allergic, and hepatoprotective effects[52]. Patients with COVID-19 are at a higher risk of hepatic injury, and therefore drugs such as nonsteroidal anti-inflammatory drugs, acetaminophen, and antibiotics, which have the potential of causing liver injury, should be given to these patients cautiously. A small number of healthy subjects presented SARS-CoV-2 vaccination-associated liver injury, predominantly immune-mediated hepatitis, were managed using corticosteroid therapy[78].
CONCLUSION
There are variable degrees of impaired liver function in patients with COVID-19. Liver damage can be complicated and varied, requiring significant investigations and continuous monitoring. Clinicians treating patients with COVID-19 must first evaluate if the liver damage is a result of underlying liver illness, therapies used to treat COVID-19, a direct result of the virus, or a complex disease course. Recent studies proposed a number of mechanisms regarding possible causes of liver damage in COVID-19 patients. This review gave an overview of the case series that are currently available, highlighted the most recent research on hepatobiliary consequences in COVID-19, and critically explained the putative mechanisms underlying liver damage. We anticipate that this review will assist medical professionals in creating more effective plans for managing such patients.
FOOTNOTES
Author contributions:All authors were equally involved in the literature search and manuscript writing and editing.
Conflict-of-interest statement:All authors report having no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORCID number:Lekha Saha 0000-0001-5925-7159.
S-Editor:Wang JJ
L-Editor:Filipodia
P-Editor:Wang JJ
 World Journal of Gastroenterology2022年45期
World Journal of Gastroenterology2022年45期
- World Journal of Gastroenterology的其它文章
- Rifabutin as salvage therapy for Helicobacter pylori eradication: Cornerstones and novelties
- Germline BRCA2 variants in advanced pancreatic acinar cell carcinoma: A case report and review of literature
- Meta-analysis on the epidemiology of gastroesophageal reflux disease in China
- Endoscopic mucosal resection-precutting vs conventional endoscopic mucosal resection for sessile colorectal polyps sized 10-20 mm
- Best therapy for the easiest to treat hepatitis C virus genotype 1b-infected patients
- Deep learning based radiomics for gastrointestinal cancer diagnosis and treatment: A minireview
