Peptide backbone-copper ring structure:A molecular insight into copper-induced amyloid toxicity
Jing Wang(王靜) Hua Li(李華) Xiankai Jiang(姜先凱) Bin Wu(吳斌) Jun Guo(郭俊)
Xiurong Su(蘇秀榕)1, Xingfei Zhou(周星飛)1, Yu Wang(王宇)4, Geng Wang(王耿)4,Heping Geng(耿和平)4, Zheng Jiang(姜政)4, Fang Huang(黃方)5, Gang Chen(陳剛)4,6,§,Chunlei Wang(王春雷)7, Haiping Fang(方海平)8, and Chenqi Xu(許琛琦)2,9,?
1Faculty of Science,Ningbo University,Ningbo 315211,China
2National Center for Protein Science Shanghai,State Key Laboratory of Molecular Biology,Institute of Biochemistry and Cell Biology,Shanghai Institutes for Biological Sciences,Chinese Academy of Sciences,Shanghai 200031,China
3School of Sciences,Changzhou Institute of Technology,Changzhou,213032,China
4Shanghai Synchrotron Radiation Facility,Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,China
5Center for Bioengineering and Biotechnology,China University of Petroleum(Huadong),Qingdao 266580,China
6School of Physical Science and Technology,ShanghaiTech University,Shanghai 200031,China
7Shanghai Advanced Research Institute,Chinese Academy of Sciences,Shanghai 201210,China
8School of Physics and National Engineering Research Center of Industrial Wastewater Detoxication and Resource Recovery,East China University of Science and Technology,Shanghai 200031,China
9School of Life Science and Technology,ShanghaiTech University,Shanghai 200031,China
Keywords: interactions between metal ion and protein, quantum chemistry calculation, protein aggregation,amyloid diseases
1. Introduction
Copper ions are closely associated with amyloid diseases,such as type 2 diabetes (T2D), Alzheimer’s disease (AD),Parkinson’s disease (PD), and amyotrophic lateral sclerosis(ALS).[1–7]Clinical findings showed that the Cu concentrations of T2D and AD patients were significantly higher than that of healthy people.[8,9]However,the mechanism of Cu2+-mediated amyloid toxicity remains obscure.[7,10]There are two prevailing views, the oxidative stress hypothesis and the oligomer hypothesis, for amyloid toxicity. In the first hypothesis, oxidative stress was suggested as a major player in the pathogenesis of amyloid diseases. The binding of redox-active Cu2+to the amyloid peptide can induce the donation of electrons from amyloid peptide to Cu2+ion to form a peptide-Cu+complex, which can stimulate oxidative stress in a toxicity manner.[11–13]In the second hypothesis,oligomers or oligomer-like structures,rather than fibrils,were proposed to be the major cause of amyloid diseases.[14,15]Cu2+can promote the oligomerization of T2D-related human islet amyloid polypeptide (hIAPP) and inhibit the fibrillation of these peptides.[16,17]Under certain conditions, Cu2+and small molecules can also promote the oligomerization of ADrelated amyloid-βpeptides(Aβ)in a concentration-dependent manner.[18–21]These different views highlight the importance of elucidating the interaction mode of Cu2+with amyloid peptides at the atomic level,which can largely promote the understanding of copper-induced amyloid toxicity.
Here,based on various biophysical approaches and theoretical analyses, we investigated the interaction of Cu2+with hIAPP(Protein Data Bank(PDB)code 2KB8),which is a typical amyloid peptide that is implicated in T2D.[22]We revealed that Cu2+can specifically bind to the backbone of hIAPP by forming a ring structure,causing the reduction of Cu2+to Cu+to produce reactive oxygen species, the inhibition of hIAPP fibrillation and the promotion of peptide oligomerization.
2. Results and discussion
2.1. Backbone-Cu ring structure of hIAPP peptide
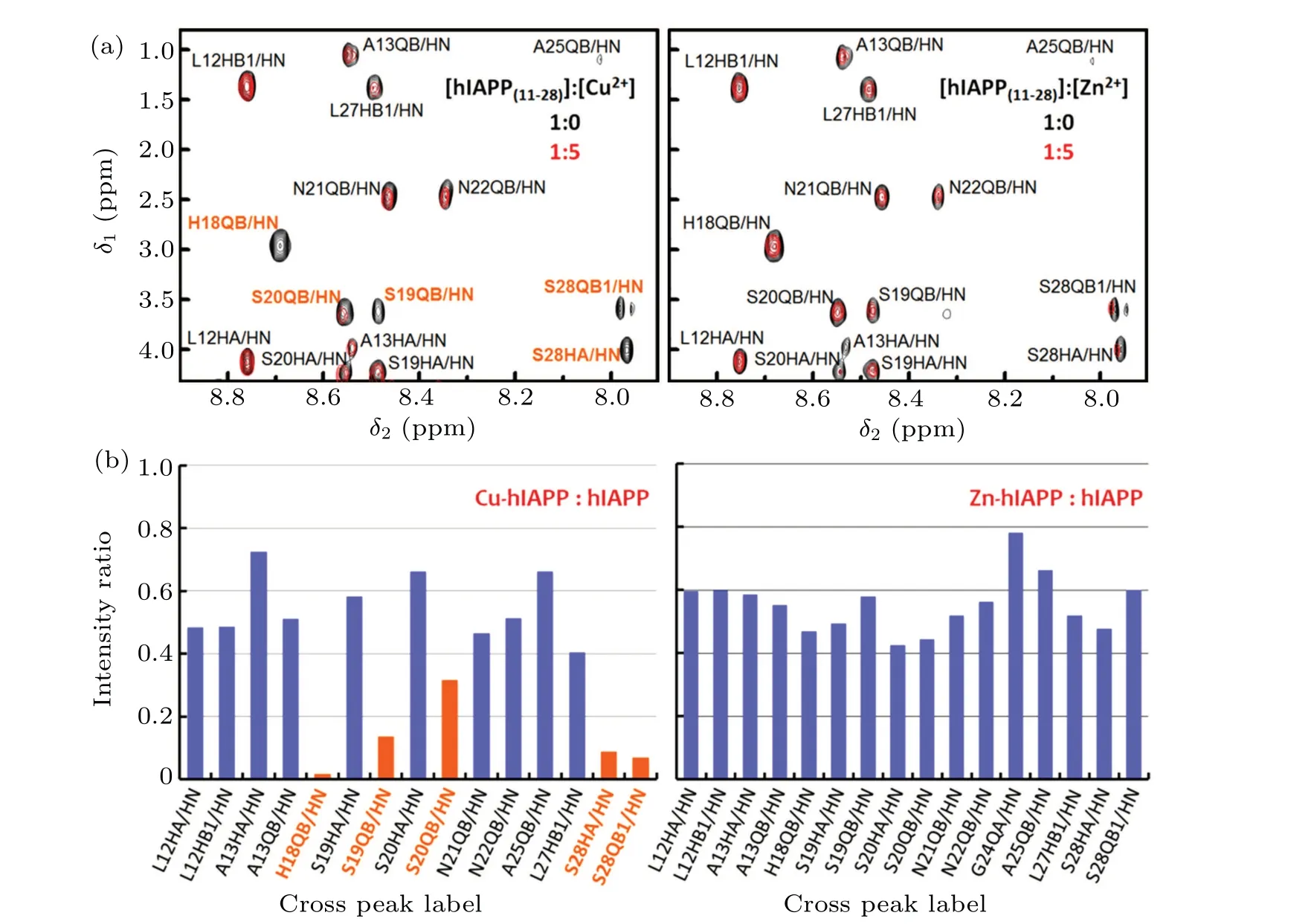
Fig.1. Specific binding of Cu2+ to the backbone of hIAPP.(a)Superimposed 1H–1H TOCSY spectra of the hIAPP(11-28)peptide in the region of the backbone amide groups. Left: with (red) and without (black) the addition of Cu2+. Right: with (red) and without (black) Zn2+. The orange labels indicate the cross peaks exhibiting a substantial change caused by the addition of metal ions. (b)Cross peak intensity changes in hIAPP(11-28)caused by Cu2+ and Zn2+. The intensity ratio between with and without the addition of Cu2+ (left)or Zn2+ (right)based on 1H–1H TOCSY spectra in the region of the hIAPP backbone amide groups. The orange denotes the cross peaks with an intensity ratio of less than 0.32.
We first performed nuclear magnetic resonance (NMR)spectroscopy to explore the specific binding sites of Cu2+in the hIAPP(11-28) peptide, which is a fragment involved in amyloid aggregation.[23]Titrating with Cu2+caused specific signal reduction of the cross peaks between Hβand the backbone amide protons of His18,Ser19,Ser20 and Ser28,as well as the cross peak between Hαand the backbone amide proton of Ser28(Fig.1). In contrast,Zn2+did not induce reductions in specific signals of the hIAPP peptide(Fig.1). These results strongly suggest that Cu2+, but not Zn2+, is able to specifically bind to the backbones of His18,Ser19 and Ser20.In addition to the backbones of His18-Ser20, peak intensity changes of sidechains were also detected. The intensities of the cross peak between the imidazole Hδ2 and Hβof His18 and the cross peak between the carboxamide NH21 and NH22 of Asn21 were specifically reduced upon Cu2+titration(supplementary Figs.1 and 2,Table 1). In contrast,the intensities of these cross peaks remained nearly unchanged with Zn2+addition.
Based on the NMR observations and considering the potential match between the size of the Cu2+ion and the space between the neighboring amide nitrogen and carbonyl oxygen atoms of the peptide backbone, we proposed that Cu2+may bind to the hIAPP backbone by forming a ring structure(Fig. 2(a)). A model peptide, HCO-Ser-NH2, was used together with a hydrated Cu2+ion([Cu(H2O)5]2+)[24](denoted as Cu(aq)2+) to simulate the interaction of Cu2+with the Ser19 or Ser20 backbone in hIAPP.As shown byab initiosimulations,Cu(aq)2+can strongly interact simultaneously with a carbonyl oxygen(–O–)and its neighboring amide nitrogen(–N–)in the backbone,thereby resulting in a ring structure composed of Cu, –N–, –O–and the neighboring carbonyl carbon C1 andα-carbon C2 in the peptide backbone(Fig.2(a)right),which is denoted as –O–Cu(aq)–N–. The binding energy of this state was calculated to be-27.65 kcal/mol (Fig. 2(c),Eq. (1)), which was much stronger than the thermal fluctuation(approximately 0.6 kcal/mol atT=300 K)and the hydrogen bond strength(approximately 5.0 kcal/mol)of water. This result strongly suggests that Cu(aq)2+is stably bound to the backbone–O–and–N–,whereas the two water molecules initially coordinated to Cu2+are released,and the amide hydrogen H initially bound to–N–is substituted by Cu2+(Fig.2(a),right). In the structure–O–Cu(aq)–N–,the distances of Cu to the nearest neighboring atoms –N–and –O–and to the next nearest neighboring atoms C1 and C2 were 1.87 ?A, 1.96 ?A,2.70 ?A and 2.81 ?A,respectively. The Cu–O distance(1.96 ?A)in–O–Cu(aq)–N–was less than the averaged distance(2.03 ?A)of Cu–O in[Cu(H2O)5]2+. Subsequent occupied orbital analyses of–O–Cu(aq)–N–showed that few electrons were present that simultaneously belonged to the Cu, –N–and –O–, suggesting weak covalent bonds of the Cu with the–N–and–O–atoms. Moreover, the Cu ion bound to the peptide backbone exhibited a Mullikan charge of only +0.94e, indicating that Cu2+is reduced to Cu+to form a peptide-Cu+complex. Additionally,the backbone-Cu ring structure–O–Cu(aq)–N–was also found in the simulations of Cu(aq)2+binding to the backbone of peptide model HCO-His-NH2for His18.
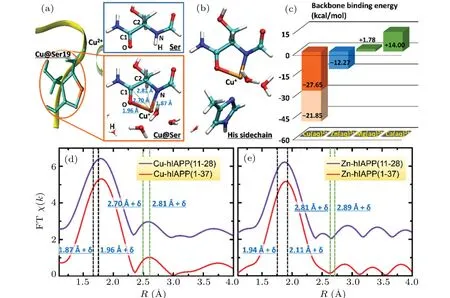
Fig. 2. Formation of backbone-Cu ring structure upon the binding of Cu2+ to hIAPP. (a)–(c) A theoretical model of Cu2+ binding to the hIAPP backbone by forming a ring structure. (a)Upon Cu2+ binding to the Ser backbone,the amide hydrogen H(in the upper panel,right)is substituted by Cu2+(in the lower panel,right),and Cu2+is reduced to Cu+. Left:The yellow ribbon,light green sticks and brown sphere stand for hIAPP backbone,Ser19 and Cu ion,respectively. Right: The model peptide HCO-Ser-NH2. The cyan,blue,red,white and brown spheres represent carbon, nitrogen, oxygen, hydrogen and copper, respectively. The labels, N, H, O, C1 and C2, indicate the amide nitrogen, amide hydrogen, carbonyl oxygen, carbonyl carbon and α-carbon of the peptide backbone, respectively. The black and green dashed lines indicate the distances of Cu to the nearest neighboring and the next nearest neighboring atoms,respectively. (b)The assistance of His sidechain to the backbone-Cu2+ binding. (c)Binding energies of the hydrated ions Cu(aq)2+, Zn(aq)2+, Mg(aq)2+ and Ca(aq)2+ with the peptide backbone,calculated by ab initio approaches. In the absence of the His sidechain, the binding strength of Cu(aq)2+ (orange column) is much stronger than that of Zn(aq)2+ (blue column). Green columns present that Mg(aq)2+ and Ca(aq)2+ cannot bind to the peptide backbone. The His sidechain can further stabilize the backbone-Cu2+ binding by providing additional binding energy(light orange column). (d)–(e)The Fouriertransformed EXAFS spectra(FT χ(k))of the M2+ ions(M=Cu or Zn)in the hIAPP(11-28)(violet)and hIAPP(1-37)(red)solutions to study the distance of the M ions to their neighboring atoms upon their binding to hIAPP.The two black and two green vertical dashed lines indicate the theoretical distances of the M to the nearest neighboring atoms N and O and to the next nearest neighboring atoms C1 and C2,respectively,in the backbone-M ring structure with a shift of δ =-0.23 ?A for M=Cu,and δ =-0.22 ?A for M=Zn. The shift δ is estimated by taking the difference between EXAFS spectra and the theoretical results of the M–O distance in the hydrated M2+ ion(supplementary Fig.4).
For comparison, we also simulated the interaction of the peptide backbone with other physiological divalent cations such as Zn2+,Mg2+and Ca2+. We calculated the binding energies of[Zn(H2O)6]2+,[25][Mg(H2O)6]2+and[Ca(H2O)6]2+with the Ser backbone using the conditions identical to those applied for [Cu(H2O)5]2+. The binding energy of Zn(aq)2+in this state was-12.27 kcal/mol, which was much weaker than the binding energy(-27.65 kcal/mol)of Cu(aq)2+in the ring structure(Fig.2(c)),indicating that a potential backbone ring structure of Zn2+is substantially less stable than that of the Cu ion. The binding energies of Mg(aq)2+and Ca(aq)2+were +1.78 kcal/mol and +14.00 kcal/mol (Fig. 2(c), green columns),respectively.The positive values indicate that Mg2+and Ca2+cannot bind to the peptide backbone to form a ring structure. The specific backbone-Cu2+binding behavior may be attributed to the ability of Cu2+, but not Zn2+, Mg2+and Ca2+, to accept an electron and be reduced to a monovalent cation. Additional simulations suggested that the His18 sidechain can enhance the stability of the backbone-Cu binding.Ab initiocalculations showed that the Cu+ion in the ring structure–O–Cu(aq)–N–can further bind to a nitrogen in the His sidechain(Fig.2(b)),whereas one water molecule initially coordinated to Cu+was displaced. The calculated binding energy of the –O–Cu(aq)–N– structure with the His sidechain(Eq.(2))reached-21.85 kcal/mol(Fig.2(c),light orange column),which enhanced the peptide binding strength of Cu2+to-49.50 kcal/mol. The stability of the backbone-Cu ring structure thus can be further enforced by the assistance of the His sidechain. Notably, the Mullikan charge of Cu coordinated with only the His sidechain was calculated to be+1.39e,indicating that,in the absence of binding to the peptide backbone,His sidechain binding alone is insufficient to reduce Cu2+to Cu+.
We then used x-ray absorption fine structure (XAFS)spectroscopy to confirm the presence of the backbone-Cu ring structure. The extended x-ray absorption fine structure (EXAFS) spectrum in real space (Fig. 2(d)) of Cu2+in the hIAPP(11-28) solution was highly similar to that in the hIAPP(1-37) solution, indicating that hIAPP(11-28) represents the major region of hIAPP(1-37)that binds Cu2+ions(more details in supplementary Fig. 3). In both spectra, the first peak was observed at 1.80 ?A,which may be related to the calculated Cu–N(1.87 ?A)and Cu–O(1.96 ?A)distances in the backbone-Cu ring structure and the Cu–O distance (2.03 ?A)in Cu(aq)2+, via a shift ofδ=-0.23 ?A.The shiftδwas estimated by taking the difference between EXAFS spectra and theoretical results of the Cu–O distance in the first solvation shell of the hydrated Cu2+ion(supplementary Fig.4). Importantly,the second peak at 2.58 ?A was consistent well with the calculated Cu–C1 (2.70 ?A) and Cu–C2 (2.81 ?A) distances in the ring structure via this shiftδ.Moreover,our previous x-ray absorption near edge structure (XANES) analysis (Fig. 3(a))further confirmed that Cu2+could be reduced to Cu+upon binding hIAPP, resulting in an hIAPP-Cu+complex.[26]The major peak of the spectrum for Cu in the hIAPP(1-37)solution was clearly observed at the same location as the peak of Cu2+in the CuCl2solution(8995.7 eV),suggesting that most of the copper ions in the hIAPP solution stay in solution. Interestingly,a shoulder peak appeared in the left region of the major peak. To present the location of this minor peak clearly and exactly, the derivative was performed over the spectrum, and a zero value corresponding to the shoulder peak was clearly observed at 8983.0 eV, which was very close to the location 8982.1 eV of Cu+in the Cu2O solution, indicating the presence of cuprous ions in the sample,following by the formation of the hIAPP-Cu complex. This result agreed well with the theoretical observation that Cu2+was reduced to Cu+upon binding the peptide backbone. Therefore, these XAFS analyses strongly support the notion of the backbone-Cu+ring structure of hIAPP. A ring structure was also observed upon Al ions binding to phosphoglycerate kinase protein.[27]In contrast, the XAFS spectra (EXAFS in Fig. 2(e) and XANES in supplementary Fig.5)of Zn2+suggest that Zn2+cannot bind to the backbone of hIAPP, and then cannot be reduced in hIAPP solution(seeing the detailed discussion in supplementary information).
3. Peptide backbone-Cu ring structure and the enhancement of reactive oxygen species
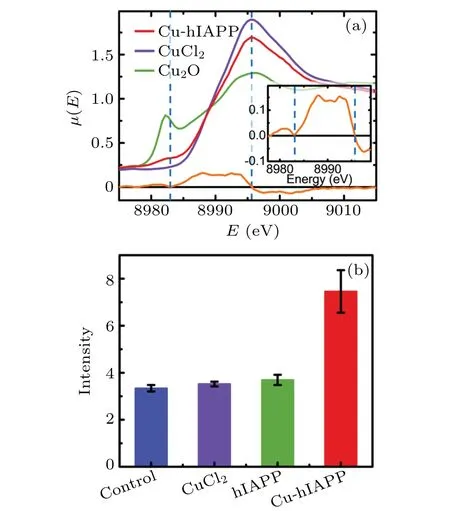
Fig. 3. Reduction of Cu2+ to Cu+ upon binding to hIAPP peptide and the enhancement of ROS level in rat insulinoma cells (INS-1) induced by the hIAPP-Cu+ complex. (a) XANES spectra of the Cu ion in hIAPP(1-37) solution. The red, violet and green curves represent the spectra of the Cu ion in the hIAPP solution,Cu2+ and Cu+ ions in aqueous solution, respectively. The blue dashed lines indicate the location of the major and shoulder peaks of the red curve. The orange curve represents the derivative of the Cu-hIAPP spectrum, and a detailed view of the Cu-hIAPP derivative spectrum is presented in the inset. (b)The blue,violet,green and red columns indicate the DCFH-DA fluorescence intensities of the control, the INS-1 cells incubated with CuCl2,hIAPP(1-37)and hIAPP(1-37)-Cu+ complex,respectively. The fluorescence intensity is proportional to the intracellular ROS level.[28]
Given that the reduction of Cu2+to Cu+can lead to the generation of reactive oxygen species(ROS),[11–13]we studied the potential effect of Cu2+binding to the peptide backbone on intracellular redox homeostasis. We used a molecular probe 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA)to detect oxidative stress in rat insulinoma cells (INS-1).The fluorescence intensity of the probe was proportional to the ROS amount.[28]When INS-1 cells were incubated with 20 μM hIAPP(1-37)or 100 μM Cu2+alone,the fluorescence intensities(Fig.3(b))were comparable to that of control(untreated)cells,indicating that hIAPP or Cu2+alone cannot induce the increase of ROS in the cells. Interestingly, when INS-1 cells were incubated with both hIAPP(1-37)and Cu2+,the fluorescence intensity was significantly stronger than that of the control, denoting that the presence of both hIAPP and Cu2+induced a significant increase in the intracellular ROS level. These results indicate that Cu2+binding to hIAPP can promote ROS generation, which is likely caused by the reduction of Cu2+to Cu+following the formation of the –O–Cu(aq)–N–ring structure.
3.1. Peptide backbone-Cu ring structure and the modulation of hIAPP aggregation
We explored the effects of the backbone-Cu2+binding on hIAPP aggregation using thioflavin T (ThT) fluorescence and atomic force microscopy (AFM) measurements. An increase in ThT fluorescence signal (Fig. 4(a)) was observed upon incubation of hIAPP alone (as a control) for approximately 200 min,meaning the formation of fibrils. In the presence of Zn2+(hIAPP + Zn2+), the hIAPP fluorescence intensity, lag time and elongation rate (Fig. 4) were comparable to those of hIAPP alone. These results indicate that Zn2+plays a modest role in hIAPP aggregation. In contrast, incubation of hIAPP with CuCl2(hIAPP + Cu2+) caused an obvious decrease in ThT fluorescence intensity compared with that of hIAPP alone. Moreover, the lag time significantly increased to 535 min(2.5-fold of the control,Fig.4(b)),and the elongation rate was reduced to 0.0035 min-1(only 60% of the control, Fig. 4(c)). A larger number of the oligomer-like structures were clearly observed in the hIAPP+Cu2+sample compared with the hIAPP alone and hIAPP + Zn2+samples(Figs.4(d)–4(f)). We attribute the remarkable difference of hIAPP aggregations between incubated with Cu2+and Zn2+to the significantly different manners of these ions binding to the peptide backbone. Namely, Cu2+, but not Zn2+, can bind to the peptide backbone by forming a ring structure. Therefore,the results above strongly indicate that the backbone-Cu ring structure plays an important role in the Cu2+modulation of hIAPP aggregation via inhibiting peptide fibrillation and promoting oligomerization.
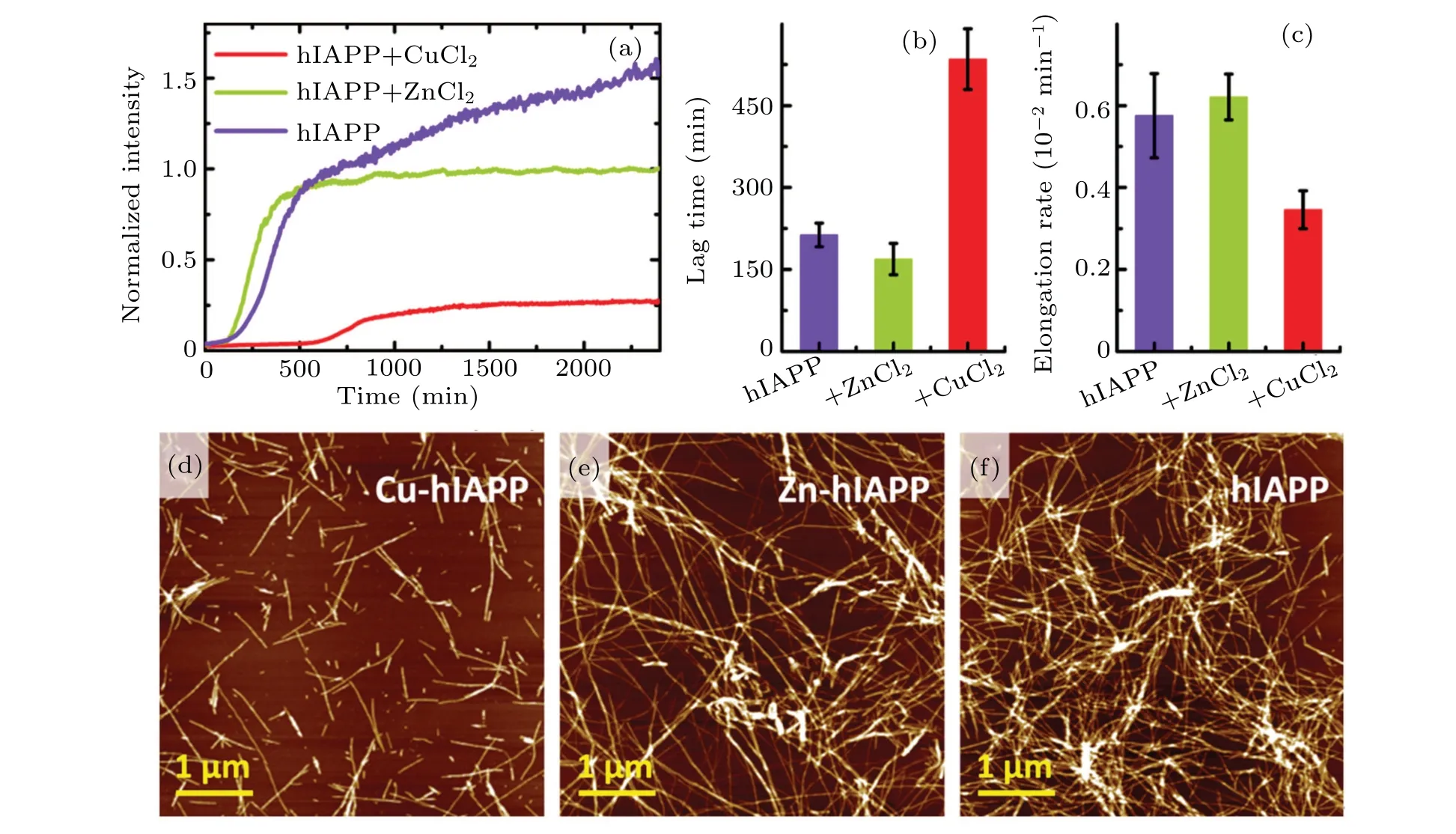
Fig.4.Cu2+-induced modulation of hIAPP aggregation.(a)–(c)ThT fluorescence intensity(a),lag time(b)and elongation rate(c)of hIAPP(1-37)in the absence and presence of CuCl2,and ZnCl2. (d)–(f)AFM images of hIAPP(1-37)in the presence of CuCl2 (d),ZnCl2 (e)and pure hIAPP(1-37)(f),following incubation for 3 days. A larger number of the oligomer-like structures clearly appear in the hIAPP+Cu2+ sample compared with the hIAPP alone and hIAPP+Zn2+ samples.
To further verify the relevance of the backbone-Cu binding model with the Cu2+-induced modulation of IAPP aggregation, we also employed Li+as a control, which has been used as a drug in clinic to slow the progress of amyloid-linked diseases.[29–31]The ThT fluorescence data (Figs. 5(a)–5(c))showed that, compared with the case of only adding Cu2+,the lag time of aggregation much significantly shortened in the case of pre-adding Li+with five minutes in advance of adding Cu2+(hIAPP + Li++ Cu2+, the ratio 1:5:5 of hIAPP, Li+and Cu2+),but the final intensity of fluorescence only weakly increased. These results indicate that the pre-addition of Li+can remarkably decrease the effect of Cu2+on the lag time of aggregation, but play a weak role in the effect of Cu2+on the final fluorescence intensity. Interestingly, in the case of only adding Li+(hIAPP + Li+), the fluorescence intensity,lag time and elongation rate were comparable to those of hIAPP alone, denoting that Li+itself has negligible influence on the peptide aggregation. To explain these complicated and surprising phenomena, we calculated the binding energies of Cu[(H2O)5]2+and[Li(H2O)4]+[32]with the carbonyl oxygen(–O–)on the Ser backbone(denoted as–O–M,M=Cu(aq)2+or Li(aq)+)(Fig.5(d)),respectively,as well as the binding energy of Li(aq)+upon forming a backbone ring structure(–O–Li(aq)–N–). The binding energies of –O–Cu(aq)2+and –O–Li(aq)+were-10.08 kcal/mol and-3.66 kcal/mol, respectively (Fig. 5(e)). Their difference was only 6.42 kcal/mol,very close to the hydrogen bond strength (~5 kcal/mol) of water, indicating that Li+and Cu2+can compete each other upon binding to the–O–in aqueous solution. The binding energy of Li(aq)+in the state –O–Li(aq)–N–was greater than+60 kcal/mol, strongly suggesting that Li+cannot form a backbone ring structure like Cu2+does. Therefore, we can understand the above experimental observations as the following. The pre-added Li+can bind to the backbone –O–.The post-added Cu2+will compete with the pre-added Li+to bind to the –O–. Due to the comparable binding strength between–O–Li(aq)+and–O–Cu(aq)2+,this competition will take a long time,which results in the much significant difference of lag time between hIAPP+Li++Cu2+and hIAPP+Cu2+. Additionally,Cu2+,but not Li+,can further replace the amide hydrogen H initially bound to–N–(Fig.2(a))to form a backbone ring structure–O–Cu(aq)–N–,reaching a more stable state (-27.65 kcal/mol). Thus the difference in the final intensity of ThT fluorescence between hIAPP+Li++Cu2+and hIAPP+Cu2+is not so significant as the difference in lag time,while Li+itself cannot obviously affect the aggregation of hIAPP like Cu2+does. Collectively,all of these results further strongly support that it is mainly through the backbone-Cu ring structure for Cu2+to modulate the hIAPP aggregation.

Fig. 5. Inhibitory effect of Li+ on the Cu2+ modulation of hIAPP aggregation. (a)–(c) ThT fluorescence intensity (a), lag time (b) and elongation rate (c) of hIAPP(1-37) in the absence and presence of CuCl2, LiCl, or both LiCl and CuCl2. The concentration ratio of 1:1 is applied for CuCl2 and LiCl. The label over the double-arrow line in the (b) and (c) panels indicates the two-tailed P value for statistically significant difference between the two groups of data: NS,*,**,and***represent P <0.05(no significant difference),P <0.05,P <0.01,and P <0.001, respectively. N =5. (d)–(e) A theoretical model of Li+ inhibiting the backbone-Cu ring formation. (d) A model peptide HCO-Ser-NH2 for the state that a hydrated ion M (M =Li(aq)+ or Cu(aq)2+) binds to the carbonyl oxygen –O–of the peptide backbone,denoted as–O–M. The cyan,blue,red,white and brown spheres represent carbon,nitrogen,oxygen,hydrogen and copper,respectively. The dashed line indicates electrostatic attraction between the–O–and M. (e)Comparison of binding energies between the hydrated ions Cu(aq)2+and Li(aq)+ in the states–O–M (left)and–O–M–N–(back-M ring structure,right),respectively. In the state–O–M,the difference of binding strength between Cu(aq)2+ and Li(aq)+ is only 6.42 kcal/mol,similar to the hydrogen bond strength(~5 kcal/mol)of water,indicating that Li+ can compete with Cu2+ upon binding to the –O–of backbone in aqueous solution. In the state –O–M–N–, the largely positive large value of binding energy for Li(aq)+ strongly suggests that the Li+ cannot form a backbone ring structure like Cu2+ does. Therefore,through the competition of the state –O–M, Li+ can inhibit/delay the formation of backbone-Cu ring structure (the state –O–Cu(aq)–N–, Fig. 2(a)),although Li+ itself is unable to form a backbone ring structure like Cu2+ does.

Fig. 6. A schematic representation for the molecular mechanism of Cu2+-induced amyloid toxicity simultaneously involving both the enhancement of ROS level and the modulation of hIAPP aggregation. (a) Without the addition of Cu2+ or Zn2+, the hIAPP peptide forms a β-sheet (middle, light blue block), as a molecular intermediate, stacking to cause hIAPP fibril formation (right, light blue column).[33] The silver ribbons represent the β strands, while the blue tube in between represents the turn region of His18-Ser20. (b) Due to the sidechain binding (supplementary Fig. 6), both Cu2+ (brown sphere) and Zn2+ (red sphere) ions can be enriched in the area close to the His18–Ser20 turn(blue sticks representing side chains). Cu2+ can bind to the backbones of His18,Ser19(and/or Ser20)by forming a ring structure(orange sticks), which can be further stabilized by the His18 sidechain (Figs. 2(b) and 2(c)). This backbone binding reduces Cu2+ to Cu+ (yellow sphere), causing the formation of a hIAPP-Cu+ complex, which can largely enhance the ROS level in cells to damage the cells (oxidative stress hypothesis).[11,12] Additionally, the backbone-Cu+ ring structure substantially disrupts the β-sheet structure of peptides, dramatically inhibiting hIAPP fibrillation and promoting oligomerization. The oligomers will contribute more to cell damage than the fibrils (oligomer hypothesis).[14,15] In contrast to Cu2+,Zn2+ cannot bind to the hIAPP backbone and is thus unable to induce the following effects on the ions and peptides as well as the consequent amyloid toxicity.
Finally, we explored the possible molecular mechanism of the Cu2+modulation on hIAPP aggregation from the aspects of the backbone-Cu ring structure and the specific Cu2+binding to the segment of His18,Ser19 and Ser20. We calculated the binding energies of[Cu(H2O)5]2+and[Zn(H2O)6]2+for the sidechains of His (through the coordination bond)and Ser (through the polar group). The binding strengths(supplementary Fig. 6) of Cu2+and Zn2+were comparable. The negative values of the binding energies indicate that these sidechains are able to bind/attract those cations. Therefore, the sidechains of His18-Ser20, particularly the His18 sidechain, can enrich both Cu2+and Zn2+in the local area(Fig.6(b),left),which should largely promote the probability of ion backbone binding. However, as demonstrated above,only Cu2+can stably form a ring structure with the peptide backbone. The stability of the backbone-Cu ring structure can be further enhanced by the His18 sidechain (Figs. 2(b)and 2(c)). This ring structure in the backbone will fix the dihedral angles between the two neighboring amide planes.Notably, Val17-Ser19 can form a turn structure that is critical for hIAPP fibrillation.[33]The backbone-Cu2+binding in the His18-Ser20 segment should prevent or destroy this turn structure, thus inhibiting the formation of an intramolecularβ-sheet structure (an intermediate molecular form for hIAPP assembly[33]). Additionally, the displacement of backbone amide protons (Figs. 1(b) and 2(a)) due to the backbone-Cu ring structure and the positive charge brought by Cu+ion in the ring structure can further reduce the number of hydrogen bonds and the hydrophobicity of hIAPP peptides,respectively,which are the driving forces of fibril formation. All of these features will result in the inhibition of hIAPP fibrillation and the promotion of peptide oligomerization(Fig.4(d)). Intriguingly, the binding energies of Zn2+with the His18-Ser20 sidechains were comparable to those of Cu2+. Thus, the lack of backbone binding is likely the primary reason for the modest effect of Zn2+on the inhibition of hIAPP fibrillation(Fig.4(e)).
4. Conclusion and perspectives
In summary,based on various biophysical approaches and theoretical analyses, we proposed a backbone-Cu2+binding model at atomic resolution for the interaction between Cu2+and amyloid peptide as well as the consequent effects(Fig.6).Redox-active Cu2+can bind to the hIAPP backbone by forming a ring structure, which caused the reduction of Cu2+to Cu+to enhance ROS levels in cells. Moreover,the backbone-Cu2+binding also dramatically inhibited peptide fibrillation and promoted oligomerization. In contrast to Cu2+,additional biologically important cations, such as Zn2+, cannot bind to the backbone and thus do not induce the subsequent effects.Furthermore, we also found that Li+considerably weakened the effects of Cu2+on hIAPP, which suggests a clue for inhibiting copper-mediated amyloid toxicity through blocking or destroying the backbone-Cu ring structure. These findings thus provide a new molecular mechanism simultaneously for Cu2+-induced ROS generation and hIAPP aggregation which are significantly involved in amyloid toxicity,potentially promoting the understanding of Cu-mediated amyloid toxicity at atomic resolution, and even the development of drug design and therapy for type-2 diabetes and other diseases.
5. Methods
Nuclear magnetic resonance spectroscopy The hIAPP(11-28)peptide was dissolved in ice-cold water to get a stock solution with the peptide concentration of 0.15 mM.This stock solution was stored at-80°C.To prepare the NMR sample,the stock peptide solution was dissolved in 10 mM sodium phosphate buffer(pH 4.3)to make the peptide concentration to be 0.12 mM.
All NMR experiments were performed at 283 K on a Bruker AVANCE spectrometer operating at 600 MHz (proton frequency)and equipped with a 5 mm,pulsed-field gradient triple-resonance probe. Two-dimensional(2D)1H–1H total correlation spectroscopy (TOCSY) spectra were acquired with a mixing time of 80 ms using the MLEV-17 spin-lock sequence to drive the coherence transfer. 2D1H–1H nuclear Overhauser effect spectroscopy (NOESY) spectra were obtained using the mixing time of 400 ms. All 2D spectra were acquired in the phase-sensitive mode. To reduce the water peak in 2D spectra,WATERGATE suppression was used. The1H resonance spin systems were assigned from the analysis of TOCSY spectra. Sequential assignments were made from the sequential connectivity information of dNNand dαNin the NOESY spectra.
NMR titration experiments were carried out by adding CuCl2or ZnCl2to 0.12 mM hIAPP(11-28)peptide dissolved in 10 mM sodium phosphate buffer(pH 4.3C).TOCSY spectra were measured at the Cu2+or Zn2+concentration of 0 mM and 0.60 mM, respectively. Peak intensities were compared for the two TOCSY spectra at the Cu2+or Zn2+concentration of 0 mM and 0.60 mM,respectively.
X-ray absorption fine structure spectra XAFS measurements (including EXAFS and XANES) were collected at Shanghai Synchrotron Radiation Facility(SSRF)beamline BL14W. A Si[111] double crystal monochromator and a set of focusing mirrors with a cut-off energy of 22.5 keV were used throughout the study. The XAFS spectra were recorded in fluorescence mode using a 32-element Ge solid-state detector. Data collected in each run were first averaged and after background subtraction,the resulting spectra were normalized. To prepare a sample for XAFS measurement, 1500 ml 20 μM hIAPP(hIAPP(1-37),hIAPP(1-11),hIAPP(11-28)and hIAPP(28-37),respectively)peptide with and without 200 μM ZnCl2or 200 μM CuCl2at pH 4.5 was incubated at 37°C for three days, respectively. In order to increase the signal/noise ratio in XAFS data, each of these samples were further concentrated to 300 ml by freeze drying.
DCFH-DA fluorescence Intracellular ROS amount was measured by ROS-sensitive probe DCFH-DA. INS-1 cells seeded in 6-well microplates at a concentration of 5×104cells/ml were cultured at 37°C in a humidified atmosphere for 48 h. Then the cell was incubated with hIAPP(1-37) (as a control,20 μM),100 μM CuCl2,or co-incubated with both 20 μM hIAPP and 100 μM CuCl2for 48 h.After that,the cells were loaded with DCFH-DA at a final concentration of 10 μM at 37°C for 60 min. The cells were washed with 10 mM PBS and then transferred to a 96-well plate. ROS generation was assessed by measuring the fluorescence intensity with a microplate reader (Thermo Scientific Fluoroskan Ascent FL)at the excitation and emission wavelengthes of 485 nm and 525 nm,respectively.
ThT fluorescence The kinetics of hIAPP(1-37)fibril formation was detected by fluorescence intensity increase upon binding of the fluorescent dye ThT to fibrils. To prepare a sample for fluorescence,the hIAPP(1-37)peptide was diluted to a final concentration of 20 μM using sodium phosphate buffer containing 20 μM ThT and 200 μM ZnCl2, CuCl2,or LiCl at pH 4.5. The pH of each sample was individually adjusted. Fluorescence was measured using a luminescence spectrometer (Thermo Scientific Fluoroskan Ascent FL) in a sealed Corning 96 well at 37°C.The excitation and emission wavelength was 440 nm and 485 nm,respectively. A common sigmoidal growth model was used to fit the time-dependent ThT fluorescence curves to extract lag time and elongation rate constants.[34]
AFM measurement 20 μM hIAPP(1-37) peptide with and without 200 μM ZnCl2or 200 μM CuCl2at pH 4.5 was incubated at 37°C for three days, respectively. Then a 5 μl incubated solution was deposited onto a freshly cleaved mica,waited for 5 min, gently rinsed with Milli-Q water and dried in air. Images were collected using a Multimode 8 with Controller V scanning force microscope(Bruker)operated in tapping mode in air. Images were simply flattened using the NanoScope analysis software.
Simulations and calculations All of simulations and calculations were performed using anab initiomethod based on the second order M?ller–Plesset perturbation theory(MP2),[35]as implemented in the Gaussian09 package with the 6-31+G(d,p)basis set and including diffuse functions was applied on all atoms.
To calculate the backbone binding energy of hydratedMion [M(H2O)n]2+(M=Cu, Zn, Mg, Ca) on the backbone of model peptide HCO-Ser-NH2(C4N2O3H8) in Fig. 2(a), we applied the following formula:

where the first,second,third and fourth terms in the right side of equation indicate the energy of the backbone ring structure,molecules H2O and H3O+, peptide HCO-Ser-NH2, and hydratedMion[M(H2O)n]2+,respectively. The indexn=5 forM=Cu,andn=6 forM=Zn,Mg and Ca.
To calculate the binding energy of the backbone-Cu ring structure and His sidechain in Fig.2(b),we applied the following formula:
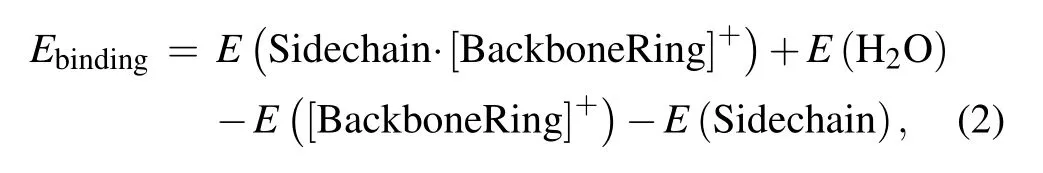
where the first, second, third, and fourth terms in the right side of equation stand for the energies of the complex of His sidechain and backbone-Cu ring, water molecule H2O,backbone-Cu ring,and His sidechain,respectively.
Acknowledgements
We thank Prof.Jun Hu and Prof.Bo Song for discussions.All the calculations were performed on the Shanghai Supercomputer Center of China. Figure 3(a) was reproduced from Ref. [25] with permission from the Royal Society of Chemistry.
Project supported by the National Natural Science Foundation of China (Grant Nos. 12074208 and 11375256),the Natural Science Foundation of Jiangsu Province (Grant No. BK20200176), the Natural Science Foundation of Jiangsu Higher Education Institutions of China (Grant Nos. 20KJB140020 and 19KJB140005), Fundamental Research Project from Changzhou Science and Technology(Grant No.CJ20200029),and the Jiangsu Province High-level Innovative and Entrepreneurial Talents Introduction Plan.
- Chinese Physics B的其它文章
- Design of vertical diamond Schottky barrier diode with junction terminal extension structure by using the n-Ga2O3/p-diamond heterojunction
- Multiple modes of perpendicular magnetization switching scheme in single spin–orbit torque device
- Evolution of the high-field-side radiation belts during the neon seeding plasma discharge in EAST tokamak
- Phase-matched second-harmonic generation in hybrid polymer-LN waveguides
- Circular dichroism spectra of α-lactose molecular measured by terahertz time-domain spectroscopy
- Recombination-induced voltage-dependent photocurrent collection loss in CdTe thin film solar cell

