Effect of the microporous structure of ammonium perchlorate on thermal behaviour and combustion characteristics
Hi-jun Zhng , Jin-xin Nie ,*, Gng-ling Jio , Xing Xu , Xue-yong Guo , Shi Yn ,Qing-jie Jio
a State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing,100081, China
b Naval Research Institute of PLA, Beijing,100161, China
c The Sixth Institute, 601 Branch of China Aeronautical Science and Technology Corporation, Hohhot, 010076, China
Keywords:Microporous structure Ammonium perchlorate Thermal behavior Porosity Combustion characteristics
ABSTRACT Ammonium perchlorate(AP)is the component with the highest content in composite propellants,and it plays a crucial role in propellant performance. In view of the effects of low-temperature AP thermal decomposition on thermal safety and combustion characteristics, porous ammonium perchlorate (PAP)samples with different mass losses were first prepared by thermal convection heating,and the structures were characterized and analysed. Second, the effects of decomposition degree on the thermal decomposition characteristics of PAP were studied by DSC-TG. Finally, the combustion characteristics of AP/Al binary mixtures were tested with high-speed photography and in a sealed bomb.The results showed that low-temperature decomposition of AP resulted in formation of porous structures for AP particles. The pores first appeared near the surfaces of the particles and began from multiple points at the same time.The pores increased in size to approximately 5 μm and then expanded,and finally,the AP particles were full of pores. After partial decomposition, the crystal structure of AP remained unchanged, but the low and high decomposition temperatures decreased obviously. The decomposition rate accelerated. Due to the porous structure of PAP,the combustion rate of the AP/Al system increased obviously with increasing decomposition of AP. The relationship between the combustion rate and the mass loss was approximately linear under open conditions, and it was exponential for a high-pressure environment. A computational model of the combustion process for the AP/Al binary system was established to explain the effects of pore structure and pressure on the combustion process.
1. Introduction
With the requirements for high power and range for ammunition, the proportion of energetic components in propellants is increasing, as is the energy level. Safety problems are becoming increasingly serious [1]. At present, the safety of rocket motors under the action of typical thermal,mechanical and shock waves is the focus of research on insensitive munitions, for which thermal stimulation is the most common accidental source[2,3].Insensitive experiments of propellants to accidental thermal stimulation were studied with fast and slow cook-off experiments. The propellant may decompose, burn or even explode (detonation), resulting in catastrophic consequences [4].
Ammonium perchlorate (AP) is the most commonly used oxidant in solid propellants[5],and it usually accounts for as much as 50%-70% of the total due to its high oxygen content, good thermal and chemical stability, and gaseous decomposition products. The thermal behaviour and combustion characteristics of AP have a great influence on the combustion and safety of the solid propellant.The reaction temperature of the composite propellant is usually between 150C and 230C in cook-off[6-8],and the AP in the propellant may partially decompose to form a porous structure[9].Once ignition occurs,the abnormal increase in the combustion surface caused by the porous structure [10] and the highly active combustible gases produced by decomposition lead to a transition from deflagration to detonation, which results in more serious consequences [11-13].
Thermal decomposition of AP can be divided into two stages:low-temperature decomposition and high-temperature decomposition. Thermal degradation of AP occurring below the orthorhombic-to-cubic phase transition temperature of 240C[14,15]is known as low-temperature decomposition[16].There is a cessation of decomposition after an approximately 30 wt%loss[17].Upon increasing the temperature, decomposition continues until complete[18].The residues of low-temperature AP decomposition are porous structures. Tolmachoff et al. studied the lowtemperature decomposition processes of AP and proposed a mechanism for low-temperature decomposition, and the morphology of porous ammonium perchlorate(PAP)was analysed by scanning electron microscopy [9,19-21]. PAP was prepared by Liu et al.,who heated AP at 230C and generated PAP to replace or partially replace the common AP in the propellant. PAP can significantly increase the combustion rates of propellants[22-24].
The porous structure formed by low-temperature decomposition of AP improves the propellant combustion rate when the propellant works normally,and the porous structure may affect the response of the propellant during the cook-off process [25]. Based on an analysis of experimental results, Essel concluded that the porous structure formed by thermal decomposition of AP may be an important factor leading to a severe response of the composite propellant [11].
In summary, it is of great significance for the combustion process and thermal safety to study the low-temperature decomposition characteristics and microstructural evolution of AP and to analyse the thermal decomposition properties of partially decomposed PAP. In this paper, AP samples exhibiting different mass losses were prepared by thermal convection heating, and the microstructures of the PAP particles were characterized by SEM and Nano-CT. Then, the effects of different PAP mass losses on combustion characteristics were investigated under open and pressurized conditions, and the mechanisms of the effects were analysed.The thermal behaviour and combustion characteristics of PAP after partial decomposition were studied. A calculated model for the combustion process of an AP/Al binary mixture system was established, and the effects of pore structure and pressure on the combustion process were explained. These results provide guidance for thermal safety research and design of propellants with low vulnerability.
2. Preparation and characterization of PAP
2.1. Materials and equipment
The average particle size(D50)of AP purchased from the Dalian potassium chlorate plant (analytical purity >99%) was 150-200 μm; it was 100 nm (D50) for aluminium powder with a purity of 99.9% (JICRON Nanotechnology Co. Ltd., Xuzhou, China).PAPs with mass losses of 9.25%,20.7%and 29.3%were prepared by heating at 230C for different times.By mixing AP/Al for 1 h,Al/AP binary mixed systems with AP mass fractions of 0.5,0.6,0.7 and 0.8 were obtained. The synchronous thermal analyser (TG-DSC,STA449F3 Jupiter?, NETZSCH, Germany) had an experimental temperature range of 30-700C,and the purge gas was argon with flow rate of 40 mL/min.A total of 3-5 mg of the sample was tiled in an AlOcrucible with a lid with a small hole. A nanoVoxel-5000 series X-ray three-dimensional microscope (Sanying Precision Instruments in Tianjin,China)with a minimum resolution of 300 nm was also used. Scanning electron microscopy was performed with an S-4800 instrument(Hitachi,Japan).The high-speed camera was from Photron (Japan). Steel grooves measured 8 × 3 × 1 cmand were homemade.
2.2. Structural characterization
The surface morphologies of partially decomposed AP particles were analysed by scanning electron microscopy,as shown in Fig.1.The surface of AP became pitted, many micropores appeared after partial decomposition of AP, and the porosity tended to increase with the degree of decomposition. The partially decomposed AP was mechanically ground and broken. The inset image shows an SEM image for the cross section of the AP particles,its location was chosen to determine the pore concentration of the particles, and the magnification used was 2.5 K.The size and spatial distributions of pores and the concentrations of pores were not very different,although the porosity increased with the degree of decomposition.
To further analyse the distribution of pores in AP particles, the PAP particles were scanned layer by layer with Nano-CT. Fig. 2 shows CT scans of AP samples with mass losses of 9.25%(Fig.2(a)),20.7%(Fig.2(b)),and 29.3%(Fig.2(c)).The grey part is AP,and the white spaces are pores. One of the particle images was extracted to analyse its pore distribution.When the mass loss was 9.25%, the pores were mainly distributed in the peripheries of the particles, and there were almost no pores in the interior. When 20.7% of the original mass was lost, the pores had increased obviously and extended inward.When 29.3%was lost,the AP particles were full of pores. The pores were separated from AP particles by image enhancement,noise processing,and threshold segmentation with AVIZO[26]software to analyse the microstructures.Fig.2(d)-Fig. 2(f) shows cross sections of the pore distributions for mass losses of 9.25%, 20.7% and 29.3% (the blue parts in the figure indicate pores).
From the results of CT experiments,when the AP decomposition degree was low, the pores were mainly concentrated near the surface and gradually expanded to the interior of the particles with increases in decomposition degree, and finally,the interiors of the particles were filled with pores. This was the same result seen by SEM for pore distributions in sections where the pores were concentrated.Therefore,it can be concluded that low-temperature decomposition of AP particles occurred first near the surfaces of particles, and multipoint decomposition began at the same time.Then, the pores increased in diameter to approximately 5 μm and then diffused to the periphery; finally, the pores filled the particle interior.This is because AP low-temperature decomposition begins with defects in the crystal,but the decomposition process depends on the emission of gas products.The gas products are easy to escape only at the decomposition point near the particle surface. The gas can be smoothly discharged when the pores expand to the region and the reaction proceeds further.
As decomposition progresses, the surface area of AP increased.The specific surface area of AP at different decompositions (mass loss of 0, 9.25%, 20.7%, and 29.3%) was measured and analysed by mercury intrusion porosimetry (MIP). As shown in Fig. 3(a), the specific surface area of the PAP consisted of two parts, particle external surface and pore surface. As the decomposition degree increased, the particle surface became rough,causing the external surface area to increase. Meanwhile, the amounts of pores caused by decomposition increased,and the surface area of the pores also increased, which had a greater effect on the surface area than the degree of surface roughness(Fig.3(b)).The specific surface areas of AP with different mass loss were 0.028 m/g,0.171 m/g,0.419 m/g,and 1.184 m/g respectively,indicating the significant increase of specific surface areas of AP.
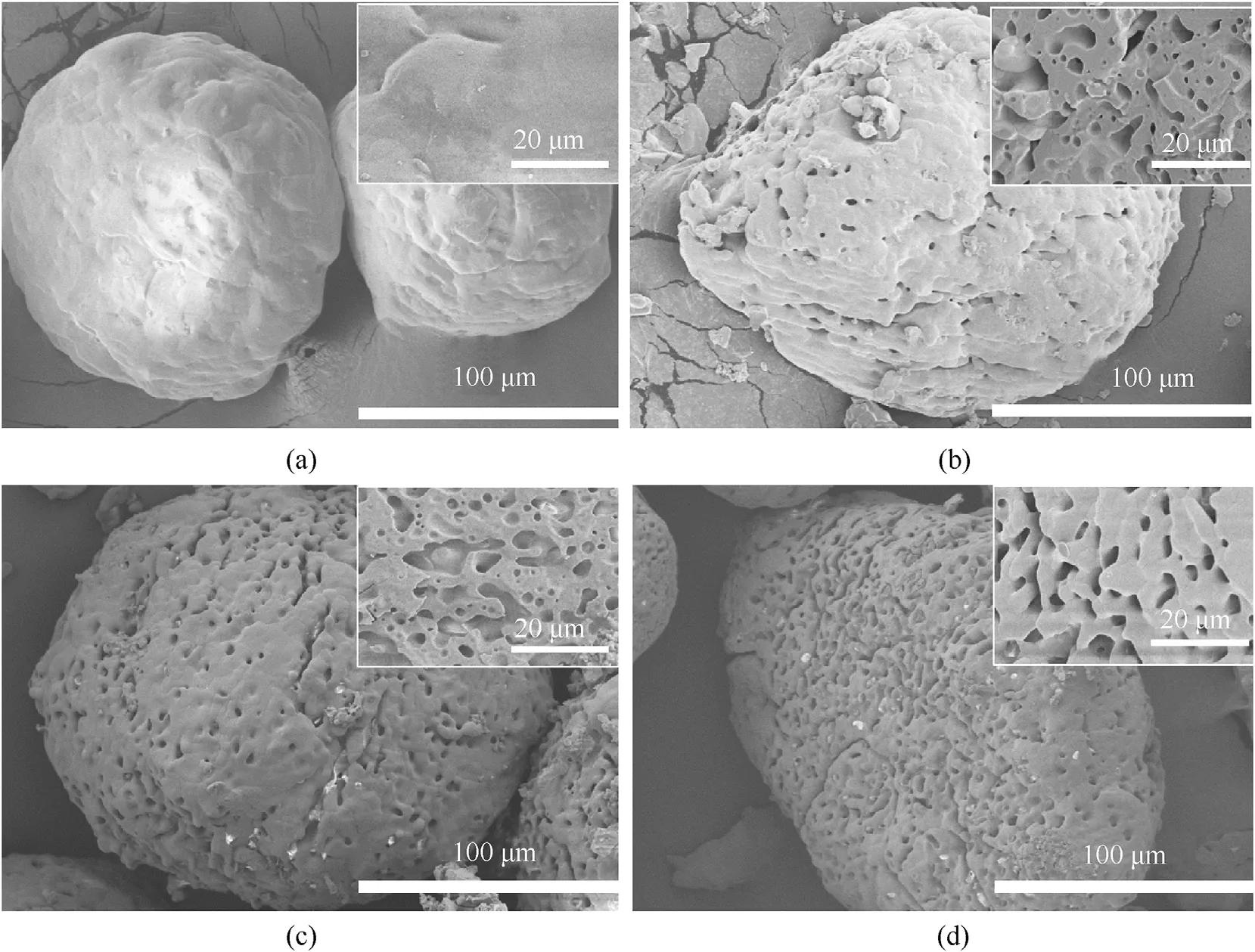
Fig.1. SEM images showing different mass losses of AP, insert, SEM image of the cross section: (a) untreated; (b) 9.25 wt%; (c) 20.7 wt%; and (d) 29.3 wt%.
3. Thermal behaviour
To study the effects of partial AP decomposition on the thermal safety performance, thermal decomposition was analysed by DSCTG. The endothermic peak seen at approximately 243C was the crystal transition temperature,and the crystallization temperature of PAP was the same as that of the original AP (Fig. 4(a)). This indicated that low-temperature decomposition of AP did not change the crystal structure. However, the low and hightemperature decomposition peaks of PAP were lower than those of the original AP and had little relation with the degree of decomposition (Fig. 4(b)). As shown in Fig. 4(c), the initial decomposition temperature of partially decomposed AP was also obviously lower,and the initial decomposition temperatures for the 20.7%and 9.25%mass loss samples were basically the same,while the initial decomposition temperature of the 29.3% mass loss sample was higher.
The decomposition processes of untreated AP and PAP samples with mass losses of 29.3% were measured at heating rates of 5C/min, 10C/min, 15C/min and 20C/min. The chemical kinetics parameters were obtained by fitting with the Kissinger method,as shown in Table 1. The activation energy for low-temperature decomposition was significantly increased after mass loss, but the activation energy for high-temperature decomposition was significantly reduced.
The first step of low-temperature decomposition by AP was proton transfer, and NHClOdissociation to form adsorbed NHand HClO[27].Low-temperature decomposition mainly involved a reaction between NHand HClOadsorbed on the particle surface.NHcannot be oxidized by the decomposition product HClOat low temperature.The remaining adsorbed NHwould cover the surface of the ammonium perchlorate. When the particle surfaces were completely covered by NH, the low-temperature decomposition was concluded, and the mass loss was approximately 30%. The high-temperature decomposition process mainly involved a reaction in the gas phase, in which adsorbed NHand HClOwere desorbed into the gas phase upon heating.In the gas phase,HClOwas further decomposed to produce oxidation products; at the same time,NHwas oxidized by an oxidation product of HClO,and the final product was formed.
When the PAP was reheated, the initial reaction temperature was decreased because of the presence of pores and the increase in specific surface area. However, as the specific surface area increased,adsorption of the unreacted NHbecame easier,and lowtemperature decomposition of AP often began with dislocations or defects;the proportion of these may decrease when AP is partially decomposed. These factors led to an increase in the activation energy for low-temperature decomposition, which may also explain the higher initial decomposition temperature for PAP with a mass loss of 29.3%. When the temperature continued to rise, the reactions of AP changed from the solid phase to the gas phase, and NHand HClOwith a larger specific surface area and lower adsorption energy were desorbed into the gas phase.Therefore,the reaction temperature and activation energy were significantly reduced.
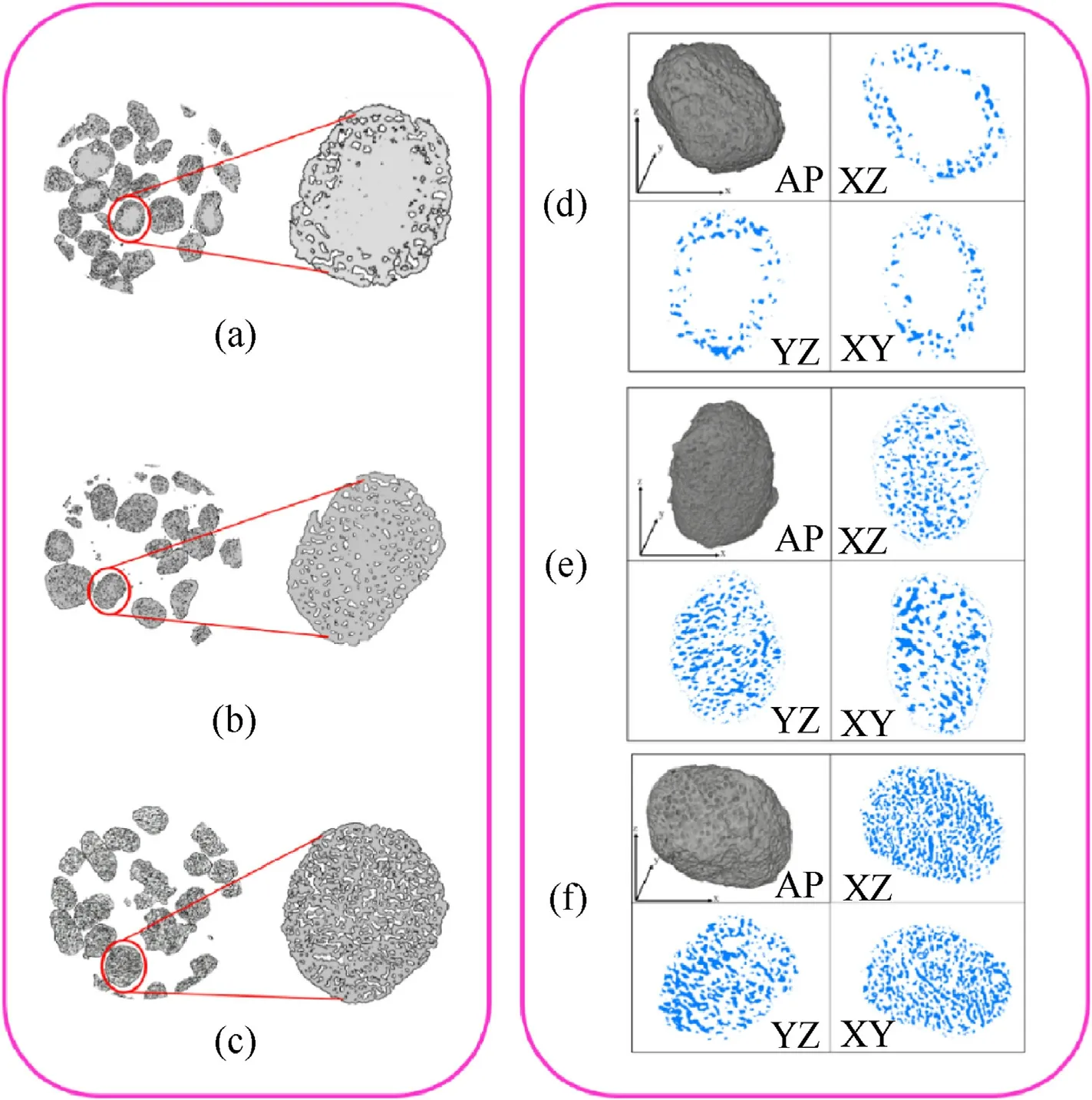
Fig.2. Nano-CT images with different AP mass losses:(a)9.25 wt%,(b)20.7 wt%,and(c)29.3 wt%.(d)-(f)Cross-section CT images for the 9.25 wt%,20.7 wt%,and 29.3 wt%samples.
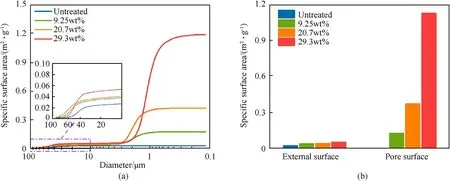
Fig. 3. (a) specific surface area of PAP by MIP; (b) the external surface and pore surface.
4. Combustion performance
To study the effects of PAP on combustion characteristics,closed bomb and high-speed photography were used,and the combustion characteristics of AP/Al binary mixtures were studied under closed and open conditions with aluminium powder fuel.
4.1. Combustion characteristics in a high-pressure environment
The closed bomb is a test system used to study the pressure change law of a gun propellant when it burns under constant volume, and it exhibits the advantages of easy operation, reliability and high repeatability.As shown in Fig.5,the experimental system included a closed tank, ignition device, pressure sensor, and a testing and data acquisition system.The volume of the closed tank was 10 mL.
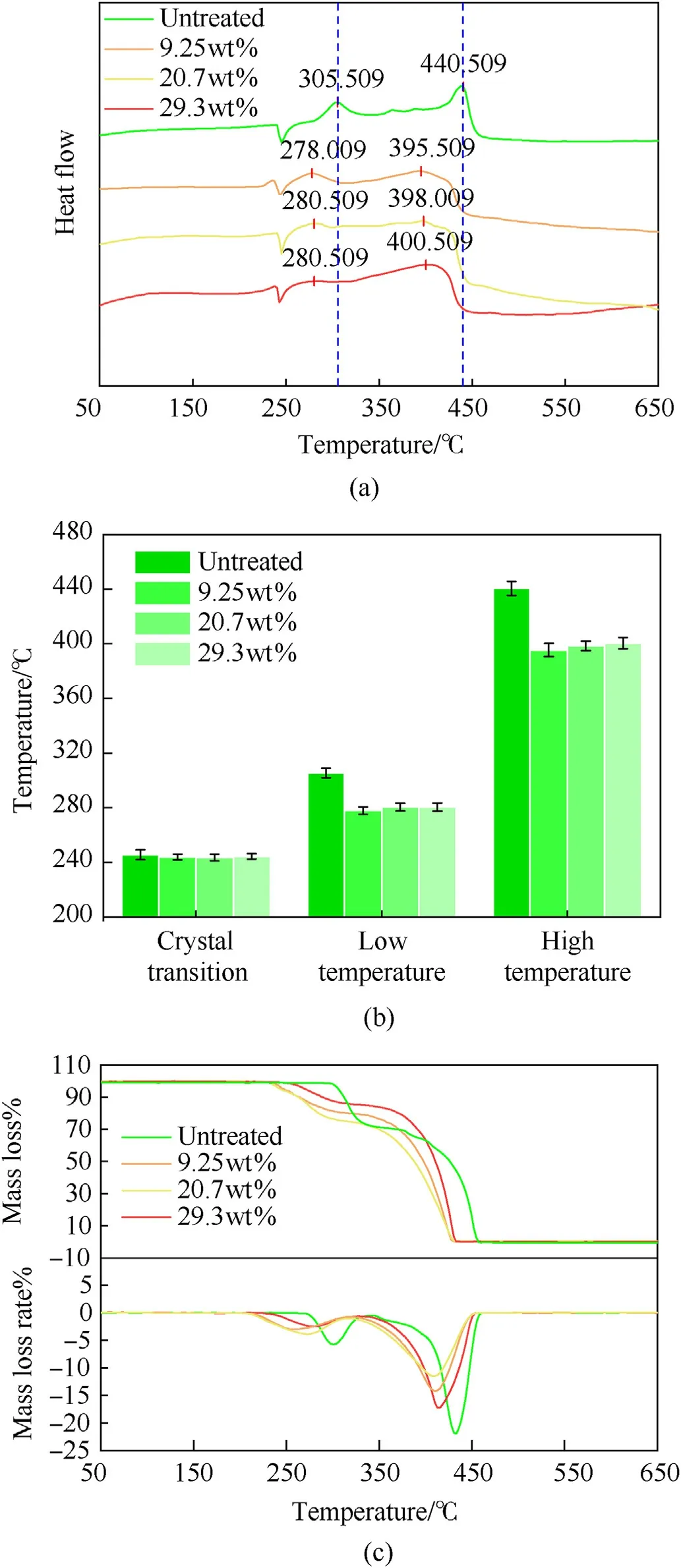
Fig. 4. AP thermal decomposition: (a) Heat flow; (b) Temperature peak for crystal transformation and decomposition temperature; (c) TG and DTG data.
The combustion characteristics of AP/Al binary mixtures were tested with different mixing ratios, as shown in Table 2. Thecombustion characteristics were measured with a negative, zero and positive oxygen balance.Combustion experiments of AP and Al mixtures were carried out with untreated AP and PAP samples with mass losses of 9.25%, 20.7%, and 29.3%, respectively. Each experiment was repeated three times.The sample was placed in the tank,and the tank was sealed and pressurized to 3 MPa.Then,the sample in the closed bomb was ignited, and the pressure change was recorded.
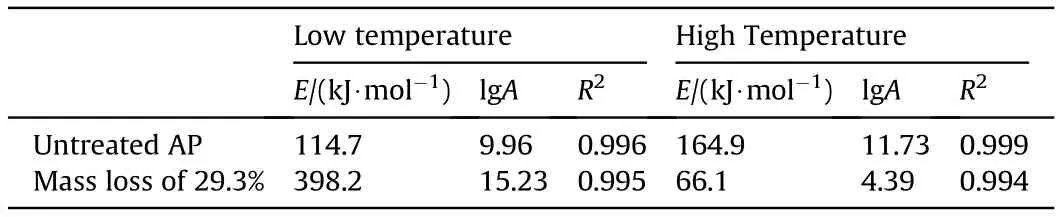
Table 1 Chemical kinetics parameters of AP with different degrees of decomposition.
Fig. 5. Schematic diagram of the closed bomb system.
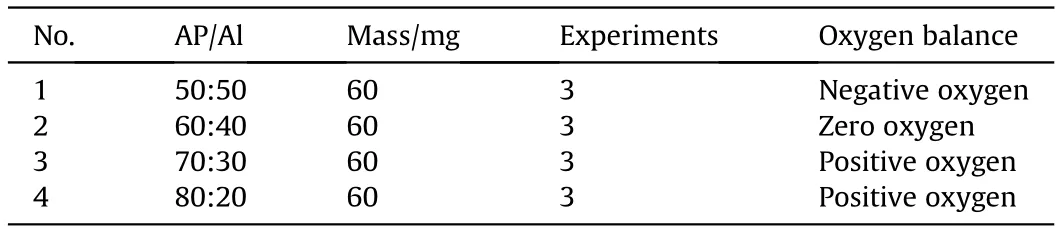
Table 2 Experimental design parameters.
Fig.6 shows Pressure-time curves for ignition of AP/Al samples with different mass losses in the closed bomb,in which the abscissa is the time and the point t=0 is the ignition moment.The ordinate shows the relative pressure (minus the initial pressure of 3 MPa).Obviously,with an increase in the AP mass loss, the peak pressure value was higher, the pressure rose faster, and the curve was steeper. Table 3 and Fig. 7 show the relationships between peak pressure,peak time,mass loss,and mixing ratio.The time required to reach the peak value was greatly affected by the porosity and less by the proportion of AP, and the higher the porosity was, the shorter the time needed to reach the peak pressure. The pore structure of PAP accelerated the combustion rate of the mixed system. However, the peak pressure was affected by both porosity and the AP ratio. The pressure peak was highest when the oxygen balance was close to zero (AP/Al = 60/40) for untreated AP with a mass loss of 9.25%. When the mass loss was 20.7%, the maximum pressure peak appeared at 70:30. When the weight loss rate reached 29.3%, the peak pressure increased with increasing AP content, and the maximum pressure occurred at 80% AP content.
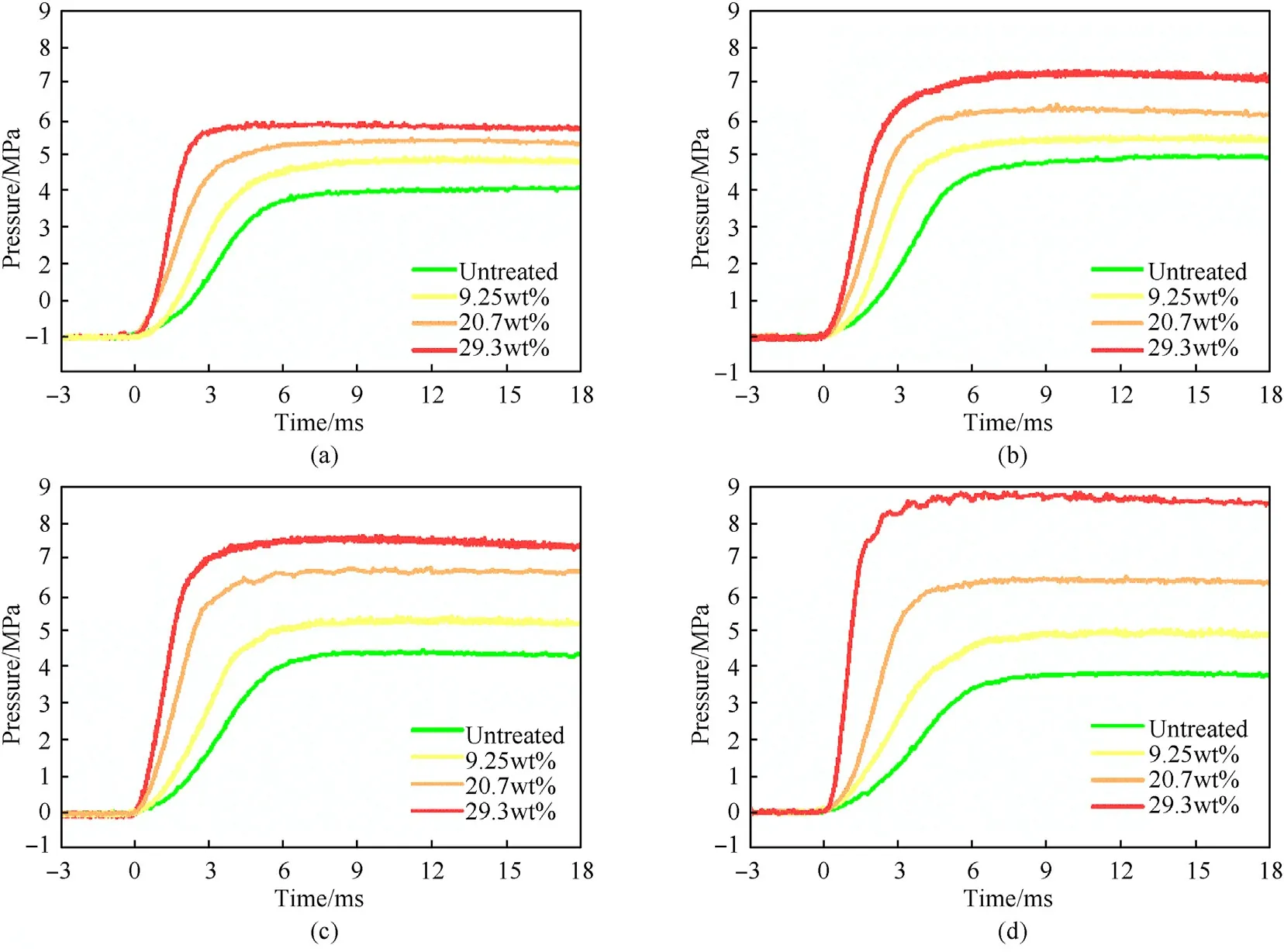
Fig. 6. Experimental results for closed bomb tests: (a) AP:Al = 50:50; (b) AP:Al = 60:40; (c) AP:Al = 70:30; and (d) AP:Al = 80:20.
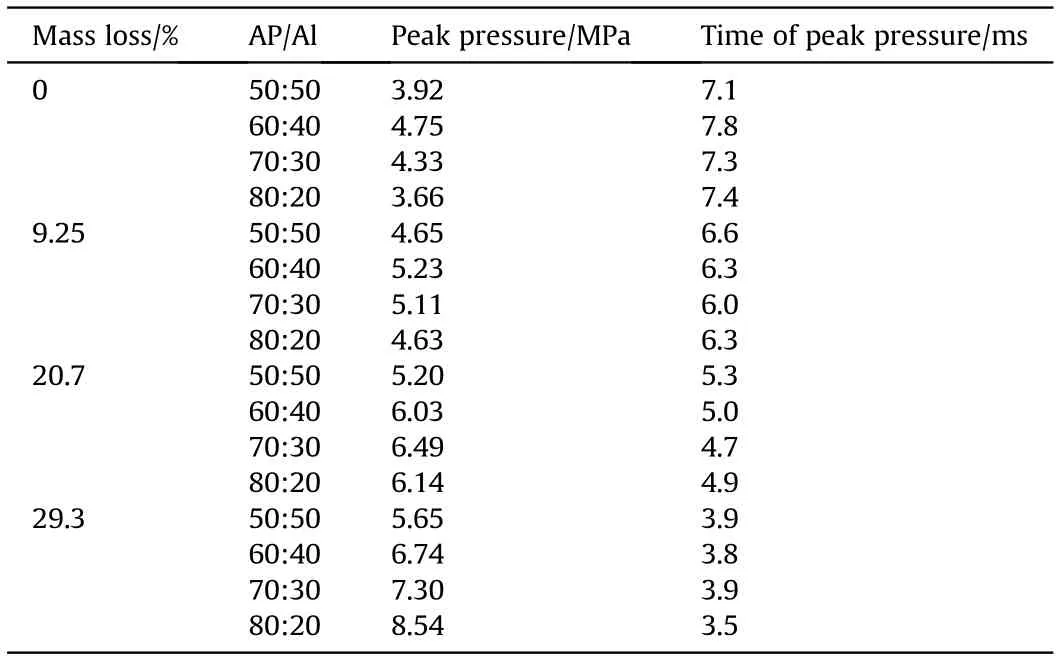
Table 3 Combustion pressure characteristics in a confined space.
The AP content in a composite propellant is usually higher than that of Al, and the composition ratio of a ternary HTPB composite propellant is binder/AP/AL = 15/68/17. The AP/Al ratio is close to 80:20; therefore, the mixed system with a mixing ratio of AP/Al=80:20 was selected for analysis.To analyse the combustion rate and the severity of the reaction,the pressure change rate dp/dt was used as a characteristic parameter of the combustion rate. As shown in Fig. 8, the tendencies for plots of dp/dt vs. t for different sample combustion processes were the same,but there were large gaps among the peak values and the times of combustion.Compared with untreated AP,the peak pressure of the 29.3%massloss system was increased by a factor of 2.3 from 3.66 to 8.54 MPa;however,the peak value for dp/dt increased by a factor of 7.9 from 861.5 to 6817.5 MPa/s. The combustion rates and peak pressures were both significantly increased. The reaction rate and mass loss showed an exponential relationship(y=754.5+111.7*exp(x/7.4)),and the reaction intensity was significantly increased.
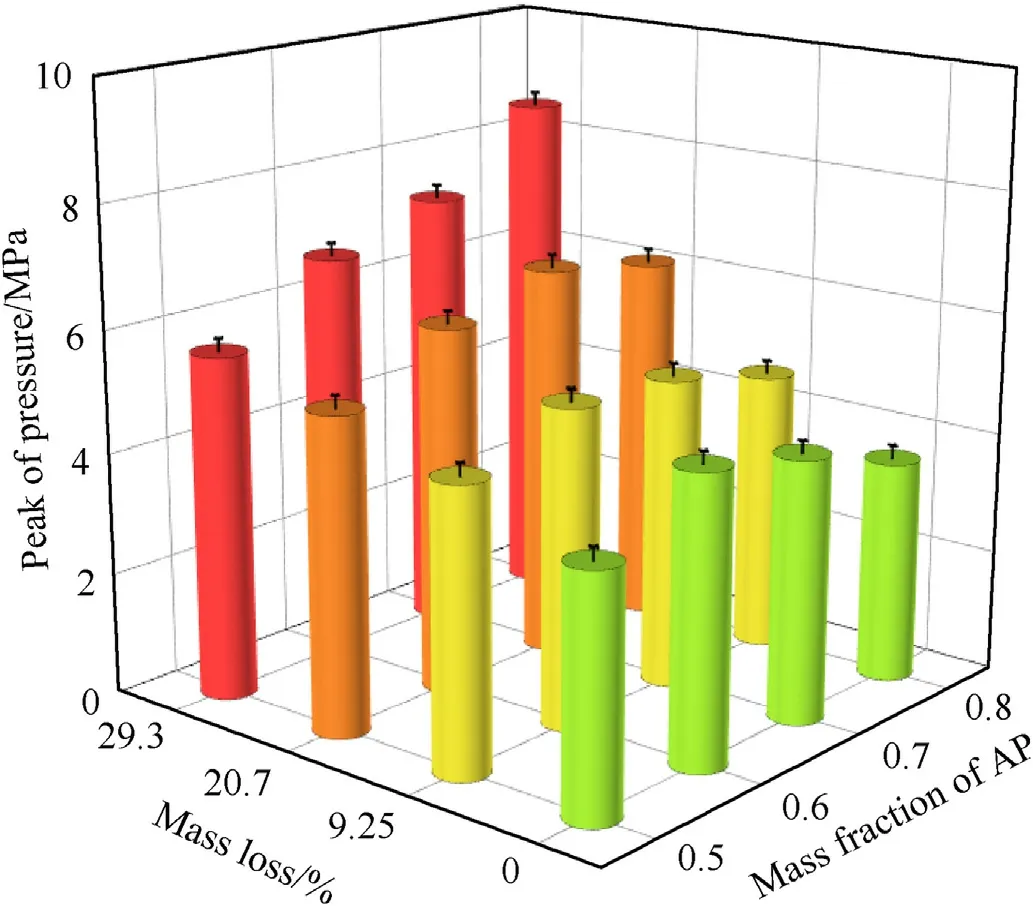
Fig. 7. Relationships among combustion peak pressure, mass loss, and AP content for AP/Al ignition in a confined space.
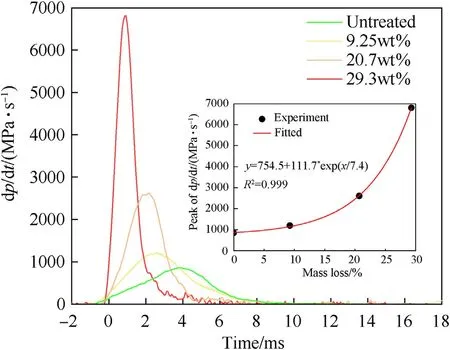
Fig. 8. Plots of dp/dt versus time for combustion processes occurring in a highpressure environment.
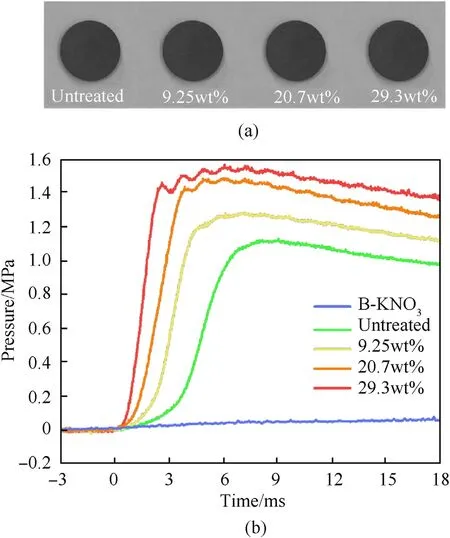
Fig.9. (a)AP/Al tablets;(b)Experimental results of AP/Al tablets for closed bomb tests.
To explore the combustion characteristics of AP/Al mixture that was compressed, the samples, adding 0.5% PTFE as the binder,compressed into 10 mm diameter tablets with 100 mg each by Tablet Press Machine at 3 MPa (Fig. 9(a)). The pressure during combustion was tested by a closed bomb experiment. The closed tank with a volume of 50 mL was used to avoid excessive pressure.To ensure reliable ignition, 20 mg B-KNOignition composition was added around the tablets. As shown in Fig. 9(b), the variation tendency of pressure was consistent with the powder samples as AP mass loss increased. However, the initial combustion rate became slow after being compressed into tablets with a 0.5-3.5 ms ignition growth period. And the ignition delay period became longer as the degree of decomposition decreases.It was mainly due to the effect of the initial specific surface area.
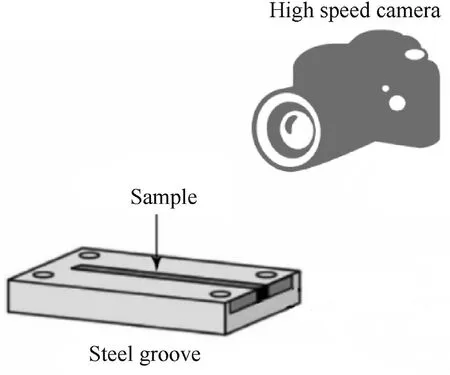
Fig.10. Schematic diagram of the combustion test with a grooved charge.
4.2. Combustion characteristics under open conditions
To observe the effects of AP pore structure on the combustion process,the grooved structure shown in Fig.10 was made,and the combustion process was observed by high-speed photography.The AP/Al mixture was naturally filled into the steel groove with a length of 2 cm. The combustion process was recorded by highspeed photography with a laser ignition instrument and a sampling frequency of 10,000 FPS.
Fig. 11 shows high-speed photography images of the flames produced during combustion of mixed AP systems with different mass losses. Taking the ignition position as the starting point of a vertical line, extracting the flame centre, and connecting them indicated that the centre of the flame was basically in a straight line,which showed that the flame propagation velocity was approximately linear, and the combustion velocity was relatively stable.The flames produced during the combustion processes were larger and brighter with higher porosities,which illustrated that both the speed of combustion and the intensity of combustion were increased.
The relationship between the average combustion rate v,v=L/t,and mass loss was obtained as shown in Fig.12.When the AP mass loss was increased from 0 to 29.3%, the average combustion rate increased from 0.13 to 0.23 m/s,and the combustion rate increased by 76.9%.
5. Results and analysis
The above results showed that partial AP decomposition accelerated the combustion rates of AP/Al binary mixed systems,and the impact on the combustion rate increased with increasing degree of decomposition. This was mainly related to the microstructures of PAP. As shown in Section 2.2, partially decomposed AP formed a porous structure, and the porosity increased obviously with the degree of decomposition. Reference [26] showed that the porosity was approximately equal to the mass loss and slightly lower than the mass loss.
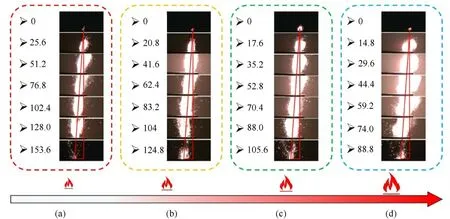
Fig.11. Combustion flames for AP/Al (80/20) mixed systems with different mass losses: (a) Untreated; (b) 9.25 wt%; (c) 20.7 wt%; (d) 29.3 wt%.
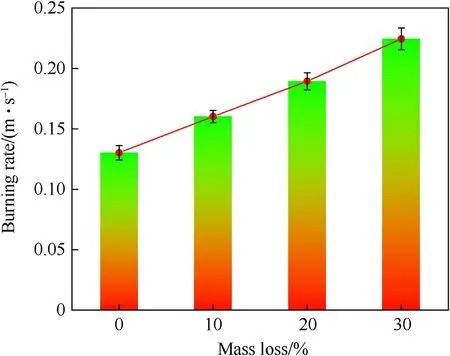
Fig. 12. AP/Al mixture systems: relationship between combustion rate and decomposition degree under open conditions.
The porous structures of PAP led to larger combustion surfaces and faster combustion speeds after ignition. On the one hand, the pore structures of PAP caused the flame to permeate into the pores during combustion,leading to increases in combustion surfaces.On the other hand, the effects of gaseous products formed at high temperature and pressure may cause the pore structure to fracture the AP particles further to increase the burning surface. We also found that the increase in combustion rate was linearly related to the mass loss when combustion occurred in the groove used for the open tests, but there was an exponential relationship when combustion occurred in the confined space where the combustion process was more affected by the degree of decomposition. This result indicated that the pressure had a strong influence on the combustion of PAP.
The combustion products of AP/Al were high-temperature and high-pressure gases. The gas equation of state follows the Noble-Abel equation:

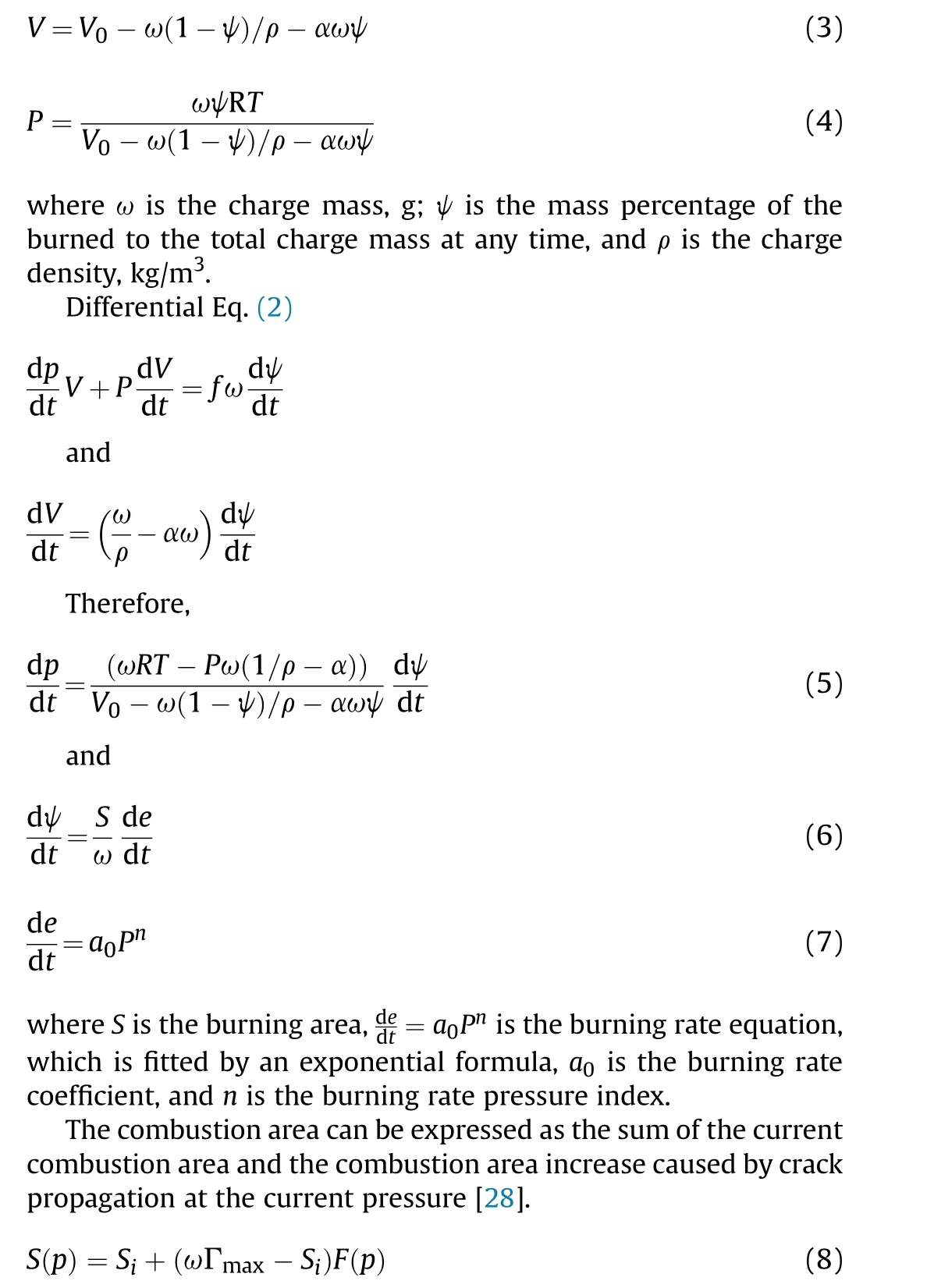
where Sis the current combustion area,the combustion area at the ignition time is determined by the characteristics of the charge,Γis the maximum specific surface area of the charge,and F(p)is the probability for expansion and evolution of the combustion crack under the action of pressure P, which is calculated by the Weibull distribution model.
In the above study,all parameters were the same except for the degree of AP decomposition,so,from Eq.(10),the combustion area S is proportional to dp/dt under a particular pressure. When burning occurs in open conditions, p = pand F(p) = 0, so the burning rate remains constant, which is the result seen via highspeed photography for the open condition. When burning under closed and pressurized conditions, p > p, and the combustion surface continues to increase. The combustion gas will cause the pressure in the closed space to increase continuously, which will cause the combustion surface to increase further and accelerate combustion. Therefore, the results for combustion acceleration under closed and pressurized conditions are presented.
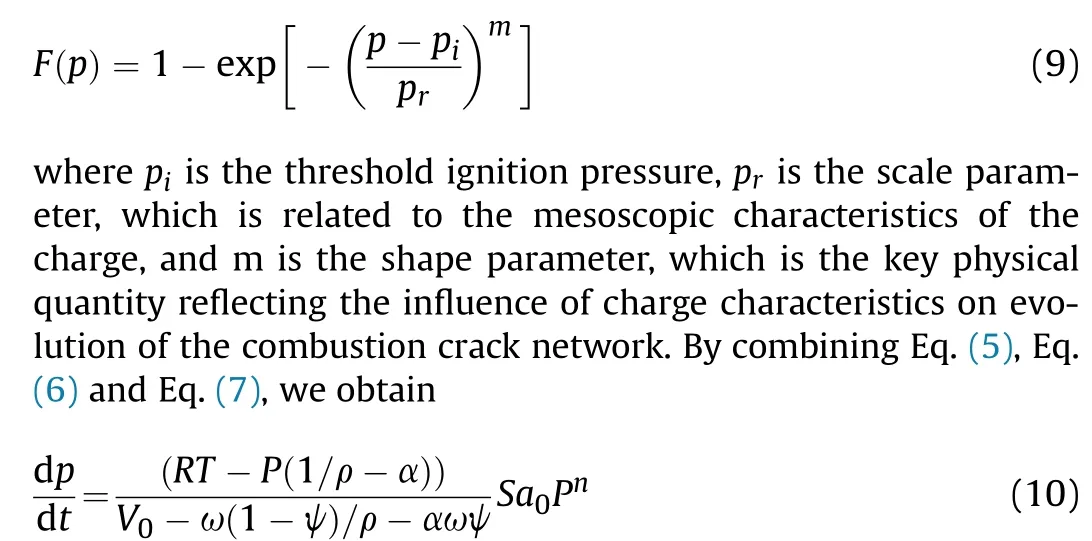
With a deepening of the AP decomposition degree,the presence of the pore structure is more likely to lead to crack propagation,which can be characterized by m and p.The effects of m and pon the combustion area can be calculated as shown in Fig.13(a) and Fig. 13(b) by Eq. (8) and Eq. (9). With increases in m and p, the burning area grows slowly,and m has a greater impact on the lowpressure section,while phas a greater impact on the high-pressure section.Assuming that the pores are spherical and of the same size,the relationship between the porosity and the specific surface area,which were obtained by geometric calculation and are shown in Fig.13(c),indicated that the specific surface area was approximately linearly related to porosity when the porosity was below 0.3. And the specific surface area increased with the decrease of the pores size. The effects of different initial combustion areas were calculated as shown in Fig.13(d), and they had a greater impact on the low-pressure section.
The larger the specific surface area of PAP was, the larger the combustion area was after ignition, which caused the faster the combustion rate and the higher the pressure rise rate.If all else was equal, only the initial specific surface area of the combustion was changed, and the pressure variation during the combustion was calculated using the established mathematical model.As shown in Fig.13(e),the combustion rate tends to accelerate with the increase of specific surface area. However, the calculated results were smaller than the experimental results,due to the fact that it became fragile after AP decomposition and the crack propagation was more likely to occur after ignition.
The P-t relationship can be determined by combining Eq. (6) -Eq. (10), and the relevant parameters of the AP/Al (80/20) binary system are shown in Table 4. Pressure-time can be calculated by adjusting S,m and p.With increases in the decomposition degree of AP,the pores of AP increased,which led to an increase in Sand decreases in m and p. These results were compared with the experimental results,as shown in Fig.14.The fit for the rising stage of the pressure was good,and the combustion growth phase of the AP/Al binary mixture system was well described.When the charge was almost exhausted,combustion weakened,and the model could not fit this process well because combustion of the powder decreased.
To verify the accuracy of the model and study the effects of the initial pressure at ignition time on the combustion characteristics,the closed bomb experiment was carried out in triplicate with the 29.3 wt% AP/Al (80/20) sample at 1 MPa. By adjusting the initial pressure in the calculation model, the pressure was calculated for various times, and the results were compared with the experimental results;this is shown in Fig.15,where the ordinate indicates the pressure increase due to combustion.The agreement was good,and the applicability of the model to combustion of the AP/Al mixed system was verified. Then, the pressures were calculated as a function of time for initial pressures of 0.1,5 and 10 MPa.The initial ignition pressure mainly affected the burning rate, and the higher the pressure was, the faster the burning rate. The initial ignition pressure had little effect on the final equilibrium pressure.
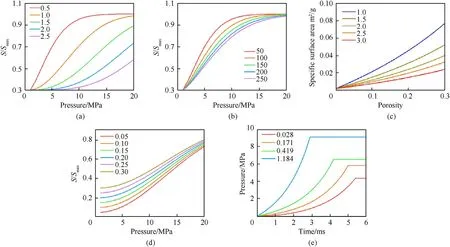
Fig.13. Influencing factors for the combustion surface:(a)Shape function m at pr =150 MPa,Si =0.3Smax;(b)scale parameter pr at m=1.0,Si =0.3Smax;(c)Influence of porosity on specific surface area; (d) Influence of initial combustion surface on expansion of the combustion surface; (e) The effect of initial specific surface area on combustion.

Table 4 Combustion performance parameters for the AP/Al binary mixed system.
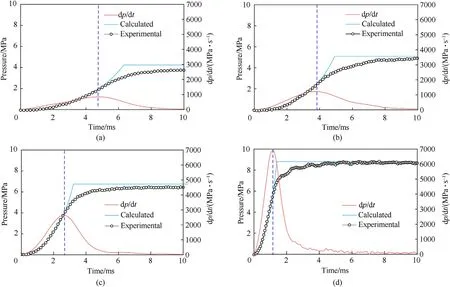
Fig.14. Comparisons of Pressure-time curves for experimental and calculated values: (a) Untreated; (b) 9.25% wt%; (c) 20.7 wt%; and (d) 29.3 wt%.
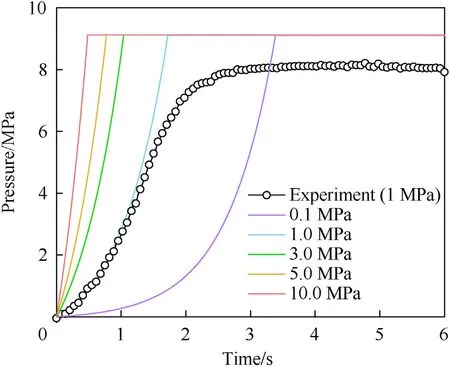
Fig.15. Verification of the calculation model and the influence of the initial pressure.
6. Conclusions
(1) Low-temperature decomposition of AP led to the formation of porous structures in AP particles.The pores first appeared near the surfaces of AP particles. With the extension of heating time,the pores developed sufficiently first and then expanded around the AP particles. Eventually, the particles were filled with pores.
(2) The crystal structure of AP remained unchanged after decomposition at low temperature. However, the low and high decomposition temperatures decreased obviously, and the decomposition rate increased. The activation energy for low-temperature PAP decomposition increased, and the activation energy for high-temperature decomposition decreased.
(3) Because of the PAP porous structure,the combustion rate for the AP/Al binary mixed system increased with increasing decomposition of AP. The relationship between the combustion rate and mass loss was approximately linear for ignition under the open condition,and it was exponential for ignition in a high-pressure environment. A computational model was established for combustion of the AP/Al binary system,and the effects of pore structure and pressure on the combustion process were explained. The calculated results were in good agreement with the experimental results.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work was supported by the National Natural Science Foundation of China (Grant No.11772058).
- Defence Technology的其它文章
- A new model for the expansion tube considering the stress coupling:Theory, experiments and simulations
- Experimental and analytical assessment of the hypervelocity impact damage of GLAss fiber REinforced aluminum
- Modeling of bistatic scattering from an underwater non-penetrable target using a Kirchhoff approximation method
- 3,6-bis (2,2,2-trinitroethylnitramino)-1,2,4,5-tetrazine. Structure and energy abilities as a component of solid composite propellants
- Rapid preparation of size-tunable nano-TATB by microfluidics
- Mechanical behavior of Ti-6Al-4V lattice-walled tubes under uniaxial compression

