Rapid preparation of size-tunable nano-TATB by microfluidics
Song Zhang, Le-wu Zhan, Guang-kai Zhu, Yi-yi Teng, Yu Shan, Jing Hou, Li Bin-dong
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
Keywords:Nano-TATB Microfluidics Energetic materials Solvent/non-solvent method Amination Simulation
ABSTRACT Nano-TATB was developed in microchannels by physical method and chemical method,respectively.The effects of total flow rate, number of microreactor plates, solvent/non-solvent ratio and temperature on the particle size of TATB in the physical method were studied.Prepared TATB were characterized by Nano Sizer, Scanning Electron Microscopy, Specific surface aperture analyzer, X-ray diffraction, Fourier transform infrared spectroscopy and Differential Scanning Calorimetry. The results show that the TATB obtained by physical method and chemical method are spherical, with average particle size of 130.66 nm and 108.51 nm, respectively. Specific surface areas of TATB obtained by physical and chemical methods are 21.37 m2/g and 21.91 m2/g, respectively. Compared with the specific surface area of micro-TATB(0.0808 m2/g), the specific surface area of nano-TATB is significantly increased. DSC test results show that the smaller the particle size of TATB,the lower the thermal decomposition temperature.In addition,by simulating the mixing state of fluid in microchannels and combining with the classical nucleation theory, the mechanism of preparing nano-TATB by microchannels was proposed.
1. Introduction
1,3,5-triamino-2,4,6-trinitrobenzene (TATB) is a kind of high explosive with extremely low sensitivity to heat,impact,shock and electric sparks [1]. As an insensitive explosive, TATB has been extensively used in the fields of atomic bomb,nuclear warhead and polymer bonded explosive, etc. [2]. Compared with explosives of conventional sizes, due to the characteristics of small particle size,large specific surface area and high surface energy, micro/nano explosives have changed their safety, combustion, and initiation properties [3-5]. Studies have shown that micro/nano TATB had better heat resisting evenness and its 5 s ignition point is advanced by 7.5 K[6];the shock initiation performance of micron,especially nano-scale TATB under high-pressure and short-duration pulse is significantly improved compared with conventional size [7,8];utilizing the excellent performance of micro/nano TATB can significantly reduce the mechanical sensitivity of high-energy explosives while maintaining high detonation performance [9,10].Therefore, the preparation of nano-TATB has received extensive attention from researchers [11]. At present, the preparation methods of micro/nano energetic materials mainly include microemulsion method [12],mechanical ball milling method [13],spray method [14], supercritical fluid technology [15] and solvent/nonsolvent method [5,6]. However, these methods have been implemented at the macroscopic level and generally suffer from prominent problems such as large online quantity of dangerous goods,low intrinsic safety, unstable process conditions, poor quality consistency and large footprint. In recent years, microfluidics has been widely used for preparing micron and nanoscale particles by taking advantage of its ability to precisely control reaction parameters,high heat/mass transfer efficiency,low reagent consumption and low amplification effect. Microfluidics provides a unique environment for investigating the synthesis process of nano- and micron-sized particles,which contributes to the formation of highquality crystals with controlled particle size and shape [16,17].
Currently,despite the popular use of microfluidics in preparing micro/nanoparticles [16,18-23], but rare literature has been reported on the refinement of energetic materials. Wang et al. prepared chrysanthemum-shaped TATB particles through microchannel oriented crystallization [24]. The length and diameter of these chrysanthemum-shaped TATB were measured to be~1 μm and~40 nm,respectively.Zhao et al.realized the controllable preparation of 91-255 nm hexanitrostibene (HNS) through the self-designed and built microfluidic platform [17]. Wu and coworkers independently designed a micro-mixer and successfully prepared 150-900 nm RDX by recrystallization [25]. Shi et al.studied the crystal morphology control of CL-20 in the microchannel and produced a micron-sized CL-20[26].In summary,the use of microfluidic technology to prepare nanoscale energetic materials is still in the laboratory exploration stage. Also, there continue to be few reports about the preparation of nano-TATB by microchannels.
To further investigate the problem of preparing nanoscale energetic materials in a microreactor, we have designed and processed independently a microreaction system (Fig. 1), which consists of a transport module, a microreaction module, and a corresponding thermostat module. In this work, we will prepare TATB in a microreactor by solvent/non-solvent crystallization and investigate the effects of the total flow rate, reactor plate number,solvent/non-solvent flow ratio and temperature on TATB particle size. In addition, we attempted to synthesize nano-TATB by chemical amination of 1,3,5-triethoxy-2,4,6-trinitrobenzene(TETNB) in microchannels.
2. Experiment
2.1. Materials
TATB (purity: 99.8%) and TETNB (purity: 99.8%) were supplied by Gansu Yinguang Chemical Industry Group Co. Ltd. Dimethyl sulfoxide (DMSO; purity: >99%), ammonium hydroxide (content:25-28%)and 1-ethyl-3-methylimidazolium acetate([Emim][OAc];purity:>98%)were purchased from Shanghai Aladdin Bio-chemical Technology Co. Ltd.
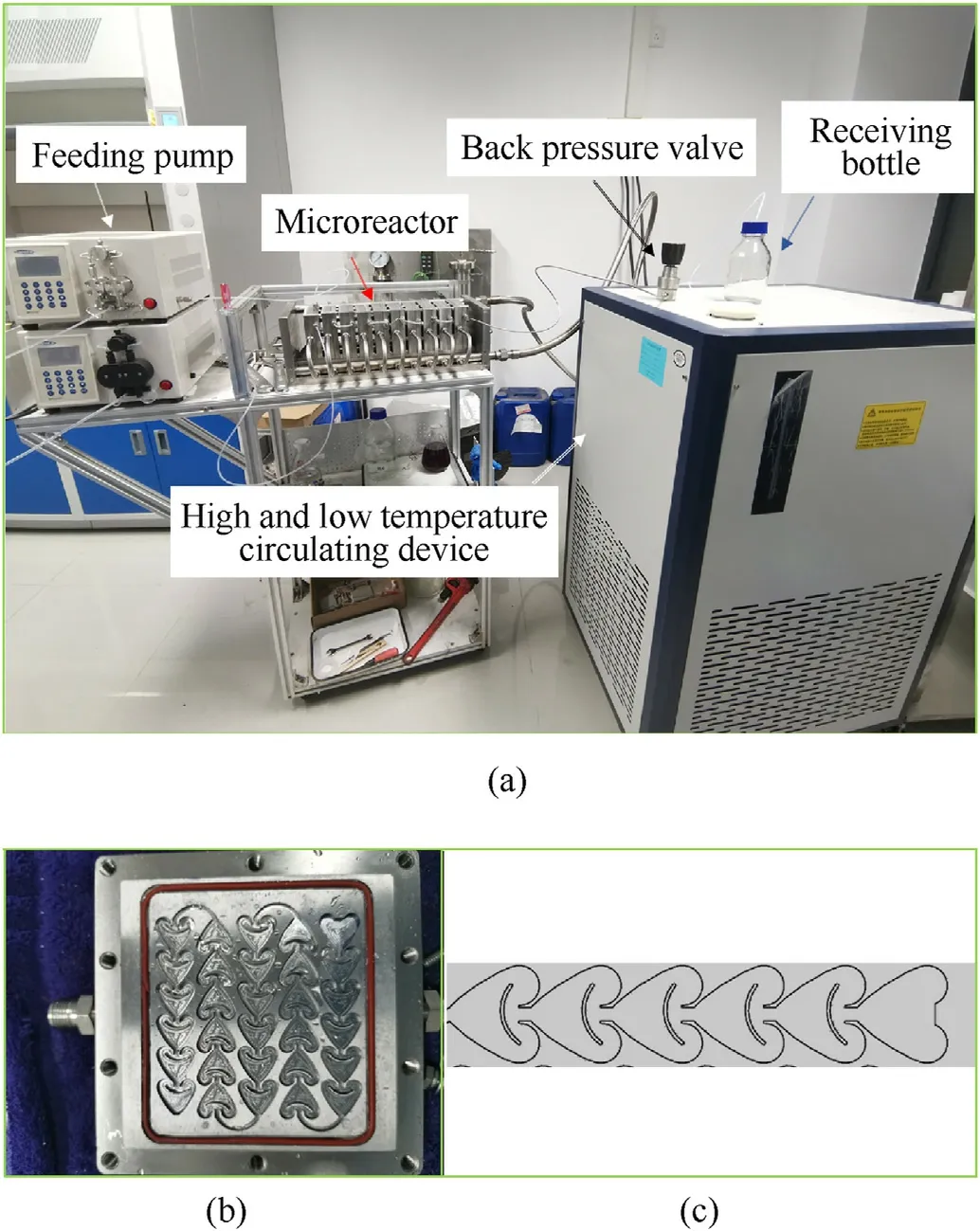
Fig. 1. Microreactor system (a), the internal structure of the microreactor (b) and Geometric model of microreactor (c).
2.2. Experimental devices
To meet the need for large-scale preparation of nanoparticles,we have developed a detachable passive mixer with a heart-shaped structure (Fig.1b), and assembled a micro-reaction device system as showed in Fig.1a. The micro-reaction device system mainly includes two plunger pumps(MPF0502C,Shanghai Sanwei Scientific Instrument Co., Ltd., China), a microreactor consisting of ten microreaction plates in series, and a high and low temperature circulation device(GDSZ-50 L/30C,Zhengzhou Ruihan Instrument Co.,Ltd.,China),a back pressure valve and a liquid collection bottle.The connection uses polytetrafluoroethylene (PTFE) tubes with an inner diameter of 1.6 mm. The total liquid holding capacity of the microreactor is 50 ml, and the main body material is HC276.
2.3. Preparation of nano-TATB by recrystallization
2.3.1. Experiment
First,the ionic liquid[Emim][OAc]and DMSO are prepared into a 100 g mixed solution at a mass ratio of 5:95, and then 0.4 g of TATB was weighed and added into the mixed solution for ultrasonic dissolution.Set the high and low temperature circulation device to make the reactor temperature up to 30C, set the pressure of the back pressure valve to 0. The prepared solution and deionized water were pumped into the reactor by the feed pump at the flow rates of 12.5 ml/min and 37.5 ml/min respectively, and the materials were collected after 1 min of discharge.Finally,centrifuge the collected liquid with water 2-3 times to remove residual DMSO and [Emim][OAC], and then the sample was dried by vacuum freeze-dryer, so as to achieve solid materials with excellent dispersion.
On this basis, the effects of total flow rates (the sum of solvent and non-solvent flow rates:20,30,40,50 and 60 ml/min),number of microreactor plates(2,4,6,8,10),solvent/non-solvent ratios(1:1,1:2,1:3,1:4,1:5) and temperatures (30, 40, 50, 60, 70C) on the particle size of TATB during recrystallization process were studied by the controlled variable method.
2.3.2. Computational fluid dynamics (CFD) simulations
The mixing effect of solvent and non-solvent determines the size distribution of nanoparticles, and CFD simulation can help evaluate the mixing effect of fluids. The geometric model in this paper(Fig.1c)was drawn using Solidworks2020 software and then meshed by HyperMesh2019 software. The mesh size is 0.1, with a total of 371,896 mesh elements. The minimum orthogonal mass reaches 0.656, which is suitable for the next CFD simulation. This paper mainly simulates the mixing of the DMSO/HO system in the microreactor, so this simulation uses an incompressible model. In addition to the mass conservation equation, momentum conservation equation, and energy conservation equation, the governing equations of the simulation also involve the diffusion and transportation conservation equations of species because DMSO and HO are mutually soluble systems.
The calculation simulates the mutual mixing of DMSO and HO at different flow ratios and different total flow rates, so a steadystate model based on pressure is selected. In addition, due to the small geometric size of the microchannels,the laminar flow model is combined with the component transport model for the simulation, and gravity is not considered. The material property parameters involved in this mixing simulation mainly include density and viscosity,the detailed values are shown in Table 1.Based on this,the material parameters in Fluent 2020 R2 are appropriately modified.In addition,the Wilke-Chang empirical formula is used to estimate the diffusion coefficient of DMSO in HO at 30C.
In the setting of boundary conditions,since the inlet adopts themass flow inlet,all the volume flow in the actual experiment needs to be converted into mass flow and input into the software. The outlet defines it as a pressure outlet,and the wall adopts a non-slip wall. In the simulation method, the pressure-velocity coupling of SIMPLE is adopted.When the space is discretized, the momentum and energy are set by the second-order upwind setting, and the other parameters adopt the default values. The residual is set to 10.

Table 1 Material property parameters.
2.4. Synthesis of nano-TATB by amination
TETNB (0.796 mmol) was dissolved into a mixed solvent of[Emim][OAc] and DMSO (60 g, 5:95). A high and low temperature cycling device was set up so that the reactor temperature reached 60C, and the back pressure valve was adjusted so that the indicated value was 0.4 MPa 5 ml/min and 0.4 ml/min of the abovementioned prepared solution and ammonia water, respectively,were passed into the microreactor for reaction. After adding the reaction mixture,the collected solids would be reacted for another 30 min in the reactor at the flow rate of 5 ml/min. The collected liquid and deionized water would be passed into the microreactor at the flow rate of 10 ml/min and 50 ml/min, respectively. Finally,centrifuge the collected liquid with water 2-3 times to remove residual DMSO and[Emim][OAC],and then the sample was dried by vacuum freeze-dryer,so as to achieve solid materials with excellent dispersion.
2.5. Characterization
Particle size distribution of TATB is measured by Malvern's ZS90 Nano Sizer and Zeta-potential Tester.Scanning electron microscopy(SEM) was conducted using an S4800 Hitachi field emission scanning electron microscope at 30 kV. To reduce the charge effect of the samples,the samples were subjected to a gold spray treatment before the SEM observation. The specific surface area of TATB was obtained by nitrogen adsorption method of Brunaure-Emmett-Teller (BET) using a TriStar II (3020) specific surface apparatus.The phases of the samples were investigated with an X-ray diffractometer (XRD, Bruker D8 Advance) using Cu Kα radiation at 40 kV and 40 mA. Fourier-transform infrared (FT-IR) spectra were collected from a Nicolet iS10 of Thermo Fisher Scientific. The differential scanning calorimetry (DSC) experiments were conducted with the DSC823E instrument from METTLER TOLEDO.All samples were weighed between 0.4 and 0.5 mg and heated from 50 to 450C with a nitrogen flow rate of 20 ml/min.
3. Experimental results and analysis
3.1. Particle size analysis
Particle size distributions of TATB during recrystallization(Fig. 2a-d) and amination (Fig. 2e) process were characterized by Nano Sizer and Zeta-potential Tester.From Fig.2a,we can see that the TATB particle size first decreases and then increases as the total flow rate increases,and when the total flow rate reaches 50 ml/min,the TATB particle size reaches the minimum value(D50=130.66 nm),and the particle size distribution was relatively narrow.The same situation is present in Fig.2c and d.It can be seen that the best solvent/non-solvent ratio and the most suitable temperature are 1:3 and 30C respectively. It can be seen from Fig. 2b that as the number of reactor plates increases, the particle size of TATB decreases.In addition,it can be seen from Fig.2e that the particle size of the TATB synthesized by amination reached 108.51 nm.
3.2. CFD simulation and analysis
This paper simulated the mixing of DMSO and HO under different total flow rates (Fig. 3a) and solvent/non-solvent ratios(Fig.3b).It can be seen from the density cloud diagram of material mixing that the material mixing process mainly occurs in the firstrow structure, and its density cloud diagram does not change significantly after flowing through the second-row structure,indicating that DMSO and HO have been basically mixed completely at this time,so the research mainly focuses on the first-row structure.In order to further quantitatively study the mixing effect, six equidistant monitoring positions were set for the first-row structure(Fig.3c),and monitoring position 7 was set at the exit position.The mass fractions of DMSO at all grid nodes in 7 monitoring positions were read,and the mixing intensity M was used to evaluate the mixing effect [27-29], it was calculated as follows:
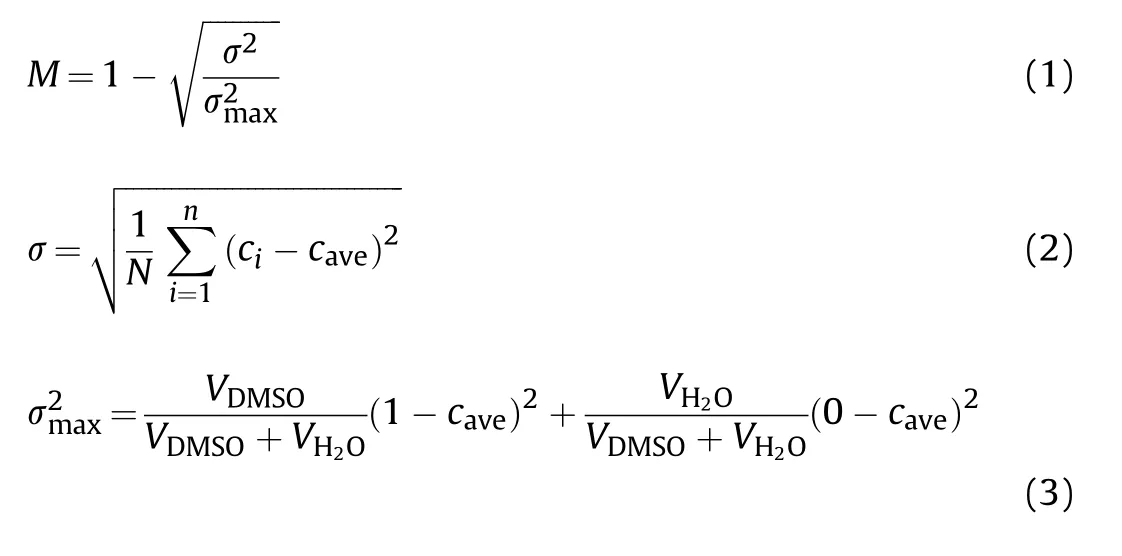
Where σ is the standard deviation of the mass fraction of DMSO at the monitoring location, σis the maximum variance of mass fraction at the monitoring position,N is the number of grid nodes in the monitoring position, namely the number of mass fraction sampling,cis the mass fraction at the monitoring node i,cis the average expectation of statistics for monitoring location mass scores,Vand Vrespectively refer to the volumetric flow of DMSO and HO during feeding (ml/min).
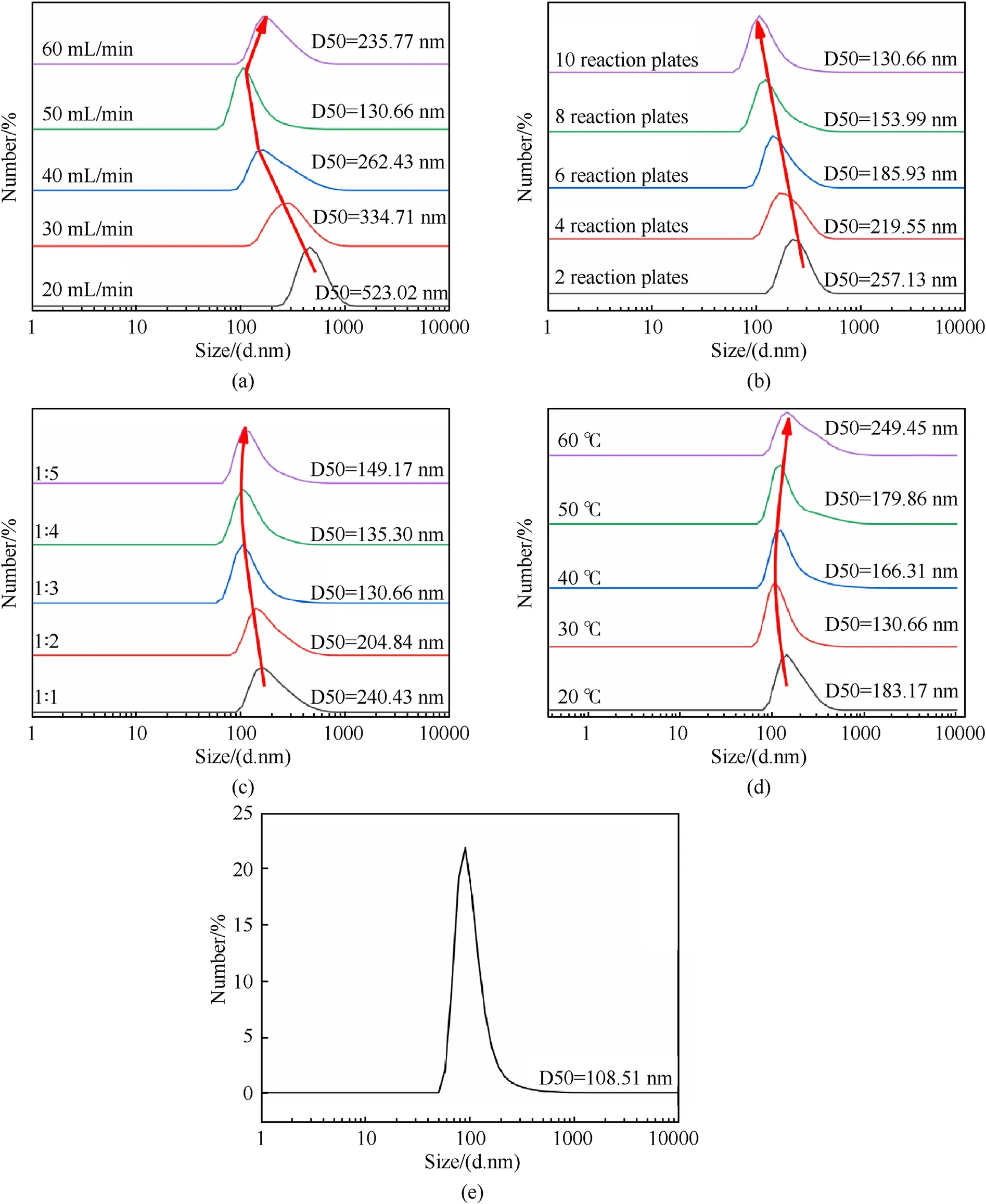
Fig. 2. Particle size distribution curves of TATB.
The obtained mixing intensity data at seven monitoring positions was graphed with the flow rates,as showed in Fig.4.As can be observed in Fig. 4, the mixing intensity of the exit (monitoring position 7)at different flow rates and solvent/non-solvent ratios all reached 1,indicating that DMSO and HO at the exit position have been totally mixed. However, from the curves of six monitoring positions that have not been completely mixed,it can be seen that the mixing intensity at the same monitoring position gradually decrease with the increase in flow rate.In order to study the relationship between mixing intensity and flow rate at the same residence time,the fluid with a flow rate of 10 ml/min within a certain period of time was set to flow to monitoring position 1, then the flow rates of 20 ml/min, 30 ml/min, 40 ml/min, 50 ml/min and 60 ml/min were set to flow to monitoring points 2,3,4,5 and 6,respectively. Based on this, curves as showed in the black dotted line in Fig.4a were obtained.As can be observed in the dashed line segment in Fig. 4a, with the same residence time, the higher the flow rate,the higher the mixing intensity.In addition,it can be seen from Fig. 4b that the mixing intensity at the same monitoring position gradually increases with the increase of the solvent/nonsolvent ratios, and the closer to the inlet, the greater the variation range is.This indicates that the larger the solvent/non-solvent ratio,the larger the velocity difference of the fluid in the microchannel,and the resulting momentum transfer is conducive to rapid mixing.
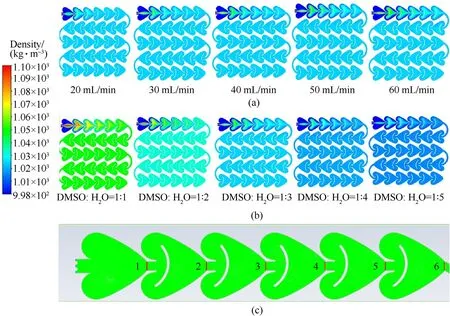
Fig. 3. The density distribution cloud maps of materials (a. flow rate; b. solvent/non-solvent ratio) and the schematic diagram of the 6 monitoring positions (c).
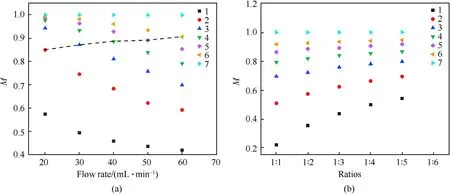
Fig. 4. Mixing intensity of materials at different flow rates and solvent/non-solvent ratios (1-7 represent seven different monitoring sites).
The synthesis process of nanoparticles includes supersaturation,nucleation and subsequent growth. According to the classical nucleation theory, the new crystal nucleus can exist stably and grow continuously only when its radius exceeds the critical size,but when it is less than the critical size, it will dissolve into the solution to reduce the total free energy. Therefore, nucleation occurs only when supersaturation is greater than a certain degree of solubility, and as nucleation progresses, supersaturation gradually decreases. When the supersaturation decreases to the critical concentration required for nucleation, nucleation will cease, but the growth process will continue until the concentration of the growth material reaches the equilibrium concentration of solute.Therefore, in the shortest time possible to nucleate in the way of explosion, increasing the number of crystal nuclei can make the nanoparticles quickly use up the reactant after nucleation, and finally get the uniform size of nanoparticles [30].
In this experiment,by increasing the flow rate of the fluid in the microchannel,the mixing intensity of the solvent and non-solvent in the same time was increased, so as to increase the supersaturation of the solution and achieve the purpose of reducing the particle size of the nanoparticles. However, the experimental results show that when the flow rate increases to 60 ml/min, the particle size of TATB is larger than that at 50 ml/min,which may be due to the excessive number of nucleation, the radius of some nuclei does not reach the critical size and dissolves,and then grows on the existing nuclei, resulting in the increase of particle size.Fig. 4b and its corresponding experiments also show a similar situation,with the optimal solvent/non-solvent ratio of 1:3.As can be observed in Fig. 2d, with the increase of temperature, the particle size of TATB first decreases and then gradually increases.This may be because the higher the temperature,the higher the solubility of TATB in solution, resulting in reduced supersaturation of the solution, lower nucleation rate and continuous crystal growth. At the same time, the higher temperature reduces the viscosity of the solution and accelerates the mass transfer rate,which leads to the accelerated growth rate of the crystal and eventually leads to the increase of particle size.In addition,when the temperature is close to the melting point of DMSO(18.4C),the viscosity of the solution increases,and the mixing efficiency in water decreases,which leads to the decrease of the nucleation rate and the increase of the particle size of TATB.
3.3. Specific surface area analysis
In order to prove the advantage of nano-TATB over micro-TATB in specific surface area, we have prepared conventional TATB with an average particle size of about 7 μm. The specific surface areas of conventional TATB, raw-TATB, recrystallization and amination are 0.0808 m/g, 16.9259 m/g, 21.3670 m/g and 21.9090 m/g,respectively.These data indicate that the smaller the particle size, the larger the specific surface area, and the specific surface area of nano-TATB is much larger than that of micro-TATB.
3.4. SEM
Surface morphology of the TATB sample was observed by SEM.Fig.5a shows our homemade conventional TATB with a particle size of about 7 μm. Fig. 5b shows raw TATB with a particle size of 270.37 nm and an irregular sheet morphology. TATB obtained by recrystallization (Fig. 5c) and amination (Fig. 5d) is homogeneous and quasi-spherical in size. As can be seen from Fig. 5a-d, the particle size of TATB gradually decreases.
3.5. XRD
The structure of the TATB crystals was characterized by XRD,as showed in Fig. 6. The XRD spectrum shows that the peaks of the TATB crystals synthesized by recrystallization and amination have the same diffraction angles as those of raw-TATB.However,from a to c in Fig. 6, the diffraction peak intensity of the crystal gradually decreases and broadens.This is because the particle size is reduced to the nanometer range, and the peak intensity of the X-ray spectrum decreases as the particle size decreases.
3.6. FT-IR
In this work, we analyzed the functional group information of the product by the IR spectrum. The IR spectrum of the raw-TATB,the nano-TATB obtained by recrystallization and amination are shown in Fig.7.From the IR spectrum,it can be observed that the IR spectrum of the product obtained by recrystallization and amination are almost the same as that of the raw-TATB. The specific functional group information is provided in Table 2. From the spectral data in the table, it can be seen that the functional group information of the three substances is almost the same.With only a displacement difference of about 2 cm, indicating that the products obtained by the two methods are TATB.
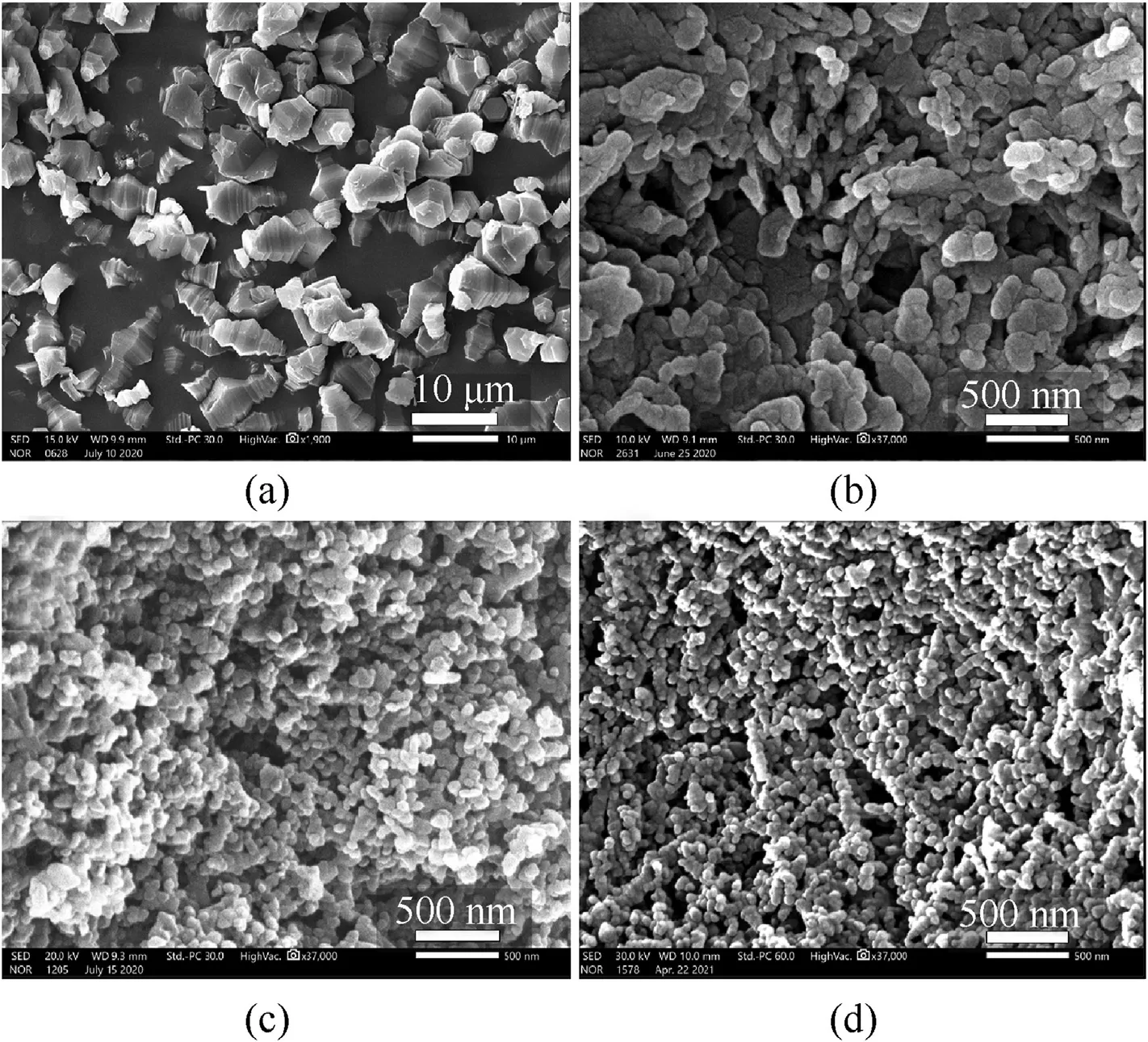
Fig. 5. SEM images of TATB samples (a. Regular size TATB; b. Raw-TATB; c. Recrystallization; d. Amination).
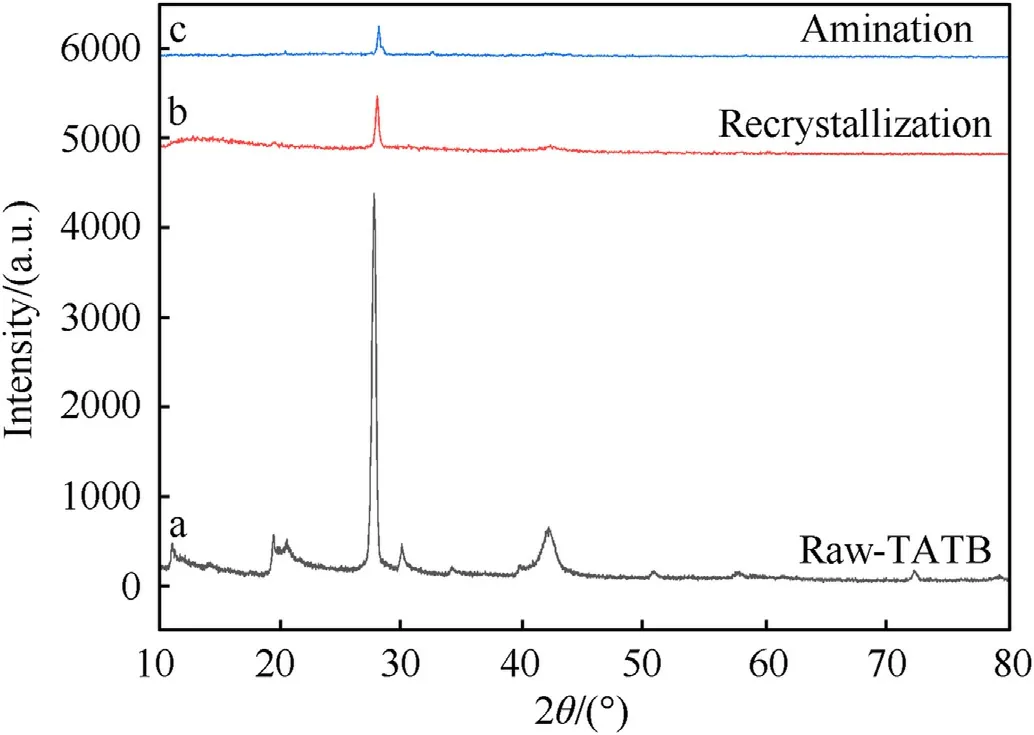
Fig. 6. X-ray diffraction pattern of TATB (a. Raw-TATB; b. Recrystallization; c.Amination).
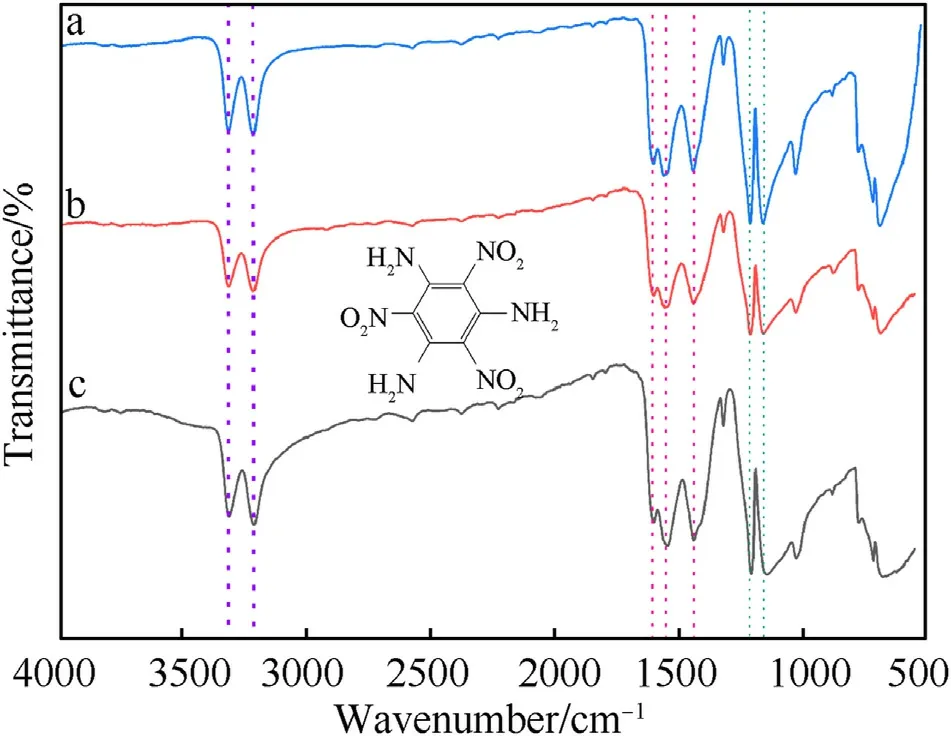
Fig. 7. IR spectra of the sample (a. Raw-TATB; b. Aminated synthetic TATB; c. TATB obtained by recrystallization).
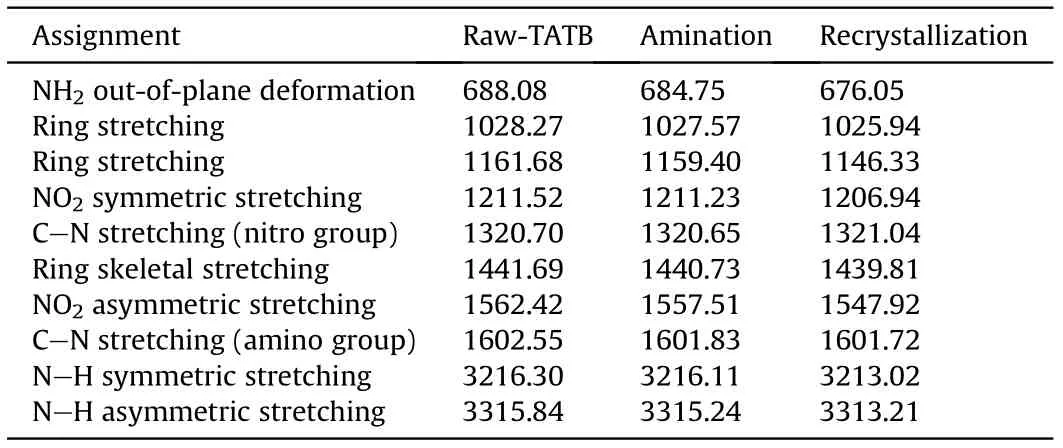
Table 2 Summary of assignment of vibrational modes/cm-1.
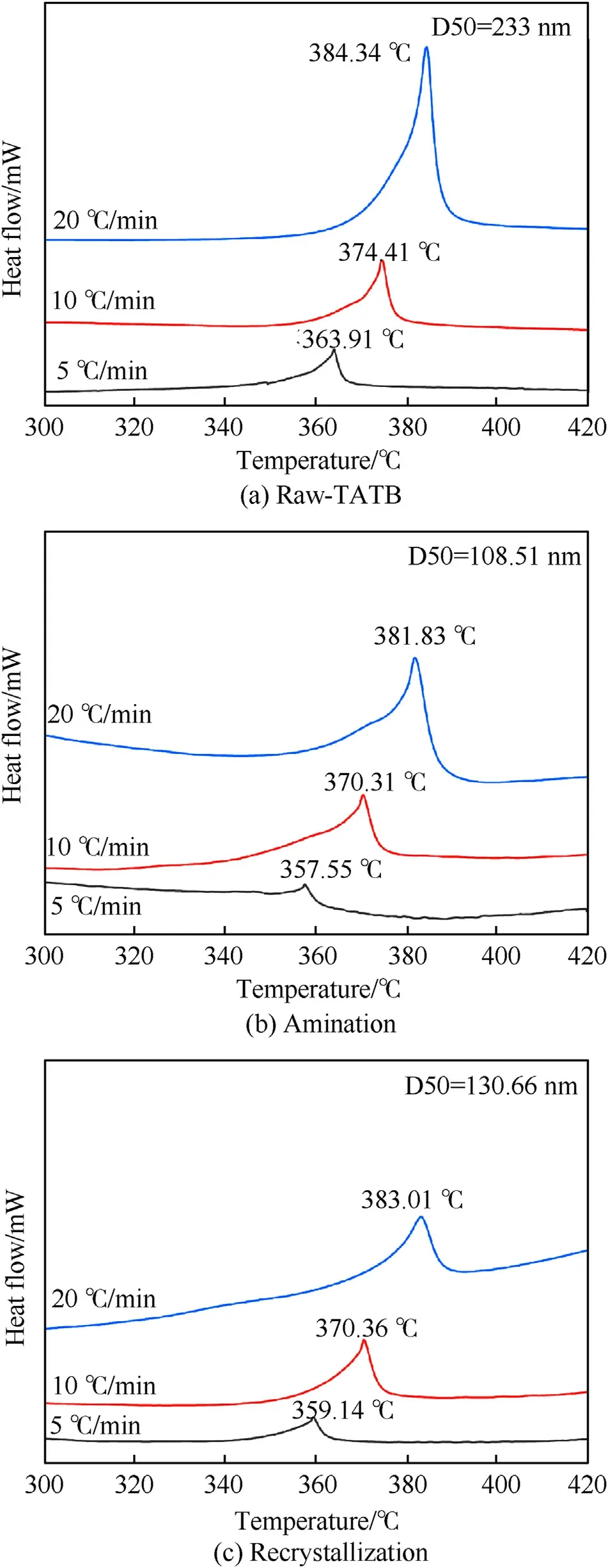
Fig. 8. DSC curves of (a) Raw-TATB, (b) Amination and (c) Recrystallization.
3.7. DSC
The thermal properties of raw-TATB (D50 = 270.37 nm), TATB obtained by amination method (D50 = 108.51 nm) and TATB obtained by recrystallization method (D50 = 130.66 nm) werecharacterized by DSC.The experimental data are presented in Fig.8 and Table 3. As the heating rate of the same sample increases, the thermal decomposition temperature of the sample increases. This is because the faster the heating rate,the shorter the residence time of the sample in this temperature range, and the greater the temperature gradient inside and on the sample. Therefore, thermal hysteresis occurs, causing the sample to decompose at higher temperatures. At the same heating rate, as the particle size of the sample decreases, the thermal decomposition temperature of the sample gradually decreases. This is explained by the fact that the smaller the particle size, the faster the heat conduction during heating, and the faster the reaction rate, which leads to the deterioration of the thermal stability of the material.
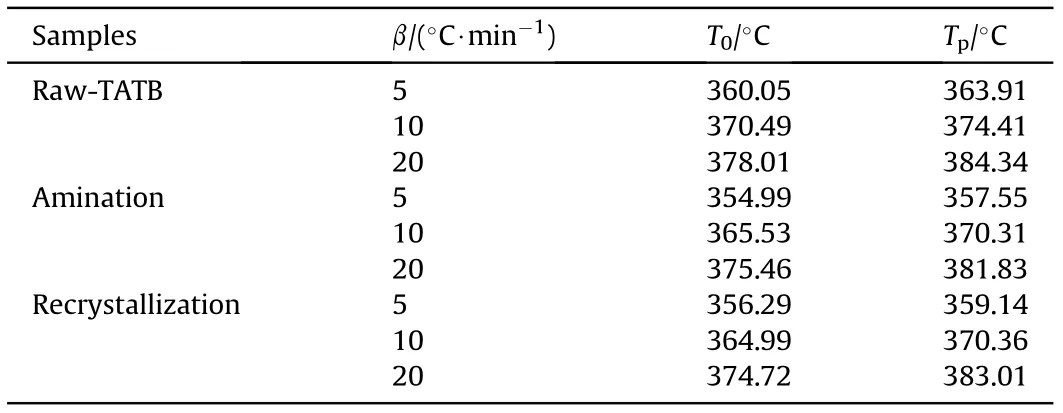
Table 3 DSC data for raw-TATB,Amination and Recrystallization.
4. Conclusions
In this paper,we successfully achieved the preparation of nano-TATB in microchannels through physical and chemical methods.The nano-TATB prepared by the microreactor has a similar spherical shape. The average particle size of TATB obtained by the physical method reaches 130.66 nm, and the particle size of TATB obtained by the chemical method reaches 108.51 nm. BET test results show that the specific surface areas of TATB obtained by physical method and chemical method are 21.37 m/g and 21.91 m/g, respectively.XRD and FT-IR analysis showed that the crystal structure of nano-TATB obtained by physical and chemical methods in microchannels was consistent with that of raw material TATB. The DSC test results show that the smaller the TATB particle size,the lower the thermal decomposition temperature. In the physical method,the effects of the total flow rate,the number of microreactor plates,the ratio of solvent/non-solvent, and the temperature on the particle size of TATB were studied.The results showed that the optimal preparation conditions for nano-TATB were the total flow rate of 50 ml/min and the number of microreactor plates is 10,the solvent/non-solvent ratio is 1:3, and the reaction temperature is 30C. In short,for the first time,we realized the controllable preparation of nano-TATB by physical and chemical methods in a 50 ml microreactor, respectively. By simulating the mixed state of DMSO and HO in the microreactor and combining the classical nucleation theory, the preparation mechanism of nano-TATB in the microchannel is proposed.This technological breakthrough is conducive to the preparation of high-quality nano-energetic materials with narrow particle size distribution,increase the intrinsic safety of the production process.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors would like to acknowledge National Natural Science Foundation of China(No.21875109)to provide fund for conducting experiments.
- Defence Technology的其它文章
- A new model for the expansion tube considering the stress coupling:Theory, experiments and simulations
- Experimental and analytical assessment of the hypervelocity impact damage of GLAss fiber REinforced aluminum
- Effect of the microporous structure of ammonium perchlorate on thermal behaviour and combustion characteristics
- Modeling of bistatic scattering from an underwater non-penetrable target using a Kirchhoff approximation method
- 3,6-bis (2,2,2-trinitroethylnitramino)-1,2,4,5-tetrazine. Structure and energy abilities as a component of solid composite propellants
- Mechanical behavior of Ti-6Al-4V lattice-walled tubes under uniaxial compression

