Discussion on the dimerization reaction of penicillin antibiotics
Qizhang Wu,Xia Zhang,Jiaxin Du,Changqin Hu,*
National Institutes for Food and Drug Control,Beijing,102629,China
ABSTRACT
Penicillins are one type of the most important antibiotics used in the clinic.Control of drug impurity profiles is an important part of ensuring drug safety.This is particularly important in penicillins where polymerization can lead to polymers as elicitors of passive cutaneous anaphylaxis.The current understanding of penicillin polymerization is based on reactions with amino groups,but no comprehensive mechanistic understanding has been reported.Here,we used theoretical calculations and column switching-LC/MS techniques to study penicillin dimerization.Ampicillin and benzylpenicillin were selected as representative penicillins with or without amino groups in the side chain,respectively.We identified four pathways by which this may occur and the energy barrier graphs of each reaction process were given.For benzylpenicillin without an amino group in the 6-side chain,dimerization mode A is the dominant mode,where the 2-carboxyl group of one molecule reacts with the β-lactam of another molecule.However,ampicillin with an amino group in the 6-side chain favors dimerization mode C,where the amino group of one molecule attacks the β-lactam of another molecule.These findings can lead to a polymer control approach to maintaining penicillin antibiotics in an active formulation.
Keywords:
Penicillins
Dimerization reaction
Theoretical calculations
LC/MS
Peer review under responsibility of Xi'an Jiaotong University.
1.Introduction
Penicillin and its analogues are the earliest and still most widely-used antibiotics in humans,but penicillins can polymerize in aqueous solution,and their polymers have long been recognized to elicit passive cutaneous anaphylaxis[1-3].Current understanding of the structure and polymerization characteristics of the polymers is based on penicillins with amino groups in the side chain such as ampicillin and amoxicillin[4-7].The polymerization mechanism is hypothesized to be where the amino group of one molecule attacks the carbonyl of the β-lactam ring of the other[4,5];thus,the amino is considered to be the key group in the formation of polymers.
The structures of dimer and trimer of ampicillin and amoxicillin have been reported in European Pharmacopoeia(EP 8.0)[8]and United States Pharmacopoeia(USP 37/NF32)[9],which also provide the reference standards of these polymers.EP 8.0 and USP 37/NF32 also contain some heterodimers whereby an amide bond,i.e.,the dimer between the amino group of 6-aminopenicillanic acid(6-APA)and the carboxyl group of oxacillin in the monograph of oxacillin sodium,and the dimer between the amino group of ampicillin and the carboxyl group of piperacillin in the monograph of piperacillin sodium(Fig.1).The linking sites of these polymers reported in pharmacopeias are related to either amino or carboxyl groups of the chemical molecules.
However,the polymerization mechanisms and polymer structures of penicillins without amino groups like benzylpenicillin have not yet been reported.Raj et al.[10]discussed the degradation reaction of dicloxacillin and stated that formic acid attacks the βlactam ring to obtain N-acetyl dicloxacilloic acid.According to this opinion,dimerization can possibly occur if the carboxyl group in one penicillin molecule attacks the β-lactam ring of another molecule in the similar way.Thus,we expand on this concept in this paper and explore the possible dimerization of penicillins both with and without amino groups in the C-6 side chain.
Ampicillin and benzylpenicillin were selected as representative penicillins with or without amino groups in the side chain,respectively,in this paper.Four possible dimerization pathways of penicillin antibiotics were proposed according to the summarization of previous reported research and evaluated in the work below:1)the reaction of a carboxyl group with the β-lactam ring,2)the reaction of a carboxyl group from an open ring product of the β-lactam ring with the β-lactam ring,3)the reaction of an amino group with the β-lactam ring,and 4)the reaction of an amino group with a carboxyl group(Fig.2).The last two pathways can only exist in penicillin antibiotics with amino groups in the C-6 side chain.
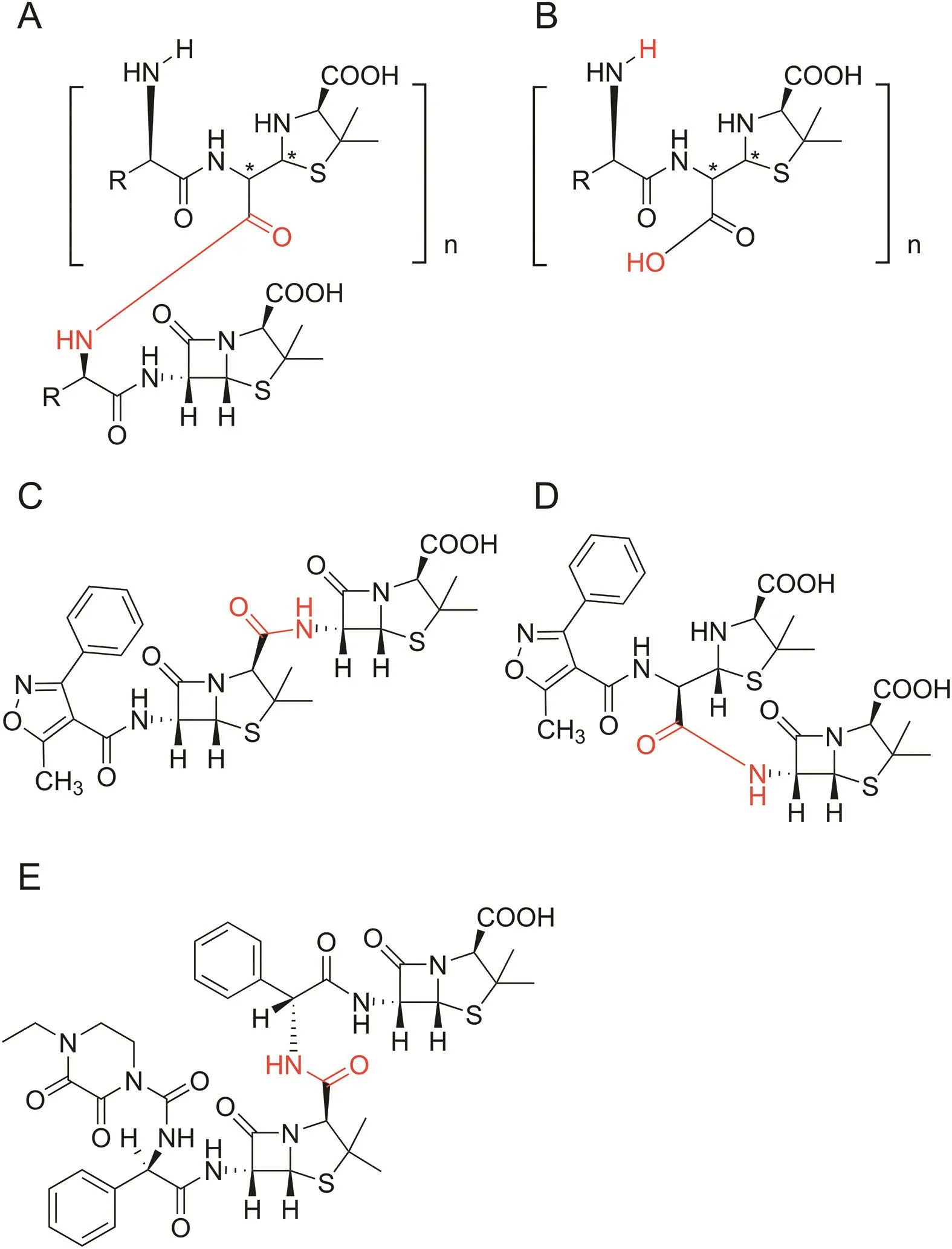
Fig.1.The structures of penicillin polymers reported in pharmacopoeia.(A and B)Ampicillin and amoxicillin polymers.(C and D)Oxacillin polymers.(E)Piperacillin polymers.
The possible mechanisms for the above different reactions were discussed through theoretical calculation in this paper.The theoretical study on reaction mechanism started in the 1980s,which simulates the chemical reaction using theoretical calculation[11].With the rapid development of computer science,quantum chemical calculation has become an important means to complement experimental technology.In this paper,the various reaction paths of dimerization were simulated via computational chemistry,and the reaction mechanism was verified to identify the dominant dimerization reaction.Density Functional Theory(DFT)on the level of B3LYP/6-311 G(d,p)calculations was used to study all the polymerization reaction mechanisms by Gaussian 09,and the reactants,the transition state and the product were optimized.In addition,the theoretical results were verified by liquid chromatograph-mass spectrometry(LC-MS)analysis via the detection of accelerated polymerization samples and actual samples.This research shows that the dimer impurities in real samples of penicillin antibiotics can be accurately and effectively controlled.
2.Experimental
2.1.Materials
Benzylpenicillin and ampicillin reference standards(RS)were provided by the National Institute for Food and Drug Control(NIFDC)of the People's Republic of China.
Benzylpenicillin sodium for injection(Pen)was from Lukang Pharmaceutical Co.,Ltd.,China(batch number 1011903008-1)and Reyoung Pharmaceutical Co.,Ltd.,China(batch number 19010501).Ampicillin sodium for injection(Amp)was provided by North China Pharmaceutical Group Co.,Ltd.,China(batch number 0701701),and Reyoung Pharmaceutical Co.,Ltd.,China(batch number 17082001).
Acetonitrile and methanol were of HPLC grade,and all other reagents were of analytical grade and came from different commercial suppliers.
2.2.Sample preparations
2.2.1.Preparation of polymerized samples
Stock solutions of polymerized samples were prepared by leaving 10 mL of 100 mg/mL water solution of Pen for 15 days and Amp for 10 days at room temperature,respectively.An aliquot of 1.0 mL of each was then taken and diluted to 50 mL prior to the HPLC analysis.
2.2.2.Preparation of real samples
Stock solutions of 20 mg/mL Pen and 10 mg/mL Amp were prepared by dissolving the respective substance in water.
2.3.Assay of dimers by HPLC-UV
The equipment used consisted of a Shimadzu LC-20AT high performance liquid chromatography system(HPLC)with a DAD detector,Labsolutions workstation,and Shiseido Capcell C18MGII column(4.6 mm×250 mm,5μm).The chromatographic system is the same as that used for the related substance method of benzylpenicillin in Chinese Pharmacopoeia(2015 Edition)[12].Mobile phase A included phosphate buffer(10.6 g potassium dihydrogen phosphate in 1 L of water,pH was adjusted to 3.4 with phosphoric acid)and methanol(72:14).Mobile phase B was acetonitrile.Detection used a spectrophotometer at 225 nm,and the column temperature was 34°C.The injection volume was 20 μL.A stepwise gradient was applied at flow rate of 1.0 mL/min starting at 86.5%A:13.5%B,keeping this over 17 min,changing to 64%A:36%B over 24 min and keeping at this over 12 min and finally changing back to 86.5%A:13.5%B in 1 min and keeping for additional 11 min.
2.4.Column switching-LC/MS analysis of dimers
Column switching analysis was performed according to the literature[13].Two systems were used.One LC-MS system consisted of a Shiseido Nanospace S1-2 HPLC(Shimadzu,Kyoto,Japan),ThermoFisher PDA detector(ThermoFisher Scientific,Waltham,MA,USA),and AB SCIEX 3200Q LC/MS/MS mass spectrometer(Applied Biosystems,Foster City,CA,USA).The chromatography software used was EZChrom Elite software(Shimadzu,Kyoto,Japan),and the mass spectrometry software was Analyst 1.4.2 software(Applied Biosystems,Foster City,CA,USA).The other system was a Dionex Ultimate 3000 HPLC with a Thermo Scientific Q Exactive Focus mass spectrometry(ThermoFisher Scientific,Waltham,MA,USA).The chromatography software employed was Chromelon Xpress(ThermoFisher Scientific,Waltham,MA,USA),and the mass spectrometry software was Thermo Xcalibur(ThermoFisher Scientific,Waltham,MA,USA).
The one-dimensional chromatographic system is described in Section 2.3.The column of the two-dimensional chromatography system was a Shiseido Capcell C18column (MGII;4.6 mm×150 mm,5μm).Mobile phase A was 0.5%(V/V)aqueous formic acid,while mobile phase B was a 0.5%(V/V)acetonitrile solution of formic acid.The column was at room temperature.A stepwise gradient for the two-dimensional chromatographic system was applied at the flow rate of 0.5 mL/min starting at 100%A:0%B,keeping this over tR(retention time of individual impurity)+5.5 min,changing to 0%A:100%B over 15 min and keeping at this to 65 min.
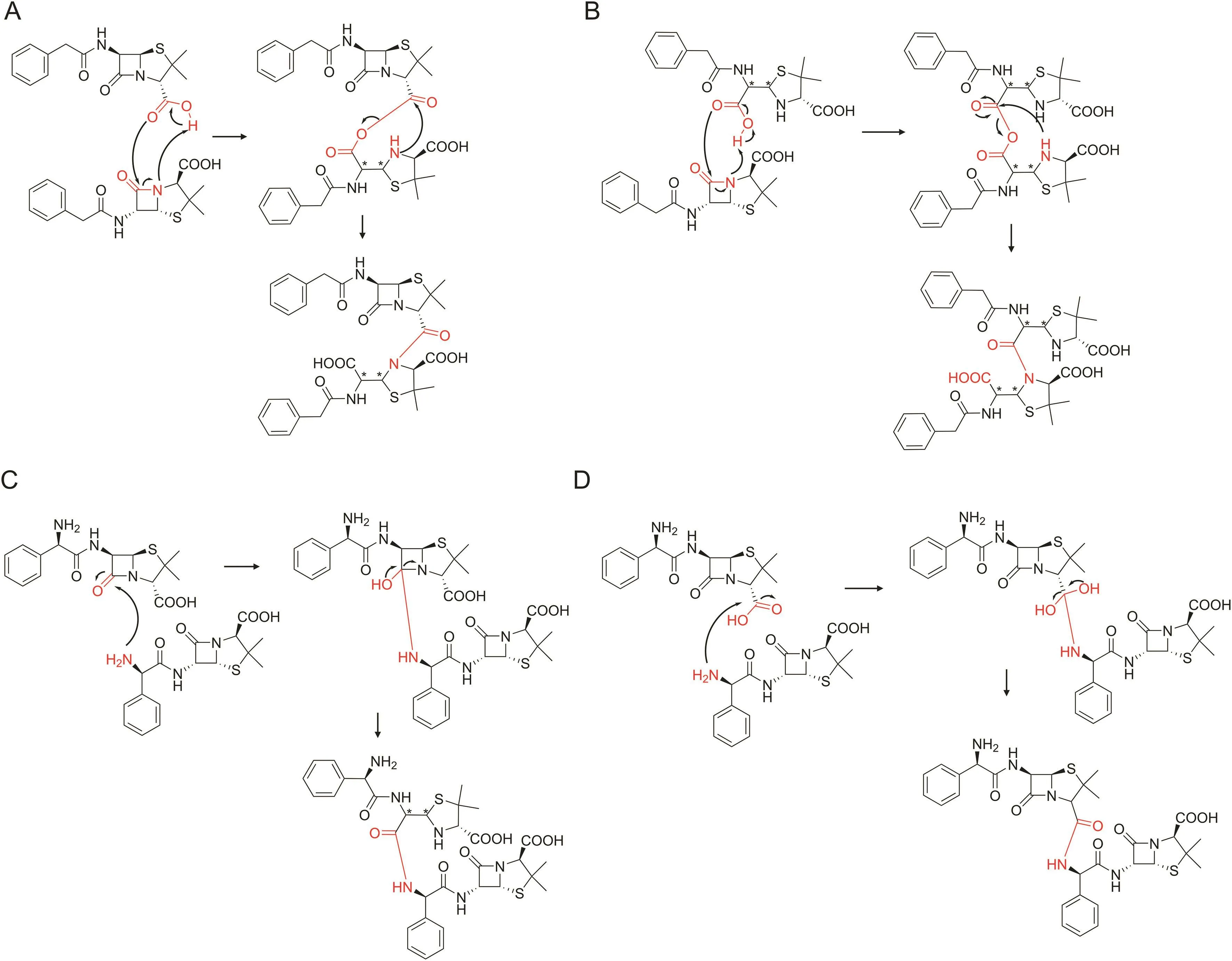
Fig.2.Different dimerization pathways of penicillin antibiotics with and without amino groups in the C-6 side chain.(A)Benzylpenicillin dimerization pathway A.(B)Benzylpenicillin dimerization pathway B.(C)Ampicillin dimerization pathway C.(D)Ampicillin dimerization pathway D.
The mass spectrometry parameters for benzylpenicillin included a ionization voltage of 4000 V,gasification temperature of 400°C,declustering potential(DP)of 28 V,collision energy(CE)of 33 V,and collision energy spread(CES)of 0 V.For ampicillin,the parameters were the ionization voltage of 5000 V,gasification temperature of 500°C,DP of 22 V,CE of 33 V,and CES of 0 V.
2.5.Theoretical simulation of dimerization reactions
The molecular structures from PubMed(https://www.ncbi.nlm.nih.gov/guide/chemicals-bioassays/)were the starting point of optimization,and the transition states of each reaction were searched using TS and QST2 methods in Gaussian software(Gaussian 09,Gaussian Inc.).At the B3LYP/6-311 G(d,p)level,the molecular geometric parameters and vibration frequencies of the reactants,transition states,intermediates,and products were all optimized[14-16].Meanwhile,the intrinsic reaction coordination(IRC)[17]algorithm identified the connection between the stable point and the transition state.The calculated energies in this study included zero-point energy corrections and the temperature when the calculations performed was 298.15 K.We then drew the energy barrier graph and calculated the activation energy of the reaction.Due to the large number of atoms in the calculated object,the solvent effect was not evaluated in order to simplify the calculation.
3.Results and discussion
3.1.Possible structure of the penicillin dimer
Penicillins can be divided into compounds without free amino groups in C-6 side chains(benzylpenicillin and sulbenicillin)and compounds with a free amino group in the C-6 side chain such as ampicillin and amoxicillin.According to the possible dimerization mechanism of penicillins(Fig.2),the former can only dimerize via the reaction of a carboxyl group with the β-lactam ring.The possible dimer of benzylpenicillin may have dimer A(Pen-A,the product formed by the reaction between the 2-carboxyl group of one molecule and the β-lactam ring of another molecule).It may also have dimer B(Pen-B,the product formed by the reaction between the carboxyl group from the opened β-lactam ring of one penicilloic acid molecule and the β-lactam ring of another penicillin molecule)(Fig.3).
Compounds with a free amino group in the 6-side chain may also be dimerized by reactions between side-chain amino groups and the β-lactam ring or carboxyl group.In the case of ampicillin,its dimer structure(Fig.3)may include dimer C(Amp-C,the product formed by the reaction between the side-chain amino group of one molecule and the β-lactam ring of another molecule)and dimer D(Amp-D,the product formed by the reaction between the side-chain amino group of one molecule and 2-carboxyl group of another molecule),in addition to dimer A(Amp-A)and dimer B(Amp-B).
3.2.Theoretical simulations of the dimerization reaction
The transition state is the first-order saddle point on the potential energy surface[18].The eigenvalue of the second-order derivative matrix of the transition state structure energy has only one negative value.Thus,the transition state only has one imaginary frequency.IRC calculations verified that the transition state connects the reactants and products through the minimum energy path at mass weight coordinates.
3.2.1.Benzylpenicillin dimerization
The reaction mechanism of the formation of dimer Pen-A(Fig.3A)is assumed as follows.The nucleophilic oxygen atom on the 2-carboxyl group of benzylpenicillin first attacks the carbonyl carbon of the β-lactam ring of another molecule accompanied by hydrogen transfer to form an intermediate.The carbon-oxygen bond is then broken,and the N on the tetrahydrothiazole ring is bonded to the exposed carbonyl carbon forming a dimerized product(Fig.2A).The entire reaction can be divided into two elementary reactions,and the corresponding transition state can be found by theoretical calculations.The dimerization reaction is endothermic.The energy barrier graph of the reaction process is shown in Fig.4A,suggesting that the second elementary reaction is the rate-limiting step.The energy barrier is 38.42 kcal/mol(setting the reactant energy as 0.00 kcal/mol).
The reaction mechanism of the formation of dimer Pen-B(Fig.3B)is assumed as follows.First,the β-lactam ring in the benzylpenicillin molecule is ring-opened to form penicilloic acid.The newly formed carboxyl group then reacts with the β-lactam ring of another molecule to form dimer Pen-B(Fig.2B).The energy barrier graph of the reaction process is shown in Fig.4A.The energy barrier of the second elemental reaction is 46.43 kcal/mol,suggesting that dimer Pen-B is more difficult to form than dimer Pen-A.
3.2.2.Ampicillin dimerization
The formation mechanisms of dimer Amp-A(Fig.2C)and dimer Amp-B(Fig.2D)are the same as those of dimer Pen-A and dimer Pen-B;the energy change in the reaction process is shown in Fig.4B.The energy barriers of the rate-limiting steps are 37.58 kcal/mol and 66.77 kcal/mol,respectively.
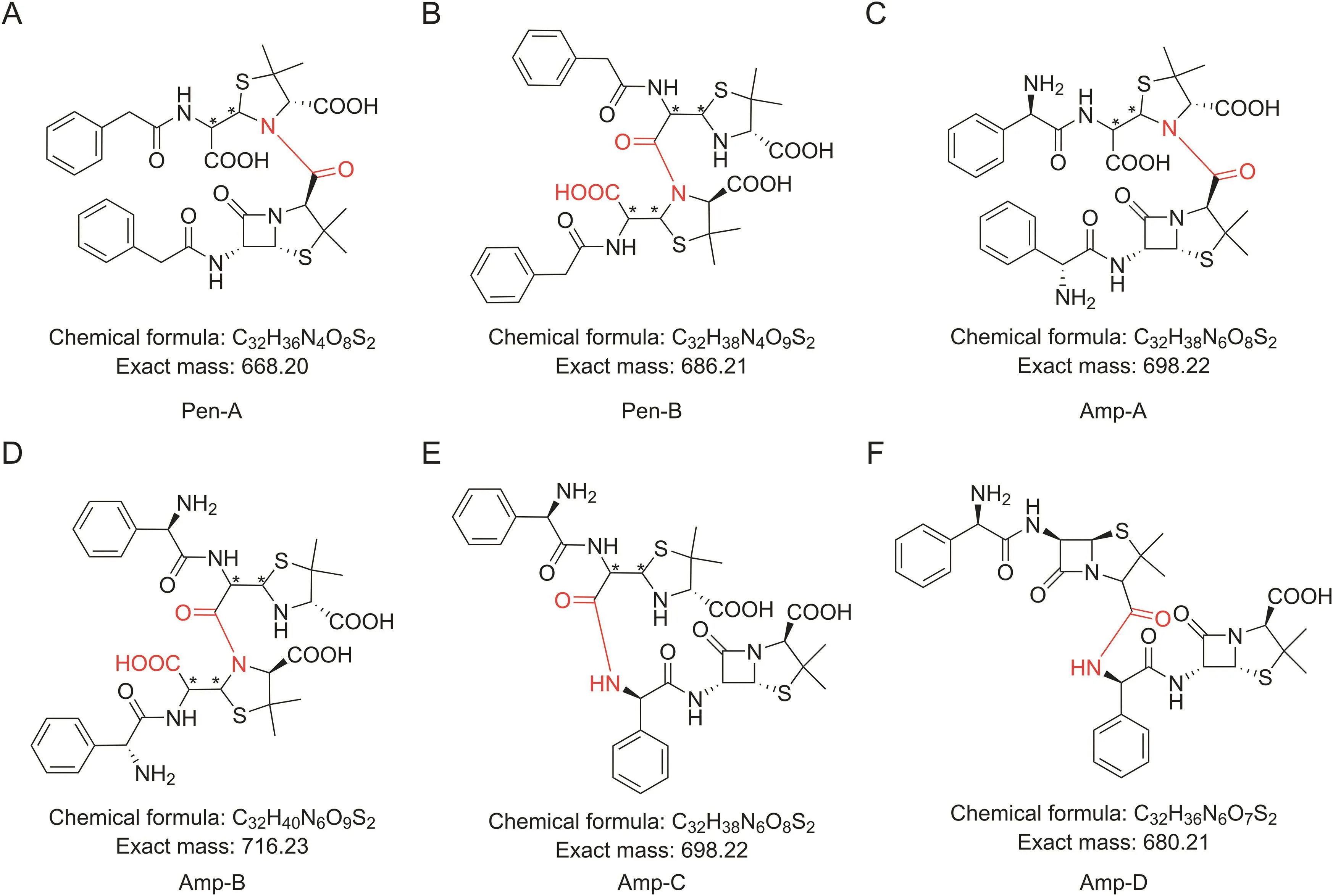
Fig.3.Possible structures of penicillin dimers:(A)Pen-A,(B)Pen-B,(C)Amp-A,(D)Amp-B,(E)Amp-C,and(F)Amp-D.
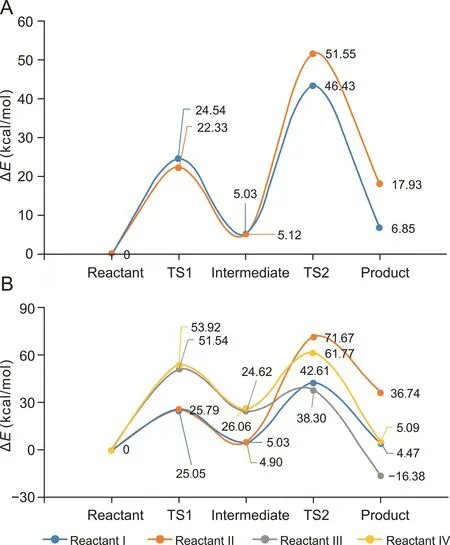
Fig.4.Energy barrier graph of polymerization process.(A)Benzylpenicillin dimerization.(B)Ampicillin dimerization.
The reaction mechanism of the formation of dimer Amp-C(Fig.3E)is assumed as follows.First,the amino group on the 6-side chain of ampicillin attacks the carbonyl carbon of the β-lactam ring of another molecule.The hydrogen on the amino group is transferred to the carbonyl oxygen to form an intermediate.The ring is then opened,and hydrogen is transferred to the nitrogen of the five-membered ring while the carbon-oxygen bond returns to a double bond(Fig.2C).The entire reaction can be divided into two elementary reactions,and the corresponding transition state can be found via theoretical calculations.The dimerization process is an exothermic reaction,which is thermodynamically beneficial to the reaction.Fig.4B indicates that the first step is the rate-limiting step with an energy barrier of 51.54 kcal/mol.
The reaction mechanism of the formation of dimer Amp-D is assumed as follows.First,the amino group on the 6-side chain of ampicillin attacks the 2-carboxyl carbon of another molecule accompanied by hydrogen transfer to form an intermediate;water removal leads to an amide bond(Fig.2D).The entire reaction can be divided into two elementary reactions,and the corresponding transition state can be found by theoretical calculation.The dimerization reaction is endothermic,and the second step is the ratelimiting step with an energy barrier of 53.92 kcal/mol(Fig.4B).Upon comparing the mechanism and energy barrier of the four dimerization,the formation of dimers Amp-A and Amp-C is more favorable.
3.3.Verification of theoretical calculation results by LC-MS analysis
3.3.1.Analysis of dimers in benzylpenicillin
The relative molecular masses of dimer Pen-A and dimer Pen-B are 668 and 686(Fig.3),respectively.The β-lactam ring is unstable,and the relative molecular mass of the ring-opened dimer Pen-A is 686.Those kinds of dimers were identified by LC-MS in the polymerized sample respectively.
HPLC analysis of the benzylpenicillin polymerized sample(Fig.5A)showed that the retention time of benzylpenicillin was about 29 min.In reversed-phase chromatography systems,the retention time of benzylpenicillin dimers should be longer than that of benzylpenicillin.Column switching analysis was performed individually for impurities with retention time longer than that of benzylpenicillin.The MS analysis revealed that the relative molecular mass of peak 10 was 668(Fig.6A),and the main mass spectrometry fragments(m/z)at 476,623,505,551,and 651 conformed to the fragmental pattern of dimer Pen-A(Fig.6B).Therefore,peak 10 represents dimer Pen-A.
The relative molecular mass of peak 4 is 686(Fig.7A),and the main mass spectrometry fragments(m/z)at 392,510,335,309,217,668,and 410 confirm the fragment pattern of dimer Pen-B(Fig.7B).In particular,fragment 410 is only produced by removing the fivemembered ring and breaking the amide bond of the side chain of dimer Pen-B.Therefore,this sample may represent dimer Pen-B.
The relative molecular masses of peaks 1-3 and 5-9 are also 686(Fig.8A),and their mass fragmental patterns are nearly identical(Fig.S1).The main mass fragments(m/z)at 309,353,335,217,and 160 also agree with the fragmental pattern of the ring-opened dimer Pen-A(Fig.8B).Therefore,these eight impurities are all probably ring-opened products of dimer Pen-A because the 5 and 6 chiral sites of the penicillin β-lactam ring are likely to epimerize after ring opening.
Attention to other impurity peaks in benzylpenicillin polymerized sample.Peak?is the maximum impurity peak after benzylpenicillin peak with a relative molecular mass of 527.The relative molecular masses of peaks?and?are both 663.The relative molecular mass of peak④is 644.These are not judged to be dimer impurities.From what has been discussed above,the chromatographic peaks 10 and 4 represent dimer Pen-A and Pen-B,respectively.Peaks 1-3 and 5-9 in Fig.5 belong to the ring-opened dimer Pen-A.Analysis of dimerized benzylpenicillin reveals that a variety of ring-opened products of dimer Pen-A isomers exist in the sample.These products suggest that benzylpenicillin dimerization pathway A is more likely to occur than dimerization pathway B,which is consistent with theoretical calculations.This might be because the β-lactam ring first hydrolyzes to form penicilloic acid in dimerization mode B,and the hydrolyzed product is only a small part in the entire molecules.Meanwhile,as a nucleophilic attack site,the carboxyl group of penicilloic acid reacts more difficultly than the 2-side chain of benzylpenicillin.
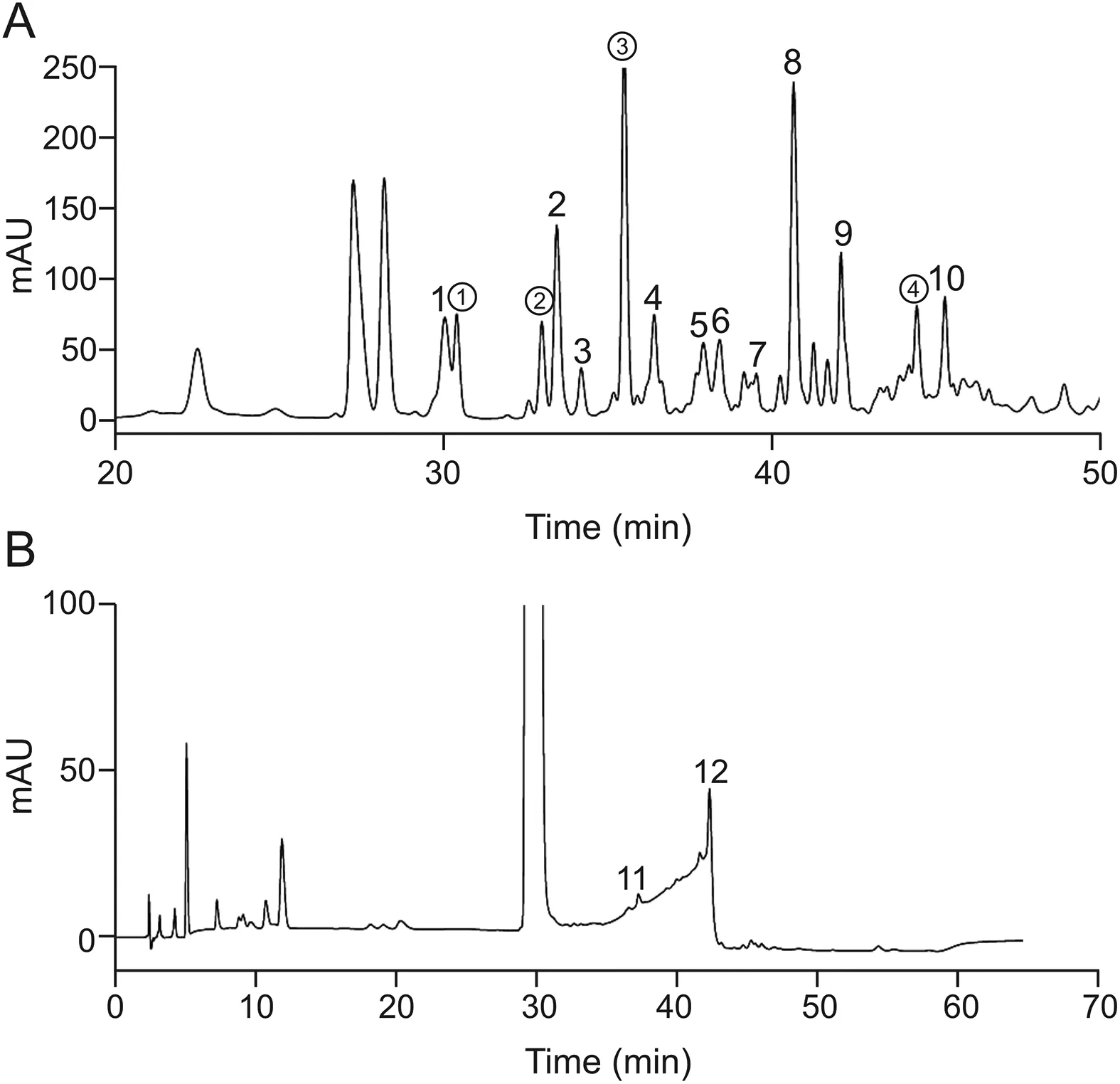
Fig.5.HPLC chromatograms of(A)benzylpenicillin polymerized sample and(B)benzylpenicillin real sample.
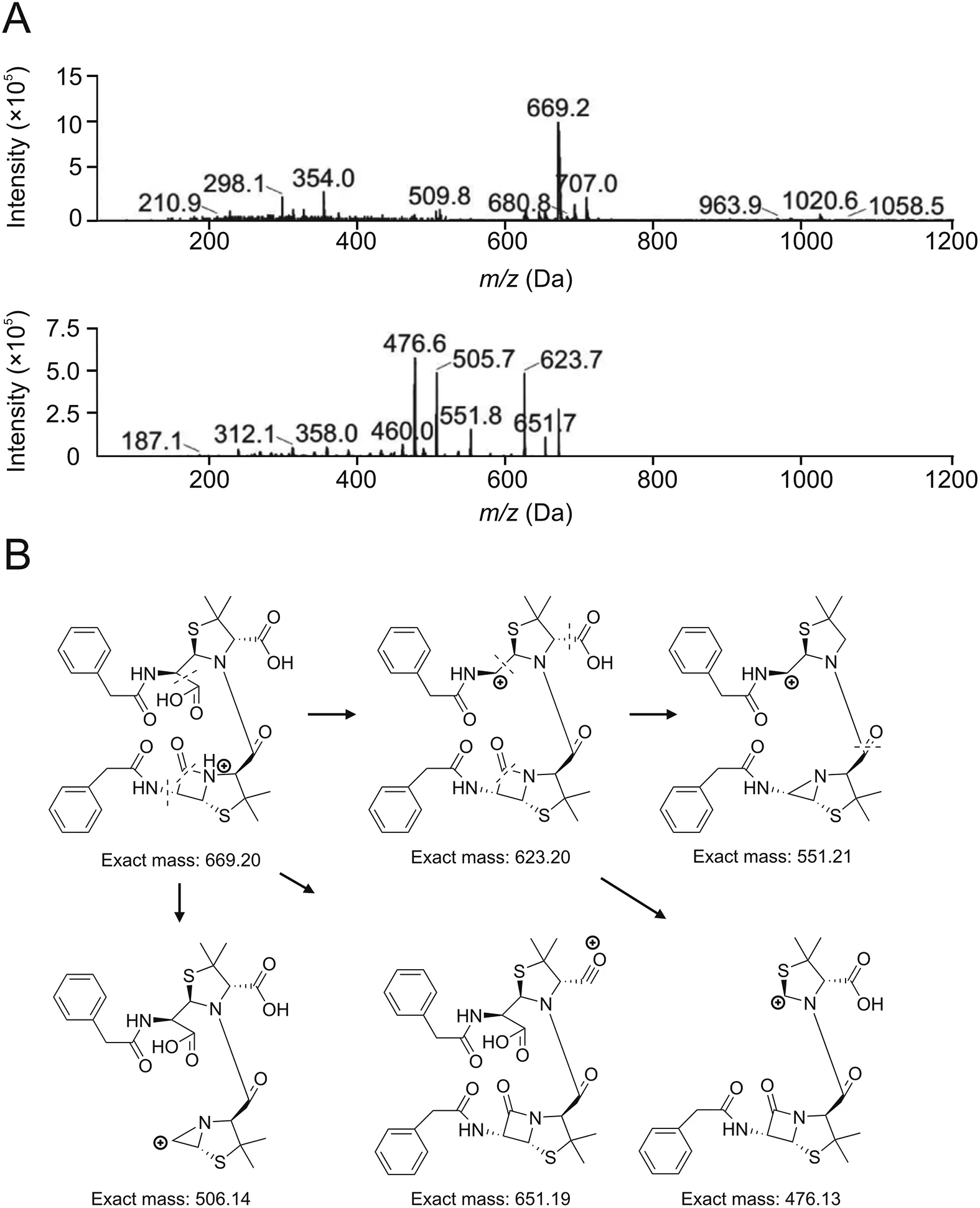
Fig.6.MS analysis results of benzylpenicillin dimers.(A)Mass spectrum of Peak 10.(B)Mass fragment pattern of dimer Pen-A.

Fig.7.MS analysis results of benzylpenicillin dimers.(A)Mass spectrum of Peak 4.(B)Mass fragmental pattern of dimer Pen-B.
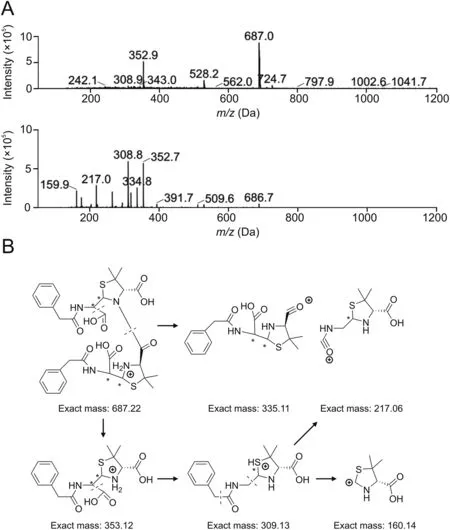
Fig.8.MS analysis results of benzylpenicillin dimers.(A)Mass spectrum of Peak 8.(B)Mass fragmental pattern of ring-opened dimer Pen-A.
These results also revealed that dimer Pen-A is likely to hydrolyze and the peak 8 with the retention time of about 41 min may be the dominant ring-opened structure.HPLC analysis of the real sample of benzylpenicillin for injection is shown in Fig.5B.Although no significant dimer peaks were found in two batches of the real sample,a small impurity peak with the retention time of about 42 min could be found.The relative molecular mass of this peak is 686(Fig.S2A),which is assumed to be the ring-opened dimer Pen-A.The other impurity peak with the retention time of about 37 min is not the dimer as the relative molecular mass of this peak is 387.0768(Fig.S2B).The results verified that dimerization A is more likely to occur and the dimer is easily hydrolyzed in real situations.
3.3.2.Analysis of dimers in ampicillin
Fig.3 shows that the relative molecular masses of ampicillin dimers A-D are 698,716,698,and 680,respectively.The β-lactam ring is easily hydrolyzed to form penicilloic acid.The relative molecular mass of the ring-opened product of dimer Amp-A is 716.The relative molecular mass of the product formed by opening one βlactam ring of dimer Amp-D is 698,and the product formed by opening two β-lactam rings of dimer Amp-D is 716.Those kinds of dimers were identified by LC-MS in the polymerized sample respectively.
HPLC analysis of the ampicillin polymerized sample is shown in Fig.9A;the retention time of ampicillin is about 3.8 min.Column switching analysis was carried out individually for impurities with retention time longer than that of ampicillin.MS analysis showed that the secondary MS fragment types for peaks 1,3,and 4 are almost the same(Fig.S3),and the relative molecular mass of peak 4 is 698(Fig.10A).Its main fragments(m/z)are 540,381,353 and 333,which is in accordance with the fragmental pattern of dimer Amp-C(Fig.10B).Four isomers will be generated theoretically after opening the ring of the dimer.Therefore,we speculate that all the three peaks are isomers of dimer Amp-C(Fig.3E),and that peak 4 with the retention time of about 43 min is the predominant isomer.
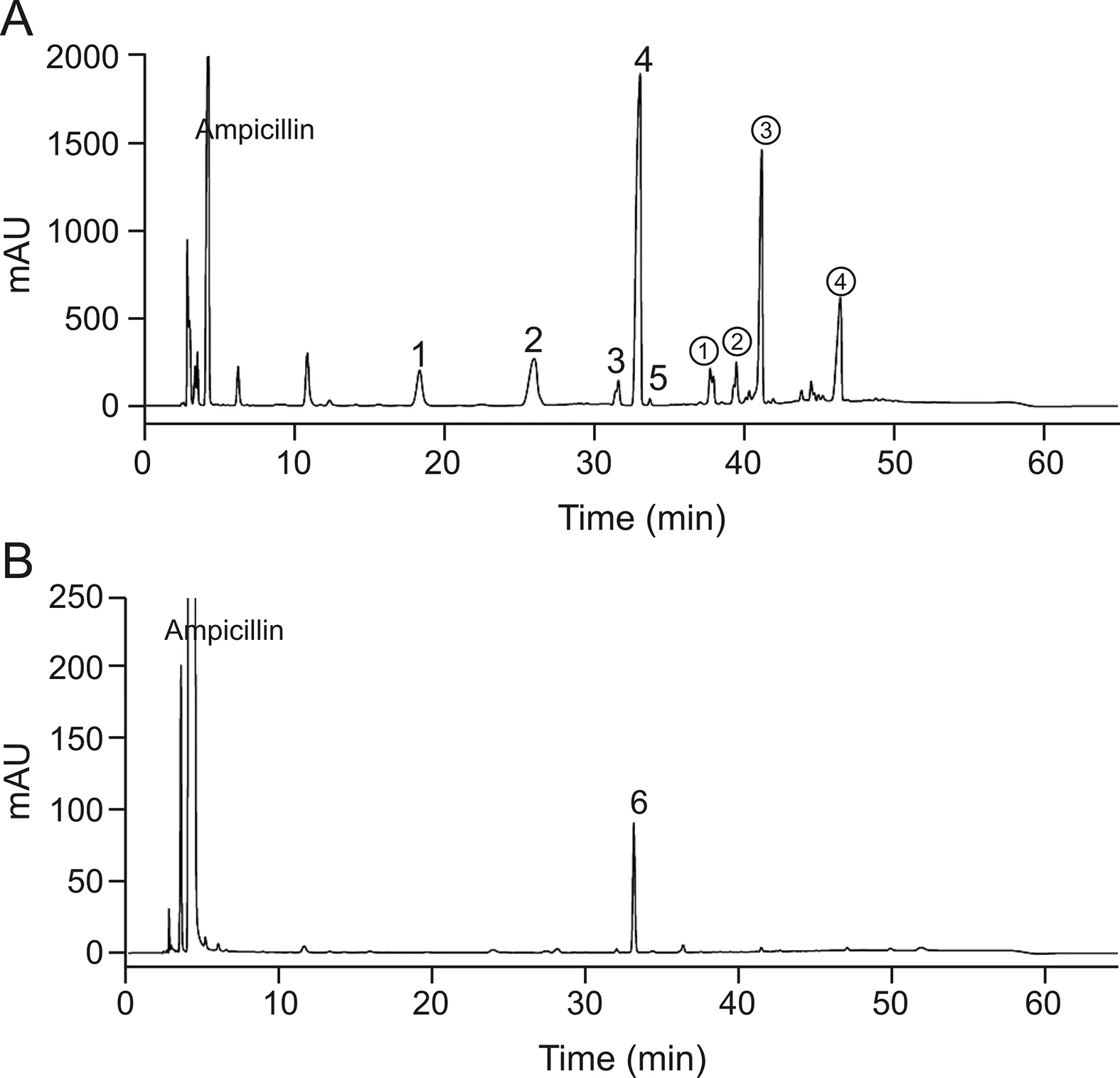
Fig.9.HPLC chromatograms of(A)ampicillin polymerized sample and(B)ampicillin real sample.
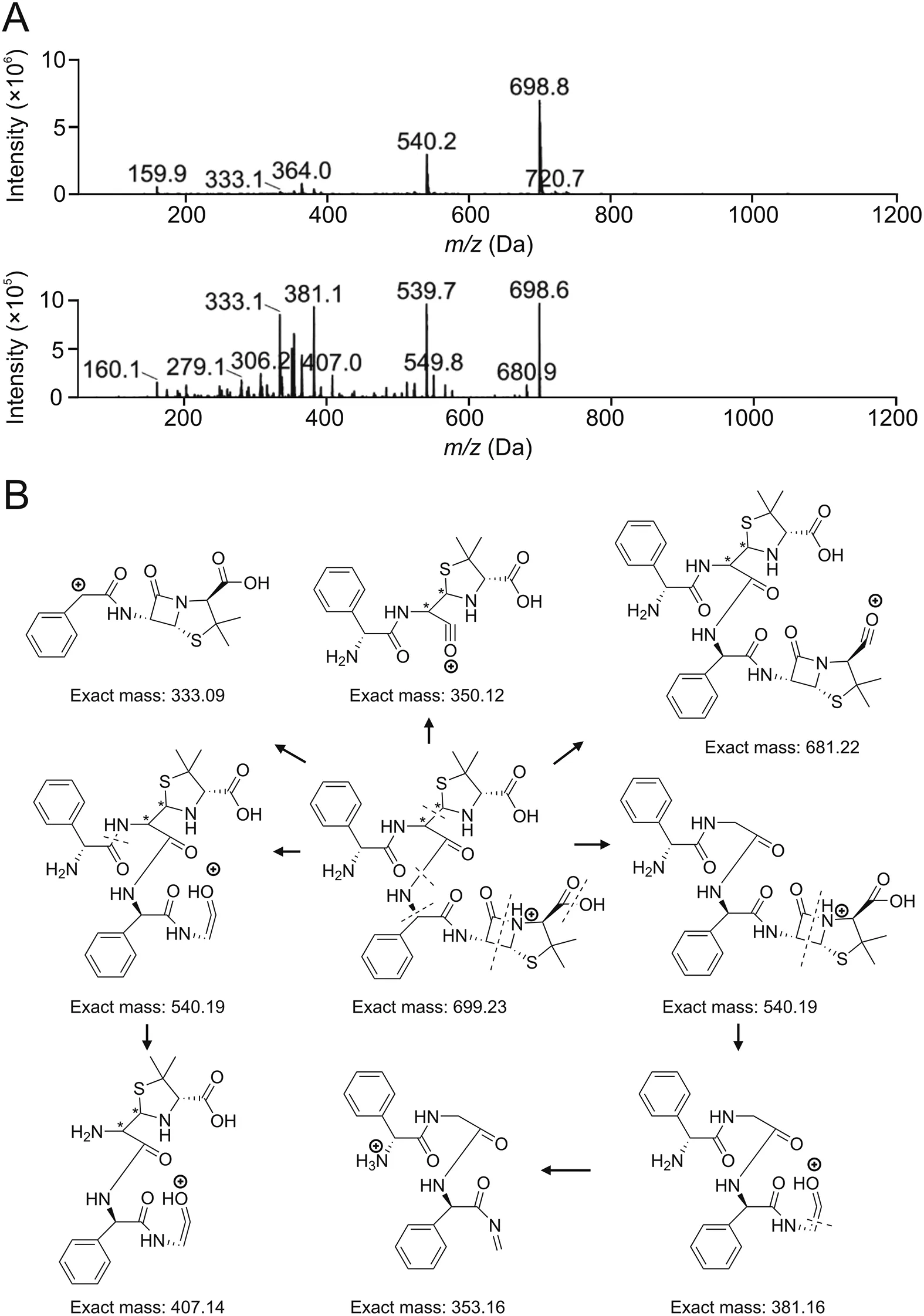
Fig.10.MS analysis results of ampicillin dimers.(A)Mass spectrum of Peak 4.(B)Mass fragmental pattern of dimer Amp-C.
The relative molecular mass of peak 5 is 698 as well(Fig.S4A);the main fragments(m/z)are 364,334,540,353,and 201,which are different from dimer Amp-C.Only cleavage on the dimerization site of ampicillin dimer Amp-D is most likely to produce 334 and 353 fragments(Fig.S4B).In tandem with the other results,peak 5 is inferred to represent the hydrolysate of dimer Amp-D(Fig.3F)that is formed by opening one β-lactam ring.
The relative molecular mass of peak 2 is 716(Fig.S5A),which is the same as those of dimer Amp-B and the ring-opened dimers Amp-A and Amp-C.Its main fragments(m/z)include 673,514,324,334 and 306.The dimer is likely to lose one five-membered ring and one carboxyl group at the same time and thus it is not the ringopened dimer Amp-A and dimer Amp-B.As dimers Amp-A and Amp-C are kinetically and thermodynamically dominant products according to the theoretical calculations,dimer Amp-C is the major dimerization product of ampicillin,and it is most probably a ringopened dimer Amp-C(Fig.3E).
Attention is paid to other impurity peaks in ampicillin polymerized sample.The relative molecular mass of peak①is 1066.The relative molecular masses of peaks②and③are both 1048.The relative molecular mass of peak④is 1079.These are not judged to be dimer impurities.
From what has been discussed above,the chromatographic peaks 1,3,and 4 in Fig.9A represent dimer Amp-C,while peak 5 is the ring-opened dimer Amp-D,and peak 2 is probably the ringopened dimer Amp-C.These analyses of the ampicillin polymerization samples revealed that dimerization C is more likely to occur in real situations and has a dominant isomer.The real samples of ampicillin were analyzed(Fig.9B),and the dimers of the two batches of the real samples are mainly dimer C dominant isomers(Peak 6,whose relative molecular mass is 698)(Fig.S5B).
4.Conclusion
Here,the possible dimerization mechanisms of penicillin dimers were analyzed theoretically and experimentally.We found that the carboxyl group participates in a dimerization reaction as a nucleophilic group.For penicillins without an amino group in the 6-side chain,dimerization mode A is the dominant mode,i.e.,the 2-carboxyl group of one molecule reacts with the β-lactam of another molecule.However,penicillins with an amino group in the 6-side chain favor dimerization mode C,where the amino group of one molecule attacks the β-lactam of another molecule.This understanding of the mechanism of penicillin dimerization facilitates analyses of polymer impurities in samples and may lead to the discovery of the dominant polymers.This is vital for targeted polymer quality control.
CRediT author statement
Qizhang Wu:Methodology,Investigation,Software,Writing-Original draft preparation;Xia Zhang:Methodology,Software,Validation,Writing-Reviewing and Editing;Jiaxin Du:Investigation;Changqin Hu:Conceptualization,Supervision,Funding acquisition.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Major Scientific and Technological Special Project for“Significant New Drugs Development”(Grant No.:2017ZX09101001-007).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2021.06.005.
 Journal of Pharmaceutical Analysis2022年3期
Journal of Pharmaceutical Analysis2022年3期
- Journal of Pharmaceutical Analysis的其它文章
- Recent advances in quantum dots-based biosensors for antibiotics detection
- Qualitative and quantitative evaluation of Flos Puerariae by using chemical fingerprint in combination with chemometrics method
- Multiple rapid-responsive probes for hypochlorite detection based on dioxetane luminophore derivatives
- Nitrogen-doped carbon@TiO2double-shelled hollow spheres as an electrochemical sensor for simultaneous determination of dopamine and paracetamol in human serum and saliva
- Compatibility and stability studies involving polymers used in fused deposition modeling 3D printing of medicines
- A preparation strategy for protein-oriented immobilized silica magnetic beads with Spy chemistry for ligand fishing
