Self-Assembled VS4 Hierarchitectures with Enhanced Capacity and Stability for Sodium Storage
Siling Cheng, Kaitong Yao, Kunxiong Zheng, Qifei Li, Dong Chen, Yu Jiang, Weiling Liu,Yuezhan Feng, Xianhong Rui* , and Yan Yu*
1. Introduction
Currently, lithium-ion batteries (LIBs) have attracted much heeds and been applied to many areas such as electric vehicles (EVs) and the grid.[1–4]Nevertheless, the limited lithium resources on Earth hinder the widespread applications of LIBs.[5]Sodium,on the other hand,not only shows similar physicochemical properties as lithium, but also has a higher natural abundance and lower cost.[6,7]Besides, the redox potential of sodium is pretty close to that of lithium.[8]Sodium-ion batteries (SIBs) are thus the most perspective substitute for the next generation LIBs, especially in sizeable energy storage areas. The ion radius of Na+(1.02 ?) is,however, large, which leads to slow diffusion kinetics, and thus results in serious capacity attenuation and penniless power performance.[9,10]Consequently,a lot of research has been directed at catching proper electrodes to boost the development and practical use of SIBs.
Transition metal sulfides (TMSs) have brought a great deal of interests recently,owing to their advantages such as unique layered structure, high reversible capacity, and long cycle life. TMSs like MoS2,[11,12]WS2,[13–15]and CoS2[16,17]display excellent electrochemical performance when used in SIBs. In particular,vanadium sulfides (VS2, VS4, V2S3, V5S8, etc.)are of great interest due to their high initial coulombic efficiency(CE),high reversible storage capacity, and the variable oxidation state of vanadium.[18–21]Compared with other vanadium sulfides, VS4has the highest theoretical capacity (1196 mA h g?1) due to its higher S content which provides abundant active sites and participates in more electrochemical reactions with sodium.[22]The crystal structure of VS4,which was first determined in 1964,consists of 1D chain-like structure made up of thedimer and two adjacent V atoms.[23,24]The chain is linked by weak Van der Waals forces with an inter-chain distance(5.83 ?) that is much larger than the ion radius of Na+(1.02 ?),making it easier for the Na+uptake/release during the charging/discharging process.[25,26]Researchers have made preliminary exploration on the application of VS4as SIB anodes.For example,Yang et al.[27]utilized a solvothermal method to fabricate a 3D VS4structure, which exhibits the superior rate performance (288 mA h g?1at 10 A g?1)and cycle performance(410 mA h g?1after 400 cycles at 1 A g?1) in the voltage window of 0.5–3.0 V.Li and other researchers[28]successfully prepared 3D VS4microspheres to demonstrate attractive electrochemical performance within the voltage range of 0.5–3.0 V for SIBs,such as providing a high capacity of 412 mA h g?1at 0.2 A g?1after 230 times. However, in these voltage ranges, only the electrochemical Na+(de)intercalation reactions are involved, and the reversible capacities are still relatively low.To increase the capacity,it is encouraging to operate the VS4anode in a wide voltage window covering both intercalation and conversion reactions. Additionally,the main problem is that the conversion reaction will cause part of irreversible capacity loss,resulting in poor cycling stability. This will be a research direction to promote the capacity and stability of VS4anode materials for SIBs.

Figure 1. The chemical composition characterization of VS4-CN-Hs.a)XRD pattern,b)The full spectrum of XPS,XPS spectra of c)V 2p and d)S 2p.
Herein, we developed 3D VS4curly nanosheets self-assembled hierarchitectures (VS4-CN-Hs) as the anode for SIBs. The growth mechanism of VS4-CN-Hs during the solvothermal reaction process was investigated. When evaluated in a wide voltage window of 0.01–3.0 V, such anode exhibits high reversible capacity of about 863 mA h g?1at 0.1 A g?1, and outstanding cycling stability at high current densities (e.g., delivering 386 mA h g?1at 5 A g?1after 1000 cycles). The superior electrochemical performance of the VS4-CN-Hs is mainly ascribed to the large exposed surface area of the nanosheets and the abundant buffer space formed in 3D architecture.
2. Results and Discussion
VS4-CN-Hs were successfully prepared via a one-step solvothermal method. XRD was utilized to evaluate the purity and study the crystal structure of the as-prepared VS4-CN-Hs, and the result is presented in Figure 1a. From the XRD pattern, all the diffraction peaks can be matched to those for monoclinic VS4(JCPDS No. 72-1294) with no observable impurity peak, confirming that the as-prepared sample is pure VS4.[19,29]To study the chemical composition of VS4-CN-Hs,energy dispersive spectroscopy (EDS), XPS, and Raman spectroscopy were used and the results are displayed in Figure 1b–d and Figures S1–S3 (Supporting Information). EDS together with XPS confirms the presence of vanadium(V)and sulfur(S)in the as-prepared sample.The carbon and oxygen detected by both techniques were attributed to surface adsorption as no obvious second phase is observed in the XRD pattern. To understand the chemical states of V and S, high-resolution XPS was done. From Figure 1c, it can be seen that there are 2 pairs of peaks, with the ones at 516.2 and 523.6 eV attributed to V4+while the pair at 513.5 and 521.1 eV ascribed to V3+.[30]The presence of V3+indicates that there is a planar interaction force between the curly VS4nanosheets.The S 2p1/2peak at 163.6 eV and the S 2p3/2 peak at 162.4 eV are assigned to S2-2and confirm the formation of VS4(Figure 1d).[31,32]Additionally, in the Raman spectrum, the peaks (139, 192, 282, 407,527,689,and 991 cm?1)that are characteristic to the stretching and bending bands of VS4are also observed(Figure S3,Supporting Information).[22,33]
SEM and TEM were used to observe the morphological and microstructural characteristics of the as-prepared samples.The SEM images displayed in Figure 2a,b show that the VS4-CN-Hs consist of micron-sized(~6 μm) hierarchitectures that are made up of curly nanosheets. A closer look at the sample(Figure 2c)reveals that the curly VS4nanosheet has a width of about 0.5–1.0 μm.And TEM images (Figure 2d,e) indicate that the nanosheets with thickness of approximately 20 nm are arranged irregularly, and the curly structure forms rich space gaps.The specific surface area of VS4-CN-Hs is determined to be 24.4 m2g?1by using Brunauer–Emmett–Teller (BET) method (Figure S4, Supporting Information). The selected area electron diffraction (SAED) pattern shows diffraction rings from the(013)and(044)planes(inset of Figure 2e),and corroborates the polycrystalline nature of the VS4.The high-resolution TEM(HRTEM)image reveals a spacing of 0.56 nm between adjacent lattice fringes (Figure 2f),which corresponds to the(110) crystal plane of VS4-CN-Hs(calculated by Bragg equation).High-angle annular dark field scanning TEM (HAADF-STEM) and energy dispersive X-ray(EDX) elemental mapping images of VS4-CN-Hs are shown in Figure 2g–i,which illustrates that V and S were evenly distributed on the curly VS4nanosheets, and further confirms the as-prepared sample is the pure VS4.
The growth process of the VS4-CN-Hs is demonstrated in Figure 3.During the initial 0.5 h of reaction, particles nucleated rapidly and grew into larger ones with numerous small nanoflakes on the surfaces(Figure 3a). As the reaction proceeds (from 2 to 6 h), the nanoflakes continue to grow and some of them acquire curly structure(Figure 3b and Figure S5,Supporting Information).As the growth continued with 8 h (Figure 3c), tensile deformation took place with the help of intermolecular force, causing the structure to bend. After 16–28 h of reaction, the bending nanosheets morphology with the presence of some incompletely transformed particles is demonstrated (Figure 3d,e). The perfect VS4-CN-Hs were obtained after a solvothermal treatment for 36 h (Figure 3f). During the whole reaction process, as illustrated by the XRD patterns (Figure S6, Supporting Information), the VS4crystal phase was always maintained.Therefore,the growth of VS4-CN-Hs follows the well-established Ostwald ripening mechanism,as illustrated in Figure 3g.

Figure 2. The morphology and microstructure characterizations of VS4-CN-Hs. a–c) SEM images, d) TEM image, e) High magnification TEM image (inset: the selected area electron diffraction spectrum), f) High-resolution transmission electron microscope (HRTEM) image, g–i) HAADF-STEM and the corresponding EDX elemental mapping images.
CR 2032 type cells were assembled to test the sodium storage properties of the VS4-CN-Hs anode for SIBs with 1 M NaPF6in DME as the electrolyte. They were cycled within a wide voltage window of 0.01–3.0 V versus Na+/Na.Initially,its electrochemical reaction behaviors were elucidated by cyclic voltammetry(CV).From the CV profiles at 0.1 mV s?1(Figure 4a),it is thinly disguised that the initial cathodic scan has multiple reduction peaks at ~2.2, ~1.8, and ~1.7 V, implying that the NaxVS4is formed via multiple steps of Na+intercalation(Equation 1).The strong reduction peak at low voltage of~0.25 V is attributed to the conversion reaction (Equation 2), resulting in the formation of V-metal wrapped by Na2S matrix and the formation of solid electrolyte interphase (SEI) layer after discharging to 0.01 V.[34–36]In the subsequent anodic cycle, the peak at ~1.9 V is ascribed to the reaction in Equation (3), where VS4is re-formed through the fast kinetics conversion reaction between V and Na2S.[37–39]In the following cyclic scans,the reactions involved are similar to those in the first cycle except that the position of the strong reduction peak is shifted due to the different position of sodium-ion intercalation.
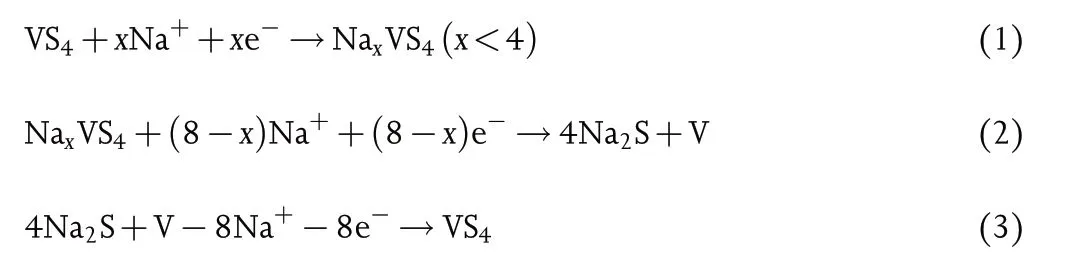

Figure 3. SEM images of intermediates collected at different reaction times: a) 0.5 h, b) 2 h, c) 8 h, d) 16 h, e) 28 h, and f) 36 h. g) Schematic illustration of the growth process of VS4-CN-Hs.
Figure 4b displays the galvanostatic charge/discharge curves at 0.1 A g?1. These curves present multiple voltage plateaus and are perfectly in tune with the CV results. High initial discharge and charge capacities of 1019 and 829 mA h g?1, respectively, were attained by the VS4-CN-Hs,with a gratifying CE of 81%.Even at the 30th cycle,it still retains good capacity (863 mA h g?1). Remarkably, VS4-CN-Hs deliver excellent rate performance as shown in Figure 4c. Capacities of 847, 735, 674, 597, 479, and 444 mA h g?1are achieved at rates of 0.2, 0.5, 1.0, 2.0, 5.0, and 10 A g?1, respectively. Figure 4d shows the charge/discharge profiles corresponding to the various rates and it can be seen that all the curves have similar shape and the charge/discharge platforms are consistent with that in Figure 4b. Such rate capability is much better than that of the reference sample (VS4particles without 3D architecture, Figures S7–S9, Supporting Information). Figure S10(Supporting Information)shows that satisfactory electrochemical performance of 701 mA h g?1after 300 cycles (0.5 A g?1) and 728 mA h g?1after 150 cycles (1 A g?1) for the VS4-CN-Hs is attained.It is worth to mention that the capacities have a sharp decline during the first several cycles, and then gradually increase to a stable state. Such phenomenon was analyzed by electrochemical impedance spectroscopy(EIS)measured under different number of cycles(the 1st,10th, 35th, 50th, and 60th cycles), and the results are shown in Figure S11 (Supporting Information). It is found that the charge transfer resistance (Rct) increases significantly during the initial cycling stage(1–10 cycles),which may be caused by the pulverization,and then the value decreases gradually during the subsequent cycles,which is consistent with the capacity variation.It is an activation process with the slow construction of the polymeric SEI surface layer.[40]In addition, the long-term cycling stability of this anode at 5 A g?1is also outstanding,expressing nearly no capacity degradation after 1000 cycles (Figure 4e),which is much higher than VS4particles(Figure S12,Supporting Information) and the most advanced VS4anodes (e.g.,~238 mA h g?1for cuboid-shaped VS4//Na foil,[39]and~309 mA h g?1for VS4hollow microspheres//Na metal[41]).And the corresponding charge/discharge curves (Figure S13, Supporting Information) show no shape change during the cycles, indicating a good reversibility. These results show that our VS4-CN-Hs is distinguished,which is superior than other reported VS4anodes or other TMSs anodes for SIBs,as listed in Table S1(Supporting Information).
To further understand the superiority of the VS4-CN-Hs anode for SIBs, its sodium storage kinetics was investigated by the CV technique(scan rate:0.2–1.0 mV s?1).As displayed in Figure 5a,it is visible that the CV curves at different scan rates have amazing similarity with only slight deviation in peak positions, indicating that the polarizability of VS4in ether-based electrolyte is relatively low. Judging by the literatures, the relationship between the current (i) and scan rate (ν) conforms to the power-law: i = aνb. The analysis of charge storage behavior can be explained by the value of b,where b = ~0.5 corresponds to diffusion-controlled reaction, and ~1.0 represents surface-controlled mechanism or capacitance.[42–44]The fitted b value of the cathodic peaks (at ~0.78 and ~0.45 V) and anodic peak (at~2.1 V) are 0.76, 1.00, and 0.7, respectively (Figure 5b), indicating that the (de)sodiation is largely controlled by the capacitive mechanism. The capacitance obtained by these two mechanisms is calculated using the formula: i(ν) = k1ν + k2ν1/2, where k1ν and k2ν1/2represent the current contribution to surface-controlled and diffusion-controlled reactions, respectively.[45]For the pseudo-capacitance contribution of the VS4-CN-Hs anode at the various scan rates (shadow area in Figure 5c and contribution ratio in Figure 5d),the value increases gradually from 53.0%to 70.7%.Thus,it can be concluded that the high capacity and excellent rate performance of the VS4-CN-Hs seems largely attributed to the high pseudo-capacitance contribution.
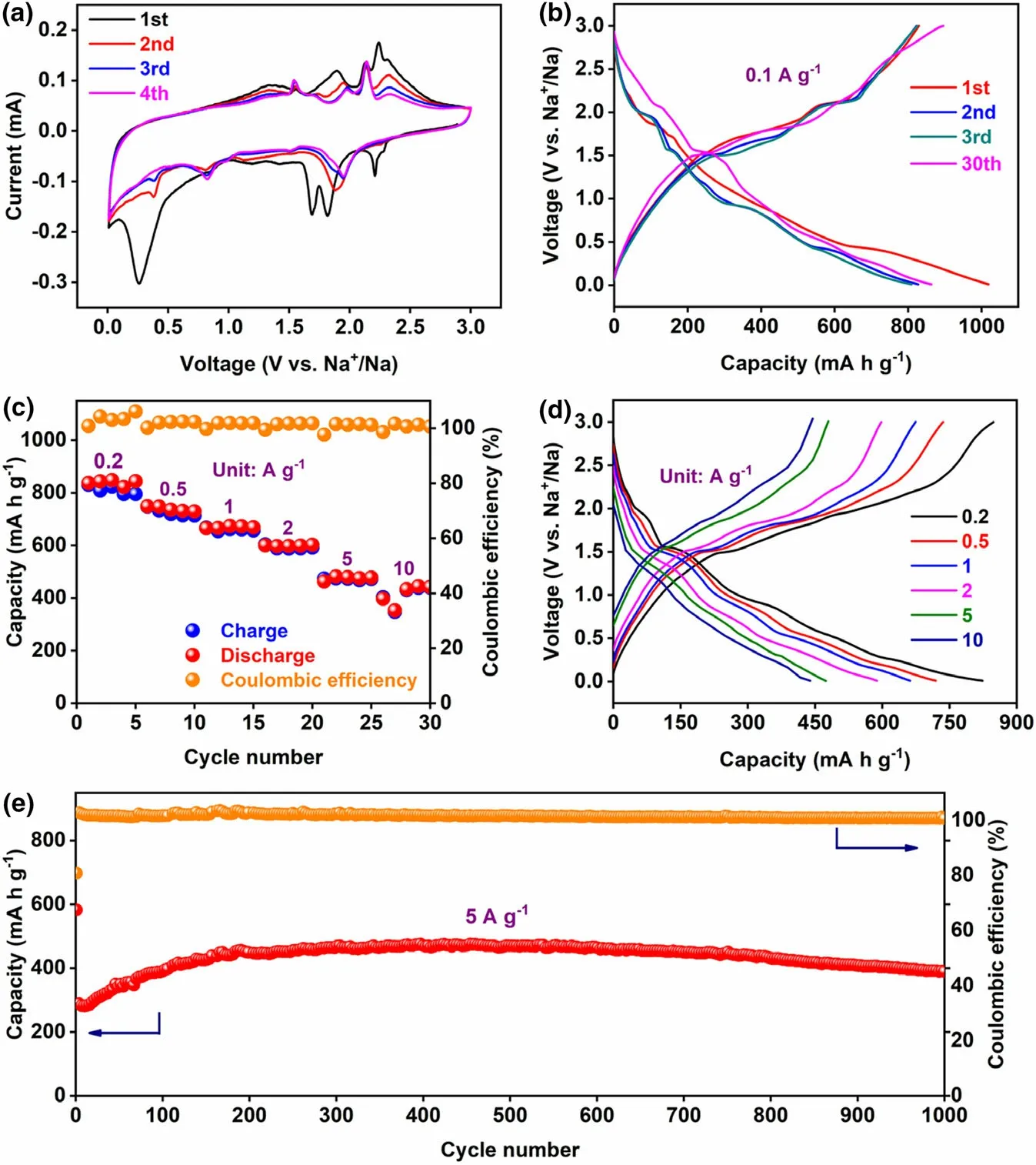
Figure 4. Electrochemical performances of VS4-CN-Hs anode. a) CV curves between 0.01 and 3.0 V at a scanning rate of 0.1 mV s?1, b) Charge/discharge profiles at a current density of 0.1 A g?1, c)Rate property at various current densities (from 0.2 to 10 A g?1), d) Galvanostatic charge/discharge curves at various current densities (from 0.2 to 10 A g?1), e) Long?term cyclic stability at a high current density of 5 A g?1.
To investigate the sodium storage mechanism in VS4-CN-Hs,ex situ TEM measurement was performed as shown in Figure 6. From Figure 6a, it seems that the curly nanosheets still maintain a good structural integrity, indicating that the Na+embedding does not cause significant structural collapse.Figure 6b,c show clear lattice stripes with d-spacing of 0.23 and 0.19 nm corresponding to the(220)and(200)planes of Na2S and metal V, respectively. Further, the diffused diffraction rings of the SAED pattern(Figure 6d)can be indexed to Na2S and metal V, and the HAADF-STEM and the corresponding EDX elemental mapping images (Figure 6e–h)display uniform distribution of Na,S,and V in the fully discharged product. These results confirm the occurrence of the conversion reaction(VS4→Na2S + V).
3. Conclusion
In summary,the VS4curly nanosheets self-assembled hierarchitectures were successfully developed through a simply solvothermal approach. Owing to VS4-CN-Hs providing large contact area and rich buffer space, it can effectively alleviate the volume expansion and improve the electrochemical reaction kinetics during the cycle.When evaluated in a wide current window of 0.01–3.0 V,VS4-CN-Hs exhibits exceptional electrochemical performance involving superior rate performance(e.g., 444 mA h g?1at 10 A g?1), outstanding cycling stability (e.g., at high current densities of 5 A g?1with the reversible capacities reach 386 mA h g?1after 1000 cycles)and high initial capacity (1019 mA h g?1at 0.1 A g?1with initial coulombic efficiency of 81%).
4. Experimental Section
Synthesis of VS4-CN-Hs: All chemicals, including ammonium metavanadate (NH4VO3, 99%), thioacetamide(C2H5NS,99%),and ethylene glycol(EG,99%),were used directly without further purification. The VS4-CN-Hs were produced by an effortless solvothermal approach. Typically, 7.5 mmol of C2H5NS was directly dissolved into 30 mL of EG under stirring to construct a faint yellow solution. Afterward, 1.5 mmol of NH4VO3was added into the above solution and the mixture was subjected to constant stirring. Subsequently, the obtained suspension was transmitted into an autoclave and heated at 160 °C for 36 h. Finally, the obtained product was collected and washed by centrifugation with water and ethanol.For comparison,VS4particles without 3D architecture were synthesized according to the previous work.[26]
Materials characterization: The scanning electron microscopy (SEM) measurements were employed by a Hitachi SU-8220 to explore the sample morphology. Transmission electron microscope (TEM) images were conducted by FEI TALOS 200X transmission electron microscopy at 200 kV to investigate the microstructure of the products. X-ray diffraction (XRD, Rigaku D/max 2500, Cu Kα,λ = 0.15418 nm)patterns were operated with scan range of 10–80°and scan rate of 20° min?1. Raman spectra were tested by LabRAM HR with laser wavelength of 532 nm.And X-ray photoelectron spectroscopy(XPS)analysis was performed by a Escalab 250 Xi at voltage of 15 kV and current of 15 mA. The charge correction is calibrated by C 1s binding energy 284.8 eV (C–C bond) as the reference standard.
Electrochemical measurements: The anode was prepared by mixing 60 wt%VS4-CN-Hs, 30 wt% carbon, and 10 wt% poly (vinylidenefluoride) in Nmethylpyrrolidone.Then,the stirred mixture coated on copper foil and cut it into a circle with a diameter of 12 mm. The 1 M NaPF6in 1,2-dimethoxyethane electrolyte (DME) solution was employed as electrolyte. The cells were convened in an argon-filled glove box.The galvanostatic charge/discharge tests were carried out in a NEWARE battery system, and the voltage window was run within 0.01–3.0 V.
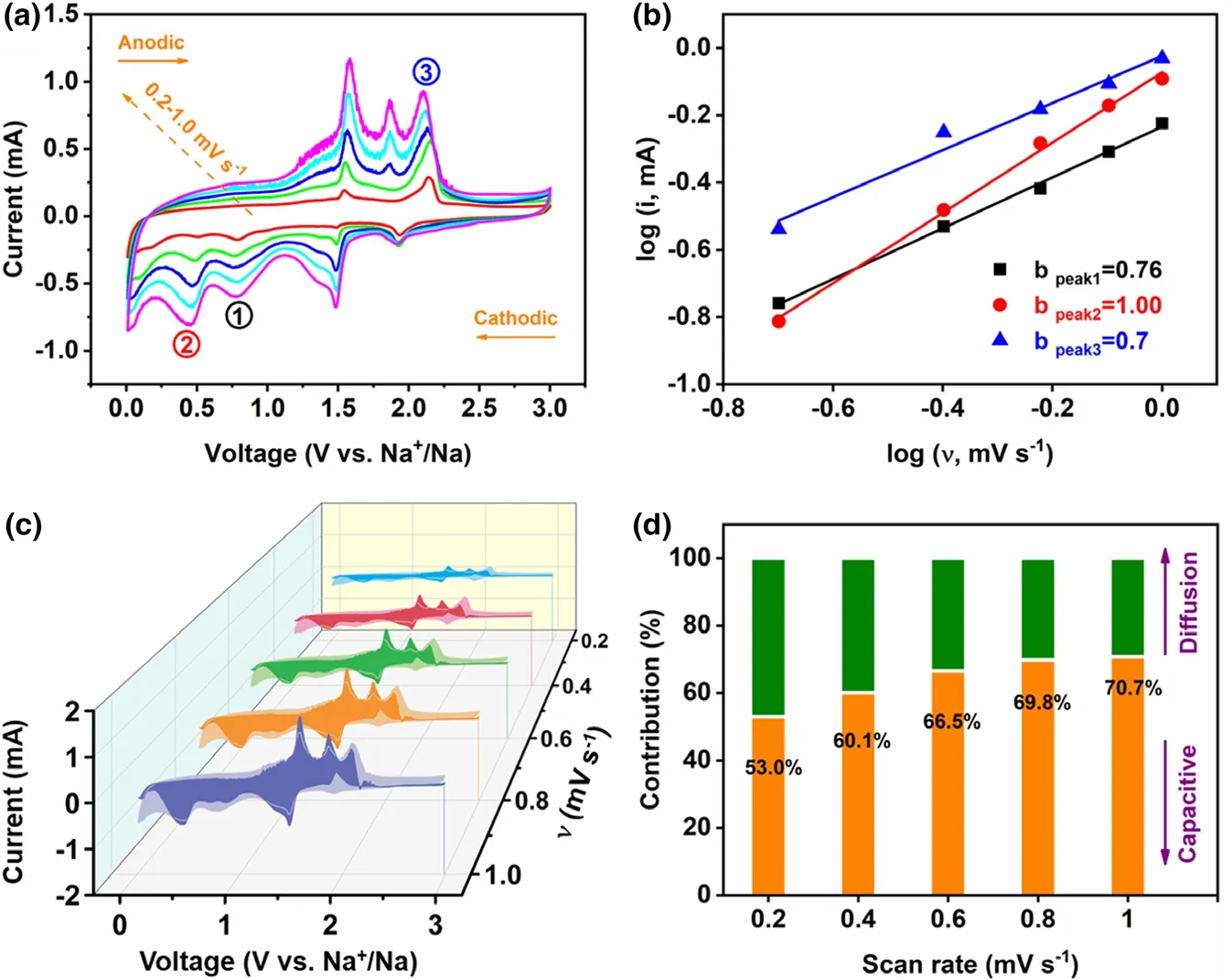
Figure 5. a) CV curves at scan rates from 0.2 to 1.0 mV s?1, b) b value issued from the relationship between peak current and CV scan rates, c) Evaluation of capacitive contribution to the total charge storage at various scan rates, d) Contribution ratio of the capacitive?controlled and diffusion?controlled capacities at various scan rates.
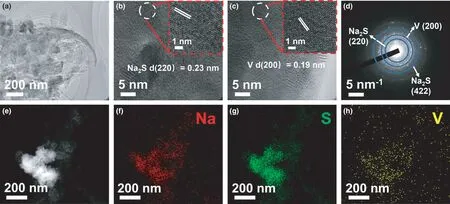
Figure 6. a) TEM image of the VS4-CN-Hs anode measured at the first full discharging state (current density: 20 mA g?1), b, c) the corresponding HRTEM images, d) SAED pattern, e–h) HAADF-STEM and the corresponding EDX elemental mapping images.
Acknowledgements
The authors gratefully acknowledge the National Natural Science Foundation of China(Grant Nos.51925207,U1910210, 51872277, 51972067, 21606003, 51902062,51802043, and 51802044), the Fundamental Research Funds for the Central Universities (WK2060140026),the DNL cooperation Fund, CAS (DNL180310), the NationalSynchrotronRadiationLaboratory(KY2060000173), and Guangdong Natural Science Funds for Distinguished Young Scholar (Grant No.2019B151502039).
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information
Supporting Informationis available from the Wiley Online Library or from the author.
Keywords
3D hierarchitecture, anode material, high rate capability,sodium-ionbattery,vanadium tetrasulfide
Received: December 22, 2020
Revised: March 9, 2021
Published online: March 23, 2021
[1] Y. Wu, W. Wang, J. Ming, M. Li, L. Xie, X. He, J. Wang, S. Liang, Y. Wu,Adv. Funct. Mater. 2019, 29, 1805978.
[2] X. Zeng, M. Li, D. A. El-Hady, W. Alshitari, A. S. Al-Bogami, J. Lu, K.Amine, Adv. Energy Mater. 2019, 9, 1900161.
[3] R. Du, X. Hu, S. Xie, L. Hu, Z. Zhang, X. Lin, J. Power Sources 2020, 473,228568.
[4] T. Ma, S. Wu, F. Wang, J. Lacap, C. Lin, S. Liu, M. Wei, W. Hao, Y. Wang,J. W. Park, ACS Appl. Mater. Inter. 2020, 12, 56086.
[5] K. Chayambuka, G. Mulder, D. L. Danilov, P. H. L. Notten, Adv. Energy Mater. 2020, 10, 2001310.
[6] V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-Gonz′alez,T. Rojo, Energy Environ. Sci. 2012, 5, 5884.
[7] L. Li, Y. Zheng, S. Zhang, J. Yang, Z. Shao, Z. Guo, Energy Environ. Sci.2018, 11, 2310.
[8] M. Chen, Q. Liu, Z. Hu, Y. Zhang, G. Xing, Y. Tang, S. Chou, Adv. Energy Mater. 2020, 10, 2002244.
[9] H. Tan, D. Chen, X. Rui, Y. Yu, Adv. Funct. Mater. 2019, 29, 1808745.
[10] X. Rui, X. Zhang, S. Xu, H. Tan, Y. Jiang, L. Y. Gan, Y. Feng, C. C. Li, Y.Yu, Adv. Funct. Mater. 2020, 31, 2009458.
[11] A. Cheng, H. Zhang, W. Zhong, Z. Li, D. Cheng, Y. Lin, Y. Tang, H. Shao,Z. Li, Carbon 2020, 168, 691.
[12] Y. Dong, Z. Zhu, Y. Hu, G. He, Y. Sun, Q. Cheng, I. P. Parkin, H. Jiang,Nano Res. 2020, 14, 74.
[13] H. Wu, X. Chen, C. Qian, H. Yan, C. Yan, N. Xu, Y. Piao, G. Diao, M.Chen, Small 2020, 16, 2000695.
[14] S. Xu, X. Gao, Y. Hua, A. Neville, Y. Wang, K. Zhang, Energy Storage Mater. 2020, 26, 534.
[15] X. Li, Y. Sun, X. Xu, Y.-X. Wang, S.-L. Chou, A. Cao, L. Chen, S.-X. Dou,J. Mater. Chem. A 2019, 7, 25932.
[16] Z. Zhao, S. Li, C. Li, Z. Liu, D. Li, Appl. Surf. Sci. 2020, 519, 146268.
[17] C. Dong, L. Guo, H. Li, B. Zhang, X. Gao, F. Tian, Y. Qian, D. Wang, L.Xu, Energy Storage Mater. 2020, 25, 679.
[18] J. Wang, N. Luo, J. Wu, S. Huang, L. Yu, M. Wei, J. Mater. Chem. A 2019, 7, 3691.
[19] L. Yu, S. Zhao, Q. Wu, J. Zhao, G. Wei, Adv. Funct. Mater. 2020, 30, 2000427.
[20] L. Shen, Y. Wang, F. Wu, I. Moudrakovski, P. V. Aken, J. Maier, Y. Yu,Angew. Chem. Int. Ed. 2019, 58, 7238.
[21] C. Yang, X. Ou, X. Xiong, F. Zheng, R. Hu, Y. Chen, M. Liu, K. Huang,Energy Environ. Sci. 2017, 10, 107.
[22] S. Ding, B. Zhou, C. Chen, Z. Huang, P. Li, S. Wang, G. Cao, M. Zhang,Nano-Micro Lett. 2020, 12, 39.
[23] R. Allmann, I. Baumann, A. Kutoglu, H. R¨osch, E. Hellner, Naturwissenschaften 1964, 51, 263.
[24] K. Shimoda, K. Koganei, T. Takeuchi, T. Matsunaga, M. Murakami, H.Sakaebe, H. Kobayashi, E. Matsubara, RSC Adv. 2019, 9, 23979.
[25] W. Li, J. Huang, L. Feng, L. Cao, Y. Liu, L. Pan, Mater. Lett. 2018, 230,105.
[26] Z. Li, S. Ding, J. Yin, M. Zhang, C. Sun, A. Meng, J. Power Sources 2020,451, 227815.
[27] F. Yang, W. Zhong, H. Wang, M. Ren, W. Liu, M. Li, L. Su, J. Alloys Compd. 2020, 834, 155204.
[28] W. Li, J. Huang, L. Feng, L. Cao, S. He, Nanoscale 2018, 10, 21671.
[29] G. Yang, H. Wang, B. Zhang, S. Foo, M. Ma, X. Cao, J. Liu, S. Ni, S. Madhavi, Y. Huang, Nanoscale 2019, 11, 9556.
[30] W. Li, J. Huang, R. Li, L. Cao, X. Li, S. Chen, L. Feng, Chemsuschem 2019,12, 5183.
[31] Y. Wang, C. Wang, X. Yi, Y. Hu, L. Wang, L. Ma, G. Zhu, T. Chen, Z. Jin,Energy Storage Mater. 2019, 23, 741.
[32] M. Ramu, J. R. Chellan, N. Goli, P. Joaquim, V. Cristobal, B. C. Kim, Adv.Funct. Mater. 2019, 30, 1906586.
[33] M. N. Kozlova, Y. V. Mironov, E. D. Grayfer, A. I. Smolentsev, V. I. Zaikovskii, N. A. Nebogatikova, T. Y. Podlipskaya, V. E. Fedorov, Chem. Eur.J. 2015, 21, 4639.
[34] D. T. Pham, B. Sambandam, S. Kim, J. Jo, S. Kim, S. Park, V. Mathew,Y.-K. Sun, K. Kim, J. Kim, Energy Storage Mater. 2019, 19, 270.
[35] S. S. Zhang, J. Mater. Chem. A 2015, 3, 7689.
[36] Y. Li, Y. Liang, F. C. R. Hernandez, H. D. Yoo, Q. An, Y. Yao, Nano Energy 2015, 15, 453.
[37] R. Tan, J. Yang, J. Hu, K. Wang, Y. Zhao, F. Pan, Chem. Commun. 2016,52, 986.
[38] R. Sun, Q. Wei, Q. Li, W. Luo, Q. An, J. Sheng, D. Wang, W. Chen, L.Mai, ACS Appl. Mater. Inter. 2015, 7, 20902.
[39] S. Wang, F. Gong, S. Yang, J. Liao, M. Wu, Z. Xu, C. Chen, X. Yang, F.Zhao, B. Wang, Y. Wang, X. Sun, Adv. Funct. Mater. 2018, 28,1801806.
[40] R. Sun, Q. Wei, J. Sheng, C. Shi, Q. An, S. Liu, L. Mai, Nano Energy 2017,35, 396.
[41] W. Li, J. Huang, R. Li, L. Cao, X. Li, L. Feng, S. Chen, Chem. Eng. J. 2019,384, 123385.
[42] Z. Wu, C. Lu, Y. Wang, L. Zhang, L. Jiang, W. Tian, C. Cai, Q. Gu, Z.Sun, L. Hu, Small 2020, 16, 2000698.
[43] Q. Li, X. Rui, D. Chen, Y. Feng, N. Xiao, L. Gan, Q. Zhang, Y. Yu, S.Huang, Nano-Micro Lett. 2020, 12, 67.
[44] X. Li, T. Liu, Y.-X. Wang, S.-L. Chou, X. Xu, A. Cao, L. Chen, J. Power Sources 2020, 451, 227790.
[45] C. Cui, Z. Wei, G. Zhou, W. Wei, J. Ma, L. Chen, C. Li, J. Mater. Chem. A 2018, 6, 7088.
 Energy & Environmental Materials2022年2期
Energy & Environmental Materials2022年2期
- Energy & Environmental Materials的其它文章
- Editor’s Note on “Frontier Outlook” Column
- Graphite Anode for Potassium Ion Batteries: Current Status and Perspective
- Green Synthesis of Nitrogen-to-Ammonia Fixation: Past,Present, and Future
- Photo-assisted Rechargeable Metal Batteries for Energy Conversion and Storage
- 3D Printing of Next-generation Electrochemical Energy Storage Devices: from Multiscale to Multimaterial
- The Transference Number
