Production, structure, and bioactivity of polysaccharide isolated from Tremella fuciformis
Hongjie Yun, Lin Dong, Zhiyun Zhng, Yn He,*, Xi M,b,*
a School of Perfume and Aroma Technology, Shanghai Institute of Technology, Shanghai 201418, China
b National R & D Center for Edible Fungus Processing Technology, Henan University, Kaifeng 475004, China
Keywords:
Tremella fuciformis polysaccharide
Structure characterization
Antioxidant activity
Moisturizing
A B S T R A C T
Tremella fuciformis polysaccharide (TFP) is an important bioactive substance in T. fuciformis, which contributes to its application as medicine and food. In the present work, TFP was extracted with enzymatic combined with wet beating and its physicochemical as well as biological activities were investigated in the study. The result showed that the purity of TFP1 reached 92.14% (m/m). The TFP1, purified by DEAE column,had a molecular weight (Mw) of 5.8 × 103 kDa and consisted mainly of mannose, xylose, fucose, glucose and glucuronic acid at a molar ratio of 1.91 : 0.1 : 2.49 : 6.23 : 0.95. The structure of TFP1 was preliminarily investigated by infrared spectroscopy, nuclear magnetic resonance and atomic force microscope (AFM)analysis. Moreover, the antioxidant assays showed that TFP1 could scavenge DPPH, ABTS+ and hydroxyl radicals. The excellent water holding capacity of TFP1 implied its application in the food, pharmaceutical andcosmetic industries.
1. Introduction
Tremella fuciformisbelongs to the order Tremellaces and the family Tremellacea. It has many common names, such as snow fungus, snow ear, silver ear fungus, white jelly mushroom and white auricularia. As a kind of famous edible and medicinal mushroom in China, it always grows on the decayed trunk of hardwood trees such as oak and willow [1]. The polysaccharides, obtained fromT. fuciformisfruit body, have been regarded as the major active component related to the nutritional and health ofT. fuciformis[2]. As the main bioactive component ofT. fuciformis, the polysaccharide has been proved to be effective in inhibiting quorum sensing [3], enhancing immunity [4],lowering blood sugar [5], anti-tumor [6], anti-aging [7], anti-mutagenic [8],anti-radiation [9]. Additionally, other literatures further indicated that theT. fuciformispolysaccharide (TFP) also had excellent moisturepreserving activity and skin protection function, and might be useful for improving the cognitive function [10-12]. As the TFPS have a positive impact on the human health, they have been widely used as health products, such as moisturizing agent and anti-wrinkle agent. Polysaccharides with biological activity differ greatly in their chemical compositions, con figurations and physical properties [13].Although the structure, conformation and bioactivity of TFPS have been extensively studied, the knowledge of the relationship between structure and biological activityd is still very limited.
Extraction is a key step for obtaining polysaccharides and has a great influence on polysaccharide yield, quality, chemical structure and biological activities [14-16]. At present, TFP were extracted mostly by hot water extraction, acid extraction, alkaline extraction,enzymatic extraction, and microwave-assisted extraction [1]. The generally adopted polysaccharide extraction method is to stir the pulverized fruiting bodies in hot water for several hours. This extraction method is easy to carry out, but requires a long extraction time, large volumes of solvents and elevated temperatures [17].Due to the violent reaction of acid and lye extraction methods, TFP are likely to cause glycosidic bond breakage and conformational changes in strong acid and strong alkali solutions, thereby destroying the structure of TFP, reducing the molecular weight of TFP and its medicinal value [18]. In addition, microwave and ultrasonic assistance have higher requirements for equipment and higher energy consumption [19]. Moreover, enzyme preparations are generally more expensive, and the extraction rates of these methods are not very high [20]. To accelerate the extraction procedure and avoid the bioactivity decreases of polysaccharides at excessively high extraction temperature, wet extraction has been used as an alternative to the extraction of TFP.
In this study, the technology of fast extraction of TFP by enzyme method combined with wet pulping was studied. We discussed the chemical structure, morphology and antioxidant activities of purified TFP1 from fruit bodies ofT. fuciformisfrom Gutian. To our best knowledge, that is the earlier report to study the biological functions of TFP1 extracted by enzyme method combined with wet pulping to evaluate the relationship between structural characteristics and biological activities TFP1.These results have significance for our understanding of potential correlation between the structural properties and biological functions of this particular polysaccharide.
2. Material and method
2.1 Materials
Fresh white fungus was harvested from Gutian County, in Fujian province, China. The fruit bodies were soon washed; sun dried and kept in plastic bags at room temperature.
2.2 Extraction of crude TFP
According to the material-liquid ratio of 1:50 (m/V), distilled water was added to 50 g fresh white fungus. Then the resulting mixture was stirred and crushed (Philips Blender, China, HR2101).Pectinase (0.8%) and cellulase (1.0%) were added respectively.In the condition of 55 ℃, enzymatic hydrolysis for 120 min. Then the solution was beaten for 7 min after heating to 80 ℃. After centrifugation, the supernatant was collected. Trypsin (1.0%)was added to the supernatant, and the digestion was carried out at 37 ℃ for 120 min. The Sevage method was used to deproteinize three times. The crude polysaccharide solution was concentrated. 95% ethanol was added to the precipitation in 4 times (V/V), and the crude polysaccharide was obtained by centrifugation. Finally, the precipitate was washed once with ethanol, reconstituted in deionized water,dialyzed, and lyophilized to obtain crude TFP.
2.3 Preparation of TFP and determination of molecular weight (Mw)
The crude polysaccharide was purified and divided into two parts by using ion-exchange chromatography of DEAE Sepharose Fast Flow (GE Healthcare, Sweden). Firstly, the crude polysaccharide was dissolved to 10 mg/mL in ultra-pure (up) water and loaded onto a column of DEAE Sepharose. As shown in Fig. 1, elution with 7 mL per tube was collected with 0.0, 0.2, 0.4 and 0.6 mol/L NaCl solutions at a flow rate of 1 mL/min. The phenol sulfuric acid method [21]was used to determine the polysaccharide content of each tube.According to the sequence of elution, the polysaccharide was dialyzed and lyophilized. The main peak polysaccharide component is the component eluted with 0.4 mol/L NaCl solution, named TFP1. TheMwwas determined by high-performance size exclusion chromatography (HPSEC). The sample and standard of dextran with differentMw(289, 410, 500, 750, 1 000 kDa) were analyzed by Waters Ultrahydrogel TM Linear gel column (7.8 × 300 mm, 10 μm)with detector (Waters 2410 refractive index detector and UV detector)and eluted with 0.1 mol/L NaNO3at a flow rate of 0.9 mL/min at 45 ℃. TheMwwas calculated by referencing a standard curve.

Fig. 1 DEAE-Sepharose Fast Flow chromatography of TFP1.
2.4 Analysis of monosaccharide composition
High performance ion chromatography (HPIC) was used for identification and quantification of the monosaccharides. 100 μL of 5 mg/mL polysaccharides were hydrolyzed with 100 μL of 4 mol/L trifluoroacetic acid (TFA) at 110 ℃ for 2 h, and then evaporated continuously by a rotary evaporator at 45 ℃ for pH up to neutrality. The hydrolysate was dissolved in 5 mL of water. Filtered with a 0.45 μm microporous membrane for monosaccharide composition analysis.
Its corresponding TFP1 was analyzed by Dionex ICS5000 system equipped with GS50 quaternary gradient pump,ED50A electrochemical detector (gold electrode), LC30column thermostat and Chromeleon 7.0 chromatography workstation. The chromatographic column Carbo Pac PA20 (ID 3 × 150 mm) was equipped with a pulsed amperometric detector (PAD), Au working electrode, and Ag/AgCl reference electrode were used. The mobile phase A: H2O; B: 250 mmol/L NaOH; C: 1 mol/L NaAc. A ternary gradient elution with a flow rate of 0.5 mL/min. The column temperature was 30 ℃ and the injection volume was 20 μL. The control standard monosaccharides were fucose, rhamnose, arabinose,galactose, glucose, xylose, mannose, fructose, galacturonic acid, and glucuronic acid.
2.5 The Fourier transform-infrared (FT-IR) spectra
TFP1 samples (4 mg) were stacked by dry potassium bromate precipitation method using Bruker Vector 22 instrument in the 400-4 000 cm?1region.
2.6 Atomic force microscope (AFM) observation
2.6.1 Preparation of test samples
10 mg of TFP1 was weighed and dissolved it in 100 mL ultrapure water. A 100 μg/mL TFP1 solution was obtained. In order to fully dissolve the solution, magnetic stirrer stirred for 12 h. The TFP1 solution was diluted with ultrapure water to 1 μg/mL. To further observe the chain conformation of TFP1 molecules. The surfactant sodium dodecyl sulfate (SDS) added at a ratio of 1 : 1 (SDS/TFP1,m/m) in 100 μg/mL TFP1 solution. Stirred to fully dissolve it.The ultrapure water was carried to dilute and obtain a 0.1 μg/mL TFP1/SDS solution.
2.6.2 AFM test
On the freshly peeled mica flakes, 5 μL of 2 mmol/L NiCl2solution dropwise was added. After 1 min, it was washed with water and dried to positively charge of the surface of the mica sheet. TFP1 solution 5 μL was taken and dropped on the Ni2+- mica flakes. Aired in the to dry it naturally. At room temperature and atmospheric environment(relative humidity not higher than 60%), the prepared polysaccharide mica flake samples were scanned under the Nano Scope IIIa atomic force microscope. Scanning adopted tapping mode to work. The force was controlled within 3-4 nN, and the scanning frequency was 2 Hz. Si3N4probe, 225 μm microcantilever and 0.6-3.7 N/m elastic modulus were used. The software Nanoscope5.30r3sr3 attached to AFM was used for image processing and analysis [22].
2.7 Antioxidant activities in vitro
2.7.1 Scavenging capacity of hydroxyl radicals
In 0.40 mL 6 mmol/L FeSO4solution, 1 mL 8.8 mmol/L H2O2solution, 1 mL 9 mmol/L salicylic acid solution and 1.60 mL distilled water were added and mixed. It was put in a water bath at 37 °C heated for 15 min (distilled water was a blank control). The the absorbance value (Ac) was recorded at a wavelength of 510 nm.Aiis the absorbance value measured by replacing 1.60 mL of distilled water with a certain concentration of sample. 1 mL of distilled water instead of hydrogen peroxide solution to determine the absorbance valueAj. The VC solution was used as a positive control, and each sample was made 3 parallel samples to calculate the scavenging rate of the samples to hydroxyl radicals.
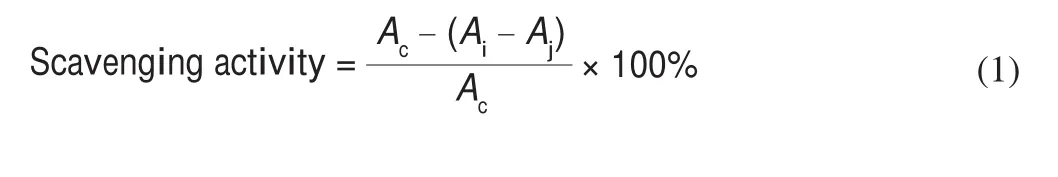
2.7.2 Scavenging capacity of DPPH
Polysaccharide samples were dissovled in distilled water to form sample solution inflnal concentrations of 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mg/mL, respectively. 2 mL of the sample solution was mixed with 2 mL of 0.2 mmol/L DPPH ethanol solution. The reaction solution was incubated for 40 min at room temperature, and the absorbance of the mixture was measured at 517 nm using the spectrophotometer.The scavenging activity of DPPH radical was calculated by the following equation.
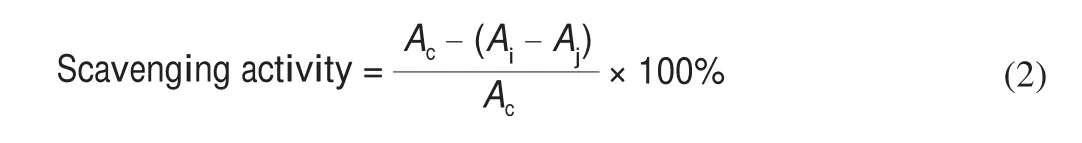
WhereAi, the absorbance of the 2 mL sample solution + 2 mL DPPH solution,Aj, 2 mL sample solution + 2 mL absolute ethanol andAc, the absorbance of 2 mL absolute ethanol + 2 mL DPPH solution.
2.7.3 Scavenging capacity of ABTS+
The ABTS+solution is diluted with phosphate buffer (10 mmol/L,pH 7.4) to make the absorbance at 734 nm wavelength of 0.700 ± 0.020 to obtain ABTS+working solution. 1 mL of a certain concentration of sample solution and 3 mL of ABTS+working solution were added to the test tube and mixed. At room temperature,it was kept in dark for 10 min, the absorbance at 734 nm was mmeasured (phosphate buffer is a blank control).Vcsolution was used as a positive control, and each sample was made 3 parallel samples.The scavenging activity of ABTS+radical was calculated according to the following equation:
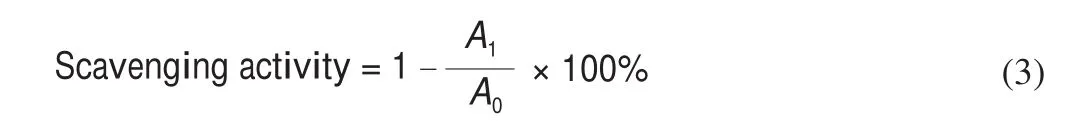
WhereA1, the absorbance of the test samples, andA0, the absorbance of blank control.
2.8 Moisture-preserving in vitro
2.8.1 Moisture absorption activity
The moisture-preserving test was referenced by Xu et al. [23]with a modification. 3 parts of 0.5 g glycerin, crude TFP and TFP1 were weighed into weighing bottles and divided into 2 groups. One set is placed in a desiccator filled with saturated Na2CO3solution (RH 43%), the other group was placed in a desiccator saturated (NH4)2SO4solution (RH 81%). At room temperature, the mass of the sample at 4,12, 24, and 48 h were measured respectively, the average value of 3 times were recorded and the moisture absorption rate was calculated as follows:
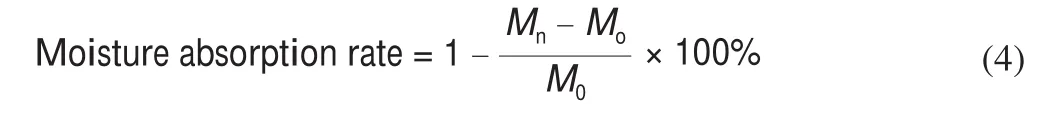
Where:Mnis the mass of the sample at different times (g);Mois the initial mass of the sample (g)
2.8.2 Moisture retention activity
3 parts each of 0.5 g glycerin, crude TFP and TFP1 into a weighing bottle were weighed and 0.2 g of distilled water was added. After the sample has fully absorbed water, it was placed in a desiccator filled with silica gel. At room temperature, it was weighed after 4, 12, 24, and 48 h, and the average value for 3 times was calculate. The moisture retention rate by the following formula was calculate:

Where:Hnis the water mass of the sample at different times (g);Hois the initial moisture mass of the sample (g).
2.9 Statistical analysis
All results were presented as mean ± SEM of three determinations and were analyzed statistically by by One-Way ANOVA. APvalue < 0.05 was regarded as statistically significant. All computations were performed using statistical software.
3. Results
3.1 Purification and Mw determination of TFP
The crude TFP obtained by enzymatic assisted wet beating was 6.5 g/10 g, and the TFP1 component was 2.3 g/10 g after column chromatography. TFP1 was made into 0.2 mg/mL solution with deionized water. After the TFP was fully dissolved, it was filtered with 0.22 μL filter membrane. The solution was scanned by UV in the wavelength range of 190-800 nm. The UV spectrum of TFP1 solution did not show the characteristic absorption peaks of protein and nucleic acid at the wavelengths of 260 and 280 nm. Therefore, it showed that there was no pigment in TFP1.
Two peaks of polysaccharide were separated from the crude polysaccharide. One was a small-molecular weight polysaccharide,the other was a macromolecule polysaccharide, TFP1. The main peak polysaccharide component was eluted with 0.4 mol/L NaCl solution and named as TFP1. TheMwof TFP1 (Fig. 2) was 5.8 × 106Da.Compared with theMwof most of the extractedTremellapolysaccharide, theMwof the TFP1 was large. In 2020, macromolecule polysaccharides (TFPB) purified by Ge et al. [24]was found to had a molecular weight (Mw) of 1.14 × 103kDa. In 2019, Lin et al. [25]compared the proportions and differences in the polysaccharides ofT. fuciformis(Berkeley) after drying them by various processes, such as 18 ℃ cold air, 50 ℃ hot air, and freeze-drying. Among the various drying processes, cold air-drying had the highest molecular weight of 2.41 × 107Da. The macromolecular polysaccharide fermented in this study has more than twice theMwof these previous extractions.However, the small molecular polysaccharide had a slightly smaller range of extraction. The absorption peak of TFP1 was a narrow single symmetrical peak. It showed that TFP1 had a large molecular weight and uniform composition.
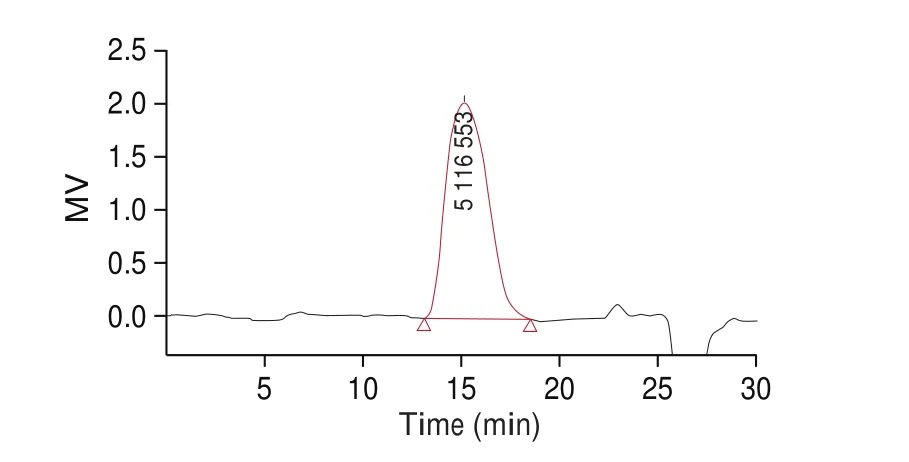
Fig. 2 The HPLC of molecular polysaccharide (TFP1).
3.2 Monosaccharide compositions
The monosaccharide compositions of TFP1 were detected through the HPIC method, and the results are shown in Fig. 3. It is indicated that TFP1 was composed of mannose, xylose, fucose,glucose and glucuronic acid with a molar ratio of 1.91 : 0.1 : 2.49 :6.23 : 0.95, respectively. Mannose has the largest proportion. A polysaccharide fraction (TPS), which was extracted by Zhang et al. [26]is a homogeneous polysaccharide consisting primarily of while consisted of rhamnose, xylose, mannose and glucose in the molar ratio of 1.13 : 1 : 4.70 : 0.81. An acidic TFP was extracted by boiling and precipitated by ethanol was also composed of mannose,glucuronic acid, xylose and fucose, but in different proportions [27].It is investigated that the monosaccharide compositions of the TFPB are different to polysaccharides extracted fromTremellafruit body.
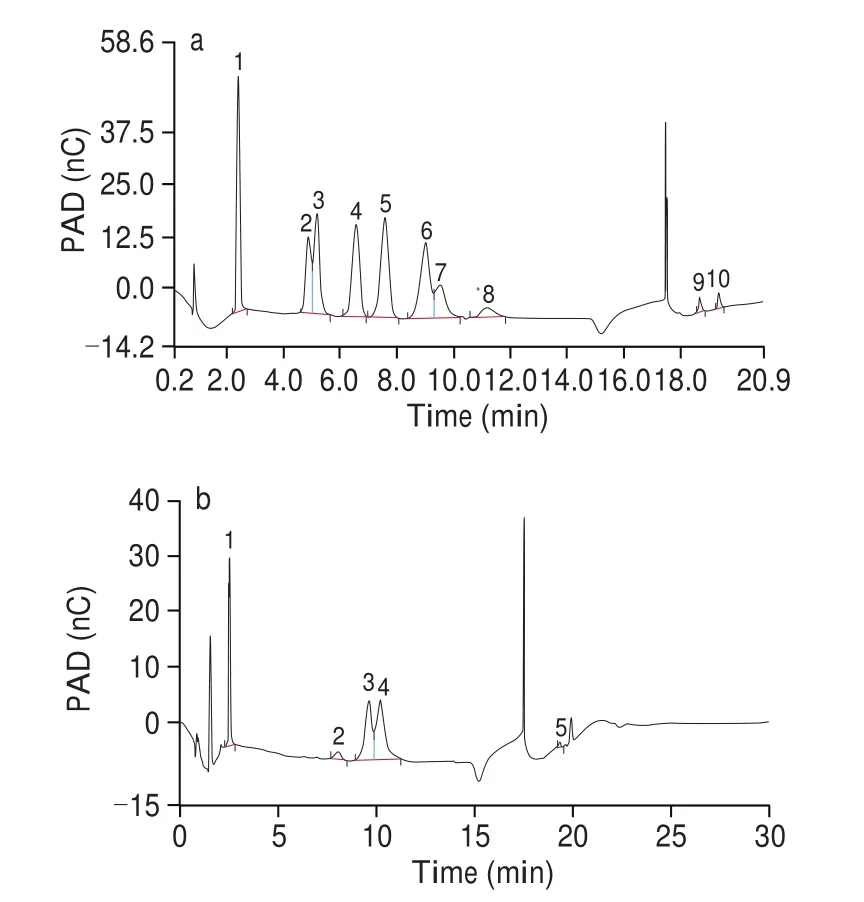
Fig. 3 (a) The HPAEC chromatograms of standard monosaccharides; (b) the HPAEC chromatograms of component monosaccharides released from TFP1(peaks; (1) fucose, (2) glucose, (3) xylose, (4) mannose, (5) glucuronic acid).
3.3 Infrared spectroscopy
The infrared spectrum is highly characteristic, so it can be used to analyze and determine some specific functional groups in samples. In the study of sugars, infrared spectroscopy can also distinguish the types of some sugar bitter bonds in furan, pyranose,and polysaccharide. At 3 440 cm?1, there was a stretching vibration peak of the O-H bond; at 2 930 cm?1, there was a stretching vibration peak of weak absorption of the C-H bond. At 1 732 and 1 641 cm?1,the absorption peaks indicate the presence of glucuronic acid at 1 080 cm?1leading to the angular vibration of alcohol hydroxyl, and the wave-shaped curve at around 800 cm?1representsα-D-mannopyranose(Fig. 4). By observingthe infrared spectrum, according to the results of monosaccharide composition, the main components include mannose,glucuronic acid, glucose, xylose, and fucose.
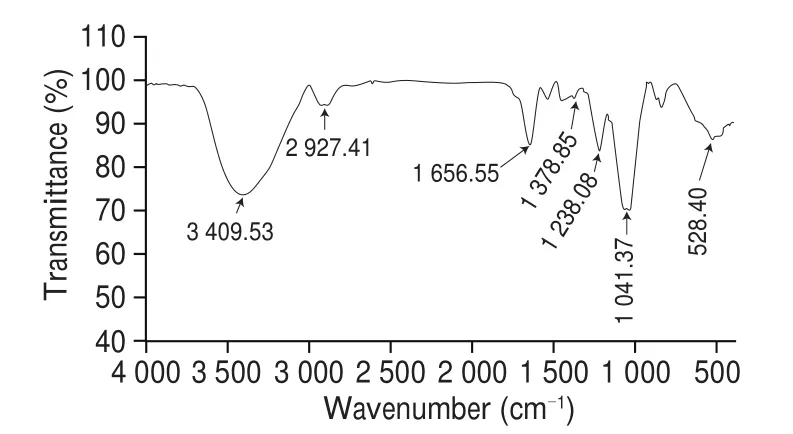
Fig. 4 FTIR spectrum of TFP1.
3.4 AFM Analysis
Fig. 5 showed the atomic force microscope images of 1 μg/mL TFP1 at different magnifications. It could be observed that the TFP1 forms cyclic and chain-like structures on the mica sheet. The polysaccharide chains were entangled with each other, and there were multiple branches, forming a local accumulation. The width of the chain was 16-90 nm, the height of the chain was 0.5-1.5 nm. It was much larger than the width of the polysaccharide chain, which is caused by the broadening effect of the scanning probe [28].

Fig. 5 AFM topographic image of TFP1 (1 μg/mL).
From the observation results, TFP1 had multiple branched chain structures. It was consistent with the related description ofT. polysaccharidesby existing researchers. Ma et al. [29]carried out the I2-KI reaction, mixed TFP1 solution with the iodine reagent and scanned it with an ultraviolet spectrophotometer in the range of 300-700 nm. No absorption peak was found at 565 nm. It is inferred thatTremellapolysaccharide may have longer side chains and more branches. AFM was used to observe the morphology ofTremellapolysaccharides, and most of the observations were irregular spherical [16,23]. The possible reason was that both mica flakes andTremellapolysaccharides were negatively charged andTremellapolysaccharides contained a large number of hydroxyl groups. In this experiment, Ni2+was used to treat the mica flakes, which reduced the molecular aggregation caused by electric charges. Therefore, some cyclic and chain structures were observed. It was observed that the height of the polysaccharide chain ofT. fuciformispartially exceeds the range of 0.1-1.0 nm of the height of a single polysaccharide molecule.Bai et al. [30]made AFM observations on pumpkin peel polysaccharide PP1. It was easy to form an intermolecular hydrogen bond between the uronic acid carboxyl group on one sugar chain and the hydroxyl hydrogen on the other chain. This uronic acid branch also existed inTremellapolysaccharide. Therefore, it was possible that multiple sugar chains inT. fuciformispolysaccharides may be twisted into strands through the interaction of van der Waals forces and hydrogen bonds.
To further observe the chain structure of TFP1. The surfactant SDS was added to the solution of TFP1 in equal proportions to promote the dispersion of polysaccharide molecules. Fig. 6 showed the atomic force microscope images of TFP1 dispersed in SDS aqueous solution at different magnifications. It is observed that SDS could effectively eliminate part of the hydrogen bonds entangled between molecules and part of the branching and aggregation between molecules. Therefore, the molecular chain and ring structure become more clearly visible. However, polysaccharide molecules still had different degrees of aggregation and entanglement in the chain structure. The width of the chain was measured to be 8-32 nm and the height is 0.4-0.8 nm. It was in the range of 0.1-1.0 nm in height of a single polysaccharide molecule [31]. Therefore, it was possible that the spatial con figuration of uronic acid branches in a single molecule of TFP interacts with each other to form intramolecular hydrogen bonds and twist.

Fig. 6 AFM topographic image of TFP1 dispersed in SDS solution (0.1 μg/mL).
3.5 Antioxidant activity of TFP1
3.5.1 Scavenging capacity of hydroxyl radicals
It had been reported that the hydroxyl radical reacts with polyunsaturated fatty acid moieties of cell membrane phospholipids to produce lipid hydroperoxide, which can be decomposed into alkoxy and peroxyl radical. Hydroxyl radicals are dangerous to living organisms, as they candamage macromolecules, such as carbohydrates and amino acids [32,33]. Therefore, it is vital to identify the scavenging capacity of hydroxyl radicals. The hydroxyl radicals scavenging capacity of TFP1 was found to be positively correlated with the linear dose (Fig. 7). The maximum hydroxyl radicals scavenging rates of VC, TFP and TFP1 were 97.22%, 21.13% and 46.39%, respectively. The IC50values of TFP and TFP1 were 9.43 mg/mL and 2.95 mg/mL, respectively. Chen [34]obtained the highest yield of TFP at extraction temperature 100 ℃ which showed good hydroxyl radical-scavenging activities (79.8%)at a concentration of 0.2 mg/mL in the reaction mixture.Wang et al. [35]preparated ATP by alkaline method which showed poorly soluble in water and weak scavenging activity.The carboxymethylation of ATP was carried out showed that CATP4 at the concentration of 1.64 mg/mL exhibited the highest ability (60.2%) to quench hydroxyl radical. Therefore, the results exhibited that TFP1 possessed the outstanding hydroxyl radicals scavenging capacity.

Fig. 7 Scavenging of TFP1 on hydroxyl radical. Values are mean ± SEM (n = 3).
3.5.2 Scavenging capacity of DPPH
The model of scavenging the stable DPPH radical is a widely used method for evaluating the free radical scavenging ability of natural compounds. Fig. 8 showed the DPPH radical scavenging activity of VC, TFP and TFP1 at different concentrations. The DPPH radical scavenging activity of TFP and TFP1 increased dependently with concentrations, and was 21.35% and 37.61% at 2 mg/mL, respectively.Obviously, the scavenging activity of TFP was significantly lower than that of TFP1. However, the scavenging activity of these polymers against DPPH radical was less than that of VC at the same concentrations. Use hot water extraction to afford TPS. In 2016, Liu et al. [36]used hot water extraction to afford TPS. The DPPH radical scavenging activity of TPS was 10.60% at 1 mg/mL.

Fig. 8 Scavenging of TFP1 on DPPH radical. Values are mean ± SEM (n = 3).
3.5.3 Scavenging capacity of ABTS+
When the concentration of TFP and TFP1 was 0.5-2.0 mg/mL,the scavenging rate of ABTS+free radicals increased significantly(P< 0.05) (Fig. 9). With the increase of sample concentration, the ABTS free radical scavenging rate of TFP and TFP1 increased slightly, but the difference between the two was significant(P< 0.05). When the concentration of TFP1 reached 3 mg/mL, the ABTS free radical scavenging rate of TFP1 reached 40.64%, while the scavenging rates of VC and TFP ABTS reached 99.84% and 18.30%.The ABTS free radical scavenging effect of TFP1 was better than TFP. Li et al. [37]in 2014 assessed for the ABTS+radical scavenging activity, of methanol extract subfractions of the edible white jelly mushroom (T. fuciformis). The ABTS+radical scavenging activity of the chloroform subfraction was the highest among all subfractions which reached to 7.89 μmol trolox/mg extract.

Fig. 9 Scavenging of TFP1 on ABTS+ radical. Values are mean ± SEM (n = 3).
3.6 Moisture absorption and retention properties
The moisture absorption and retention properties of glycerin,crude TFP and TFP1 was shown in Figs. 10 and 11. From the figures,the moisture absorption rate (Ra) of all the samples at 81% RH and 43% RH increased significantly in the first 12 h but slowly after that.At the same time, glycerin, crude TFP and TFP1 exhibited the higher moisture absorption rate at 81% RH than 43% RH, which was similar to Wang et al.’s study in 2015 [35]. Comparing the three, the order of moisture absorption rate was: glycerol > TFP1 > crude TFP. Glycerin,crude TFP and TFP1 all contain hydroxyl groups. Glycerin contains more hydroxyl groups per unit mass than the other two substances,so it had the highest moisture absorption rate. Because of crude TFP’s impurities, its moisture absorption rate was lower than that ofTremellaphysically purified polysaccharide TFP1.

Fig. 10 Moisture absorption rate of different samples at RH of 43% (a) and at RH of 81% (b) (n = 3).
Fig. 11 showed the moisture-retention properties of all samples were placed and evaluated in a water-humidified chamber to absorb moisture for 48 h at 43% RH. As time goes by, the moisture retention rate of glycerin, crude TFP and TFP1 all decreased. The moisture retention rate of crude TFP declined the fastest, and the moisture retention rate was the worst among the three. These results indicated the significant moisture-retention capacity of TFP1. In this study, TFP1 showed the significant moisture absorption and retention properties because of the carboxyl group in uronic acid.The capability was better than many other reported polysaccharides,like the hot water extracted polysaccharide from bodies ofT.fuciformis[23]and the alkaline extraction from fromT.fuciformis[35].TFP was reported due to its functions in cosmetics in previous study.It may be due to that the uronic acid and hydroxyl group in TFP1 structure could form the hydrogen bond with H2O, that contributed to moisture preservation. Another reason could be that the flexible chains of TFP intertwined with each other creating a spatial network in solution to enhance water retention. Based on this, TFP1 may have a use as a possible supplementin the food, pharmaceutical and cosmetic industries.
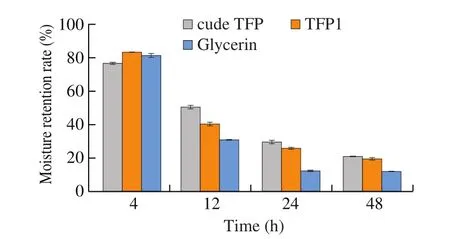
Fig. 11 Moisture retention by samples at RH = 43% (n = 3).
4. Discussion and conclusions
The different extraction methods to prepare polysaccharides have differences in its physical and chemical properties and biological activities [14,38]. The biological activity of polysaccharides is affected by their structural characteristics and solution conformation [23,39,40]. In recently, there has been an increasing interest in the utilization and study of TFP, particularly the establishment of structure-function relationships, for various novel applications owing to their biocompatibility, biodegradability, and non-toxicity, and also for some specific therapeutic activities.
In summary, we extracted the polysaccharide from fruit bodies ofT. fuciformisin Gutian of China with enzymatic combined with wet beating, and explored the chemical structure antioxidant activities and moisture absorption and retention properties of purified TFP1. Our results demonstrated that TFP1 was an acidic heteropolysaccharide consisting offive monosaccharide groups of mannose, xylose, fucose,glucose, and glucuronic acid with mannose as the main chain and theMwwas 5.8 × 103kDa. AFM observation of TFP1 showed that polysaccharide molecules formed cyclic and chain-like structures on the mica sheet. The polysaccharide chains were entangled with each other, and there are multiple branches, forming a local accumulation.SDS surfactant treatment eliminates the influence of some intermolecular hydrogen bonds. A clearer cyclic and chain structure of the polysaccharide molecular chain through local magnification and different degrees of molecular entanglement and aggregation on the polysaccharide chain were observed.
These results provided further guidance on the structure-function potential relationship of polysaccharides in the food industry.Moreover, the TFP1 has shown good moisture-preserving capacityin vitroand high DPPH, ABTS+and hydroxyl radical scavenging activities. Thus, TFP extracted by enzymatic combined with wet beating may be applied as a possible supplementin the food,pharmaceutical and cosmetic industries.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgement
We gratefully acknowledge the the Open Project Program of National R & D Center for Edible Fungus Processing Technology(20200110).
- 食品科學(xué)與人類健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study

