Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study
Junjie Lin, Hun Xing, Dongxio Sun-Wterhouse, Chun Cui,b,*, Wei Wng
a College of Food Science and Technology, South China University of Technology, Guangzhou 510640, China
b Guangdong Wei-Wei Biotechnology Co., Ltd, Guangzhou 510640, China
Keywords:
Seabuckthorn seed meal
Protein extraction
Deep eutectic solvent
Alkaline extraction and acid precipitation
Amino acids
A B S T R A C T
Seabuckthorn seed meal (SSM) is a waste of oil extraction industry that rich in protein. In order to seek suitable protein extraction method, three different deep eutectic solvents (DESs) (including choline chlorideglycerol, choline chloride-oxalic acid and choline chloride-urea) were developed for extracting protein from SSM and compared with alkaline. Result indicated that alkaline could effectively extract 56.9% protein from SSM and its protein content was 73.1%, higher than DES at 31.0%-41.4% and 64.3%-67.5%, respectively.However, compared to alkali, DES led to a product with less β-sheet, more β-turn, more essential amino acids,higher total amino acid content, especially choline chloride-urea which extracted protein showing an integrated and similar protein weight distribution compared to SSM. Also, this protein extracted chloride-urea showed a highest digestibility in vitro (by pepsin) (54.2%). These results indicated that choline chloride-urea extraction is better than alkaline extraction for SSM.
1. Introduction
Seabuckthorn (Hippohae rhamnoidesL.), belongs to the family Elaeagnaceae. Seabuckthorn berries have attracted growing attention due to their medicinal activity (such as anti-stress, anti-atherogenic,anti-tumor, immunomodulatory, hepatoprotective, radioprotective,anti-microbial and tissue regeneration) and nutritional potential with abundant intrinsic bioactive substances (such as proteins, lipids,polyphenols and vitamins) [1]. In particular, seabuckthorn seeds contain a relatively high protein content (37.79%), and are considered as a great source of complete protein (total amino acid content in the protein is approximately 83%, with about 68% being essential amino acids) [2]. It has been demonstrated that seabuckthorn seed proteins (SSP) has significant hypoglycemic and anti-inflammatory effects [3]and anti-diabetic function [2,4]. The perceived benefits of seabuckthorn consumption and plant-based proteins (including the under-researched SSP) motivates this current study to utilize seabuckthorn seed meal (SSM), which is the by-product of seabuckthorn seeds after oil extraction and often contains a protein content up to 20% [5].
To maximally extract the naturally occurring bioactive substances from novel food materials and retain their desired bioactivities for further nutraceutical or functional food applications, the development of an effective, industrially feasible and eco-friendly extraction method is particularly important. At present, SSP extracted from SSM is mainly through alkaline extraction (pH 11, 60 °C, in water)and acid precipitation (at pH 5) after the removal of lipids (using petroleum ether) and flavonoids (using ethanol) from seabuckthorn seeds [3]. Such an alkaline extraction-acid precipitation procedure is the conventional approach for extracting proteins [6]. However,the heavy use of acid, alkali and water in this approach has caused environmental problems and operational difficulties [7,8]as well as product quality issues (such as pH-induced protein denaturation and dissolution of other unwanted substances in protein extracts [6,9].Deep eutectic solvent (DES), an eutectic mixture of two or three inexpensive and self-associated components with a melting point lower than that of each component, is regarded as an environmentfriendly and green solvent for effective extraction of bioactive compounds from plant materials [10,11]. The feasibility of tuning the properties of DESs (including conductivity, density, freezing point and viscosity) according to their applications via selecting proper biodegradable/recyclable hydrogen bond donor (HBD) and hydrogen bond acceptor (HBA) makes DESs even more attractive [12].Recently, DESs have been used to extract proteins from oilseed cakes,cod skins and brewer’s spent grain included chloride-glycerol (ChCl-Gly), choline chloride-oxalic acid (ChCl-OA), and choline chlorideurea (ChCl-urea), respectively [8,13,14]. The results obtained in these studies suggested the selectivity of DESs to proteins during extraction.Published studies, though limited in number and preliminary in their conclusions, have shown that the structural characteristics of the DES-treated protein products may or may not be altered,depending on the type of DES and other conditions (including matrix composition of the raw material, pH of the mixture, temperature and pressure). Accordingly, it is of high interest to investigate further how the extraction with DES affects the physicochemical and nutritional characteristics of the extracted proteins when compared with the conventional protein extraction method.
This research aimed to make a contribution to the above-mentioned knowledge gaps. Three different DESs (ChCl-Gly, ChCl-OA, and ChCl-urea) were prepared for extracting proteins from SSM along with alkali. The obtained SSM proteins (SSMPs) were characterized by solid state13C nuclear magnetic resonance (NMR) and Fourier transform infrared spectroscopy (FTIR) as well as sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). To our knowledge, this is the first report on the extraction of proteins from SSM using different DESs, and the differences in the characteristics of the extracted proteins.
2. Materials and methods
2.1 Materials
SSM were obtained from Qinghai KangPu biotechnology Co.,Ltd. (Qinghai, China), which was a product of seabuckthorn seed after extracting oil by supercritical CO2at 40 °C. Choline chloride (≥98.0%) was purchased from Shanghai Source Biological Technology(Shanghai, China). SDS-PAGE Gel Quick Preparation Kit (P0012AC),protein standard markers (275 kDa) and Coomassie Blue Fast Staining Solution (P0017) were purchased from Shanghai Beyotime Biotechnology Co., Ltd. (Shanghai, China). All other chemical reagents used were of analytical grade and purchased from CapitalBio Corporation (Shanghai, China).
2.2 Sample preparation
SSM powder was obtained by pulverization of SSM with a pulverizer, and the resulting particles then passed through a 40 ×sieve. DES was prepared by mixing choline chloride separately with glycerol, oxalic acid or urea at a molar ratio of 1 : 2, 1 : 1 and 1 : 2,respectively, under continuous stirring at 60 °C until the mixtures became clear and colorless liquids.
2.3 Protein extraction from SSM powder
2.3.1 Alkali extraction
Alkali extraction of SSMP referred to Yuan et al. [3]with a slight modification. Brie fly, 50 g of SSM powder was mixed with distilled water at a mass ratio 1 : 9 and the pH of the water solution stabilized at pH = 11 by 1 mol/L NaOH solution, before a 3 h incubation at 60 °C and a shaking speed of 250 r/min. These resulting mixtures were centrifuged at 10 000 ×gand 25 °C for 10 min. The supernatants were collected and adjusted to pH 5 using 1 mol/L HCl solution. The precipitate was obtained via centrifugation under the above-described centrifugation conditions, and then washed using distilled water until its pH reached neutral. Finally, the neutralized precipitate was freezedried for 48 h and this dried protein-rich fraction was regarded as SSMP-A.
2.3.2 DES extraction
DES extraction of SSMP referred to previous methods with a slight modification [8,13,14]. 50 g of SSM powder was mixed with DESs at a mass ratio 1:9 and incubated at 60 °C for 3 h with a shaking speed of 250 r/min. These resulting mixtures were centrifuged at 10 000 ×gand 25 °C for 10 min. The obtained DES fractions were mixed with ethanol (ChCl-OA) or distilled water (ChCl-Gly and ChCl-urea) at a mass ratio of 1 : 40. The precipitate generated was obtained via centrifugation under the above-described centrifugation conditions, and then washed using distilled water until its pH reached neutral. Finally, the neutralized precipitates were freeze-dried for 48 h and the SSMP fractions generated with ChCl-Gly, ChCl-OA or ChCl-urea termed SSMP-G, SSMP-O, and SSMP-U, respectively.
2.4 Determination of crude protein content and recovery
Crude protein content was determined according to the previous method with a minor modification [15]. The nitrogen content of protein was measured by the Dumas combustion method using a TruMac N system (Leco Corporation, St. Joseph, MI, USA). Crude protein content in a sample was calculated using a protein factor of 6.25 to convert the nitrogen content to protein content. Crude protein recovery was calculated as followed:
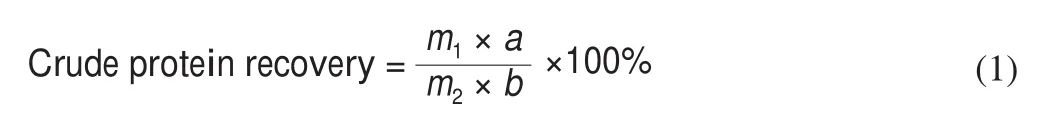
In this equation:m1,a,m2,brepresents SSMP fractions, crude protein content of SSMP fractions, SSM mass, crude protein content of SSM, respectively.
2.5 SDS-PAGE
SDS-PAGE was performed following the method of Grudniewska et al. [13]with some modifications. Brie fly, 1.5 mg of a sample was suspended in 1 mL of distilled water, and 50 μL of this solution was mixed with 200 μL of the sample buffer. The mixture was boiled for 10 min. An aliquot (20 μL) of protein standard markers and the prepared samples were loaded onto the gel (15% separating gel and a 5% stacking gel). The gel was run at 80 V, and stained with Coomassie Blue Fast Staining Solution before being destained with distilled water.
2.6 Amino acid composition analysis
The amino acid composition of SSMP was analyzed according to the method of Zhang et al. [16], using an automatic amino acid analyzer (membra Pure A300, Germany). Brie fly, SSMP fractions (15 mg) were hydrolyzed with 6 mol/L HCl solution for 24 h in vacuum hydrolysis tubes prior to analysis. The results were expressed as“Percentage of each amino acid to the total amino acids or protein”.
2.7 In vitro protein digestibility
In vitroprotein digestibility was preliminarily evaluated by the pepsin digestibility assay described by Zhang et al. [16]with a slight modification. SSMP fractions (0.2 g each) were dispersed in 20 mL of distilled water before pH adjustment to 2 with 0.5 mol/L HCl solution. Then, 4% pepsin (m/m, based on protein) was added. The resulting mixture was incubated for 1 h at 37 °C with a shaking speed of 150 r/min, before the enzyme was deactivated in a boiling water bath for 10 min. These mixtures were centrifuged at 10 000 ×gand 4 °C for 10 min, and the supernatants were collected for the measurement of protein content by the Dumas combustion method using a TruMac N system (Leco Corporation,St. Joseph, MI, USA). Protein digestibility was evaluated using the following equation:

2.8 FTIR analysis
SSMP samples were mixed with KBr and the obtained mixtures were pressed into a tablet. FTIR spectra of the samples were acquired in the range of 400-4 000 cm-1and at a resolution of 4 cm-1(with 32 total scans), using a Vertex 70 FT-IR spectrometer(Bruker Corporation, Billerica, MA, USA). The FTIR spectral data was analyzed using Ominc 8.0 software (Thermo Fisher Nicolet,America). The relative amounts of secondary structures of four SSMPs were calculated according to relative areas of the resolved amide I region by means of Peakfit 4.0 (SPSS Inc.). The relative contents ofα-helix,β-sheet,β-turn, and random coil in SSMP were determined according to the peak area at 1 650-1 658, 1 610-1 640,1 660-1 700, and 1 640-1 650 cm?1, respectively [17].
2.9 Solid-state 13C cross polarization-magic angle spinning(CP-MAS) NMR spectroscopy
Solid-state13C CP-MAS NMR spectroscopy was used to evaluate SSMP fractions according to the method of Grudniewska et al. [13].The NMR spectra was acquired using a 400 MHz Bruker Avance III HD model spectrometer operated under the following conditions:Spinning rates (12 000 ± 2) Hz, contact time 1 ms, optimized recycle delays 5 s, numbers of scans 0-280 MHz. Chemical shifts were reported with respect to TMS.
2.10 Statistical analysis
All experiments were performed in triplicate, unless indicated otherwise. The results were reported as “mean ± standard deviation”,with the differences from the mean consisted of analysis of variance(ANOVA) with Duncan’s multiple range test in SAS 8.0 (SAS institute Inc. USA).P< 0.05 was considered as significant.
3. Results and discussion
3.1 Crude protein content and recovery of SSMP fractions
The crude protein content of SSM was 30.9% (date not shown), which was higher than that reported previously for SSM,probably due to the differences in the material source, handling and processing [18]. This crude protein content in SSM was relatively high, as compared with some other food wastes (such as rubber seed meal, 26%; jatropha kernel meal, 27.2%; palm kernel meal,16.6%), indicating the potential of SSM as a very attractive low-cost protein source [18,19]. Crude protein contents of SSMP fractions exceeded more than two-fold that of SSM: 73.1% for SSMP-A,64.3% for SSMP-G, 65.0% for SSMP-O and 67.5% for SSMP-U and corresponding protein recovery were 56.9%, 41.4%, 31.0% and 32.3%, respectively (Fig. 1). These results suggested that these four protein extraction methods were effective in extracting proteins from SSM, especially alkaline method. The extracting effect ChCl-Gly and ChCl-urea (higher the crude protein content and recovery) were better than previous studies which extracted proteins from oilseed cakes and Brewer’s spent grain, respectively [8,13,14]. However,pervious study reported the effect of ChCl-urea was similar even better than alkaline method (pH = 11) in extracting protein from brewer’s spent grain, press cake and wheat bran, opposite with this study [8]. It might be related to the high solid-liquid ratio (10%) in our study, resulting in a mass of DES to adhere to solid residue after centrifuge and thus decreased protein recovery. Wahlstr?m et al. [8]reported that the protein yields decreased with 10%-13%-points with the increase of solid-liquid ratio (ChCl-urea as solvent)from 5% to 10%. In addition, the different acidity and properties of oxalic acid, glycerin and urea could cause different protein loss at washing process and further affect protein recovery. On the other hand, DES extracted SSMP showed different colour(as shown in supplemented Fig.1), particularly SSMP-U which showed a fossil gray and a higher acceptability compared to dark brown SSMP-A which was reported to be related to the lignin content [20]. This might be due to that alkaline was not selective and easy to caused carbohydrates and lignin dissolution together with protein recovery [9]. Therefore, these results indicated DES could be regarded as an effective solvent for extracting protein from SSM, especially ChCl-urea.
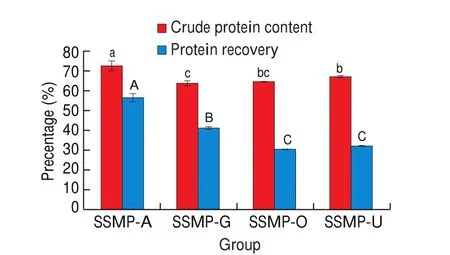
Fig. 1 Comparison of crude protein contents and recovery of SSMP-A,SSMP-G, SSMP-O and SSMP-U. Different letters (a, b, c; A, B, C) indicate significant differences (P < 0.05).
3.2 SDS-PAGE
SDS-PAGE was used to estimate the molecular weights of proteins in SSM and SSMP fractions. As shown in Fig. 2, SSM had proteins in the relative molecular weight range of 11-75 kDa, with the highly intense bands distributed around 17, 25, 30, 35, 48, and 63 kDa. SSMP-U had similar bands to SSM, whilst the band around 63 kDa was absent for SSMP-A and very weak for SSMP-G and SSMP-O.It might be due to that alkaline pH could degrade some amino acids and disul fide bonds, and thus resulting in a decrease of molecular weight [21].Moreover, there were unseparated bands (indistinguishable units)at 11-35 kDa for SSMP-O. This might be due to high acidity of ChCl-OA resulted in change of protein structure. The similar result was observed at high-temperature treatment and high concentration alkali treatment for extracting protein from evening primrose cake protein and rice residue, respectively [6,13]. These results indicated that ChCl-urea was a better method which successfully extract and preserve the native protein units in SSM.

Fig. 2 SDS-PAGE of Raw material, SSMP-A, SSMP-G, SSMP-O and SSMP-U. Column A: Standard markers; Column B: raw material (SSM);Column C: Column SSMP-A; Column D: SSMP-G; Column E: SSMP-O;Column F: SSMP-U.
3.3 Amino acid component of SSMP
The four extracted SSMP fractions had similar percentages for the total amino acid content in protein (Table 1). These SSMP fractions had acidic, basic, hydrophilic, and hydrophobic amino acids in the ranges of 35.60%-38.35%, 22.33%-23.32%, 66.83%-68.81% and 26.47%-28.67% (in total amino acid), respectively, with relatively high contents of glutamic acid (~26%), aspartic acid (~11%) and arginine (~17%), a typical characteristic of most seed storage proteins.The detected values of SSMP herein were even higher than those for many storage proteins of seeds such asCamelliaseed cake and chickpea [22-24], and was similar to the amounts of essential amino acids recommended by World Health Organization (WHO) (except for lysine and sulphur-containing amino acids) [25]. Seabuckthorn protein is a good plant protein, and the SSMP products generated in this study are of high nutritional value.
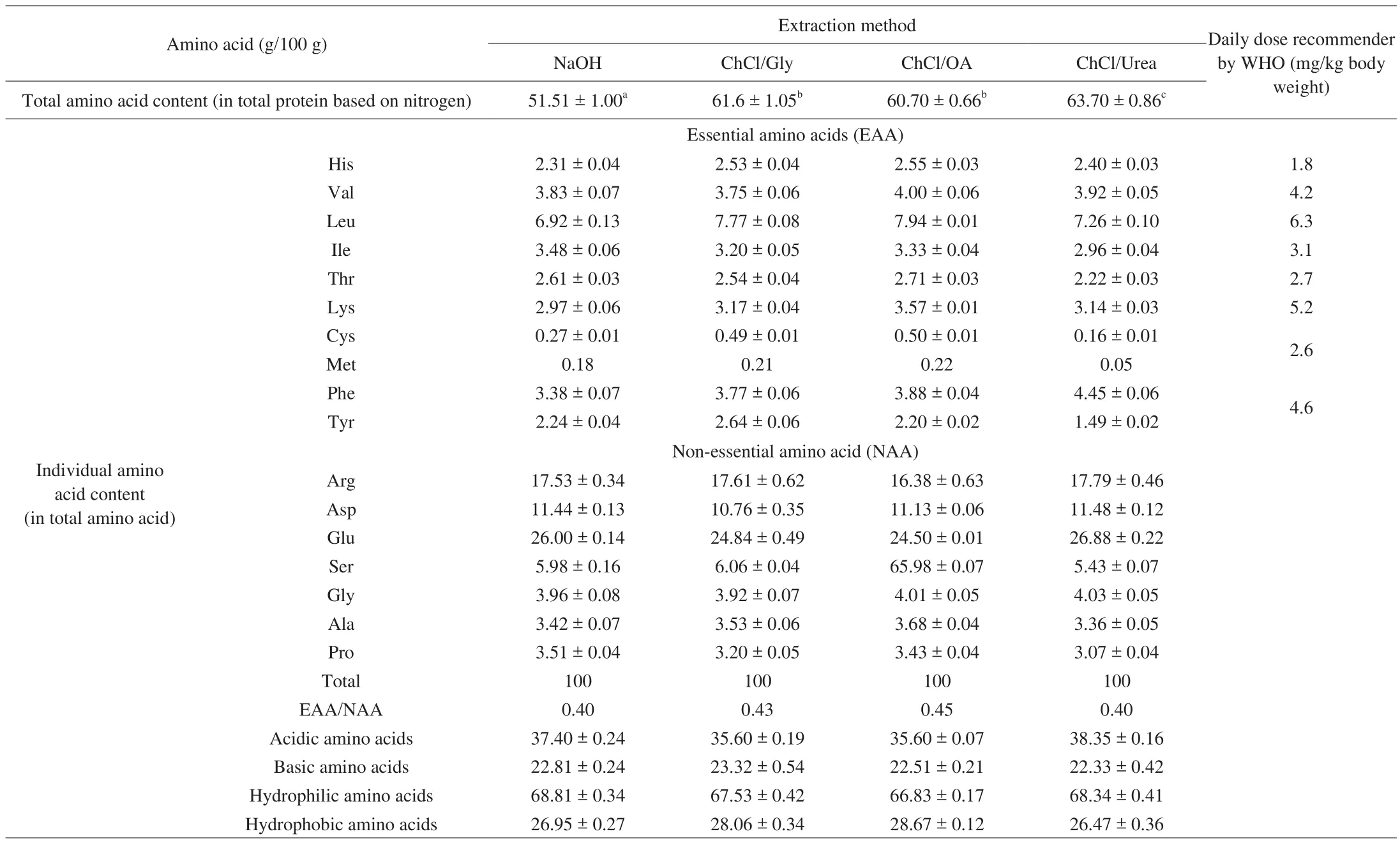
Table 1Amino acid composition of the different sea buckthorn protein products.
It is well-known that Kjeldahl method provided information about total nitrogen compounds including proteins nitrogen (amino acids)and nonproteins nitrogen [18]. The fact that SSMP-A had a much lower content of total amino acids content in total protein based on nitrogen,compared with the other SSMP products (Table 1) indicated the presence of abundant non-protein nitrogen in SSMP-A. Hou et al. [6]reported that solubilization of proteins with alkali could cause dehydrogenation and desulfurization of -OH and -SH, and further interacted with lysine residues (to form new amino acids such as lysinoalanine), leading to the decreases in contents of some amino acids. The relative low contents of lysine and cysteine and low essential amino acid (EAA)/non-essential amino acid (NAA) ratio for SSMP-A further indicated the reduced nutritional value of SSMP-A as compared with the other SSMP products produced using DESs.Accordingly, DES extraction might be advantageous over the alkaline extraction for SSM.
3.4 Preliminary evaluation of in vitro protein digestibility
Protein digestibility is an important index to evaluate the nutritional value of protein [26]. As shown in Fig. 3, thein vitrodigestibility (evaluated by the pepsin digestibility assay) of the SSMP fractions decreased in the order of SSMP-U (54.2%) >SSMP-A (42.3%) > SSMP-G (35.6%) > SSMP-O (25.5%) (P< 0.05).These results indicated that SSMP-U might have good digestibility(> 50%), which was similar to the digestibility of soy isolate protein(56%) and higher than rice residue protein isolates (< 50%) [16,27].The highest digestibility of SSMP-U indicated that the seabuckthorn proteins extracted by ChCl-urea might be easier degraded in gastric juice. This result might be attributed to the lower content of ordered structures (likeβ-sheet), in agreement with the result of secondary structure. Previous reported that the decrease ofβ-sheet content has been associated with a linear increase of digestibility [28]. However,the contradictory result was observed at SSMP-G and SSMP-O with a lowerβ-sheet content and digestibility than SSMP-A. These could be due to the presence of phenolics in SSMP-G and SSMP-O fractions,such as proanthocyanidins which could inhibit digestive enzymes activityin vitro[29]. Previous study reported that SSM is a biomass rich in anthocyanins and ChCl-OA and ChCl-Gly were effective extraction solvent for anthocyanins [30,31].

Fig. 3 Preliminary evaluation of in vitro protein digestibility of SSMP-A,SSMP-G, SSMP-O and SSMP-U. Different letters indicate significant differences (P < 0.05).
3.5 SSM and SSMP composition and structural analysis
Solid-state13C CP-MAS NMR spectroscopy has been used to evaluate the composition and structure characteristics of biomass. As shown in Fig. 4, the spectra of SSM (raw material) and SSMP fractions roughly resembled, indicating the similarity in overall composition and chemical structures among these samples (in the main carbon atom signals of proteins: (172-174, 131-155, 65?48, and 16-58) ×10-6,corresponding to carbonyl carbon (C=O) in the peptide bond,aromatic carbons of amino acids,α-carbons in protein, and aliphatic amino acids, respectively [13,37,38]. For protein-rich samples, the band at (20-25) × 10-6has been assigned for LeuCδ, LeuCγ, ArgCγand/or Val Cγ, 30 × 10-6for ProCβand/or Val Cβ, 39 × 10-6for GlyCα, LeuCβ, IleCβand/or TyrCβ, 43 × 10-6for Gly Cα, 54 × 10-6for LysCα, SerCα, ArgCα, LeuCαand/or Ala Cα), 62 ppm for ThrCαand/or SerCβ, 69 ppm for ThrCβ, (72-73) × 10-6HyProCγ, 108 × 10-6for spinning side bands arising from the carbonyl group, 132 × 10-6for Tyr Cδand/or CysCδ, and 154 × 10-6for TyrCεor ArgCε[39,40].However, differences in the actual location, intensity and shape of some spectral peaks among these SSM and SSMP fractions are still significant, especially the signal peaks around (172-174, 105-108,72, 62 and 54) × 10-6(assigned toβ-,γ- andδ-carbons) of SSM being quite different from those of the SSMP fractions. This might be attributed to the different conformation and amino acid content of protein from different extraction method.

Fig. 4 Solid-State 13C CPMAS NMR spectra of raw material (SSM).
In terms of the signal around (172-174) × 10-6(assigned to the carbonyl carbon C=O in the peptide bond, a slight shift was observed suggesting that some changes in protein secondary structures might have taken place: SSM (172 × 10-6), SSMP-A (172 × 10-6), SSMP-G(174 × 10-6), SSMP-O (174 × 10-6), and SSMP-U (174 × 10-6) (172 × 10-6and 174 × 10-6are normally associated with proteins inβ-sheet, and disordered/random coil structures, respectively) [41]. For solid-state13C CP-MAS NMR spectroscopy, the resonance of COO–, COOH and COOCH3differ slightly: (175-176) × 10-6, 173 × 10-6, and (170-172)× 10-6, respectively [40]. Accordingly, the small shift around(172-176) × 10-6might re flect the different effects on SSM (alkaline or DESs), causing some alterations in protein secondary structures.Compared to SSM, SSMP-A had a much higher intensity at 172 × 10-6,probably due to the higher protein content of SSMP-A. The similar result was observed by Pizzoferrato et al. [42].
Additionally, it is worth to note the contribution from other non-protein substances in SSM and SSMP fractions (given their protein content < 71%). For example, the characteristic signals at(108-106) × 10-6and 54 × 10-6also represented the arabinan C-1 and cellulose C-1, and CH3O of lignin respectively. Compared to SSM, all SSMP showed a decrease at 105 × 10-6(even disappear) and increase at 54 × 10-6. This might be attributed to the presence of lignin at SSMP, especially for SSMP-A. Pan et al. [43]reported that protein might be tightly attached to alkaline lignin, thus, they were extremely difficult to be separated. DESs such as ChCl/Gly may co-extract substances like lignins and hemicelluloses [13,44]. The specific composition of SSMP might be further studied.
3.6 Protein secondary structure analysis by FTIR
FTIR is a well-established technique to track the changes on protein’s functional groups and conformational characteristics. As shown in Fig. 5A, the FITR spectra roughly resembled, containing signal peaks mainly around 3 442, 2 960, 1 642, 1 539 and 1 233 cm-1;(corresponding to the bending vibrations of free and bound OH and NH groups (amide A), asymmetric -CH2and symmetric -CH3and-CH2stretching (amide B), C=O stretching (amide I), N-H bending and C-N stretching (amide II), and C-N stretching and N-H vibration(amide III), respectively [32]. These results indicated that these extraction methods had very similar effects on the peptide bonds of seabuckthorn proteins. In terms of amide-I (the most sensitive spectral region that shows the changes in protein secondary structures),significant differences were found in the overall peak area and the ratio of amide A to amide B, with SSMP-A having the smallest amide-B. This indicated that SSMP obtained by alkali and DES might have different conformation.
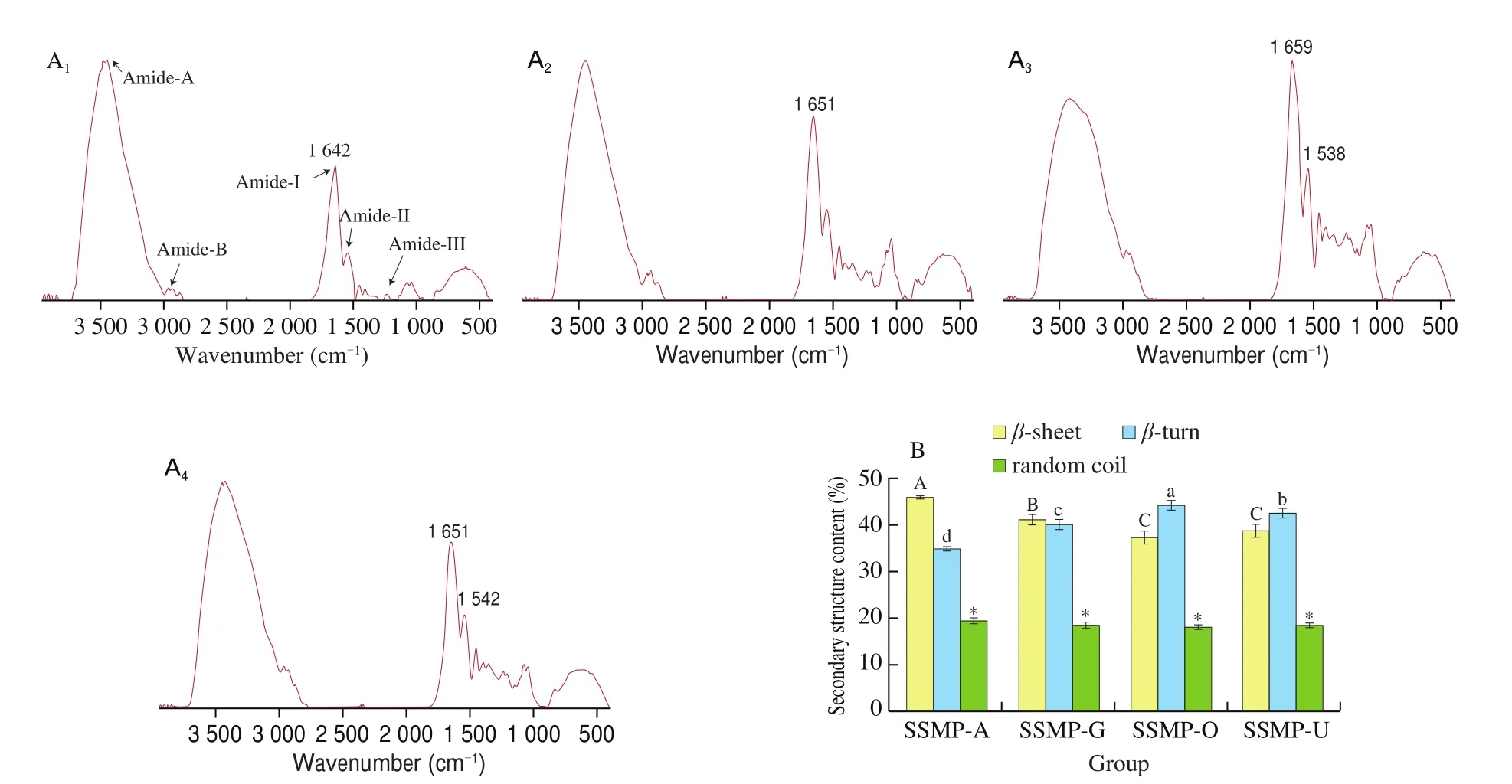
Fig. 5 FTIR analysis of SSMP-A, SSMP-G, SSMP-O or SSMP-U: (A) FTIR spectra; (B) Secondary structure content (%). A1, SSMP-A; A2, SSMP-G;A3, SSMP-O; A4, SSMP-U; Different letters (a, b, c; A, B, C) and asterisk (*) indicate significant differences (P < 0.05).
According to the result of amide-I, further determination resolved three types of secondary structure in all SSMP includingβ-sheet,β-turn, and random coil (Fig. 5B). Compared to SSMP-A, SSMP extracted by DESs (SSMP-G, SSMP-O and SSMP-U) had higherβ-turn, lowerβ-sheet.β-sheet structure was mainly stabilized via hydrogen bonding formed between -C=O and -NH2groups of the same or adjacent peptide(s) [33].β-turns impose restrictions on the conformational entropy of peptide chain, formed by a direct hydrogen bond between the -C=O of the first residue and -NH of the fourth residue [34]. The decrease ofβ-sheet and increase ofβ-turn increased protein structure flexibility and the amount of unordered structure [28,35]. These results suggested that the current extraction methods could cause small but significantly different changes in the secondary structures of seabuckthorn proteins, leading to modification of the proportions of these secondary structures. This might be due to different protein extraction mechanism of alkaline and DES. Alkaline extraction of protein was mainly based on electrostatic repulsion under high pH which promote exposure of hydrophobic group and protein aggregation by hydrophobic interaction [36]. Also, these hydrophobic groups would be further strengthened by the electrostatic attractions under isoelectric point (pH = 5, protein precipitate), and thus leading to a tight protein structure [36]. Protein extraction by DES mainly depended on formation of hydrogen bond between DES and amino or carboxyl groups of protein and different DESs showed different the binding amino acid. Bai et al. [14]found that the of ChCl-OA could effectively extract collagen peptides from cod skins by forming hydrogen bond between ammonium salt and imino in collagen. This mechanism was completely different with alkaline extraction which can’t form hydrogen bond, and thus obtaining loose protein by DES method.
4. Conclusion
This study showed that DESs (ChCl-Gly, ChCl-OA and ChClurea) as green solvent could effectively extract proteins from SSM.Compared to the conventional extraction method using alkaline,ChCl-urea could successfully extract and preserve the native protein units in SSM raw material. Also, ChCl-urea extracted protein showed a higher content of total amino acids, a higher EAA/NAA ratio and a better digestibilityin vitrothan alkali extracted SSM product.Accordingly, ChCl-urea might be considered as an alternative method for extracting the proteins from SSM.
Conflict of interest
The authors declare there is no conflict of interest.
Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (No. 31201416) and Science and Technology Research Program of Guangdong Province(No. 2017A01010502).
- 食品科學與人類健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Isolation of a novel characterized Issatchenkia terricola from red raspberry fruits on the degradation of citric acid and enrichment offlavonoid and volatile profiles in fermented red raspberry juice

