Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
Li Zeng, Fngwei Zhong*, Zhiling Chen, Gengxi Li, Qi Zhu,*
a School of Pharmacy, Shaoyang University, Shaoyang 422000, China
b School of Laboratory Medicine, Shaoyang University, Shaoyang 422000, China
Keywords:
Polygonatum sibiricum polysaccharide
High-fat diet
Obesity
Non-alcoholic fatty liver disease
Lipid metabolism
Rat
A B S T R A C T
Polygonatum sibiricum is a traditional medicinal and dietary plant of the family Liliaceae. The main functional macromolecules of P. sibiricum are polysaccharides, which function in antioxidation and regulating immunity.Previous studies have shown that insulin resistance (IR), oxidative stress, and inflammation are important factors in the induction of lipid metabolic diseases such as obesity. Therefore, in this study, we established a high-fat diet-induced rat model of obesity and nonalcoholic fatty liver disease (NAFLD) to explore the potential protective effect of P. sibiricum polysaccharides (PSPs) and the mechanisms behind it. After 4 weeks of high-fat diet feeding to induce obesity, the rats were treated with different doses of PSP solution or distilled water for 6 weeks. Compared with untreated obese rats, PSP-treated obese rats showed a decrease in body weight, serum total cholesterol, triglyceride, and low-density lipoprotein cholesterol levels, hepatic aspartate aminotransferase and alanine aminotransferase activity, hepatic malondialdehyde content, and hepatic levels of the pro-inflammatory factors tumor necrosis factor-α, interleukin-1β, and interleukin-6, as well as increased serum high-density lipoprotein cholesterol levels and hepatic superoxide dismutase, catalase,and glutathione peroxidase activity. Pathological analysis and immunoblotting of the liver tissues indicated that mechanistically, PSPs reduced obesity and NAFLD in rats by upregulating insulin receptor expression,increasing adenosine monophosphate-activated protein kinase phosphorylation, and downregulating sterol regulatory element-binding protein 2 and low-density lipoprotein receptor expression, thus promoting lipid metabolism, decreasing body weight, and reducing inflammation and oxidative stress caused by lipid accumulation. Based on these results, PSPs may have the potential to reduce obesity and NAFLD associated with a high-fat diet.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is characterized by liver steatosis that occurs without a history of alcohol consumption [1].Obesity significantly increases the incidence of NAFLD [1,2]; with the accelerated pace of modern life and changes in eating habits, the incidences of chronic diseases such as obesity are increasing rapidly,creating global public health concerns [3]. In people with obesity,NAFLD is more prone to eventually develop into non-alcoholic steatohepatitis, liver cirrhosis, and even hepatocellular carcinoma [4].Therefore, identifying healthy, safe, and effective dietary regimens and drug treatments to improve obesity-induced NAFLD is a public health priority [5,6].
The body’s metabolic system contains a variety of complex nutritional metabolic pathways, including the insulin-glucagon system, which regulates glucose levels. However, in case of nutritional imbalance, a disturbance of the interaction between glucose and insulin is notable in metabolic syndrome. Insulin can no longer promote muscle glucose uptake, and gluconeogenesis is inhibited in the liver, resulting in decreases in cell function and insulin resistance (IR) [7]. During IR development, the decomposition and resynthesis of peripheral fat by hepatocytes increases hepatic fat levels, which results in more degeneration and a subsequent increase in lipid peroxidation. IR also plays an important role in inducing obesity-associated NAFLD and is associated with its pathogenesis [8]. According to Méndez-Sánchez et al. [9], NAFLD is a dynamic process that occurs at the intersection of metabolic changes in the liver and its periphery. During this process, hepatic steatosis and IR are mutually enhanced.
Liver triglycerides accumulate in hepatocytes in large amounts,which induces liver inflammation, fibrosis, and cirrhosis through the actions of inflammatory cytokines and oxidative stress (known as the“second strike theory” of NAFLD pathogenesis) [10]. Chen et al. [11]predicted that oxidative stress is the main factor influencing liver injury and disease progression in NAFLD. Reactive oxygen species disrupt cellular homeostasis, which promotes metabolic dysfunction and non-adaptive inflammatory responses. During oxidative stress,reactive oxidation products accumulate, reducing the stability of cellular proteins and eventually leading to hepatocyte fibrosis [12].Given the close relationship between the insulin receptor (INSR) and IR [13], it is important to study the potential mechanisms by which natural products affect lipid metabolism disorders such as obesityinduced NAFLD by examining the expression levels of INSR and oxidative stress-related and inflammatory factors.
Polygonatum sibiricumpolysaccharides (PSPs) are the main functional ingredients ofP. sibiricum, a traditional medicinal and dietary plant of the family Liliaceae, and it is also the only quality control component stipulated in the 2015 edition ofChinese Pharmacopoeia[14]. Previous studies have shown that PSPs have a complex structure, which is mainly composed of galactose,arabinose, rhamnose, xylose, glucose and so on [14]. PSPs exhibit strong antioxidant activity bothin vivoandin vitro[14,15]. It can reduce blood lipids in mice by promoting lipid metabolism through the PPARs/SREBP-1c pathway [16], and strengthen immunity by inhibiting the increase of pro-inflammatory factors (tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, etc.) caused by external adverse stress [17,18]. These activities are closely related to the prevention and treatment of obesity and NAFLD; however,studies on the treatment of related diseases with PSPs have not been conducted. In this study, a rat model of obesity was established by feeding of a high-fat diet (HFD), and the effects and potential molecular mechanisms of PSPs on improving obesity and obesityinduced NAFLD were explored by analyzing body mass, blood lipid parameters, hepatic enzyme functions, oxidative stress, inflammatory factors, and pathology to provide a basis for developing PSP-based functional products.
2. Materials and methods
2.1 Materials and reagents
PSPs (batch number: J001982) were purchased from Shanghai Jianglai Industrial Co., Ltd. (Shanghai, China). As described previously [16,17], the total sugar content of polysaccharides was determined using the phenol-sulfuric acid method. Using glucose as a standard reference substance, absorbance as the ordinate and polysaccharide concentration as the abscissa, the absorbance was determined with an ultraviolet spectrophotometer (Shimadzu Co.,Ltd., Japan) at a wavelength of 493 nm. The standard curve equation wasY= 0.047 1X+ 0.024 6 (r= 0.999 7). After being treated using the water-soluble alcohol precipitation method, the mass fraction of PSPs was 90.4%. Triglyceride (TG; A110-1-1), total cholesterol (TC;A111-1-1), high-density lipoprotein-cholesterol (HDL-C, A112-1-1),low-density lipoprotein-cholesterol (LDL-C; A113-1-1), alanine aminotransferase (ALT; C009-2-1), aspartate aminotransferase (AST;C010-2-1), superoxide dismutase (SOD; A001-3-2), malondialdehyde(MDA; A003-1-2), catalase (CAT; A007-1-1-1), and glutathione peroxidase (GSH-Px; A005-1-2) were purchased from Nanjing Jiancheng Institute of Biological Engineering (Nanjing, China).Enzyme-linked immunosorbent assay kits for detecting TNF-α (E-ELR2856c), IL-1β (E-EL-R0012c), IL-6 (E-EL-R0015c) were purchased from Wuhan Boshi Biological Co., Ltd. (Wuhan, China). The BCA protein quantification kit (item no. P0010) and antibodies against glyceraldehyde 3-phosphate dehydrogenase (GAPDH; AF0006),INSR (AF7287), adenosine monophosphate activated protein kinase(AMPK; AF6195), phosphorylated (p-)AMPK (AF5908), and lowdensity lipoprotein receptor (LDLR; AF1438) were purchased from Shanghai Biyuntian Biotechnology Co., Ltd. (Shanghai, China). The anti-sterol regulatory element binding protein-2 (SREBP-2) antibody was purchased from Abcam (ab30682; Cambridge, UK). Secondary antibodies (CSB-PA573747, CSB-PA00220E1Rb) were purchased from Wuhan Huamei Biological Engineering Co., Ltd. (Wuhan,China). ECL luminescent agent (item no. 29050) was purchased from Beijing Yingeen Biological Co., Ltd. (Beijing, China). All other reagents were of pure analytical grade and produced in China.
2.2 Experimental design
Specific-pathogen-free male Sprague Dawley rats (n= 40;weighing 145-160 g) were obtained from Hunan Shrek Jingda Experimental Animal Co., Ltd. (Changsha, China; production license number: SCXK [Xiang]2016-0002) and housed in separate cages at (23 ± 2) °C and (50 ± 5)% humidity with a 12-h light-dark cycle and free access to food and water. After 7 days of adaptation eating normal chow, the rats were randomly divided into normal control (NC;n= 8) and HFD (n= 32) groups. The NC group was fed normal chow,whereas the HFD group was fed a diet containing 63.6% normal chow, 20.0% sucrose, 15.0% lard, 1.2% cholesterol, and 0.2% sodium cholate. After 4 weeks of feeding, 32 HFD rats were randomly divided into HFD, 200 mg/kg PSPs (PSP200), 400 mg/kg PSPs (PSP400),and 800 mg/kg PSPs (PSP800) groups (n= 8 for each group). Rats in the NC and HFD groups were tube-fed with distilled water, whereas rats in the PSP groups were tube-fed with different doses of PSP solution. Rats were treated daily for 6 weeks, and their body mass was measured weekly. At the end of the experiment, the rats were fasted for 12 h and then anesthetized with sodium pentobarbital. Immediately afterward, the blood of each rat was extracted via the abdominal aorta,and the liver was dissected. After the collected blood was allowed to stand for 45 min, the serum was separated by centrifugation at 3 000 ×gfor 15 min (Michael Experimental Instruments Co., Ltd., Changsha,China). Serum and liver samples were stored at -80 °C (Zhongke Meiling Cryogenic Technology Co., Ltd., Hefei, China). Treatment of the experimental animals conformed to the regulations of the Animal Experimental Ethics Committee of Shaoyang University and was strictly implemented in accordance with the 8th edition of the US Guidelines for the Care and Use of Laboratory animals.
2.3 Detection and analysis
2.3.1 Preparation of liver tissue sections
Hematoxylin and eosin (H&E) and Oil Red O tissue staining are often used to examine the pathological status of the liver[19,20]. Three livers were randomly selected from each group.The same portion of all selected livers was immediately fixed in 10% paraformaldehyde. The specimens were embedded in paraffin, and H&E and Oil Red O-stained sections were prepared as previously described [19]. The prepared sections were imaged at 200 × magnification using an optical microscope (Leica Instrument Co., Ltd., Wetzlar, Germany) to evaluate pathological differences among the liver tissues from each group. As described in previously published studies [19], we assessed the degree of pathological changes induced by HFD in the livers of obese rats.
2.3.2 Detection of liver function and oxidation indicators,serum lipid levels, and hepatic inflammation factor release
An enzyme labeling instrument (Bio Tek Instruments, Inc.,Winooski, Vermont, USA) was used to measure serum ALT and AST activity levels according to the manufacturer’s instructions. Liver tissue homogenates were prepared by combining 1 g of liver tissue with 9 mL of normal saline. After centrifugation (12 000 ×g, 10 min),the supernatant was used to determine SOD, CAT, GSH-Px, and MDA levels according to the manufacturer’s instructions.
Serum lipid levels of TC, TG, HDL-C, and LDL-C were detected according to instructions provided with each kit. A microplate reader was used to measure the release of TNF-α, IL-1β, and IL-6 from the liver tissue homogenates. The procedures were performed according to the instructions provided with the enzyme-linked immunosorbent assay kits.
2.3.3 Western blot analysis
Total protein was extracted from the collected liver tissues and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described by Deng et al. [21]. The samples were transferred to membranes, and INSR, AMPK, p-AMPK, SREBP-2,and LDLR were detected by immunoblotting. GAPDH was used as an internal reference. After development, the optical density values were calculated (Media Cybernetics, Inc., Rockville, MD, USA).
2.4 Statistical analysis
Experimental data were analyzed using GraphPad Prism 7 software (GraphPad Software, Inc., San Diego, CA, USA). All data are expressed as the mean ± standard deviation. Groups were compared byt-tests and one-way analysis of variance.P< 0.05 was considered to indicate statistically significant results.
3. Results
3.1 Effect of PSPs on the bodyweight of obese rats
Compared with the NC group, the body weight of rats in the HFD group was significantly increased at week 4 (P< 0.01),indicating successful establishment of the obesity model (Fig. 1A).The PSP400 and PSP800 groups showed significantly reduced body weights compared to that of the HFD group (P< 0.05 andP< 0.01,respectively; Fig. 1B).
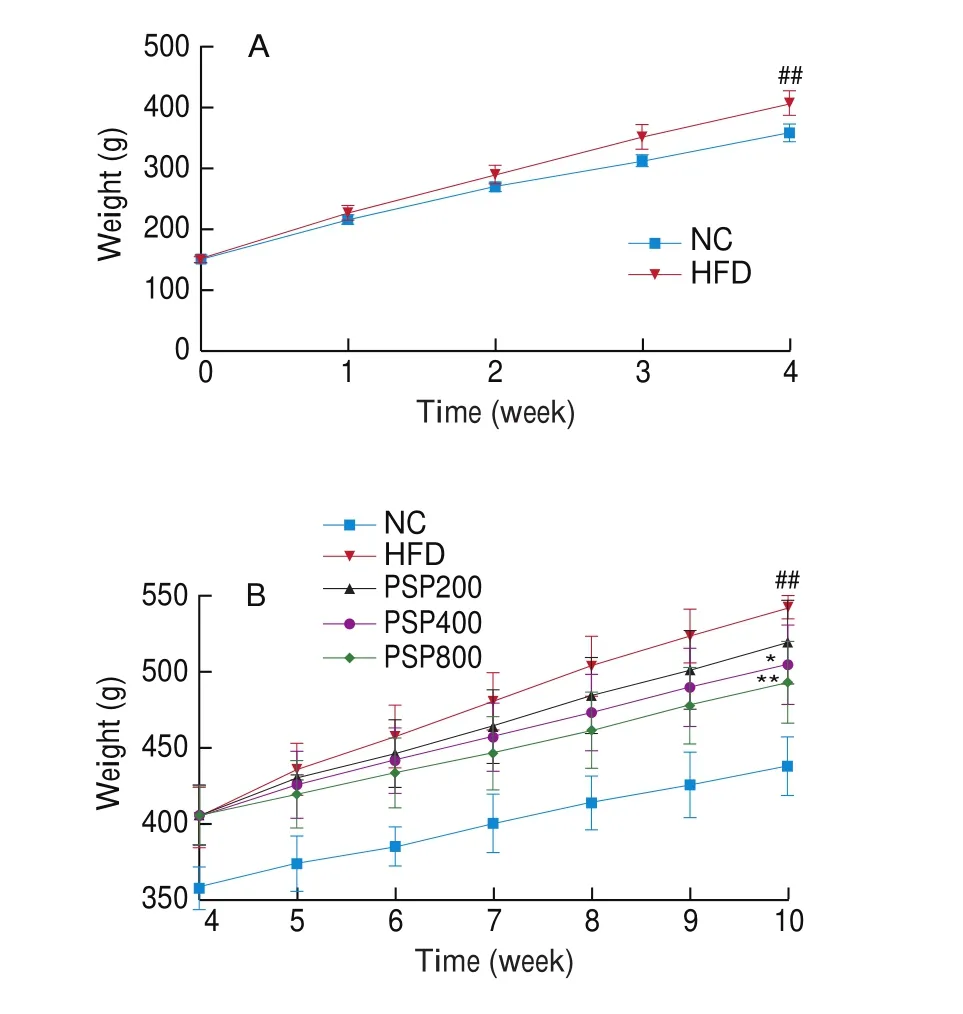
Fig. 1 Effects of different diet treatments on the body weights of rats. (A)Changes in the body weight of rats in NC group (n = 8) and HFD group(n = 32) in 4 weeks. (B) Changes in the body weight of rats after PSPs intervention for 6 weeks (n = 8 per group). *P < 0.05, **P < 0.01 compared with the HFD group; #P < 0.05 or ##P < 0.01 compared with the NC group.
3.2 Pathological analysis of rat liver sections
H&E-stained liver sections from rats in the NC group showed normal hepatocyte morphology without obvious degeneration, and no lipid droplets were observed after Oil Red O staining (Fig. 2).In contrast, H&E-stained sections from the HFD group displayed obvious symptoms of lipid degeneration, such as large vacuoles and cytoplasmic porosity (black arrow), and Oil Red O staining revealed marked lipid droplet deposition. PSP improved these phenotypes in a dose-dependent manner with noticeable decreases in liver vacuolation,particularly in the PSP800 group.

Fig. 2 Results of H&E staining and Oil Red O staining in liver tissues of rats in each group (n = 3 per group) (A) H&E staining and Oil Red O staining sections of liver tissue (200 × magnification). (B) Degree of hepatic vacuolization. (C) Degree of hepatic lipid accumulation. The black arrows show vacuole. *P < 0.05,**P < 0.01 compared with the HFD group; #P < 0.05 or ##P < 0.01 compared with the NC group.
3.3 Effect of PSPs on liver enzyme functions
The levels of ALT and AST were significantly higher in the HFD group than in the NC group (P< 0.01; Fig. 3), indicating liver dysfunction in the HFD group. Following PSP treatment, the levels of ALT and AST were significantly lower than those in the HFD group (P< 0.01),indicating that PSP ameliorated liver dysfunction in obese rats.

Fig. 3 Comparison of (A) ALT and (B) AST contents in groups of rats fed different diets (n = 8 per group). *P < 0.05, **P < 0.01 compared with the HFD group; #P < 0.05, ##P < 0.01 compared with the NC group.
3.4 Effect of PSPs on serum lipid levels
The serum TC, TG, and LDL-C contents in the HFD group were significantly higher than those in the NC group (allP< 0.01),whereas the HDL-C content was significantly lower (P< 0.01;Fig. 4). Serum TC and TG levels in the PSP400 and PSP800 groups were significantly reduced compared with those in the HFD group(P< 0.01), as was the serum LDL-C level in the PSP800 group(P< 0.01). In contrast, the serum HDL-C levels of rats in the PSP200,PSP400, and PSP800 groups were increased significantly (P< 0.05 in groups PSP200, PSP400, andP< 0.01 in group PSP800) compared to that of the HFD group. These results indicate that PSP can dosedependently improve blood lipid levels in a HFD-induced rat model of obesity.
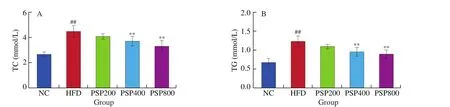
Fig. 4 Effects of different diets on serum lipid contents (n = 8 per group). *P < 0.05, **P < 0.01 compared with the HFD group; #P < 0.05, ##P < 0.01 compared with the NC group.
3.5 Effect of PSPs on hepatic antioxidant enzyme activity and MDA content
The MDA content was significantly higher in the HFD group than in the NC group (P< 0.01; Fig. 5), and significantly lower in the PSP800 group compared to the HFD group (P< 0.01). Hepatic SOD,GSH-Px, and CAT activities were significantly decreased in the HFD group compared to the NC group (P< 0.01). Compared to the HFD group, GSH-Px and CAT activities were significantly increased in the PSP200 group (P< 0.05), and the levels of all three antioxidant enzymes were significantly increased in the PSP400 (P< 0.05) and PSP800 (P< 0.01) groups.

Fig. 5 Effects of different diet treatments on liver antioxidant enzyme activities and MDA content (n = 8 per group). *P < 0.05, **P < 0.01 compared with the HFD group; #P < 0.05, ##P < 0.01 compared with the NC group.
3.6 Effect of PSPs on hepatic TNF-α, IL-1β, and IL-6 release
The release of TNF-α, IL-1β, and IL-6 was significantly higher in the livers of the HFD group compared with those of the NC group(P< 0.01; Fig. 6). PSP treatment resulted in dose-dependent decreases of these inflammatory factors. These decreases were all significant,except for that of IL-1β in the PSP200 group.

Fig. 6 Effects of different diet treatments on the levels of hepatic inflammatory factors (n = 8 per group). *P < 0.05, **P < 0.01 compared with the HFD group;#P < 0.05, ##P < 0.01 compared with the NC group.
3.7 Effect of PSPs on hepatic expression of key lipid metabolism proteins
Hepatic INSR and p-AMPK levels were significantly downregulated (bothP< 0.01) in the HFD group compared with those in the NC group, whereas SREBP-2 and LDLR were significantly upregulated (bothP< 0.01; Fig. 7). INSR and p-AMPK levels increased significantly following treatment with increased concentrations of PSPs (P< 0.01), whereas SREBP-2 and LDLR levels decreased significantly (P< 0.01). However, there were no significant effects on the hepatic levels of key lipid metabolism proteins in the low-dose (PSP200) group.
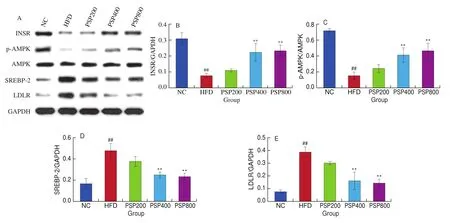
Fig. 7 Effects of different diet treatments on hepatic levels of INSR, AMPK, p-AMPK, SREBP-2, and LDLR (n = 3 per group). *P < 0.05, **P < 0.01 compared with the HFD group; #P < 0.05, ##P < 0.01 compared with the NC group.
4. Discussion
Modern epidemiological studies have shown that NAFLD is the most common chronic liver disease worldwide [22]and that the incidence of obesity-induced NAFLD is rapidly increasing [23].Abnormal blood lipid levels (including TC, TG, LDL-C, and HDL-C)are common metabolic markers of obesity [24], as blood lipid contents re flect the state of systemic lipid metabolism [25]. Increased fat intake results in increased free fatty acids and a gradual accumulation of TG in the body. Once the liver storage limit is exceeded, toxic lipid metabolites are produced, which can lead to liver dysfunction and damage [26]. Our results show that PSPs not only decrease the body mass of obese rats, but also decrease serum TC, TG, and LDL-C levels, and increase serum HDL-C. This suggests that PSPs promote lipid metabolism and regulate blood lipid levels. In this study, the degrees of revealed liver injury and fat accumulation suggest that PSPs improve obesity-induced NAFLD.
A healthy liver is fundamental to the maintenance of normal nutrition and metabolism [27]. AST and ALT are common indicators of liver metabolism and transport function. ALT is recommended by the World Health Organization as the most sensitive indicator of liver dysfunction [28]; when 1% of hepatocytes are necrotic, the serum ALT content doubles [29]. During the development of HFD-induced obesity, excessive reactive oxygen species are produced because of hepatic accumulation of TG and free fatty acids. Balanced free radical metabolism in the body is primarily maintained by antioxidant enzymes such as SOD, GSH-Px, and CAT [30]. MDA, the main product of lipid peroxide degradation, promotes inflammation.Therefore, promoting the activities of SOD, CAT, and GSH-Px and inhibiting the level of MDA in the liver can promote lipid metabolism and prevent obesity-induced NAFLD [31]. Hepatic lipid accumulation and the presence of inflammatory cells are closely related [32].Obesity not only makes hepatocytes prone to lipid peroxidation, but also induces excessive secretion of inflammatory factors, increasing the possibility of NAFLD, liver fibrosis, liver cirrhosis, and even hepatocellular carcinoma [33,34]. The disordered production of“aggressive” pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, is closely involved in the pathogenesis of metabolic syndrome and promotes the occurrence of IR [35,36]. In addition, chronic inflammation induced by obesity can exacerbate metabolic disorders,thus accelerating the development of obesity [37]. Therefore,regulating the antioxidant and immune capacities of the body may actively improve obesity-induced NAFLD [31,33]. Previous studies indicated that PSPs has many activities [14], including the ability to alleviate oxidative stress injury [38]and enhance immune ability [17].Our study provides mechanistic insight into how PSPs alleviate liver function damage in rats with HFD-induced obesity.
The liver is an important organ in lipid metabolism. The presence of IR in patients with obesity-induced NAFLD is closely related to INSR activation and downstream signal transduction [13,39].Gan et al. [40]demonstrated that long-term HFD-induced IR can be regulated by activating proteins related to INSR signaling to promote lipid metabolism Therefore, INSR may be the key factor for maintaining lipid metabolism homeostasis and inhibiting obesity-induced NAFLD [41]. In this study, INSR was upregulated in the livers of rats treated with PSPs, suggesting that PSPs play a lipid-lowering role in regulating glucose and lipid metabolism by controlling INSR expression. In diet-induced IR, INSR was downregulated and AMPK phosphorylation was decreased [42,43].AMPK is an important cellular energy sensor with an important role in regulating the balance of cellular energy metabolism [44].SREBP-2 primarily regulates the expression of genes related to cholesterol synthesis and uptake, and its abnormal expression can lead to disorders of lipid metabolism (particularly cholesterol metabolism), resulting in excessive tissue deposition [45,46]. Shah et al. [47]found that depletion of adiponectin receptor 1 resulted in decreased AMPK expression, significantly increased SREBP-2, and increased intracellular cholesterol biosynthesis. Activation of AMPK has been suggested to inhibit the regulation of cholesterol metabolism by SREBP-2. Plasma LDL-C enters the cell by interacting with LDLR, which is directly regulated by SREBP-2 and accumulates in hepatocytes [48,49]. In NAFLD, abnormal upregulation of SREBP-2 results in a significant increase in exogenous cholesterol uptake by LDLR [49,50]. Thus, the INSR/AMPK/SREBP-2/LDLR signaling axis is a potential key pathway promoting lipid metabolism and maintaining its homeostasis in the liver. Our data revealed that PSPs upregulated INSR expression, activated AMPK signaling (shown by its increased phosphorylation), and inhibited SREBP-2 and LDLR expression. This may be the key mechanism by which PSPs inhibit cholesterol biosynthesis, promote lipid metabolism, and reduce lipid accumulation in the body.
However, the body is a complex system, and signaling pathways related to lipid metabolism are intricate and finely tuned. A previous study reported that PSPs promotes lipid metabolism in the mouse liver induced by an egg yolk emulsion through the peroxisome proliferatoractivated receptor and SREBP-1c pathways [16], whereas we found that PSPs reduce cholesterol synthesis and uptake by inhibiting SREBP-2. This indicates that the signaling pathways upstream of SREBP-1c and SREBP-2 overlap or regulate each other and that SREBP-2 dysregulation plays an important role in the generation and development of NAFLD. The molecular mechanism by which PSPs protect against obesity-induced NAFLD is very complex and requires further analysis [51]. Our future studies will focus on clarifying the molecular mechanism of PSPs to prevent and treat obesityinduced NAFLD in HFD-fed rats through metabolomic technology and network pharmacology analysis to provide a scientific basis for advancing the processing and application of PSPs. In addition, Roy et al. [52]explored the role of the intestinal microflora in NAFLD by transplanting intestinal microorganisms into mice. Interestingly,they found that different microorganisms caused different metabolic responses and that transplanted micro flora prevented the development of inflammation and IR. The effects of PSPs on the intestinal microorganisms inhabiting obese rats should also be further examined.
5. Conclusion
In summary, PSPs effectively decrease body mass and inhibit the progression of NAFLD in rats with HFD-induced obesity by reducing liver oxidative stress injury and chronic inflammation, maintaining the normal liver function, and promoting hepatic lipid metabolism through the INSR/AMPK/SREBP2/LDLR signaling pathway. These effects improve blood lipid levels and prevent toxic reactions caused by high levels of lipid peroxidation (Fig. 8). In the concentration range used in this study, the effects of treatment with PSPs were positively correlated with the dose, with the 400 and 800 mg/kg doses showing relatively better therapeutic effects on HFD-induced obesity and NAFLD in rats compared to the 200 mg/kg dose. The molecular mechanism by which PSPs regulate lipid metabolism should be further elucidated.
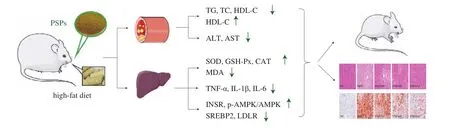
Fig. 8 Possible mechanism by which PSPs confer weight loss and alleviate NAFLD in rats with HFD-induced obesity.
Conflict of interest
The authors declare no competing financial interest.
Acknowledgments
This work was financially supported by the Natural Science Foundation of Hunan Province, China (2019JJ40272); the Scientific Research Foundation of Hunan Provincial Education Department,China (20C1676); and the Scientific Research Foundation of Shaoyang College, China (2020HX122).
 食品科學(xué)與人類(lèi)健康(英文)2022年4期
食品科學(xué)與人類(lèi)健康(英文)2022年4期
- 食品科學(xué)與人類(lèi)健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study
- Isolation of a novel characterized Issatchenkia terricola from red raspberry fruits on the degradation of citric acid and enrichment offlavonoid and volatile profiles in fermented red raspberry juice
