Chitin oligosaccharides alleviate atherosclerosis progress in ApoE-/-mice by regulating lipid metabolism and inhibiting inflammation
Hongmin Zhen, Qiojun Yn, Yiho Liu, Ynxio Li, Shoqing Yng, Zhengqing Jing,*
a Key Laboratory of Food Bioengineering (China National Light Industry), College of Food Science & Nutritional Engineering,China Agricultural University, Beijing 100083, China
b Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Engineering, China Agricultural University, Beijing 100083, China
Keywords:
Chitin oligosaccharides
Atherosclerosis
Lipid metabolism
In flammation
ApoE-/- mice
A B S T R A C T
Atherosclerosis is driven both by hyperlipidemia and inflammation. Chitin oligosaccharides (NACOS) have shown pharmacological effects on multiple diseases via hypolipidemic and/or anti-inflammatory activities.The present study aims to evaluate whether NACOS treatment can prevent atherosclerosis induced by a highfat-diet (HFD) in Apolipoprotein E-knockout (ApoE-/-) mice. Results showed that 300 and 900 mg/kg b.w./day NACOS supplementation for 14 weeks significantly decreased atherosclerotic lesions up to 45% and 67% in compared with the HFD (P < 0.05), as measured in the valve area of the aortic root. Further, NACOS supplementation significantly reduced the serum hyperlipidemia and circulating proinflammatory cytokines including interleukin-1β, interleukin-6, monocyte chemoattractant protein-1 and tumor necrosis factor-α.NACOS decreased the hepatic Hmgcr to reduce cholesterol synthesis, activated the genes involved in reverse cholesterol transport to enhance cholesterol efflux and excretion, and reduced the intestinal Npc1L1 to lower cholesterol absorption. Additionally, NACOS enhanced cecum short chain fatty acids production and intestinal integrity. Thus, NACOS supplementation ameliorated atherosclerosis via altering lipid metabolism and reducing inflammation. These findings indicate that NACOS may be a potential functional food material for attenuating atherosclerosis development.
1. Introduction
Atherosclerosis is the most common underlying cause of ischemic heart disease and stroke and accounted for 31% of deaths worldwide [1]. The progress of atherosclerosis is initiated by the accumulation of cholesterol-carrying low-density lipoprotein (LDL)particles in the arterial wall, modification of these lipoproteins, and receptor-mediated uptake by macrophages leading to foam cell generation [1]. The accumulated foam cells promote inflammation for migration and proliferation of more leukocytes into the plaque area and contribute to the necrotic core of atherosclerosis plaque,which is further exacerbated by systemic inflammation [2].Therefore, hypercholesterolemia and inflammation are the two major risk factors of atherosclerosis [3]. Although current strategies for reducing atherosclerosis are powerful to delay the development of atherosclerosis, such as taking platelet aggregation inhibitors and statins, the risk of cardiovascular occurrences in those patients are still existing due to the unsolved inflammation [4]. Therefore,novel approaches that can efficiently decrease inflammation and hyperlipidemia offer a promising therapeutic target for atherosclerosis.
Dietary administration of chitin oligosaccharides (NACOS) is one potential applicant, as NACOS exhibit low toxicityin vivo[5].NACOS and chitosan oligosaccharides (COS) are the hydrolytic products of chitin, which is commonly found in the exoskeletons of arthropods and insect cuticles and fungal cell wall [6]. NACOS are the linear oligosaccharides ofN-acetylglucosamine linked byβ-1,4 linkages with a degree of polymerization (DP) of 2-10, while COS are the deacetylated NACOS [6]. Many studies have shown that COS have various biological functions, including anti-inflammation [7], antiobesity [8], anti-diabetic [9], anti-oxidation [10], antimicrobial [11],immunostimulation and antitumor [12]. Several studies have revealed that COS possess atherosclerosis protective activity, which is connected with the enhanced hepatic expression of low density lipoprotein receptor (LDLR) and scavenger receptor B1 (SR-B1),macrophage SR-B1 and ATP-binding cassette transporter1(ABCA1) and promoted reverse cholesterol transport (RCT) [13,14].Furthermore, COS supplementation at dose of 250 mg/kg b.w./days or 1 000 mg/kg b.w./days in dyslipidemic Apolipoprotein E-knockout(ApoE-/-) male mice for 12 weeks decreased the lipid deposits in the aorta and reduced the hepatic convertase subtilisin/kexin type 9 protein levels compared with high-fat diet (HFD)-fed mice [14,15].This activity may be attributed to the combination changes of hepatocyte nuclear factor-1α and sterol-responsive element binding protein 2 after COS treatment [14,15]. Compared to the extensive studies on the COS’s functions and underlying mechanisms, few works have been reported on the function of NACOS. It has been reported that acetyl group is essential for NACOS’s functions with different target proteins in plants and animals [16-18]. For example, the recognition of theN-acetyl moieties allows At Chitin Elicitor Receptor Kinase 1 to distinguish NACOS from COS and oligoglucan [16-18].NACOS have also showed application potential in the treatment of various diseases, including cancer, gastritis and Alzheimer’s disease[19,20]. A systematic review and meta-analysis with the inclusion of 22 prospective cohort studies and a least 3 years’ follow-up period found an inverse connection between total dietary fiber intake(including insoluble fiber and fiber from cereal and vegetable sources)and risk of CVD and coronary heart disease [21]. Moreover, human and animal experiments have revealed that chitin-glucan fiber had beneficial effects in reducing the development of atherosclerosis by decreasing plasma oxidized low-density lipoprotein (ox-LDL) or improving the antioxidant status [22,23]. Additionally, a recent research indicated that NACOS supplementation alleviated dyslipidemia and inflammation responses, thus attenuated HFD-induced metabolic syndrome in mice [24]. However, it is still unknown whether NACOS can alleviate the development of atherosclerosis.
Given the cholesterol-lowering and anti-inflammatory capacity of NACOS, we hypothesized that NACOS can reduce the atherosclerosis development in a well-established animal model of atherosclerosisApoE-/-mice by regulating lipid metabolism and inhibiting inflammation. Therefore, we explored the role of NACOS on the progress of atherosclerosis by investigating the effect of NACOS in HFD-fedApoE-/-mice and the underlying mechanisms.
2. Materials and methods
2.1 Reagents
NACOS were obtained using the chitinase hydrolysis methods as previously described [9,25]. Antibodies for anti-matrix metalloproteinase-2 (MMP-2) and anti-vascular cell adhesion molecule-1 (VCAM-1) were purchased from Proteintech (Rosemont,USA). Antibodies for anti-LDLR and anti-SR-B1 and all secondary antibodies used for western blotting were brought from Abcam(Cambridge, MA, USA).
2.2 Animals
7-week aged maleApoE-/-mice on C57BL/6J background (Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing,China) had free access to chow diet and water during the one-week accommodation. Then the mice were randomly divided into four groups: Normal diet (ND) group, fed with a normal diet; HFD group,fed with a HFD (45% anhydrous milkfat kcal diet with 0.2% total cholesterol (TC)); NACOS-L group, fed with the same HFD plus 300 mg/kg b.w./day NACOS in drinking water, refreshed daily;NACOS-H group, fed with the HFD plus 900 mg/kg b.w./day NACOS in drinking water. This HFD (TD10885) was obtained from KeAo XieLi Feeds Co., Ltd. (Beijing, China). According to the drinking volume daily, it is equivalent to approximately 32.97 and 98.91 mg/kg b.w./day for humans, respectively. The animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals(NIH Publications No. 8023, revised 1978) and approved by the Subcommittee of Experimental Animal Welfare, China Agricultural University, China (permission number: 20185001-3).
The body weight and food intake of mice were monitored once a week. After 14 weeks of intervention, mice were fasted for 12 h and then sacrificed for organs and tissues collecting. The blood samples were collected by enucleating the eyeball before cervical dislocation and kept at 4 °C for at least 4 h. Blood samples were then centrifuged at 3 500 r/min for 20 min at 4 °C, and the supernatant was collected as serum in non-pyrogenic tubes.
2.3 Aortic plaque lesion analysis by hematoxylin-eosin(H&E) staining and immunohistochemical analysis
After collection of blood samples, the circulating system was rinsed with phosphate-buffered saline (PBS) and then fixed with 4% phosphate-buffered paraformaldehyde. The hearts of these mice were excised and fixed in 4% phosphate-buffered paraformaldehyde,dehydrated in 70% ethanol and embedded in paraffin, and then crosssectioned (5 μm) perpendicular to the axis of the aorta throughout the aortic root area, starting from the appearance of open aortic valve leaflets [26,27]. From the appearance of open aortic valve leaflets the 20thsection was stained with H&E staining kit (Beijing Solarbio Science & Technology, Beijing, China). The quantification of lesion area and size were analyzed using Image J software (NIH,Bethesda, Maryland, USA) as in a previous study [28]. And the data analysis was verified by a second researcher who was blinded to the experimental groups.
For immunohistochemical experiments, sections of aortas were incubated with MMP-2 and VCAM-1 antibodies overnight at 4 °C,separately. Sections were then incubated with biotin-labeled goat antirabbit immunoglobulin G (IgG) secondary antibody at room temperature for 30 min. For quantitative analysis of images, 5 random fields were captured from different areas of a single section, and the intensity of positive staining was analyzed by Image Pro Plus 6.0 (Media Cybernetics,Silver Spring, USA) software and calculated as the integrated optical density (IOD) per stained area (IOD/area) in each field.
2.4 Serum lipids and inflammatory cytokines
The contents of triglyceride (TG), TC, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol(HDL-C) in the serum were quantified using assay kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The TG were checked with glycerol phosphate oxidase-P-aminophenazone method(catalog number: A110-1-1) and TC with cholesterol oxidase phenol 4-aminoantipyrine peroxidase method (catalog number: A111-1-1).The LDL-C were checked with different detergents and chemicals to specific block or solubilize of lipoprotein classes with homogeneous assay for direct determination method (catalog number: A113-1-1)and HDL-C with phosphotungstic acid-Mg2+method (catalog number: A112-1-1). Serum very low-density lipoprotein (VLDL)(catalog number: H249) [29], ox-LDL (catalog number: H248) and lipopolysaccharide (LPS) (catalog number: H255) were determined by competitive ELISA kits (Nanjing Jiancheng Bioengineering Institute,Nanjing, China), in which the target sample and a biotin-conjugated peptide bind competitively to a capture antibody. All experiments were performed according to the manufacturer’s instructions. Serum tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1(MCP-1), interleukin-1β (IL-1β) and IL-6 were analyzed by Mouse Inflammation Panel (BioLegend, San Diego, USA) on a flow cytometer (BD LSR II, Liberty lake, USA).
2.5 Liver lipids and histological examination of liver tissues
The liver lipids including TG and TC were measured using commercial assay kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) following the manufacturer’s instructions.After euthanasia, the collected liver tissues from the same site were fixed in 10% neutral buffered formalin and dehydrated. Then the fixed samples were sliced into 5 μm-thick sections, and stained with H&E staining kits [30]. The pathological changes were imaged by an optical microscopy (BM Optical Instrument Manufacturing Co. Ltd.,Shanghai, China). These representative pictures were taken from three individual liver samples in each group.
2.6 Real-time quantitative PCR (RT-qPCR) analysis
Total RNA of aorta, liver and small intestinal samples was extracted with a TRIzol Reagent kit (Life Technologies, Carlsbad,USA) and reverse transcribed into cDNA using a GoScript Reverse Transcription System (Promega, Madison, USA). The mRNA expression of each gene was detected using the TB Green Premix Ex Taq kit (Takara, Japan), with glyceraldehyde-3-phosphate dehydrogenase (Gapdh) which is a housekeeping gene used in many previously studies [13,14,31]serving as the internal reference, on a CFX Connect Real-Time PCR Detection System (Bio-rad, Hercules,USA). The primers used are shown in Table 1. The 2–ΔΔCtmethod was employed to determine the relevant gene expressions.

Table 1List of oligonucleotide primers used in this study.
2.7 Western blotting
About 100 mg of frozen liver tissues were lysed with 0.9 mL RIPA lysis buffer supplemented with 1/200 dilution of protease and phosphatase inhibitor cocktails (Roche Diagnostics Ltd.,Mannheim, Germany) for 30 min on ice. Protein in lysates was isolated through centrifugation (15 000 r/min, 4 °C, 15 min),quantified by bicinchoninic acid assay (Biomiga, San Diego, USA),and then separated by SDS-PAGE. The proteins were transferred to a nitrocellulose filter membrane. After being blocked with 5% skimmed milk for 1 h at room temperature, the membrane was incubated with LDLR or SR-B1 antibodies overnight at 4 °C,separately. The membrane was further incubated with the horseradish peroxidase-labeled goat anti-rabbit IgG secondary antibody at room temperature for 1 h. Protein bands were visualized by enhanced chemiluminescence substrate (Bio-rad, Hercules, USA) and quantified using Image J software.
2.8 Analysis of cecal short-chain fatty acids (SCFAs)
The contents of SCFAs in cecum content were examined by high-performance liquid chromatography (HPLC) equipped with an Agilent Hi-Plex column (250 mm × 4.6 mm, Agilent Technologies,Wilmington, DE, USA) as previously described [32]. Briefly,100-200 mg of cecum content was homogenized in 0.8 mL of concentrated sulfuric acid, and then centrifuged at 12 000 ×gfor 10 min. The supernatant was collected, filtered (0.22 μm) and then subjected to HPLC analysis. The acetic acid, propionic acid and butyric acid (Sigma Aldrich, St. Louis, MO, USA) were used as standards. The contents of SCFA are expressed as milligram per gram cecum content.
2.9 Statistical analysis
The results were expressed as the means ± SEM. Statistical analysis was performed using GraphPad Prism 8.0 (GraphPad Software, San Diego, USA). Statistical differences were measured using an unpaired two-sided Student’st-test or one-way ANOVA with Bonferroni correction for multiple comparisons. Normality was checked using the Kolmogorov-Smirnov test. A nonparametric test(Mann-Whitney) was used when the data did not pass the normality test. A value ofP≤ 0.05 was considered as statistically significant.
3. Results
3.1 NACOS attenuated atherosclerosis progress
As illustrated by representative images in Fig. 1A, H&E staining of aorta roots demonstrated that HFD induced much more severe atherosclerotic lesions inApoE-/-mice than that of ND. However,ApoE-/-mice administered with NACOS both at low and high doses showed significantly reduction of atherosclerotic lesion area (by 40.5% and 61.9% for NACOS-L and NACOS-H, respectively)and size (by 45.3% and 66.6% for NACOS-L and NACOS-H,respectively) when compared to HFD (Fig. 1B-C,P< 0.05). These results demonstrated HFD obviously accelerated the development of atherosclerosis, while the low and high doses of NACOS attenuated the development of atherosclerosis in HFD-fedApoE-/-mice.
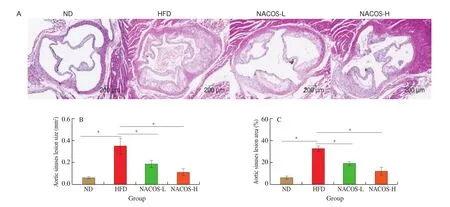
Fig. 1 NACOS attenuated the development of atherosclerosis. 7-week old ApoE-/- mice were randomly divided into 4 groups (n = 8): ND, HFD,NACOS-L, and NACOS-H for 14 weeks as described in methods and materials. (A) Representative images of H&E-staining of aortic cross-sections. Scale bar,200 μm. Quantitative analyses of the lesion area (B) and size (C) in the aortic root; Data are presented as means ± SEM; n = 5. * P < 0.05 was considered to be statistical significance.
3.2 NACOS improved the proatherogenic microenvironment in atherosclerotic plaque
The protein expressions of inflammatory cytokines and monocyte recruitment factors including MMP-2 and VCAM-1 that are associated with the progress of atherosclerosis in the aorta ofApoE-/-mice were analyzed by immunohistochemistry [33]. HFD significantly enhanced the protein expression of MMP-2 and VCAM-1 in the aorta ofApoE-/-mice compared with the ND (Fig. 2A-C,P< 0.05). However, NACOS at the high doses significantly decreased the protein expressions of MMP-2 and VCAM-1 in the aorta of the HFD-fedApoE-/-mice (Fig. 2A-C,P< 0.05). Also, HFD significantly enhanced the aortic gene expression of IL-6, MMP-2, and ICAM-1 compared with the ND (Fig. 2D-F,P< 0.05). However, NACOS treatment both at low and high doses significantly decreased the aortic gene expressions of IL-6, MMP-2, and ICAM-1 compared with the HFD, while no significantly change was observed between two doses(Fig. 2D-F,P< 0.05). Thus, these results suggested that NACOS could improve the proatherogenic microenvironment of atherosclerosis.
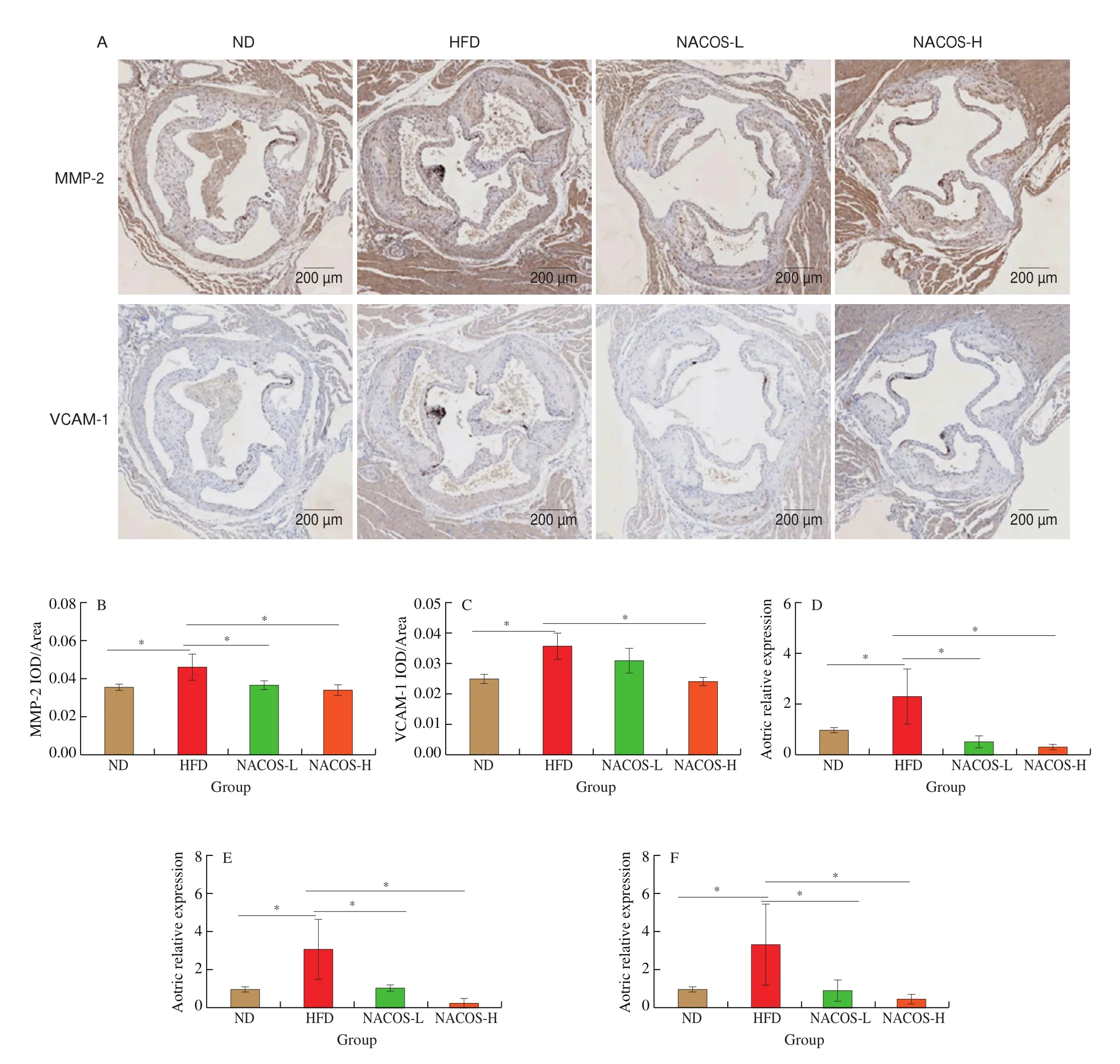
Fig. 2 NACOS improved the proatherogenic microenvironment in atherosclerotic plaque. (A) Representative images of immunohistochemical staining of MMP-2 and VCAM-1 in the aortic root. Scale bar, 200 μm. Analysis of IOD/Area for immunohistochemical staining of MMP-2 (B) and VCAM-1 (C) in the aortic root.mRNA levels of reverse cholesterol transport genes including (D) IL-6, (E) MMP-2, and (F) ICAM-1 in the aortas. Data are presented as means ± SEM; n = 5.*P < 0.05 was considered to be statistical significance.
3.3 NACOS improved hyperlipidemia and systemic inflammation
Since hyperlipidemia is one of the major risk factors of atherosclerosis [34], serum lipid profiles were examined. Serum levels of TC, TG, HDL-C, LDL-C, VLDL and ox-LDL were significantly elevated in HFD-fedApoE-/-mice compared with these serum lipid elements of ND-fedApoE-/-mice (Fig. 3A-F,P< 0.05).However, serum TC and LDL-C levels were significantly decreased
in the NACOS administration groups (decreased by 20.9% and 26.7% of TC, and 21.0% and 23.9% of LDL-C in the NACOS-L and NACOS-H groups, respectively) compared with HFD groups, whereas no significant change of the HDL-C level was observed (Fig. 3A-D,P< 0.05). The serum TG levels were reduced by 14.6% and 19.7% in the NACOS-L and NACOS-H groups compared with HFD groups,respectively (Fig. 3B,P< 0.05). The serum VLDL and ox-LDL levels were also significantly reduced in the NACOS group mice (reduced by 23.7% and 26.0% of VLDL, and 27.9% and 57.7% of ox-LDL in the NACOS-L and NACOS-H groups, respectively) (Fig. 3E-F,P< 0.05), when compared with those in the HFD group. Circulating proinflammatory cytokines such as IL-1β, IL-6, TNF-α and MCP-1 play a role in the progress of atherosclerosis [33]. The serum levels of IL-1β, IL-6, TNF-α and MCP-1 were significantly elevated in HFD-fedApoE-/-mice compared with theApoE-/-mice fed ND (Fig. 3G-J,P< 0.05). In contrast, supplementation of NACOS both at low and high doses significantly decreased circulating levels of inflammatory cytokines, and the IL-1β, IL-6, TNF-α and MCP-1 concentrations were decreased by 72.4% and 53.8%, 49.2% and 62.4%, 20.6% and 47.2%, and 78.4% and 76.4% in the NACOS-L and NACOS-H groups compared with those of HFD group, respectively(Fig. 3G-J,P< 0.05).

Fig. 3 NACOS improved hyperlipidemia and systemic inflammation. Serum (A) TC, (B) TG, (C) LDL-C, (D) HDL-C, (E) VLDL and (F) ox-LDL levels (n = 8).Serum levels of inflammation cytokines (G) IL-1β, (H) IL-6, (I) TNF-α, and (J) MCP-1 (n = 5). Data are presented as means ± SEM. *P < 0.05 was considered to be statistical significance.
3.4 NACOS altered hepatic lipid metabolism
To explore the involvement of hepatic lipid metabolism in the antiatherosclerotic function of NACOS, body weight, diet intake,water intake and liver weight were examined. HFD led to significantly higher body weight and liver weight, whereas lower food and water intake than those of the ND (Figs. 4A-D,P< 0.05). However,treatment with NACOS at both doses significantly decreased the body weight, liver weight and food intake (Figs. 4A-D,P< 0.05), while had no obviously changes on water intake compared with HFD-feeding.HFD significantly increased the hepatic TG and TC compared with ND feeding, while NACOS at both doses significantly reduced the TG and TC contents in the liver tissues compared with HFD(Figs. 4E,F,P< 0.05). Moreover, H&E staining of liver tissues showed swollen hepatic cells and large lipid droplets, indicating hepatic fat accumulation in HFD-induced atherosclerosis mice. However, hepatic lipid accumulation was alleviated and liver cell degeneration was lessened after NACOS feeding for 14 weeks (Fig. 4G). Next, several genes and proteins associated with lipid and cholesterol metabolism in hepatic tissue were analyzed to explore the underlying mechanism of NACOS in suppressing atherosclerotic development (Fig. 4H-P).Compared with HFD group, gene expression of Hmgcr were significantly reduced by the NACOS supplementation both at low and high doses (Fig. 4H,P< 0.05). NACOS both at low and high doses enhanced the gene expressions ofLdlrandSr-b1and protein expressions of LDLR and SR-B1 in hepatic tissues compared with HFD (Figs. 4I-P,P< 0.05), while no significantly difference was observed between two doses (Figs. 4I-P).Furthermore, NACOS both at low and high doses significantly enhanced the gene expressions ofCyp7a1, ATP-binding cassette subfamily G member 5 (Abcg5) and Abcg8 compared with HFD(Figs. 4K-M,P< 0.05).

Fig. 4 NACOS altered hepatic lipid metabolism. (A) Body weight (n = 8). (B) Diet intake (n = 8). (C) Water intake (n = 8). (D) Liver weight (n = 8). (E) Liver TG (n = 8). (F) Liver TC (n = 8). (G) Representative images of H&E staining of liver tissues (n = 3). Scale bar, 50 μm. mRNA levels of hepatic gene expression including (H) Hmgcr, (I) Ldlr, (J) Sr-b1, (K) Cyp7a1, (L) Abcg5 and (M) Abcg8 (n = 5). (N) Protein expressions of LDLR and SR-B1 in the liver (n = 3).(O) LDLR and (P) SR-B1 in the liver tissues (n = 3). The Gaphd level was serving as the internal reference. β-actin was used as loading control. Densitometry quantification of the expressed protein bands Results are presented as means ± SD. *P < 0.05 was considered to be statistical significance.
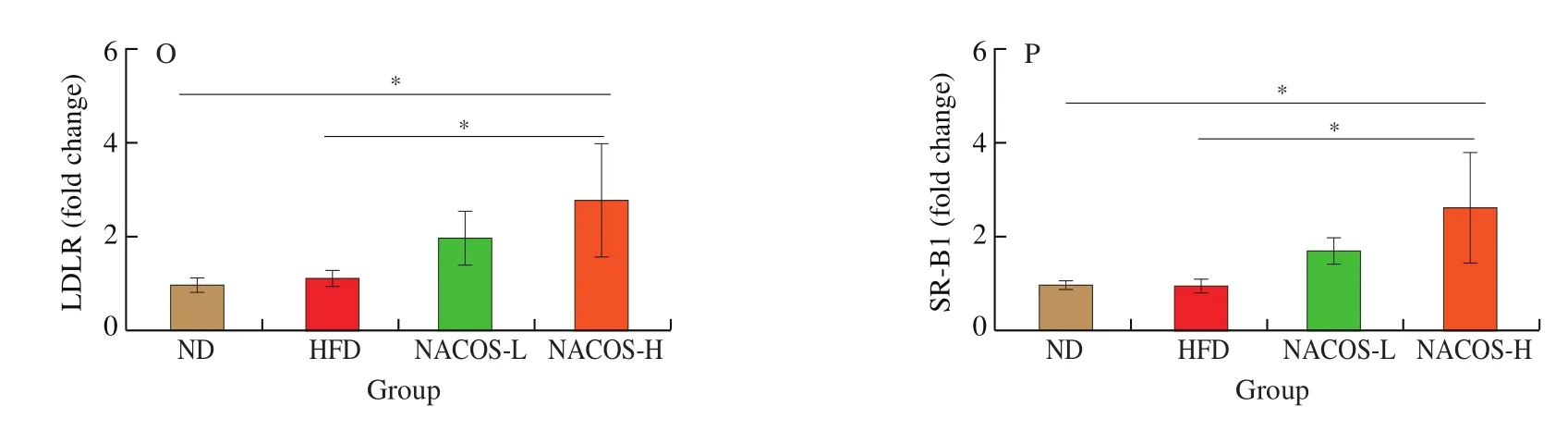
Fig. 4 (Continued)
3.5 NACOS changed gene expressions of RCT in the aorta and markers of intestinal cholesterol absorption and efflux
The effects of NACOS on the expression of genes involved in RCT (Abca1andAbcg1in the aorta) were analyzed. There were no significantly difference of these two genes in the aorta ofApoE-/-mice between HFD group and ND group (Figs. 5A,B). However, treatment with NACOS both at low and high doses significantly upregulated the gene expression ofAbca1andAbcg1in the aorta in HFD-inducedApoE-/-mice compared with HFD (Figs. 5A,B,P< 0.05). To further clarify the potential function of NACOS on intestinal cholesterol uptake and efflux in atherosclerotic animals, the expressions of several typical genes were confirmed by RT-qPCR analysis. The intestinal gene expressions of niemann-pick c1-like 1 (Npc1l1) which is responsible for intestinal cholesterol uptake was not significantly changed in HFD-fedApoE-/-mice compared with ND-fedApoE-/-mice, whereas this gene expression in the intestine was significantly decreased by NACOS treatment both at low and high doses(Fig. 5C,P< 0. 05). The intestinal gene expression ofAbcg5andAbcg8which are involved in cholesterol efflux were not changed after HFD feeding compared with ND. However, gene expressions ofAbcg5andAbcg8was significantly was enhanced by treatment with NACOS(Figs. 5D-E,P< 0.05).
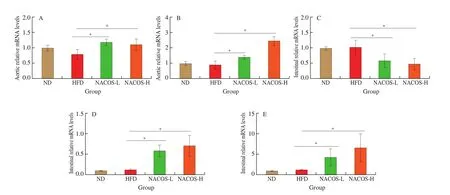
Fig. 5 NACOS changed gene expressions of RCT in the aorta and markers of intestinal cholesterol absorption and efflux. mRNA levels of reverse cholesterol transport genes including (A) Abcg1 and (B) Abca1 in aorta and mRNA of cholesterol metabolism and cholesterol absorption including (C) Npc1l1, (D) Abcg5 and(E) Abcg8 in the small intestine of ApoE-/- mice. The Gapdh level was serving as the internal reference. Results are presented as means ± SD; n = 5. *P < 0.05 was considered to be statistical significance.
3.6 NACOS improved cecum SCFA contents and intestinal integrity
As recent studies have shown that SCFA reduce inflammation[35,36], SCFA in the contents of cecum of the mice were measured.A significant reduction was found in the contents of cecal SCFA in HFD-fedApoE-/-mice compared with ND-fed mice (Fig. 6A-C,P< 0.05). However, NACOS treatment significantly increased the abundance of the contents of cecal SCFA including acetic acid (by 32.8% in the NACOS-H group), propionic acid (by 17.6% and 31.3% in the NACOS-L and NACOS-H groups, respectively) and butyric acid (by 66.0% and 43.6% in the NACOS-L and NACOS-H groups,respectively) compared with the HFD (Fig. 6A-C,P< 0.05). To investigate the role of NACOS in regulating the intestinal integrity of HFD-fedApoE-/-mice, mRNA levels of two major tight junction proteins Zona occludens 1 (Zo-1) and Occludin in the small intestinal were determined. HFD had reduced intestinal gene expression of bothZo-1andOccludincompared with ND. In contrast, the gene expressions of these two genes were considerably upregulated in small intestine of NACOS treated mice compared with those of HFD (Fig. 6D-E,P< 0.05). Moreover, the repair of gut integrity by NACOS treatment led to a significant decrease of circulation LPS level in HFD-fedApoE-/-mice (Fig. 6F,P< 0.05).

Fig. 6 NACOS improved cecum SCFA contents and intestinal integrity. Cecum SCFA including acetic acid (A), propionic acid (B) and butyric acid (C) were measured (n = 8). mRNA levels of the major tight junction proteins (D) Zo-1 and (E) Occludin in the small intestine of ApoE-/- mice (n = 5). (F) Serum LPS levels(n = 5). Results are presented as means ± SD. *P < 0.05 was considered to be statistical significance.
4. Discussion
NACOS have been reported to possess both cholesterollowering effects and anti-inflammatory effects, and can facilitate the prevention of multiple metabolic diseases [7,24]. So far, numerous researches have been focused on the biological effects of COS [16].However, there is few information on the biological functions and underlying mechanism of NACOS. The present study investigated the antiatherosclerotic effects of NACOS on HFD-treatedApoE-/-mice and explored the possible underlying mechanisms. The present study demonstrated that NACOS attenuated HFD-induced deterioration of atherosclerosis inApoE-/-mice (Fig.1) by regulating lipid metabolism and inhibiting inflammation.
High plasma levels of LDL-C and lipoprotein, as well as low plasma level of HDL-C, are the major risk factors for atherosclerosis [37,38].In the current research, NACOS supplementation improved hyperlipidemia by significantly reducing the serum TC, LDL-C,TG, VLDL, and ox-LDL levels inApoE-/-mice (Fig. 3A-F), which is in accordance with the results in a previous study that NACOS decreased the serum lipids in type 2 diabetes mice [9]. The lower serum lipid profiles after NACOS treatment might be connected with the decreased cumulative food intake and changes of lipid metabolism(Fig. 4). In addition, histological examination of liver tissue, decreased liver weight and liver lipids indicated that NACOS alleviated hepatic lipid accumulation and liver cell degeneration (Fig. 4).Taken together, these results further indicated that NACOS can improve lipid metabolism by attenuating hepatic lipid accumulation and lowering serum lipid levels.
RCT pathway plays a crucial role in cholesterol metabolism [34,39].In this pathway, accumulated cholesterol is transported from foam cells in the vessel wall to the liver for excretion in the bile, thus preventing atherosclerosis [34,39]. ABCA1 and ABCG1 are the main transporters of HDL for transferring cholesterol from the peripheral macrophages to plasma, and hepatic SR-B1 plays a key role in HDL cholesterol uptake from plasma to the liver [40]. However, LDL receptor is responsible for uptake of LDL cholesterol [34]. Then the cholesterol in the liver for bile acid synthesis or excretion in the feces was regulated by ABCG5 and ABCG8. Another essential enzyme involving RCT is CYP7A1, the rate-limiting enzyme for conversion of cholesterol into bile acids for their consequent biliary secretion [41].Hence, in the present study, we investigated the effects of NACOS on the gene expressions of the typical members of RCT. NACOS inhibited the hepatic gene expression ofHmgcrwhich is a ratelimiting enzyme in cholesterol biosynthesis, suggesting that NACOS decreased the hepatic cholesterol synthesis (Fig. 4). NACOS also increased the hepatic gene expressions ofSr-b1,LdlrandCyp7a1and the hepatic protein expressions of SR-B1 and LDLR, suggesting NACOS enhanced bile acid synthesis (Fig. 4). Its functions in HFD-induced atherosclerosis mice are similar to the results of previous studies that oral administration of functional oligosaccharides such as COS attenuated atherosclerosis and reduced plasma non-HDL levels, which might be involved in cholesterol metabolism, including enhanced expressions of hepatic LDLR and SR-B1, and macrophage ABCA1 [13,14]. Experiments employed functional oligosaccharides such as alginate oligosaccharide also demonstrated lower plasma levels of LDL-C attributed to upregulation of hepatic LDLR expression [42]. Adjustments of the activities of major transporters or receptors in RCT pathway, such as ABCA1, ABCG1, ABCG5,ABCG8 and SR-B1, have been reported to be able to lower the incidence of atherosclerosis [40,43]. The gene expressions ofAbca1andAbcg1in the aorta with atherosclerotic lesions were significantly increased in the NACOS-treated group when compared to the HFD-fedApoE-/-group (Fig. 5). Furthermore, in these atherosclerotic mice, hepatic gene expressions ofAbcg5andAbcg8(Fig. 4) and small intestinal gene expressions ofAbcg5andAbcg8(Fig. 5) were significantly enhanced by NACOS intervention accompanied by an elevated serum HDL-C ratio (data not shown). NPC1L1 is a polytopic transmembrane protein that is essential for intestinal cholesterol absorption by facilitating the uptake of un-esterified cholesterol from the intestinal lumen into absorptive enterocytes [44]. In the present study, NACOS decreased intestinalNpc1l1expression (Fig. 5),resulting in the reduction of intestinal cholesterol uptake. All these results indicated that the hypocholesterolemia function of NACOS mainly depend on decreasing cholesterol synthesis and elevating the cholesterol excretion, as well as the reduction of LDL-C mediated by LDLR and decrease of cholesterol uptake by Npc1L1. In the future,studies should be performed to investigate the molecular mechanisms by which NACOS enhanced RCT.
Except for the modifications in lipid and cholesterol metabolism,inflammation also leads to the development of atherosclerosis [45,46].Therefore, anti-inflammatory interventions have emerged as a novel promising therapy to alleviate atherosclerosis, such as inhibiting proinflammatory cytokines [2,3]. In this study, NACOS administration not only ameliorated the progress of atherosclerosis but also improved local aorta inflammation (protein expressions of MMP-2 and VCAM-1 in the aorta) (Fig. 2) and systemic inflammation chemokines (serum levels of IL-1β, IL-6, TNF-α and MCP-1) (Figs. 3G-J). The results suggested that the antiatherosclerotic effect of NACOS is partially ascribed to its anti-inflammatory activity, which is compatible with the recent investigations that NACOS suppressed serum inflammatory cytokines (TNF-α and IL-1β) and gene expressions involved in inflammatory (IL-6, TNF-α and MCP-1) in abdominal adipose tissue in HFD-fed mice [18,24]. However, treatment with up to 1 000 mg/kg/day of COS for 12 weeks inApoE-/-mice showed no significant change of plasma TNF-α and IL-6. These differences might be attributed to the difference of molecular distribution of oligosaccharides, the duration of treatment and dietary pattern.Furthermore, NACOS both at low and high doses dramatically lowered the serum LPS in HFD-inducedApoE-/-mice (Fig. 6F). The results are agreeing with the conclusion that long-term circulating LPS can accelerate the development of atherosclerosis [47-49]. It has been reported that the serum LPS which initially originated from gut gram-negative bacteria is regulated by tight junction proteins of the intestine [47,48]. In the present study, NACOS significantly enhanced the gene expressions of two major intestinal tight junction proteins (Zo-1 and Occludin) (Fig. 6), indicating that NACOS may prevent LPS perforation by maintaining the intestinal barrier integrity,thus ameliorate inflammation of aorta tissues of HFD-fedApoE-/-mice. SCFA is crucial microbial fermentation metabolites that can directly regulate host health via mechanisms involved in gut barrier integrity, glucose homeostasis, anti-inflammatory properties, appetite adjustment, and obesity [36,50]. NACOS treatment significantly enhanced the contents of cecal SCFA in the atherosclerotic mice(Fig. 6), suggesting that the antiatherosclerosis effect of NACOS may be partially associated with the augmentation of cecal SCFA amounts which may be attributed to regulation of gut microbiota [24], but the detailed correlation should be established in future research.
There were certain limitations in the present study. Previous studies have proved that the microbiota and their products specifically butyrate affects the intestinal epithelial barrier and defenses functions by regulating related gene and protein expressions [51,52]. Although,a previous study indicated that NACOS at a dose of 1 mg/mL in drinking water improves HFD-induced metabolic syndrome by rebuilding the structure of the intestinal microbiota and enhancing gut integrity [24]. However, there is still a lack of direct evidence of whether NACOS can influence the colon SCFA amount in this HFD-induced metabolic syndrome model. Whether NACOS increased the colon SCFAs via the intestinal microbiota or interacted with receptors to regulate atherosclerosis progression needs to be further explored.Additionally, considering the high dose of NACOS in the present study, the small sample size, the lack of direct connection of NACOS on gut microbiota, and other influencing parameters, further research is highly demanded to verify the current results.
5. Conclusions
NACOS supplementation ameliorated atherosclerosis induced by HFD inApoE-/-mice by altering cholesterol metabolism and reducing inflammation. NACOS facilitated the anti-atherosclerosis intervention through the decrease of hepatic HMGCR to reduce cholesterol synthesis, activation of genes involved in RCT to enhance cholesterol efflux and excretion, and reduction of intestinal NPC1L1 to lower cholesterol absorption. Moreover, NACOS significantly decreased the circulating LPS and inflammation chemokines levels by enhancing intestinal integrity. These results demonstrated that NACOS should be a potential functional food for inhibiting atherosclerosis development.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This research was financially supported by the National Science Found for Excellent Young Scholars (No. 31822037) and National Natural Science Foundation of China (No. 21576283).
- 食品科學(xué)與人類健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study

