In fluence of particle size and ionic strength on the freeze-thaw stability of emulsions stabilized by whey protein isolate
Hao Lai, Fuchao Zhan, Yujie Wei, Abel W.S.Zongo, Sha Jiang,Haomin Sui, Bin Li, Jing Li,*
a College of Food Science and Technology, Huazhong Agricultural University, Wuhan 430070, China
b Key Laboratory of Environment Correlative Dietology (Huazhong Agricultural University), Ministry of Education, Wuhan 430070, China
Keywords:
Emulsion
Freeze-thaw stability
Whey protein isolate
Particle size
Ionic strength
A B S T R A C T
The influence of particle size and ionic strength on the freeze-thaw (FT) stability of emulsions stabilized by whey protein isolate (WPI) was investigated in this study. The destabilization of emulsions during the FT process could be suppressed in a way by decreasing the particle size of the initial emulsions, which was the result of retarding the coalescence between oil droplets. To further improve the FT stability of emulsions,different amounts of NaCl were added before emulsification. The emulsions with the ionic strength at 30-50 mmol/L exhibited good FT stability. Notably, the ionic strength in this range would not lower the freezing point of emulsions below the freezing temperature used in this study. Salt addition could improve the structural properties of proteins, which was available to strengthen the rigidity and thickness of interfacial layers, sequentially building up the resistance that the destruction of ice crystals to emulsions. Moreover,stronger flocculation between emulsion droplets could promote the formation of a gel-like network structure dominated by elasticity in the emulsion system, which might effectively inhibit the movement of droplets, and improve the FT stability of emulsions eventually. The result was of great significance for the preparation of emulsion-based foods with improved FT stability.
1. Introduction
Oil-in-water (O/W) emulsions are ubiquitous in the food system,such as cheese, cream sauces, and some beverages, etc. Many emulsion-based foods often need to be frozen before consumption and unintentionally undergo the repeating processes of freezing and thawing. However, most O/W emulsions are uns table after the freeze-thaw (FT) treatments, resulting in the generation of physical instability, such as creaming or oiling-off [1-6]. Therefore,an emulsion system with good FT stability is of great significance for frozen foods. In the process of freezing, the formation of ice crystals forces oil droplets to get closer and promotes droplet-droplet interactions. Furthermore, the ice crystals may also penetrate the oil droplets and destroy the interfacial layers of emulsions, thus making the oil droplets merge after thawing. In the process of thawing, the stability of emulsions mainly depends on their composition and structure, as well as their thermal and mechanical histories [3]. At present, different approaches in improving the FT stability of O/W emulsions have been intensively investigated, including the control of ice crystal growth [4], the promotion of aqueous phase vitrification [7],the change of shearing conditions [8], and the adjustment of the emulsion composition [9].
Many proteins are surface-active [10]and often used as emulsifiers, which can adsorb on the surface of newly formed oil droplets, and prevent further coalescence of droplets during homogenization [11]. Like other emulsifier-stabilized emulsions,protein-stabilized emulsions also face the problem of instability after the FT treatment. To solve this problem, some strategies were developed. For instance, heat pretreatment of proteins was used to improve the FT stability of protein-stabilized emulsions [12]. It has been reported that the emulsions stabilized by heated soybean protein isolate (HSPI) and whey protein exhibited better FT stability than that of the unheated proteins [13]. Considerable research efforts have been devoted to improve the FT stability of emulsions by modified proteins. The FT stability of emulsions stabilized by whey protein isolate (WPI)-dextran conjugates was much higher than the emulsions stabilized by WPI owing to the overlap of the thicker interfacial films between dextran and oil droplets, which enhanced the repulsive steric force between oil droplets [14]. Zang et al. [15]have reported that the FT stability of emulsions could be remarkably improved by enzymatic modification of SPI. They have also revealed that the emulsions stabilized by SPI which was enzymatically hydrolyzed by papain or by the combination of papain and phytase exhibited favorable FT stability [16]. Additionally, salt addition was also applied to improve the FT stability of protein-stabilized emulsions. Zhu et al. [17]have illustrated that appropriate ionic strength (100-200 mmol/L NaCl)remarkably improved the FT stability of emulsions stabilized by HSPI nanoparticles, which was attributed that the presence of NaCl strengthened the interfacial protein or protein nanoparticle films coating on the droplets. Another research group have reported that the FT stability of emulsions could be optimized by the combination of thermal pretreatment (80 °C, 30 min) and salt addition (200 mmol/L NaCl) [18]. At present, it is commonly known that salt addition is an effective approach to improve the FT stability of protein-stabilized emulsions. However, from a healthy perspective, reducing the amounts of salt in foods has always been pursued by food scientists and enterprises. Whether the emulsions could exhibit excellent FT stability on the premise of minimizing the salt added as much as possible deserves to be concerned. Consequently, we hypothesized that the physical instability of emulsions during the FT process might be improved by the combination of decreasing the particle size of the initial emulsions and adding a small amount of salt.
In our study, the influence of particle size and ionic strength on the FT stability of emulsions stabilized by WPI were investigated. In the first part, the FT stability of emulsions with different particle sizes was characterized, in terms of the variations in visual appearance,particle size, microstructure, stability index and interfacial protein adsorption. In the second part, some properties were evaluated on the emulsions with NaCl addition. In addition, the structural properties of proteins and the interfacial adsorption kinetics were performed on the aqueous dispersions. This study has a potential significance in understanding the influence mechanism of the particle size and ionic strength on the FT stability of protein-stabilized emulsions, and enlarging the application of emulsion-based frozen foods.
2. Materials and methods
2.1 Materials
WPI with a total purity of 90% was purchased from Yuanye Biology (Shanghai, China). Soybean oil was purchased from the local supermarket (Wuhan, China). Medium chain triglyceride (MCT)was obtained from the Sigma-Aldrich (Shanghai, China). All other experimental reagents were of analytical grade and purchased from Sinopharm Chemical Reagent Company (Shanghai, China).
2.2 Preparation of emulsions
WPI stock aqueous dispersion (4%,m/V) was prepared by dispersing WPI powders in deionized water and sodium azide(0.02%,m/V) was added as an antibacterial agent. The protein aqueous dispersion was magnetically stirred at room temperature for 2 h and hydrated overnight at 4 °C. Emulsions were prepared by a two-step homogenization process. Firstly, WPI stock aqueous dispersion (4%,m/V) and soybean oil (oil volume fraction,φ= 40%)were blended by using a high-speed homogenizer (T18 digital Ultra Turrax, IKM Instruments Ltd, Germany) at 20 000 r/min for 5 min to obtain the primary emulsions. Afterward, the primary emulsions were homogenized at 60 MPa for 0, 3, 5 or 7 times by high-pressure micro fluidizer (Micro fluidics M-110L, Micro fluidics Corp., Newton,MA, USA) to obtain the initial emulsions with different particle sizes.
The influence of ionic strength on emulsions was examined by adding different amounts of NaCl into WPI stock aqueous dispersions(4%,m/V) and magnetically stirred for 2 h. The final concentrations of NaCl in WPI dispersions were 10, 30, 50, and 70 mmol/L,respectively. The emulsions with different ionic strengths were prepared by the same two-step homogenization process described above. In the second step, the primary emulsions were homogenized at 60 MPa for 5 times by high-pressure micro fluidizer.
2.3 FT treatment cycles
Aliquots of emulsions were loaded into the sealed glass tubes(20 mL) and frozen at -30 °C for 24 h. Subsequently, the frozen emulsions were thawed at 25 °C for 2 h until completely melted. The FT treatment was repeated thrice and physicochemical properties of emulsions were measured after each cycle of FT treatment.
2.4 Characterization of initial and FT emulsions
2.4.1 Particle size, flocculation index (FI) and coalescence degree (CD)
The particle size was determined by laser diffraction using the Malvern Mastersizer 2000 analyzer (Malvern Instruments,Worcestershire, UK). The deionized water or 1% (m/V) sodium dodecyl sulfate (SDS) solution was used as a dispersant in the measurement. The relative refractive index of the oil phase and aqueous phase were 1.470 and 1.330, respectively. The rotating speed of the agitator was 2 000 r/min. All determinations were carried out at least in triplicate. The particle size was expressed as the volumeweighted mean diameter (d4,3).
The fiand CD were calculated according to a previous method of literature [13]. After each cycle of FT treatment, the test samples were chosen from the middle of the emulsions layer after the oiling-off being removed. The results were calculated by measuring the particle size dispersed in deionized water and 1% (m/V) SDS solution. The calculated formulas as follow:
Whered4,3waterandd4,3SDSrepresented thed4,3of emulsions measured in deionized water and 1% (m/V) SDS solution,respectively.
Whered4,3SDS,Iandd4,3SDS,F-Trepresented thed4,3of the initial emulsions and the emulsions after the third cycle of FT treatment,respectively, when 1% (m/V) SDS solution was used as a dispersant in the measurement.
2.4.2 Microstructure
The micrographs of magnified emulsions before and after three cycles of FT treatment were evaluated by using a Leica DM LB Microscope (CX40, Wetzlar, Allemagne). A drop of emulsion (5 μL)was put on the slides and covered with cover slips. The samples were observed under a 40 × lens and the images were recorded by software installed on a computer.
2.4.3 Adsorbed protein (AP)
The AP was measured according to the method reported by literature [19]. Briefly, initial emulsion (1 mL) was centrifuged at 10 450 ×gfor 30 min and the subnatant was sucked out using a 0.45 μm filter needle. After the subnatant was centrifuged again at 10 450 ×gfor 30 min, the collected subnatant was diluted 100 times with deionized water. The protein concentrations in the subnatant (Cf)and the initial WPI aqueous dispersion (Cs) were determined using a bicinchoninic acid (BCA) protein assay kit. The equation used to calculate AP as follows:

2.4.4 Differential scanning calorimetry (DSC)
The phase transition temperature of the initial emulsions was evaluated by DSC using a calorimeter (204F1, NETZSCH, Germany)according to a previous study [18]. The initial emulsion (5-8 mg)was poured into an aluminum crucible and covered with a matching aluminum cap. Subsequently, the aluminum crucible was placed into the measuring chamber and an empty crucible was used as control.The samples were frozen from 40 °C to -40 °C at a cooling rate of 10 °C/min. After being equilibrated for 1 min, the samples were heated from -40 °C to 40 °C at the same rate. Nitrogen was used as blanket gas and the flow rate was 25 mL/min. The freezing point was analyzed using Proteus Analysis Software (Version 5.0, NETZSCH,Germany) installed on the computer.
2.4.5 ζ-potential
Theζ-potential of emulsions was determined by the Zetasizer Nano ZS instrument (Malvern Instrument Ltd, Malvern, UK).Before each measurement, the emulsions were appropriately diluted using a deionized water with the same pH and ionic strength. The determinations were conducted at least in triplicate.
2.4.6 Rheological properties
The strain-controlled rheometer (AR2000ex, TA Instruments,USA), associated to a parallel plate geometry, was used to characterize the apparent viscosity, elastic modulus (G’) and viscous modulus (G’’) of the initial emulsions. The rheological properties were examined according to a reported literature [17]. The plate diameter was 60 mm, and the distance between plates was 0.5 mm.The linear viscoelastic region was determined by the oscillating strain sweep mode at the 1 Hz scanning frequency, and the strain value was fixed at 1% according to the measurement results. The steady-state shear mode was used to measure the apparent viscosity of emulsions in the range from 1 to 100 s-1. The measurement ofG’ andG’’adopted the frequency sweep mode at a frequency varying from 0.1 to 100 rad/s. All experiments were carried out at 25 °C.
2.4.7 Interfacial pressure
Interfacial pressure (π) of proteins at the oil-water interface was measured using an automatic drop tensiometer (Tracker-H, Teclis,France) according to the method of literature [20]. The test model of raising hanging drop was adopted. To avoid the interference of impurities in soybean oil on the results, MCT was used instead of soybean oil in this study [21]. The MCT and WPI dispersions were added to the syringe and cuvette, respectively. The volume of the oil droplet was set to 10 μL. The WPI dispersions were prepared by adding different amounts of NaCl with the final concentrations of 0, 10, 30, 50, and 70 mmol/L into WPI stock aqueous dispersions(4%,m/V), respectively, and then magnetically stirred at room temperature for 2 h. Afterward, the protein dispersions were diluted to 0.01% (m/V) with 10 mmol/L phosphate buffer at pH 7.0 and then magnetically stirred for 1 h to ensure dispersion. The density of each sample was measured with an ordinary density bottle (1 mL)before the experiment. The diffusion rate (Kdiff) was the slope of the adsorption curve after the beginning of 100 s adsorption. The test duration was 2 h and all measurements were carried out at 25 °C. π was calculated as follows:
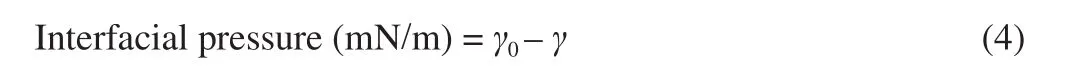
Whereγ0(mN/m) was the interfacial tension of deionized water on the surface of MCT (26.5 mN/m) andγ(mN/m) was the interfacial tension of protein dispersions with different ionic strength on the surface of MCT.
Furthermore, to analyze the rate of penetration and rearrangement of particles at the interface, the first-order equation was used to fit the data.

Wherekiwas a first-order rate constant,π0,π7200, andπtrepresented interfacial pressure of oil droplets at the beginning of adsorption time for 0, 7 200 s, and any time, respectively.
In general, the first slope is usually considered as a first-order rate constant of penetration rate (Kp), while the second slope takes to a first-order rate constant of molecular rearrangement rate (Kr).
2.5 Characterization of structural properties of proteins
2.5.1 Particle size and ζ-potential
The particle size (Z-average diameter) andζ-potential of WPI particles with different ionic strengths (0, 10, 30, 50, and 70 mmol/L NaCl) were determined by the Zetasizer Nano ZS instrument (Malvern Instrument Ltd., Malvern, UK). Before each measurement, all test samples were diluted 50 times in deionized water with the same pH and ionic strength, so that its concentration was 0.8 mg/mL. The measurements were carried out at 25 °C, and the results were taken as the average of three parallel samples.
2.5.2 Protein solubility
The protein solubility was measured according to a reported method with corresponding modifications [22]. Different amounts of NaCl were added into WPI stock aqueous dispersions (4%,m/V), and magnetically stirred for 2 h at room temperature to obtain initial WPI dispersions. The final concentrations of NaCl in protein dispersions were 0, 10, 30, 50, and 70 mmol/L, respectively. Firstly, each protein dispersion was centrifuged at 12 000 ×gfor 20 min at 4 °C, and then the supernatant was filtered through glass wool. The content of soluble protein in the supernatant and the initial WPI dispersion was measured using the BCA protein assay kit. The protein solubility was calculated as follows:

2.5.3 Surface hydrophobicity
The surface hydrophobicity of proteins with different ionic strengths (0, 10, 30, 50, and 70 mmol/L NaCl) was determined according to the fluorescence probe 1-anilino-8-naphthalene sulfonate(ANS) method with corresponding modifications [23]. Each protein sample was diluted to five concentrations (0.004%-0.02%,m/V)using 10 mmol/L phosphate buffer at pH 7.0. The ANS solution(8 mmol/L) was prepared using the same phosphate buffer and dissolved away from light. Twenty microliters of ANS solution were added to 4 mL protein diluents and vortex blended at 30 r/min for 5 s. The fluorescence intensity of samples was measured using a fluorescence spectrophotometer (F4500, Hitachi, Japan). The photometry mode was adopted. The excitation wavelength and emission wavelength were 365 nm and 484 nm, respectively. The background fluorescence was calibrated using the same phosphate buffer. The initial slope was calculated by linear analysis to the fluorescence intensity-protein concentration curve, and its value can be regarded as the surface hydrophobicity of proteins.
2.6 Statistical analysis
SPSS statistical software (Version 21.0, IBM Co., USA) was used for data analysis. Duncan’s multiple range test at a significance level of 0.05 was applied to estimate the statistical difference between samples. Each measurement was carried out by three parallel samples and the results were reported as mean ± standard deviation.
3. Results and discussion
3.1 Characterization of emulsions with different particle size
3.1.1 Visual appearance and microstructure
The particle size is one of the important factors affecting the stability of conventional emulsions (emulsions stored in quiescent conditions at room temperature) [24]. The gravitational separation rate decreases with the decrease of particle size, thereby the instability of emulsions could be retarded by reducing the particle size of the initial emulsions during homogenization [3]. To study whether the particle size would affect the FT stability of emulsions, the initial emulsions with particle size (d4,3) of 8.92, 1.28, 0.83, and 0.65 μm were prepared,which were marked as EuA, EuB, EuC, and EuD, respectively. All initial emulsions none showed any evidence of creaming before freezing (Fig. 1A). The EuA with a relatively bigger particle size(8.92 μm) exhibited a heavy oiling-off after the first cycle of FT treatment. Oil float formed in the EuB after the second cycle of FT treatment. The oiling-off occurred in the EuC and EuD only after the third cycle of FT treatment. As shown in Fig. 1B, the particle size (d4,3) of all emulsions except the EuA increased with increasing the cycle number of FT treatment, which might be the result of moderate flocculation or coalescence between droplets. Since the EuA exhibited a heavy oiling-off after the first cycle of FT treatment,the measurement results could not truly reflect the particle size of emulsions (data not shown).

Fig. 1 Visual appearance (A), De Brouckere, volume-weighted, mean diameter (d4,3) (B) and optical micrographs (C) of emulsions prepared by different homogenization process before and after different cycles of FT treatment. The different uppercase letters above the columns represent significant differences(P < 0.05) in the particle size of different emulsions after the same cycle of FT treatment. The different lowercase letters above the columns represent significant differences (P < 0.05) in the particle size of the same emulsion after different cycles of FT treatment.

Fig. 1 (Continued)
The microstructure images of emulsions were shown in Fig. 1C. It can be observed that the particle size of the FT EuA was much smaller than the initial EuA. As a result of the structure of the EuA was completely destroyed after three cycles of FT treatment, lots of WPI aggregates and small amounts of oil droplets can be observed in the microscopic vision. As the cycle number of FT treatment increased,larger droplets in the EuB and EuC were observed. As the partial destruction of protein layers on the surface of the EuB and the EuC,a growing number of oil droplets coalesced together to form larger oil droplets. Instead, there was an indistinctive increase in the particle size of the EuD after repeated FT treatments, and the droplets seemed to flocculate together. The moderate degree offlocculation might be conducive to resisting the dramatic variations of environmental factors during the FT process, which was beneficial to maintain the stability of emulsions [18]. The above results elucidated that decreasing the particle size of the initial emulsions could effectively enhance the FT stability of emulsions.
3.1.2 Flocculation index and coalescence degree
The emulsions would flocculate or coalesce during the FT process. The moderate degree of flocculation between droplets could improve the FT stability of emulsions, due to the movement of emulsion droplets would be inhibited in a way [3]. After being subjected to repeated FT treatments, the flocculation index (FI) of all emulsions increased gradually (Fig. 2A). Since that the particle size of the EuA measured in 1% (m/V) SDS solution was much larger than that measured in deionized water, the actual results of the ficould not be obtained (data not shown). Generally, as the flocculation reaches a certain degree, the coalescence would appear. Eventually,the extensive coalescence would result in creaming and oiling-off.It would happen when the size of oil droplets was large enough to enhance the rate it rose to the surface, which might be caused by the changes of density differential between the oil phase and aqueous phase [25]. As can be seen from Fig. 2B, with decreasing the particle size of the initial emulsions, the coalescence degree (CD) declined gradually after repeated FT treatments, implying that the stability against coalescence of emulsions was improved.

Fig. 2 fi(A) and CD (B) of emulsions with different particle size after different cycles of FT treatment. The different uppercase letters above the columns represent significant differences (P < 0.05) in fiand CD of different emulsions after the same cycle of FT treatment. The different lowercase letters above the columns represent significant differences (P < 0.05) in fiof the same emulsion after different cycles of FT treatment.
3.1.3 Interfacial protein adsorption
For protein-stabilized emulsions, the adsorbed protein (AP) at the oil-water interface is an important parameter to affect the particle size,surface charge, microstructure and the stability against coalescence of emulsions [26]. The AP gradually increased with decreasing the particle size of the initial emulsions (Fig. 3), which was attributed to the increase in the specific surface area of oil droplets. The AP of the EuA greatly reduced after the first cycle of FT treatment,but the decreasing trend slowed down with further decreasing the particle size of the initial emulsions. The reason may be ascribed that the large droplets were generally less capable to resist the stress imposed by the formation of ice crystals during the frozen process,because they were confined in a reduce volume of unfrozen aqueous phase [3,27]. Therefore, the emulsions with larger particle size would increase the possibility of creaming and oiling-off during repeated FT treatments, which was detrimental to maintain the stability against coalescence of emulsions.

Fig. 3 AP of emulsions with different particle size before and after the third cycle of FT treatment. The different uppercase letters above the columns represent significant differences (P < 0.05) in AP of different emulsions after the same cycle of FT treatment. The different lowercase letters above the columns represent significant differences (P < 0.05) in AP of the same emulsion after different cycles of FT treatment.
3.2 Characterization of emulsions with different ionic strength
It can be observed from the above results that even the EuD with the smallest initial particle size exhibited a mild oiling-off after the third cycle of FT treatment. To further improve the FT stability of emulsions, we considered that the structural properties of proteins adsorbed on the interface need to be changed appropriately, which may greatly improve the interfacial stability, and perhaps would improve the FT stability of emulsions. The speculation was a good agreement with our research, which the ionic strength in the range of 10-70 mmol/L NaCl indeed has a positive effect on the structural properties of proteins. Moreover, the fact that salt addition has an important influence on the stability of conventional emulsions has been con firmed [27]. When ionic strength is within a certain range,the emulsions will rapidly flocculate and form a network structure due to the occurrence of electrostatic interactions, ion bridging or ion binding [28]. Simultaneously, the emulsions anchored by proteins with lower surface charge density are more prone to aggregate, while moderate flocculation between emulsions is beneficial to improve the FT stability of emulsions. Identical conclusions were obtained in some studies that salt addition would improve the FT stability of emulsions [13,17-18]. It was noteworthy that the effective ionic strength was often higher than 100 mmol/L in their studies. From the perspective of reducing salt for health, it is worth exploring whether the possibility to improve the FT stability of emulsions by the combination of decreasing the particle size of the initial emulsions and adding a small amount of salt. In this part, different concentrations of NaCl were added into WPI stock aqueous dispersions (4%,m/V)before emulsification. After that, the emulsions with different ionic strengths (10-70 mmol/L NaCl) were obtained by the same preparation method as the EuC, which were marked as EuC-10,EuC-30, EuC-50, and EuC-70, respectively.
3.2.1 Visual appearance and microstructure
The visual appearance of the emulsions with different ionic strengths were presented in Fig. 4A. After the second cycle of FT treatment, the emulsions of EuC-30, EuC-50 and EuC-70 were relatively stable except the EuC-10. As the cycle number of FT treatment increased, a heavy oiling-off occurred in the EuC-10 and a slight creaming appeared in the EuC-70, as for the EuC-30 and EuC-50, no oiling-off was observed. The microstructure images of emulsions were shown in Fig. 4B. After repeating FT treatments,the increase in particle size of all emulsions was observed in the visual field. This was attributed that the repeated FT treatments promoted the coalescence between oil droplets, resulting in the formation of larger oil droplets. There were some massive lump aggregates in the upper part of the EuC-70. In contrast, fewer aggregates can be observed in the EuC-30 or EuC-50. With the increase of the number of FT cycles, the particle size of emulsions increased while theζ-potential further decreased when the ionic strength reached 70 mmol/L. Larger droplets and less electrostatic repulsion are more susceptible to generate gravitational separation and accelerate the aggregation of emulsions.

Fig. 4 Visual appearance (A) and optical micrographs (B) of emulsions with NaCl addition (10-70 mmol/L) before and after different cycles of FT treatment.

Fig. 4 (Continued)
3.2.2 DSC analyses
The ionic strength in aqueous phase will affect the freezing point of emulsions, further affecting the FT stability of emulsions [29]. The influence of ionic strength on the crystallization of the oil phase and aqueous phase were evaluated in this section. As shown in Fig. 5,there was an obvious exothermic peak for all samples in the cooling stage. The temperature of the exothermic peak can be regarded as the freezing point of samples, which were between -17 °C and -20 °C.The addition of salt (50 mmol/L NaCl) reduced the freezing point of emulsions, illustrating that the EuC-50 would be crystallized later compared with the EuC during the frozen process. It should be pointed out that the freezing point of all samples was higher than the frozen temperature (-30 °C), demonstrating that both oil phase and aqueous phase have been crystallized completely at the frozen conditions set in this study. Undeniably, the actual cooling conditions (such as the cooling rate, emulsions volume, etc.) within the freezer and DSC pan were different, which might affect the accuracy of the determination results. We can ensure that the cooling conditions of different samples were consistent in the measurement, thus minimizing the errors.

Fig. 5 DSC thermograms of the emulsions without and with 50 mmol/L NaCl. The thermograms of WPI stock aqueous dispersion (4%, m/V) and soybean oil were also recorded. Means with different lowercase letters represent significant differences (P < 0.05).
3.2.3 Particle size and ζ-potential
It can be seen from Fig. 6A that the particle size of emulsions increased gradually after repeated FT treatments. Simultaneously,the particle size of emulsions increased with the increase of ionic strength on the same FT cycle treatment, while the particle size of EuC-50 decreased slightly during the third cycle of FT treatment.This was supported by the result of the optical micrograph (Fig. 4B).Some aggregates can be seen in microscopic vision of the EuC-30 and EuC-70, which may be resulted by large amounts offlocculation or coalescence between emulsion droplets. When ionic strength exceeded 30 mmol/L, the particle size of emulsions measured in 1% (m/V) SDS solution exhibited fewer variations in comparison with that measured in deionized water (Fig. 6B), indicating that the emulsion droplets mainly flocculated rather than coalesced during the FT process. Due to the heavy oil occurred in EuC-10, after the third cycle of FT treatment, the particle size of the EuC-50 was the smallest among EuC-50, EuC-30 and EuC-70 except the EuC-10. However,a significant increase in particle size was observed in the EuC-70,suggesting that the emulsion droplets began to transform from flocculation to coalescence with further increasing ionic strength.This speculation was consistent with the observation from the fiand CD (Fig. 7). Consequently, the ionic strength with 50 mmol/L NaCl could promote flocculation between emulsion droplets, which can effectively inhibit the movement of emulsion droplets.
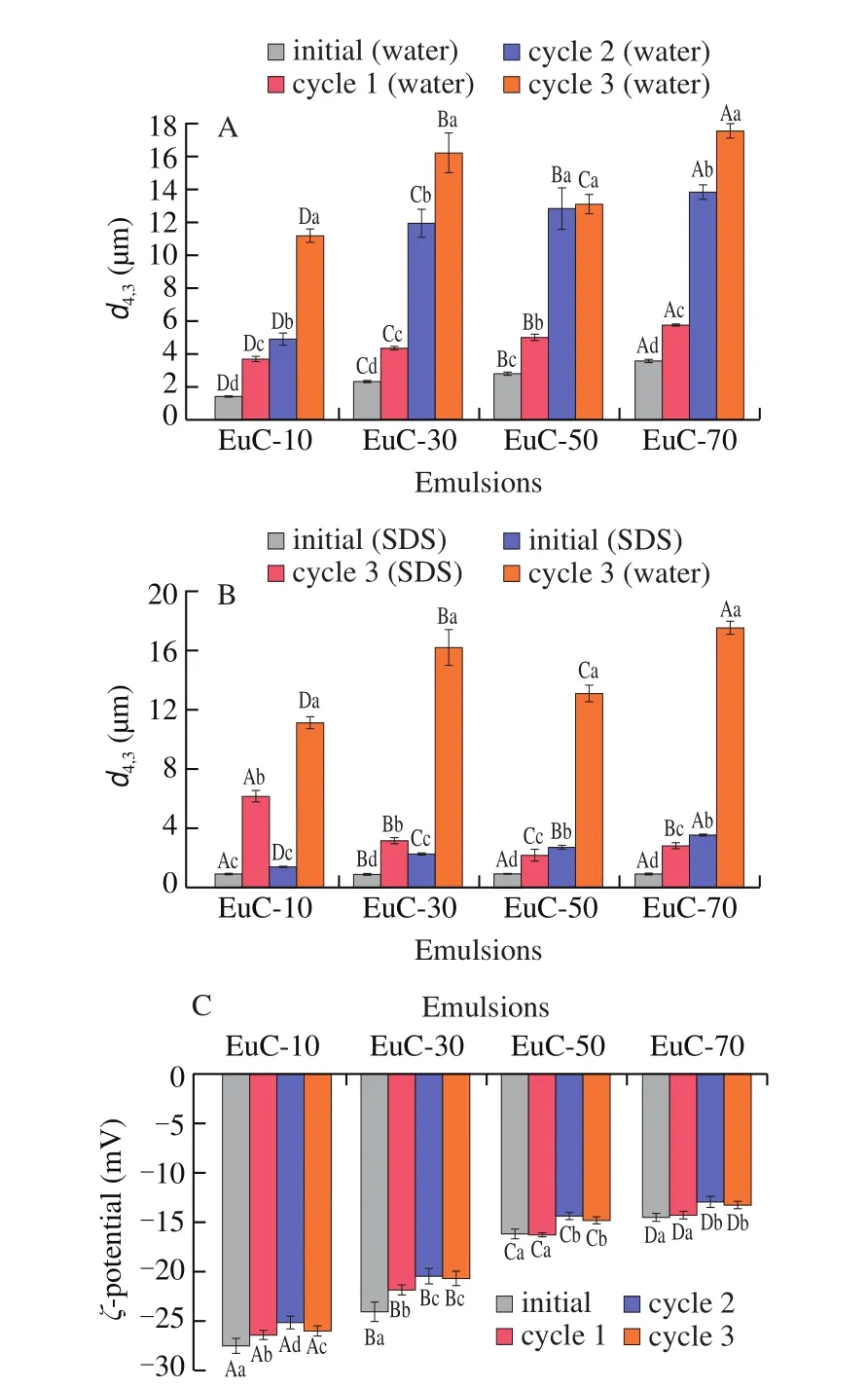
Fig. 6 De Brouckere, volume-weighted, mean diameter (d4,3) measured in deionized water (A), with comparison in deionized water and in 1% (m/V)SDS solution (B), and ζ-potential (C) of emulsions with NaCl addition(10-70 mmol/L) before and after different cycles of FT treatment. The different uppercase letters above the columns represent significant differences(P < 0.05) in the particle size or ζ-potential of different emulsions after the same cycle of FT treatment. The different lowercase letters above the columns represent significant differences (P < 0.05) in the particle size or ζ-potential of the same emulsion after different cycles of FT treatment.
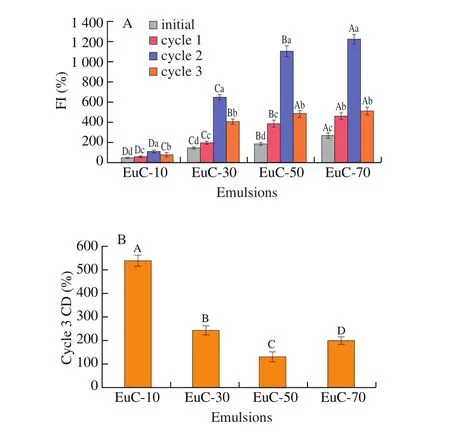
Fig. 7 fi(A) and CD (B) of emulsions with NaCl addition (10-70 mmol/L)after different cycles of FT treatment. The different uppercase letters above the columns represent significant differences (P < 0.05) in fiand CD of different emulsions after the same cycle of FT treatment. The different lowercase letters above the columns represent significant differences (P < 0.05) in fiof the same emulsion after different cycles of FT treatment.
ζ-Potential can characterize the intensity of mutual repulsion or attraction between emulsion droplets [30]. It can be seen from Fig. 6C that theζ-potential of the initial emulsions significantly decreased(P< 0.05) with increasing ionic strength, indicating the effective electrostatic screening with salt addition. The FT treatments had little influence on the ζ-potential of emulsions, that its absolute value reduced slightly with the cycle number of FT treatment, suggesting that moderate ionic strength can maintain the good stability of the emulsion system, and make it effectively resist the destruction of ice crystals to emulsions.
3.2.4 Flocculation index and coalescence degree
The fiof the initial emulsions increased with increasing ionic strength, suggesting that salt addition promoted the flocculation between emulsion droplets. However, the fidecreased for all emulsions after the third cycle of FT treatment (Fig. 7A), which might result from the destabilization offlocs by coalescence between droplets. The fiof the EuC-10 was significantly lower (P< 0.05) than other emulsions, whereas the CD was just the opposite (Fig. 7B). The results clarified that the ionic strength with 10 mmol/L NaCl could not make the emulsions form a stable interfacial structure, whose rigidity and thickness was not enough to resist the destruction of ice crystals to emulsions. When the emulsion system being gradually frozen, one crystallizing droplet might penetrate into the fluid region of another droplet, leading to the formation of irregular aggregates. As the emulsions thawed, the melt of partly crystallized droplets would merge together, resulting in a greater degree of coalescence [31].
3.2.5 Rheology properties
The influence of ionic strength (10–70 mmol/L NaCl) on the rheology of emulsions was evaluated. As shown in Fig. 8A, the apparent viscosity of emulsions reduced gradually with increasing shear rate, illustrating that there was a strong shear thinning behavior in the emulsions. Notably, the apparent viscosity increased with increasing ionic strength, indicated that the intermolecular forces in the aqueous system were greatly enhanced (including the interactions of protein-salt, protein-water and water-salt, etc.), which would affect the structure of emulsion system [32]. The elastic modulus (G’) was larger than loss modulus (G”) for all emulsions (Fig. 8B), indicating the formation of a gel network structure dominated by elasticity in the emulsion system. Moreover, bothG’ andG” enhanced with the increase of ionic strength, which was ascribed to the strengthening of interactions between interfacial proteins [33]. The highestG’ value(36.1 Pa) andG” value (4.4 Pa) at 1 Hz scanning frequency were examined in the EuC-70 (Fig. 8C). The presence of NaCl reduced the electrostatic interactions between emulsions droplets due to effective electrostatic screening, resulting in their flocculation, which was beneficial to form a gel-like network structure between aggregated emulsion droplets, thereby inhibit the movement of emulsion droplets [18,29]. Consequently, the ability of emulsions to resist dramatic variations of environmental factors could be improved.
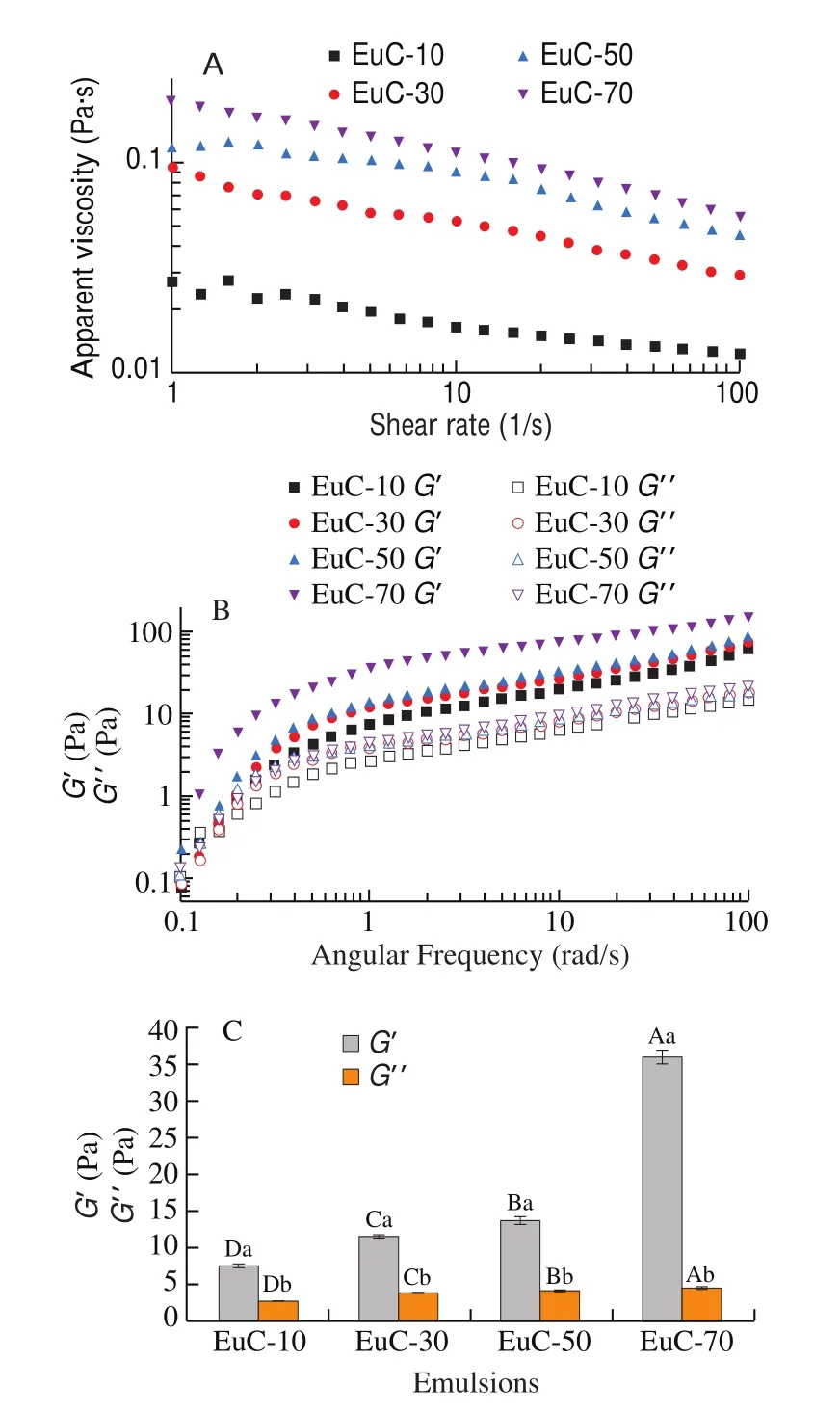
Fig. 8 Rheological properties of the initial emulsions with NaCl addition(10-70 mmol/L). The variation of the apparent viscosity of emulsions with shear rate ranging from 1 to 100 s-1 (A), the frequency sweep curve at stain value (1%) with angular frequency ranging from 0.1 to 100 rad/s (B) and the values of G’ and G” at frequency 1 Hz (C). The different uppercase letters above the columns represent significant differences (P < 0.05) in G’ or G” of different emulsions, and different lowercase letters above the columns represent significant differences (P < 0.05) between G’ and G” of the same emulsion.
3.2.6 Interfacial adsorption kinetics
Generally, the dynamic adsorption behavior of proteins at the oilwater interface was mainly composed of diffusion, penetration and rearrangement [34]. A previous study has shown that the stability of protein-stabilized emulsions was closely related to the adsorption kinetics at the oil-water interface [35]. It can be seen from Fig. 9A that the proteins with NaCl addition exhibited a fast adsorption rate from bulk to the oil-water interface, especially the changes of the interfacial pressure (π) was relatively constant in the first 100 s.TheKdiffof proteins with different ionic strengths (0, 10, 30, 50 and 70 mmol/L NaCl) were 0.462 4, 0.511 2, 0.644 7, 0.676 7, and 0.800 8 mN/m,respectively. TheKdiffincreased with increasing ionic strength, which might be related to the increase of adsorption quantity of interfacial proteins. When 50 mmol/L NaCl was added, the particle size of initial emulsion varied from 0.83 μm to 2.91 μm, and the AP increased by approximate 30% (Fig. 9C). At the same emulsification conditions,the increase in particle size of the initial emulsions might be ascribed to strengthen the rigidity and thickness of interfacial layers, which was also the result of the increase inKdiff.
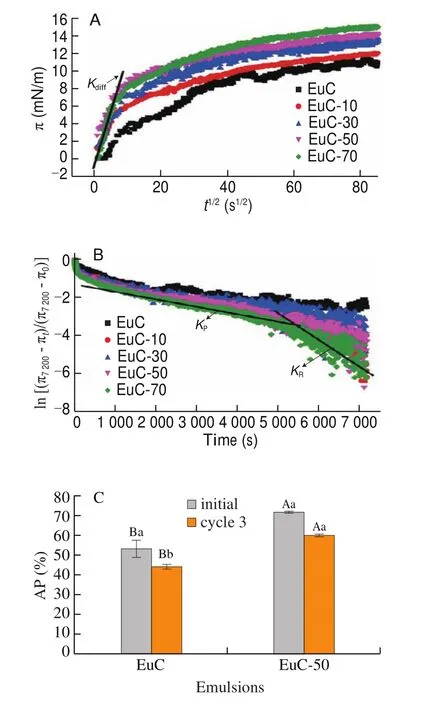
Fig. 9 Interfacial pressure (π) as the function of square root of adsorption time (t1/2) in the adsorption process (A) and the penetration and rearrangement curve of WPI aqueous dispersion without and with NaCl addition(10–70 mmol/L) at the oil-water interface (B). AP of emulsions without and with 50 mmol/L NaCl before and after the third cycle of FT treatment (C). The different uppercase letters above the columns represent significant differences(P < 0.05) in AP of different emulsions after the same cycle of FT treatment.The different lowercase letters above the columns represent significant differences(P < 0.05) in AP of the same emulsion after different cycles of FT treatment.
Notably, two linear regions could be observed in Fig. 9B. The slopes of the first region and second region were regarded asKpandKr,respectively. As shown in Fig. 9B, theKpandKrof the EuC-70 were -4 × 10–4and -6 × 10–4s-1, which were much higher than other emulsions, but its FT stability slightly declined. The reason was attributed that moderate ionic strength (30-50 mmol/L) can promote flocculation between emulsions to form a gel-like network structure,thereby inhibit the upward movement of oil droplets. However,higher ionic strength (70 mmol/L NaCl) might lead to an increase in the particle size of the initial emulsions, which was not conducive to resisting the coalescence between emulsion droplets during repeated FT treatments [17].
The above results illustrated that NaCl addition could enhance the adsorption, penetration and rearrangement rate of proteins at the oilwater interface, and improve the FT stability of emulsions eventually.Moderate ionic strength could weaken the electrostatic repulsion between proteins adsorbed at the oil-water interface. Therefore, the closer packing of proteins at the oil-water interface would promote more resistance to rupture of emulsions during the frozen process [36].It can be seen from Fig. 10A that the ζ-potential of protein particles decreased gradually with increasing ionic strength, suggesting that more negative charges on the surface of proteins were neutralized,thus accelerating aggregation between interfacial proteins [18].Simultaneously, the particle size of proteins decreased with increasing ionic strength (Fig. 10A), which can be attributed to the protein folding. Due to the ions effect, the hydrophobic groups on the surface of proteins would partially fold into the molecular interior, promoting protein molecules to form a more compact structure, which was more effective in preventing denaturation [37]. This was supported by the result of the surface hydrophobicity (H0) (Fig. 10B). It can be observed that theH0decreased first at low NaCl concentrations(e.g. < 50 mmol/L), and increased upon the ionic strength above 50 mmol/L, while theirH0was lower than unsalted protein. This demonstrated that proteins became more hydrophilic with the reduction of nonpolar groups on the surface. Therefore, the amounts of structural water molecules bound to proteins would increase significantly, which coinciding with the result of protein solubility(Fig. 10B). The ionic strength in the range of 30-50 mmol/L promoted the dissolution of proteins. Possibly, the adsorption concentrations of interfacial proteins might enhance with the increase of protein solubility, which was facilitated to improve the ability of interfacial layers to resist the puncture of ice crystals during freezing process [25,38]. The preferential hydration might be also the consequence that the perturbation of the surface free energy at the protein-water interface induced by salt addition [32]. At relatively high concentrations (e.g. 70 mmol/L), the structure of proteins might be altered with the change of hydrophobicity and electrostatic interactions between proteins, thus the combination of proteins to water molecules would relatively reduce [39,40]. Consequently, the improvement of the FT stability was primarily consequence that the interfacial stability of emulsions was enhanced, induced by salt addition.

Fig. 10 Particle size (Z-average diameter) and ξ-potential (A), and protein solubility and surface hydrophobicity (B) of WPI particles without and with NaCl addition (10-70 mmol/L). The different uppercase letters above the columns represent significant differences (P < 0.05).
4. Conclusion
In this study, the influence of particle size and ionic strength on the FT stability of emulsions stabilized by WPI were characterized.The results revealed that decreasing the particle size of the initial emulsions could improve the FT stability of emulsions by retarding the coalescence between oil droplets. The addition of NaCl to the WPI aqueous dispersion (4%,m/V) before emulsification would alter the structural properties of proteins. The formation of proteins with smaller particle size and lower surface charge could accelerate their aggregation at the oil-water interface and promote closer interfacial packing. The decrease in the surface hydrophobicity of proteins improved their solubility in the aqueous dispersions, which was beneficial to enhance the interfacial concentrations of proteins.Consequently, when proteins adsorbed from bulk to the oil-water interface, it was facilitated to enhance the interfacial stability of emulsions by strengthening the rigidity and thickness of interfacial layers, effectively resisted the puncture of ice crystals to emulsion droplets during the FT process. Simultaneously, NaCl addition could promote stronger flocculation between emulsion droplets, thus the formation of a gel-like network structure in the emulsion system that dominated by elasticity between emulsions might availably inhibit the movement of droplets, and improve the FT stability of emulsions eventually. It must also be mentioned that the improvement in the FT stability of emulsions stabilized by WPI might be achieved at lower ionic strength (30-50 mmol/L NaCl). The knowledge provided a viable source for the preparation of emulsion-based frozen foods with improved FT stability.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgment
This work was financially supported by National Natural Science Foundation of China (31871844 & 31501530).
- 食品科學(xué)與人類健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study

