Fractionation of edible bird’s nest glycoprotein hydrolysates:characterisation and antioxidative activities of the fractions
Poh Kei Chong, Sue Lin Mun, Lee Sin Chng,b,*, Abdul Slm Bbji, Seng Joe Lim,c,*
a Department of Food Sciences, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor, Malaysia
b Department of Food Science and Nutrition, Faculty of Applied Sciences, UCSI University Kuala Lumpur, No.1, Jalan Menara Gading, UCSI Heights 56000 Cheras, Kuala Lumpur, Malaysia
c Innovation Centre for Confectionery Technology (MANIS), Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor, Malaysia
d Centre for Innovation and Technology Transfer (INOVASI-UKM), Chancellery, Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor, Malaysia
Keywords:
Fractionates
Gel permeation chromatography
Hydrolysates
Peptide content
Sialic acid
A B S T R A C T
E dible bird’s nest (EBN) hydrolysates have been proven to exhibit enhanced bioactivities. However, being a macromolecule, fractions with different molecular weights would have different properties and bioactivities.Hence, this research was aimed to determine the chemical properties and antioxidant activities of freezedried (EBNFD) and spray-dried EBN (EBNSD) hydrolysates fractionated using gel permeation chromatography(GPC). Overall, two well-separated fractions were identified (EBNfFD1, EBNfFD2, EBNfSD1 and EBNfSD2). EBNFD demonstrated significantly higher (P ≤ 0.05) peptide (3.6%), total carbohydrate (27.7%) and sialic acid(18.2%) contents than that of EBNSD. Similar trend was observed in low molecular weight fractionates (EBNfFD2 and EBNfSD2). Meanwhile, the first fractionates (EBNfFD1 and EBNfSD1) exhibited significantly higher (P ≤ 0.05)hydroxyl radical (·OH) scavenging activity. Fourier transform infrared (FTIR) spectroscopy demonstrated that all EBN fractionates have similar spectrum, except in the region of N H (amide II) and C H alkyl group.In conclusion, EBN fractionates with different molecular weights showed different chemical properties and antioxidant activities.
1. Introduction
Edible bird’s nest (EBN) is a well-known delicacy among Chinese community, produced from nest that is made up from solidified saliva of insectivorous swiftlets. According to Wong et al. [1], the secretary of Sarawak’s Bird Nest Supplier Society, the price of processed EBN cost as much as 2 200–2 500 USD/kg and unprocessed EBN cost at least 1 200–1 500 USD/kg in the year of 2017 and these values are expected to increase continuously. This is due to the recognition of the public regarding its nutritional benefits and the presence of essential trace element as a health-improving food.
EBN, essentially a glycoprotein, composed mainly of proteins(62.0%–63.0%) and carbohydrates (25.6%–27.3%) [2]. The carbohydrates are made up of 9% sialic acid, 7.2% galactosamine,5.3% glucosamine, 16.9% galactose and 0.7% fucose [3,4]. Meanwhile,EBN contained all the 9 essential amino acids (43%) and 9 nonessential amino acids (57%) that accommodate 30.17 g/100 g of EBN samples, which are needed for vitalin vivoprocesses [5]. EBN has been reported to prevent influenza virus infection by neutralising the infection and inhibiting the agglutination of red blood cell, which was caused by the infection of the virus [6]. A study by Matsukawa et al. [7]showed that EBN exhibited the ability to reduce bone density loss by improving calcium concentration and slowing down skin aging. EBN was also found to synergistically induce cell proliferation which is important in cell regenerating and wound healing [8]. EBN extract was reported to exhibit neuroprotective effect by enhancing memory and inhibit neuroinflammatory in Wistar rat, in which the sialic acid from EBN could be useful against neurodegenerative diseases [9]. All these studies demonstrated the great interest of EBN in enhancing the health status of consumer which are beneficial for development and improvement of immune system.
It is prevalence that protein hydrolysates contain a great deal of small fraction of peptides that functions physiologically by exhibiting bioactive properties. Several studies reported that enzymatic hydrolysed EBN exhibited higher free radical scavenging activities [10,11],antihyperglycemic property [12], antimicrobial property [13]and angiotensin conversion enzyme inhibition (ACE-i) activity [14]as compared to unhydrolysed EBN. However, all these findings were conducted on the cleaned EBN hydrolysate, whereby these hydrolysates contain bioactive peptides that can be further fractionated based on the molecular weight, which has not been studied. From the point of view of application and economic production of bioactive peptides, the main consideration is to obtain the bioactive peptide fraction through purification, separation and isolation in order to be applied as functional ingredient. For instance,the bioactive peptide fraction that exhibited ACE-i property was obtained from molecular weight fractionated casein hydrolysate and was used to develop antihypertensive functional food in Japan [15].Meanwhile, fractionation of fish protein hydrolysate was reported to obtain calcitonin gene-related bioactive peptide [16]. Two novel antioxidant peptides were also isolated and identified from the EBN pepsin-trypsin hydrolysate [17]. Therefore, this creates a great interest to study the potential of EBN hydrolysate purified fraction in order to understand the activities and structure of the peptides.
Hence, the aim of this study was to produce EBN hydrolysate through different drying methods, and subsequently separated using gel permeation chromatography (GPC) to purify the EBN fractionates based on different molecular weights. The EBN hydrolysates and collected fractionates were analysed for chemical properties, antioxidant activities and subjected to Fourier transform infrared (FTIR) spectroscopy analysis. It was hypothesised that the different EBN fractions with different molecular weights will exhibit different activities. This research is beneficial to the EBN processing industry as it broadens the application of EBN especially in food and pharmaceutical industries according to its respective activities.
2. Materials and methods
2.1 Materials
Cleaned white EBN was purchased from Mobile Harvesters (M)Sdn Bhd (Selangor, Malaysia). Alcalase fromBacillus licheniformis(Novozyme Corps, Denmark) was used for enzymatic hydrolysis.Chemicals used in all analysis were analytical grade and were purchased from Sigma-Aldrich, UK.
2.2 Enzymatic hydrolysis of EBN
Preparation of EBN hydrolysate was carried out according to method described by Khushairay et al. [18]with slight modifications.EBN (3 g) was soaked in 100 mL distilled water and maintained at(4 ± 2) °C for 16 h. Subsequently, the solution was heated using double boiling method at (90 ± 2) °C for 30 min and then cooled to(60 ± 2) °C. Alcalase (1.8 g) was added to the solution and incubated at (60 ± 2) °C for 60 min in an incubator (Hotech Instruments Corp.,Taiwan). Next, the solution was brought to boil for 5 min to deactivate the enzyme. The hydrolysate was centrifuged (Kubota 2420, Japan)at 2 610 ×gfor 10 min at (4 ± 1) °C and the supernatant was filtered using a Whatman No. 1 filter paper.
2.3 Drying of EBN hydrolysate
The EBN hydrolysate solution (200 mL) was freeze-dried(EBNFD) using a benchtop freeze dryer (Biobase NFD-S1 Standard Chamber Freeze Dryer, China). The solution was frozen at–(18 ± 2) °C for 5 h prior to freeze drying at –(70 ± 2) °C for 18 h at 1 Pa. Meanwhile, spray-dried EBN hydrolysate (EBNSD) was produced using an industrial used spray dryer (Agridon, Malaysia).The inlet temperature and outlet temperature were (180 ± 2) °C and(100 ± 2) °C, respectively, with feed flow rate of 13 L/h.
2.4 Gel permeation chromatography
Dried EBN hydrolysates (EBNFDand EBNSD) were separated and purified based on its molecular weight using GPC technique. It was performed using an AKTAPrime Plus instrument (GE Healthcare,USA) equipped with a Sephadex G-25 superfine column HiTrapTM5 mL desalting column (GE Healthcare, USA). EBN hydrolysate(50 mg) was made to 5 mg/mL concentration with distilled water.The solution was filtered through a 0.45 μm poly tetra fluoroethylene syringe filter (Minisart Sartorious, Germany). One mL of solution was injected into the system and eluted with a linear gradient of elution buffer (made up of 50 mmol/L NaHPO4, 0.5 mol/L NaCl and 20–300 mmol/L imidazole) at a flow rate of 1.0 mL/min [19]. The elution was monitored at 280 nm and the system was operated at 1 MPa. The eluted fractionates were collected and freeze-dried. There were 2 fractionates from each sample, labelled as EBNfFD1, EBNfFD2,EBNfSD1and EBNfSD2.
2.5 Chemical analyses of EBN hydrolysates and its fractionates
2.5.1 Peptide content
The peptide contents were determined using spectroscopy method [20]with slight modification. EBN hydrolysates and fractionates(50 μL, 1.0 mg/mL) were mixed with 2 mL ofO-phthalaldehyde (OPA)reagent (25 mL 100 mmol/L sodium tetraborate mixed with 2.5 mL 20 g/100 mL of sodium dodecyl sulphate added with 40 mg OPA and 100 μLβ-mercaptoethanol) and incubated for 2 min at ambient temperature. It was then measured using an EpochTMMicroplate Spectrophotometer (Bio Tek, Vermont, US) at 340 nm wavelength.Leucine at 100–1 000 mg/L was used to plot standard curve.
2.5.2 Total carbohydrate content
The total carbohydrate content was determined using phenolsulphuric acid method [21]. EBN hydrolysates and fractionates(50 μL, 1.0 mg/mL) were added with 50 μL 5% phenol solution and 150 μL of concentrated sulphuric acid. The mixture was left for 30 min and read at 490 nm using microplate spectrophotometer. A standard calibration curve was obtained from glucose solution (100–1 000 mg/L)and calculate using equation below.
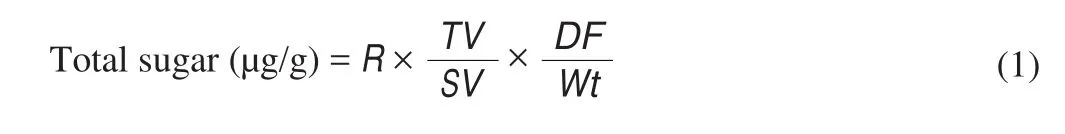
where,Rrepresents the sugar content from calibration curve (μg),TVrepresents the sample volume (mL),SVrepresents the sample volume on microplate (mL),DFrepresents the dilution factor,Wtrepresents the sample mass (g).2.5.3 Sialic acid content
Sialic acid content was determined using the resorcinol method [11].EBN hydrolysates and fractionates (250 μL, 1.0 mg/mL) were added with 250 μL resorcinol reagent (0.22 g of resorcinol was added with 10 mL of distilled water, 80 mL concentrated hydrochloric acid and 0.25 mL 0.1 mol/L copper sulphate then topped up to 100 mL with distilled water) and incubated at 100 °C for 15 min using test tubes covered with cooled marbles. Subsequently, 1 mL 1-butanol was added and vortexed for 10 s and cooled down with ice water for 10 min. The mixture solution (200 μL) was read at 580 nm andN-acetylneuraminic at concentrations of 100–1 000 mg/L was used as calibration curve.
2.6 FTIR spectroscopy
A FTIR spectrometer (Perkin Elmer Precisely, Spectrum 400,FT-IR/FT-NIR, Massachusetts, USA) was used to identify the presence of functional groups in EBN hydrolysate and fractionates.The attenuated total re flection (ATR) Spectrum Universal Accessory with a wavelength of 4 000–650 cm-1was fitted into the system. A total of 4 scans were performed on the samples at a resolution of 4 cm-1[22]. The vibrational spectra were collected and analysed using Essential FTIR?1.1.0.0 software (Madison, USA).
2.7 Antioxidant activities of EBN hydrolysates and its fractionates
2.7.1 Free radical scavenging activity assay
Free radical scavenging activity was determined according to the scavenging ability of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals [23].Samples (0.5 mL, 1.0 mg/mL) were mixed with 0.5 mL 0.05 mmol/L methanolic DPPH solution. The mixture was left in the dark for 30 min and the absorbance was measured at 517 nm using a microplate spectrophotometer. Ascorbic acid (0.1–1.0 mg/mL) was used to plot a standard calibration curve. Free radical scavenging activity was calculated using equation below.
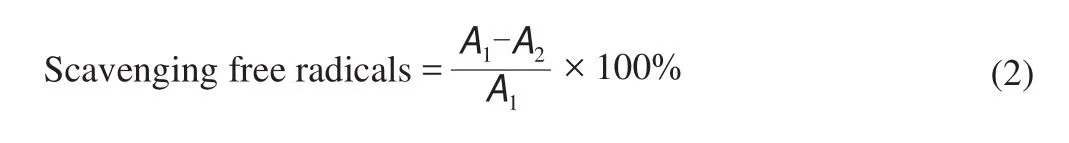
where,A1is the blank DPPH absorbance andA2is the sample absorbance. The DPPH radical scavenging activity was expressed in terms of ascorbic acid equivalent (AEAC) per 100 mL.
2.7.2 Hydroxyl radical (·OH) scavenging activity assay
Hydroxyl radicals were generated from Fenton’s reaction, where 0.5 mL 9 mmol/L iron (II) sulphate (FeSO4) was mixed with 1.0 mL 8 mmol/L hydrogen peroxide (H2O2). This mixture was added with 1.0 mL samples (1.0 mg/mL). Next, 0.2 mL 9 mmol/L salicylic acid was added and another series of mixtures were prepared without the addition of salicylic acid. All the mixtures were incubated at 37 °C for 60 min and read at 510 nm using a microplate spectrophotometer [24].Hydroxyl radical scavenging activity was calculated using the equation below.
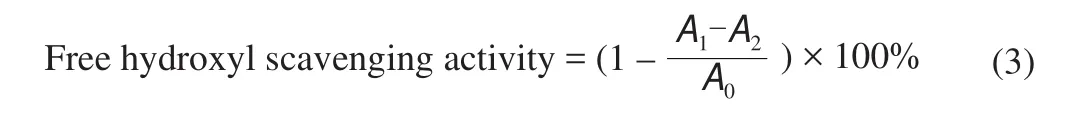
where,A0is the blank absorbance,A1is the absorbance of sample with salicylic acid andA2is the absorbance of sample without salicylic acid.
2.8 Statistical analysis
Samples were analysed in triplicate and presented as mean ±standard deviation. One-way analysis of variance test was performed followed by Tukey’s test to determine the significant differences among the samples at 95% con fidence interval. The factors that were considered were the effects of drying (freeze-dried vs spray-dried),effects of purification (hydrolysate vs fractionate) and the separated fractions based on molecular weight (fraction 1 vs fraction 2). Minitab software version 17.0 (Minitab Pty Ltd., Sydney) was employed to conduct the statistical test.
3. Results and discussion
3.1 Fractionation of EBN hydrolysate using GPC
Fig. 1 shows the chromatogram of the EBN hydrolysates fractionated from GPC. It was found that fractionation of EBN hydrolysate dried using freeze drying (Fig. 1a) and spray drying(Fig. 1b) methods showed 2 peaks, indicating 2 fractionates with different molecular weights were separated. Both EBNFDand EBNSDhydrolysates showed 2 similar fractionates with similar retention times, indicating the different drying methods did not affect the hydrolysate compositions. Based on the principal of GPC [25], the first fractionate (retention time of 0.7–3.0 min) which was eluted out first has the higher molecular weight in comparison to the second fractionates (retention time of 3.0-4.0 min) (Fig. 1). The first fractionate for EBN hydrolysate produced from freeze drying and spray drying was collected and labelled as EBNfFD1and EBNfSD1,respectively, while the corresponding second fractionates were labelled as EBNfFD2and EBNfSD2, respectively. Molecular weight is one of the important parameters in determining protein hydrolysis and correlating with hydrolysate protein biological activities [26].Two peptide fractions of different molecular weights were also obtained using GPC separation from fish hydrolysate [27], indicating the feasibility of separation based on molecular weight using GPC technique. In this study, the EBN hydrolysates and fractionates collected were analysed further to have better comprehension of their intrinsic properties and activities.
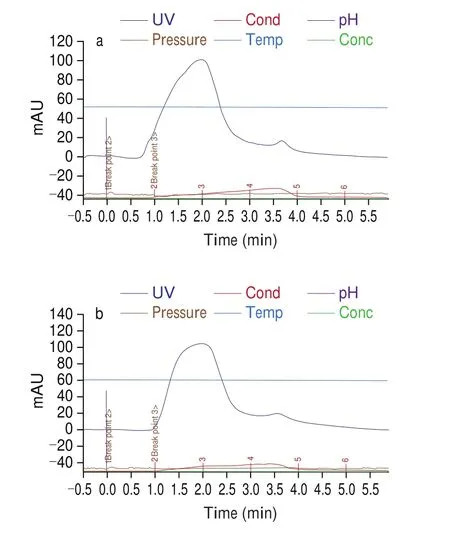
Fig. 1 GPC chromatograms of fractionation of EBN hydrolysate dried using (a) freeze drying and (b) spray drying technique.
3.2 Peptide Content
Table 1 shows the peptide contents of EBN hydrolysates and the fractionates. The results showed that the peptide contents were ranged between 20.57 and 26.33 mg/g for all samples. EBNFDhas the highest peptide content ((26.33 ± 0.12) mg/g) which was significantly higher(P≤ 0.05) than that of EBNSD((25.38 ± 0.12) mg/g), indicating that freeze drying method is milder towards the peptides in the molecule compared to that of spray drying method. This might be due to heat treatment used in spray drying which causes the occurrence of protein denaturation and thus, reducing the peptide content in the sample. Exposure to high temperature (190 °C) during atomisation might cause destabilisation of protein structure by decomposition of disulphide bond. In addition, EBN hydrolysates exhibited significantly higher (P≤ 0.05) peptide contents than the fractionates. However,opposed finding was reported in Picot et al. [16]in fish protein hydrolysate where the protein content in hydrolysate was lower than filtered fractionates.
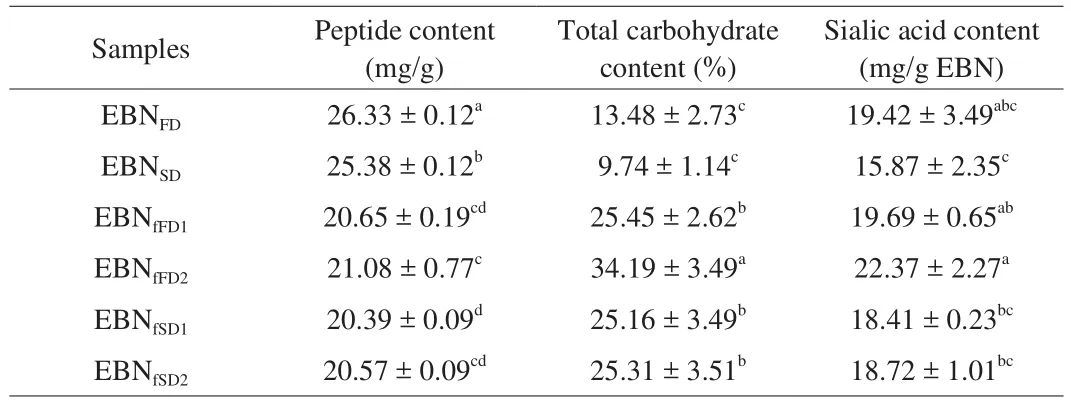
Table 1Peptide, total carbohydrate and sialic acid content for EBN hydrolysate (EBNFD and EBNSD) and fractionates (EBNfFD1, EBNfFD2, EBNfSD1 and EBNfSD2) (n = 3).
Fractionates wise, EBNfFD2has the highest peptide content((21.08 ± 0.77) mg/g) while EBNfSD1contained the lowest peptide content ((20.39 ± 0.09) mg/g). Overall, the second fractionates(EBNfFD2and EBNfSD2) with lower molecular weights exhibited higher peptide contents as compared to the first fractionates (EBNfFD1and EBNfSD1) with higher molecular weights. This indicates small molecular weight compound is associated with higher peptide content.A study conducted by Li et al. [27]showed that the fractionate of glycoprotein from fish hydrolysate separated using GPC technique exhibited a lower protein content in the first fractionate (52.72%)as compared to the third fractionate (55.42%), indicating large molecule weight fraction has low protein content. Similar trend was also reported in soybean peptide system, in which the fraction with lower molecular weight (< 1 000 kDa) had a higher peptide content(37.8 mg/mL) as compared to the fraction with higher molecular weight (> 5 000 kDa, 1.12 mg/mL) [28].
3.3 Total carbohydrate content
Total carbohydrate contents for EBN hydrolysates and fractionates are shown in Table 1. In this study, the total carbohydrate content for all the EBN samples were ranged from 9.74% to 34.19%. These values were in consistent with the values reported by Ma et al. [3]and Saengkrajang et al. [29]in EBN which were 10.63%–27.26% and 25.4%–31.4%, respectively. The difference of total carbohydrate content could be due to the geographical area and conditions [29].Freeze-dried EBN hydrolysate exhibited higher total carbohydrate content ((13.48 ± 2.73)%) as compared to spray-dried EBN hydrolysate ((9.74 ± 1.14)%). This could be due to the heat exposure during spray drying which increases the chance of sugar degradation and reduces the availability of total carbohydrate in the spray-dried sample [30].
Meanwhile, EBN fractionates showed significantly higher(P≤ 0.05) total carbohydrate content in comparison with EBN hydrolysates. This might be due to the EBN hydrolysate was purified after GPC elusion [31]. A study conducted by Li et al. [27]demonstrated that total carbohydrate content in fish protein hydrolysate was lower than the isolated fractionate. Meanwhile, EBNfFD2recorded the highest total carbohydrate content ((34.19 ± 3.49)%)significantly (P≤ 0.05) and followed by EBNfFD1with (25.45 ±2.62)%. The second fractionates (EBNfFD2and EBNfSD2) with lower molecular weight showed a higher total carbohydrate content as compared to the first fractionates (EBNfFD1and EBNfSD1) with higher molecular weight. This result is consistent with finding reported by Li et al. [32], which concluded that fractionates with lower molecular weight contained higher total carbohydrate content than fractionate with higher molecular weight in dates (Zizyphus jujube). Higher carbohydrate content was also reported in low molecular weight fraction (24.3%) as compared to high molecular weight fraction(14.2%) in fish hydrolysate [27]. This could be due to the pathway of synthesis and the extraction conditions of the poly- or monosaccharides that may account the variation of the carbohydrate content in the fraction [33]. Besides, the varying temperature at which the drying process was carried out may also contribute to different polysaccharides content in the fraction [33].
3.4 Sialic acid content
Table 1 shows the sialic acid content for EBN hydrolysates and fractionates. The EBN samples contained 15.87–22.37 mg/g of sialic acid content. The EBN samples in the current study have higher sialic acid contents as compared to work reported by Norhayati et al. [34]and Kathan et al. [4]in EBN, at 0.7%–1.5% and 9%, respectively.Although the average sialic acid content in EBNFD((19.42 ± 3.49) mg/g)was higher in comparison to that of EBNSD((15.87 ± 2.35) mg/g),but the value had no significant difference (P> 0.05), indicating sialic acid was relatively heat-stable regardless of the drying methods used. Up to date, no study has been reported on the retention of sialic acid from different drying methods. However, Gan [24]reported that low temperature used in IR-UVC drying resulted in better retention of sialic acid in EBN as compared to hot air drying that used higher drying temperature.
According to Table 1, it was evident that the respective fractionates have higher sialic acid contents compared to that of the EBN hydrolysates. This is expected as the total carbohydrate content of fractionate was higher than hydrolysate and sialic acid is considered as a compound that made up of carbohydrate.This may be due to the increased purity of the glycoproteins after the GPC elution of the samples [31]. EBNfFD2showed the significantly (P≤ 0.05) highest sialic acid content, which was(22.37 ± 2.27) mg/g. For both freeze- and spray-dried fractionates,the second fractionates (EBNfFD2and EBNfSD2) exhibited higher sialic acid content as compared to the first fractionates (EBNfFD1and EBNfSD1). No study has been reported on the relationship between the molecular weight with the sialic acid content. Current work showed that fractionate with lower molecular weight was associated with higher sialic acid content.
3.5 Fourier Transformer Infrared Analysis (FTIR)
Fig. 2 displays a typical absorption spectrum of EBN hydrolysate,similar as reported in Guo et al. [36]and Hamzah et al. [37]in pure and authentic EBN, respectively. The FTIR spectrum (Fig. 2) reveals that a total of twelve functional groups are recorded and listed in Table 2. A broad absorption band at the range of 3 267.22–3 275.29 cm-1was detected and was identified as hydrogen bonded (O-H) stretching vibration [38]indicating more moisture content in EBNFD. This could be due to EBNSDwas exposed to high heat treatment during spray drying which caused greater loss of water.
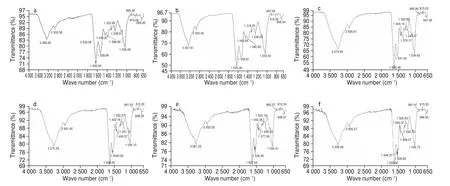
Fig. 2 FTIR spectrums for EBN hydrolysate and fractionates. (a) EBNFD; (b) EBNSD; (c) EBNfFD1; (d) EBNfFD2; (e) EBNfSD1; (f) EBNfSD2.
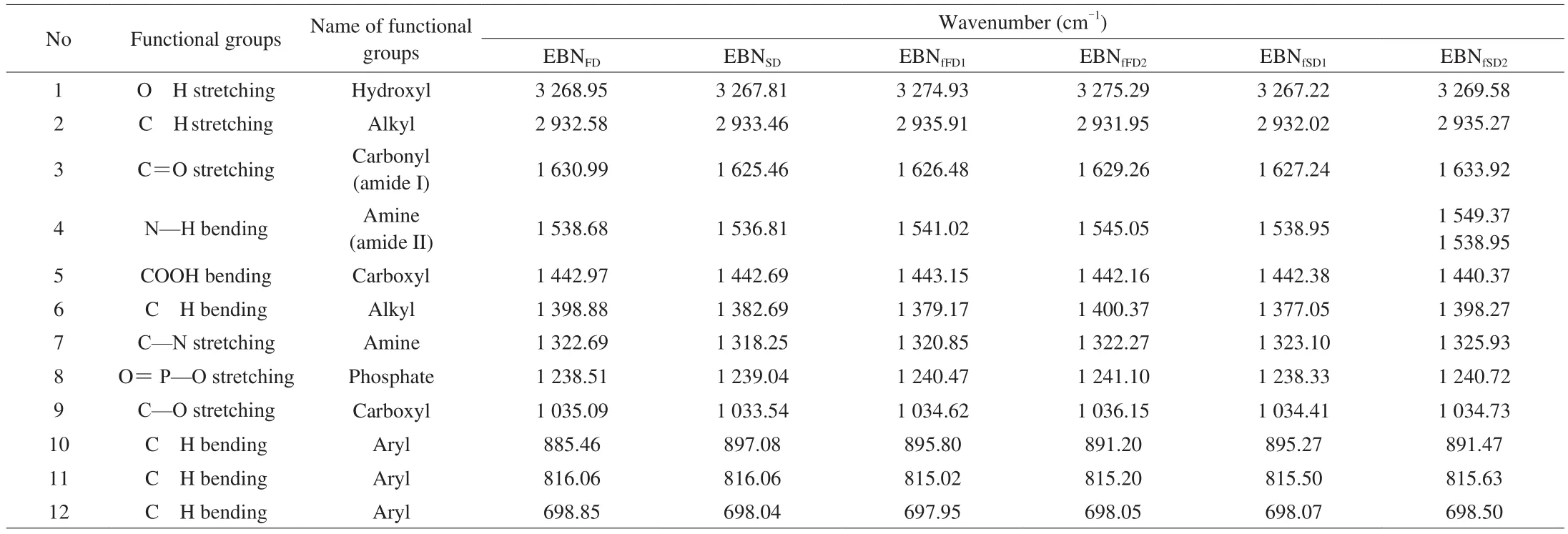
Table 2FTIR spectrum analysis result for EBN hydrolysate (EBNFD and EBNSD) and fractionates (EBNfFD1, EBNfFD2, EBNfSD1 and EBNfSD2).
The absorption peak around 2 932.58–2 935.91 cm-1is attributed to the alkyl C—H stretching vibration [38]fucoidan was isolated from S. binderi (yield 7.5%, purity 89.63%, which may be due to methyl(CH3) stretching [37]or methylene (CH2) stretching [40]. Meanwhile, the absorption band around 1 625.46–1 633.92 cm-1is assigned to the carbonyl(C=O) stretching vibration (amide I) while 1 536.81–1 549.37 cm-1is resulting bending vibration of amine, N-H bond (amide II) [37,40].Similar functional groups were detected in authentic EBN and EBN grade 2A to 5A by Guo et al. [36]and Hamzah et al. [37],respectively. Set [41]demonstrated that major functional groups in protein have peaks between 1 640 and 1 520 cm-1.At this region, EBNfFD2and EBNfSD2exhibited a higher transmission as compared to the EBNfFD1and EBNfSD1. This result was expected as the analysis of peptide contents (Table 1) indicates that the lower molecular weight fractions contained higher peptide content than the higher molecular weight fractions. It is noticeable that at the region of N—H bending vibration (amide II), the samples of EBNfFD2(Fig. 2d), EBNfSD1(Fig. 2e), and EBNfSD2(Fig. 2f) exhibited slightly different pattern of spectrum, which the EBNfSDdemonstrate a few vibration signals as compared to the smooth spectrum of EBNSD(Fig. 2b). Additionally, symmetric bending vibration of carboxylic group (COOH) with wavenumber from 1 442.69–1 443.15 cm-1was detected, which was also reported by Hamzah et al. [37]in EBN samples. Meanwhile, all samples showed a peak closed to 1 400 cm-1which indicates the presence of polysaccharides, i.e the bending vibration of the C-H (CH2) group [40]. It was notable that there was a slight different of the spectrum demonstrated by the fractionate 1 and 2,in which the first fractionate displayed a sharp peak (Fig. 2c and 2e)while the second fractionate (Fig. 2d and 2f) exhibited a flat signal.Also, all the samples demonstrated a peak near the wavenumber of 1 318.25 cm-1which is the stretching of amine group (C-N) [40].
Phosphate (O=P-O) functional group was found at the wavenumbers of 1 238.51–1 241.10 cm-1due to the stretching vibration of phosphate in nucleic acid. Similar result was reported by Guo et al. [36]and Han et al. [42]in authentic EBN. All EBN samples exhibited absorbance from 1 033.54–1 036.15 cm-1, which was identified as stretching vibration of carboxyl group (C-O) of the C-OH groups of carbohydrates [40]. From Fig. 2, polysaccharides absorption band in EBNfFD2(Fig. 2d) and EBNfSD2(Fig. 2f) exhibit a higher transmission as compared to EBNfFD1(Fig. 2c) and EBNfSD1(Fig. 2e). This observation is corresponded to the result of total carbohydrate content(Table 1), where the second fractionate contained higher total carbohydrate content than the first fractionate.
There was weak signal detected at the region of 897–697 cm-1due to out of plane bending of aromatic C H bonds [40]. A previous study conducted by Guo et al. [36]stated that the presence of peak at the wavenumber of 875–698 cm-1indicated the EBN was originated from cave nest as the house nest EBN did not exhibit any peak at this region. Generally, all the FTIR spectra denoted the main compounds presence in EBN hydrolysate and fractionates are protein and carbohydrates. Overall, the shape, position and pattern of EBN hydrolysate and fractionates were almost similar except that in the fingerprint of N-H amine bending vibration (amide II) and C-H alkyl bending vibration, which different pattern was observed in the EBN fractionates. Only small differences were observed in the FTIR spectra of almond gum and its fractions, that was obtained from separation using GPC, indicating the molecular weight of the fractions did not differ in the functional group but affect the intensity of the peaks [43].
3.6 DPPH radical scavenging activity
Fig. 3 shows the DPPH free radicals scavenging activity for EBN hydrolysates and fractionates. Generally, the DPPH radical scavenging activity ranged from (69.26 ± 3.64) AEAC mg/100 mL (EBNSD)to (78.83 ± 1.75) AEAC mg/100 mL (EBNfFD1), indicating EBN is a good source of antioxidant. Ramachandran et al. [44]reported that the highest DPPH radical scavenging activity (46.15%) when EBN was hydrolysed at 100 °C for 180 min. There was no significant difference (P> 0.05) between the freeze-dried and spray-dried EBN hydrolysate and fractionates, indicating the drying method did not affect the scavenging activity. No reported study on the DPPH radical scavenging activity between freeze-dried and spray-dried hydrolysate and fractionates. However, Gan [35]reported that the DPPH of higher temperature air-dried EBN was lower than IR-UVC drying.
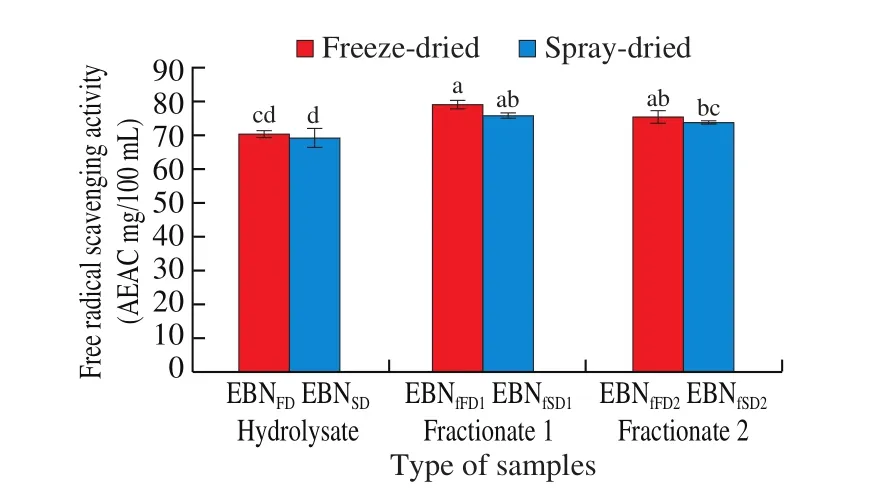
Fig. 3 DPPH radical scavenging activity of the freeze-dried and spray-dried EBN hydrolysates and EBN fractionates (n = 3).
Meanwhile, significantly higher (P≤ 0.05) DPPH radical scavenging activities were recorded in EBN hydrolysate fractionates as compared to EBN hydrolysates, signifying fractionates has higher capability in scavenging DPPH radicals than EBN hydrolysate.Fractionation of EBN through GPC helps to purify the fractionates and hence, EBN fractionates with higher DPPH radical scavenging properties were obtained. This may be due to exposure of amino acid to capture DPPH radical [26]and also the presence of sialic acid content (section 3.4) which acts as an antioxidant agent [45]. This agreed with studied by Ghassem et al. [17]that EBN hydrolysate had lower DPPH radical scavenging capacity than EBN fractionates that fractioned using ultrafiltration cut-off membranes. Slightly higher DPPH scavenging activities were recorded in the first fractions than the second fractionate. However, the difference was not significant(P> 0.05), indicating the molecular weight did not influence the DPPH radical scavenging activity. However, opposed finding was reported by Ghassem et al. [17]which a higher antioxidant capacity (DPPH assay) was exhibited by the ultrafiltrated EBN fractionate with lower molecular weight. The correlations between antioxidant capacity with the proteins and polysaccharides content of fractionates of fish hydrolysate have been studied [27]and the result reported that DPPH radical scavenging activity of the fractions showed positive correlation with protein content but negatively correlated with carbohydrate content. However, the DPPH radical scavenging activity was depending on the ratio of different monosaccharides in the composition of fractions and the enzyme specificity [27], which may led to different scavenging activities of the fractions.
3.7 Hydroxyl radical scavenging activity
The EBN under study exhibited hydroxyl radical scavenging activity (·OH) of 20.81%–37.91%. Fig. 4 shows that significantly higher (P≤ 0.05) ·OH scavenging activity was recorded in EBNFD((34.15 ± 1.53)%) as compared to EBNSD((20.81 ± 1.02)%),indicating freeze-dried hydrolysate exhibited higher antioxidant activity. Similar trend was obtained in EBN fractionates. The lower scavenging activity for spray-dried samples could be due to exposure of heat during drying [35]. Report from Gan [35]showed EBN produced from IR-UVC (lower temperature) exhibited higher antioxidant activities than that of hot air-drying (higher temperature).
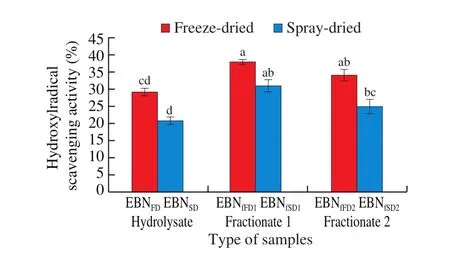
Fig. 4 Hydroxyl radical scavenging activity of the freeze-dried and spraydried EBN hydrolysates and EBN fractionates (n = 3).
In this study, ·OH scavenging activities exhibited by EBN fractionates were higher as compared to EBN hydrolysates. This may be due to the purification of hydrolysate produce fractions with mixed peptides/amino acids which had higher ability to quenching and reduce the ·OH through peptide chelation [46]. Peng et al. [46]indicated that whey protein isolate (WPI) hydrolysate fraction separated using Sephadex G-10 exhibited a higher ·OH radical scavenging activity than WPI hydrolysate sample. The electron spin resonance spectrum test showed that larger signal of ·OH was inhibited by WPI hydrolysate fraction, indicating the peptide size and composition of fractions affected the ·OH quenching activity. Meanwhile,the first fractionates showed significantly higher (P≤ 0.05)·OH scavenging activity in comparison to second fractionates,indicating higher molecular weight fraction is associated with higher·OH scavenging activity. A study conducted by Li et al. [47]also demonstrated similar result, in which the first fractionate separated using gel filtration column (Sephadex) had higher ·OH scavenging activity than second and third fractionates in chickpea protein hydrolysate. Both peptides size and composition of fractionates influenced the scavenging activity which small peptide inhibits of ferric ion contributes to ·OH reduction [47].
4. Conclusion
EBN hydrolysates have been scientifically proven to exhibit several bioactivities. From the current study, EBN hydrolysates produced from freeze drying and spray drying methods were further purified by separation according to different molecular weights using GPC. Overall, hydrolysate obtained from freeze drying showed desirable chemical properties and antioxidant activities than that of spray drying method. Both hydrolysates were further separated, and the GPC results indicated two distinct fractions that were successfully recovered from the EBN hydrolysate. There is a significant difference between the EBN hydrolysates and EBN fractionates, in which the EBN fractionates have higher peptide, total carbohydrate and sialic acid contents, and antioxidant properties as compared to the unfractionated EBN hydrolysates. The lower molecular weight fraction (fraction 2) has the highest total carbohydrate and sialic acid contents. On the other hand, the higher molecular weight fraction(fraction 1) exhibited the highest DPPH radical scavenging activity and hydroxyl radical scavenging activity. FTIR spectroscopy analysis showed that EBN fractionates exhibited different pattern in the region of N-H (amide II) and C-H alkyl group. In conclusion, the results supported the hypothesis that different EBN fractions that possess different molecular weights exhibited different chemical properties and antioxidant activities. Further research are recommended to study the composition and structure of the isolated bioactive fractions.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This project was funded by the International Collaboration Fund (IF0119A1053) provided by the Ministry of Energy, Science,Technology, Environment and Climate Change (MESTECC),Malaysia, and the Fundamental Research Grant Scheme(FRGS/1/2019/WAB01/UKM/02/1) provided by Ministry of Higher Education Malaysia. The authors would like to acknowledge the Department of Food Sciences, Faculty of Science and Technology,Universiti Kebangsaan Malaysia and Mobile Harvesters Malaysia Sdn. Bhd. for providing the necessary facilities and samples for this research.
- 食品科學(xué)與人類健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study

