Tissue distribution of Lycium barbarum polysaccharides in rat tissue by fluorescein isothiocyanate labeling
Chao Yang, Hui Xia, Huali Tang, Ligang Yang, Guiju Sun*
Key Laboratory of Environmental Medicine and Engineering of Ministry of Education, and Department of Nutrition and Food hygiene, School of Public Health,Southeast University, China-DRIs Expert Committee on Other Food Substances, Nanjing 210009, China
Keywords:
Lycium barbarum polysaccharides
Fluorescein isothiocyanate
Tissue distribution
A B S T R A C T
To date, in vivo investigations of polysaccharide’s pharmacokinetics are significantly restricted by the difficulty in their detection. This study was conducted to establish the quantitative determination of Lycium barbarum polysaccharides (LBPs) based on fluorescein isothiocyanate (FITC) pre-labeling and to investigate their tissue distribution in rat. We obtained the calibration curves linear over the range of 0.0–25 μg/mL in rat tissue samples with correlation coefficients greater than 0.99. The inter-day and intra-day precisions (RSD, %)were within 15%, and the relative recovery ranged 95.2%–102.4%, with RSD range 1.48%–9.58%, indicating that this experiment was suitable for the determination of LBPs. The fluorescence intensity was measured after 24 h storage at room temperature, 3 times of freeze-cycle and cryopreservation at –20 °C for 15 day,these results indicated that the stability of the samples was good. LBP-FITC was mainly absorbed by the small intestine and stomach, and mainly excreted in the urine through the kidney; this distinct difference in the tissue distribution of LBPs could be attributed to the size of these LBPs in relation to the pore sizes of the vascular beds in the kidney and liver. Results showed in this study enable us to comprehensively understand the biological effects of LBPs following its oral ingestion.
1. Introduction
Lycium barbarumis used as medicine, or as medicinal and functional food due to its health benefits, including anti-aging,antioxidant, antidiabetic, anticancer, cytoprotective, neuroprotective,and immunomodulatory effects [1-7].L. barbarumpol ysaccharides(LBPs) are the main components isolated fromL. barbarumand have been responsible for the biological activities ofL. barbarum.As a naturally occurring chemical, LBPs are a group of water-soluble glycoconjugates with a molecular weight (Mw) of 3–2 300 kDa [8-10].Due to their various pharmacological effects, including anti-oxidation,anti-aging, immune regulation, improvement of gut microbiota and reproductive protection [11-16], LBPs have been widely exploited in healthy foods and medicines in China. Although the comprehensive pharmacological studies of LBPs have been performed, there is still only limited information on the tissue distribution study of these compoundsin vivogiven their high molecular size. Therefore, a detailed knowledge of its mechanism is important for understanding its biological activities.
Most of the published studies on the pharmacokinetics and tissue distribution of polysaccharides used many analytical methods for tracking polysaccharidesinvivo[17]including fluorescence labeling combined with chromatography [18,19], isotope labeling [20,21],spectrophotometry [22,23], fluor spectrophotometry [24,25]and biological assay [26]. Recently, techniques based on the detection of fluorescence have been employed in drug microanalysis because of their specificity, sensitivity, and low detection threshold [27].Fluorescein isothiocyanate (FITC) is a common fluorescent probe in fluorescence immunoassay [28], and is widely used for fluorescent labeling of proteins. FITC is the used tracer which conjugate with other types of biomaterials or biomolecules for imaging [29-32].LBPs are kinds of water-soluble protein polysaccharide complex,polymerized by glucose, mannose and galacturonic acid. FITC is widely used for protein labeling due to the high reactivity of isothiocyanate groups (N=C=S) with amino groups [17]. Modification of the free amino groups in LBPs with FITC yielded soluble copolymers having covalently-bound fluorescein and glycosyl groups [33]. We have successfully synthesized a FITC-labeled LBPs according to the method of high-performance gel permeation chromatography fluorescence detection (HPGPC-FD) method [34],and the Mv of a fluorescent labeled product (LBP-FITC) did not increase compared to LBPs [35,36].
In this study, the quantitative determination of LBP was established based on FITC pre-labeling and followed by an investigation of the tissue distribution in rat. Understanding the tissue distribution of LBPsinvivoand the potential effect-targets is of high importance, which is conducive to the further study of their pharmacological activity.
2. Material and methods
2.1 Chemicals and reagents
LBPs were purified by Sevage method [34], and the preparation method of LBPs has been reported [36,37].
FITC (purity > 99.0%) was purchased from the American Sigma Corporation. Sodium bicarbonate, sodium dihydrogen phosphate,disodium hydrogen phosphate and sodium chloride were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).Anhydrous ethanol was procured by Wuxi Yasheng Chemical Co.,Ltd (Wuxi, Jiangsu). Ethylenediamine was obtained from Jinan Reagent Factory (Jinan, China). 0.45 μm microporous membrane was procured by Hangzhou Fuqiang Chemical Instrument Co., Ltd(Hangzhou, China). Ultrapure water was obtained from a Milli-Q water purification system (Millipore, Billerica, MA). All other chemicals were of high analytical grade.
2.2 Animals
Male Sprague-Dawley (SD) rats with body weight of (180 ± 20) g were obtained from Shanghai Jiesijie Experimental Animal Co., Ltd.(Animal License No.: SCXK (Shanghai)-2013-0006). The rats were fed in the animal laboratory of School of Public Health, Southeast University and kept at room temperature (21 ± 1) °C with relative humidity 60%–100% and 12-h dark-light cycle. Tap water and standard laboratory food were providedad libitum. All rats were acclimated in the laboratory for 3 days prior to the experiment.
All animal experiments were conducted in accordance with the“Principles of laboratory animal care” of NIH and the protocol for animal study of Animal Management Committee and Animal Ethical Committee of Jiangsu Province, and approved by Animal Experimental Ethical Committee of Southeast University (No. 2015-1025-010).
2.3 Instrumentation
Fluorescence spectrophotometer (Cary eclipse) was purchased from Varian China Co., Ltd (California, USA). HPLC (Agilent 1260), Fluorescence detector (Agilent 1260), Gel chromatography column (SB-804 HQ) were obtained from Agilent Technologies Co.,Ltd. Gel chromatography column (SB-804 HQ) was purchased from Agilent Technology Co., Ltd. Centrifuge (L-550) was purchased from Hunan centrifuge instrument Co., Ltd. Micro vortex mixer (WH-3)was obtained from Shanghai Huxi Analytical Instrument Factory Co., Ltd. Portable high speed homogenizer (S10) was obtained from Ningbo Xinzhi Biotechnology Co., Ltd. Magnetic stirrer (85-1) was procured from Jintan medical instrument factory. Vacuum freeze dryer(FD-1) was procured from Beijing Boyaikang Experimental Instrument Co., Ltd. PH meter (PHS-25) was purchased from Shanghai Instrument &Electronics Co., Ltd. Electronic analytical balance (BSA124S) was obtained from Mettler Toledo, Switzerland. Ultrasonic cleaner (TM-010)was obtained from Kunshan Ultrasonic Instrument Co., Ltd.
2.4 Establishment of quantitative analysis method for LBP-FITC
2.4.1 Fabrication of LBP-FITC
Five hundreds mg LBPs were dissolved in 25 mL pure water, and pH was adjusted to 8.0 with 0.5 mol/mL NaHCO3, then 25 mg FITC were added. After stirring for 24 h at room temperature and dark conditions, the reaction solution was filtered and anhydrous ethanol was added to the filtrate until the final concentration of ethanol was 80% (V/V). Precipitation occurred, and the supernatant was discarded after centrifugation. The precipitate was re-dissolved in water, and then precipitated again with absolute ethanol which repeated for three times. Then the precipitate was washed repeatedly with anhydrous ethanol until no fluorescence was recorded in the supernatant. After freeze-dried precipitation, LBP-FITC, which is the fluorescent marker of LBPs, was obtained [35].
2.4.2 Preparation of LBP-FITC standard solution
Standard stock solution preparation: 100 mg LBP-FITC sample was accurately added into a 100 mL volumetric flask, and then PBS solution was added to dilute to the scale. The 1 mg/mL LBP-FITC standard stock solution prepared was stored at 4 °C.
LBP-FITC series concentration standard solution preparation:the appropriate amount of LBP-FITC standard stock solution was accurately measured, and then 0.2, 1, 2, 4, 10, 20, 50, 100, 500,1 000 μg/mL LBP-FITC standard solutions with PBS solution were prepared, which were used right after they were ready.
2.5 Tissue sample collection
The rats were access to the diet and water freely before the experiment, and the experiment was carried out three days after adaptation to the environment. The rats were placed in a metabolic cage and administered with LBP-FITC in a single cage 12 h before death, urine and feces were collected meanwhile. The rats were executed by decapitation after taking blood from femoral artery.The liver, kidney, spleen, heart, stomach, small intestine, large intestine and muscle tissues were collected. The residual blood on the tissue surface and contents of stomach and intestine were washed out in a container containing 0.01 mol/L PBS. The liquid on the surface was dried with absorbent paper, weighed, wrapped with tin foil, and placed in a clean plastic centrifuge tube for experiment or stored at –70 °C.
2.6 Tissue sample determination
2.6.1 Determination conditions
According to our previous study [35], the excitation wavelength(Ex) and emission wavelength (Em) of LBP-FITC were 495 nm and 518 nm, respectively.
2.6.2 Tissue sample determination
Tissue samples were accurately weighed, and 0.01 mol/L PBS were added at ratio of 1 : 3. Then the mixture was homogenized at high speed and centrifuged at 4 000 r/min for 10 min. 200 μL supernatant extracted was mixed with 0.01 mol/L PBS to 1.8 mL.The suspension was centrifuged at 12 000 r/min for 10 min, and determined the fluorescence intensity of supernatant.
2.7 Fabrication of standard curve
Two hundreds μL supernatant of each blank tissue homogenate and 200 μL LBP-FITC series standard solution was accurately measured out to prepare tissue samples with concentration of 1, 5, 10, 25 μg/mL.
Blank tissue samples with the same volume of PBS instead of LBP-FITC standard solution were prepared. The serial concentrations of LBP-FITC in tissue were used as the abscissa (X), and the difference between the fluorescence intensity measured and that of blank tissue was taken as the ordinate (Y). A standard curve (linear regression equation) was obtained using the weighted least squares method (W= 1/x2). The preparation process was carried out in a dark environment.
2.8 Method validation
Two hundreds μL of rat blank tissue homogenate supernatant was taken, and LBP-FITC series standard solution was accurately added.
2.8.1 Precision
In order to evaluate the precision of the method, five samples of quality control (QC) with three concentration levels of low (0.5 μg/mL),medium (5 μg/mL) and high (25 μg/mL) were prepared. According to the corresponding standard curve of that day, the determination concentration of QC sample was calculated, and the precision of the method was obtained by comparing with the prepared concentration.Five replicate analyses were performed on each sample for 5 consecutive days. The intra-day and inter-day relative standard deviations (RSD) were calculated. Precision results were expressed by the equation as follow: RSD (%) = standard deviation/mean × 100.The intra- and inter-day precision (RSD, %) should not exceed 15%.
2.8.2 Stability
The stability index should evaluate the stability of the test object at each step of the method, which generally includes short-term,long-term, freeze-thaw and autosampler stabilities. The short-term,autosampler and long-term stabilities were evaluated by measuring QC samples at room temperature for 24 h and at –20 °C for 15 days,respectively. The freeze-thaw stability was measured by three freezethaw cycles on consecutive days.
2.8.3 Recovery rate and matrix effect
In order to evaluate the recovery rate, five samples of each QC samples (low, medium and high concentrations) were analyzed.The extraction recovery was measured by comparing the peak areas of the extracted (pre-spiked) QC samples with those of the unextracted biological samples at an equivalent concentration.The results were expressed as mean ± standard deviation (SD).The matrix effect was evaluated by comparing the peak area of the analyte added into the post-extracted blank tissue samples (n= 5)with the peak area obtained from corresponding standard solution at all QC levels.
2.9 Tissue distribution study
2.9.1 Administration method
LBP-FITC samples were prepared at 100 mg/mL solutions with normal saline. Eighteen male Sprague Dawley rats were randomly divided into 3 groups of 6 rats each (1 h observation group, 6 h observation group and 24 h observation group). The rats were given a dose of 100 mg/kg LBP-FITC solution through intragastric administration after fasting and freely accessing to water for 24 h.
2.9.2 Sampling method
After a single oral gavage, one group was undertaken blood collection from canthus at 1, 6 and 24 h. Then the rats were killed by cervical dislocation immediately, and the heart, liver, spleen,kidney, stomach, small intestine, large intestine and skeletal muscle tissues were taken. The blood was centrifuged at 4 °C, 3 500 r/min for 10 min, and plasma was transferred into EP tube by micropipette, and stored in –70 °C refrigerator for testing; tissue samples were rinsed with normal saline to remove residual blood and contents on the surface, then dried with filter paper, wrapped with tin paper and put into sealed bag, stored at –70 °C.
2.10 Sample determination
Detailed methods on determination of tissue samples were described as section 2.6.
2.11 Data processing of tissue distribution
All the pharmacokinetic parameters are expressed as the mean ± SD.Statistical analyses of main pharmacokinetic parameters were analyzed for significance using the independent sample Student’st-test. The histogram of the tissue distribution of interventions after different administration time was drawn. The Origin software was used to process the experimental data.
3. Results
3.1 Establishment and validation of quantitative analysis method
3.1.1 Standard curve drawing
The samples obtained by adding LBP-FITC standard solution and PBS with the same volume were determined, and the fluorescence intensity was recorded. According to the standard curve, the linear range of LBP-FITC in heart, liver, spleen, kidney,stomach, small intestine, large intestine, and skeletal muscle was in the range of 0–25 μg/mL. The standard curve of each major tissue is showed in Table 1. The correlation coefficients of the linear regression equation of each biological tissue sample were greater than 0.99, which indicated that the linear equation meets the requirements of pharmacokinetic study.

Table 1The linear regression equation and the correlation coefficients of biological samples.
3.1.2 Precision
According to the QC sample, the precision of the experiment was calculated. The experimental results of the QC sample of the LPB-FITC were in line with the relevant requirements for biological sample determination. The intra-day and inter-day precisions(% RSD) of LBP-FITC in rat visceral tissue were lower than 15%.Table 2 indicated that the intra-day relative standard deviation ranged between 1.85% and 4.18% for LBP-FITC in heart tissue, while the inter-day ranged between 5.42% and 2.86%. The intra-day precisions range was between 3.15% and 4.94% in liver tissue, and the intra-day precisions rangedbetween 4.28% and 7.68%.

Table 2Precision of LBP-FITC in rat tissues (n = 5).
It could be seen that the intra-day precision of 3 levels in kidney tissue was 1.53% to 4.82%, and the inter day precision was 2.69% to 6.05%; the intra-day precision in the stomach tissue was 1.78% to 4.63%, and the inter-day precision was 3.02% to 5.89%; the intra-day relative standard deviations of the concentrations in spleen tissue was 2.90% to 5.62%, and the inter-day standard deviations were 3.85% to 6.78%. Similarly, the intra-day and inter-day precisions of LBP-FITC in rat intestine tissue and muscle tissue were lower than 15%. The method proved to be precise for the analytes, since relative standard deviation (% RSD) no more than 15% were observed.
3.1.3 Stability
The fluorescence intensity was measured after 24 h storage at room temperature, 3 times of freeze-thaw and cryopreservation at–20 °C for 15 days. The stability of LBP-FITC was evaluated by analyzing the above three conditions, as shown in Table 3. The concentration of QC samples was calculated according to the plasma standard curve, and the RSD value was calculated by SPSS software.It could be seen from the table that the RSD value of the sample concentration of rat heart, liver, kidney, stomach, spleen, small intestine, large intestine and muscle tissue was within the acceptable range (below 15%) under these conditions, indicating that the stability of the samples was good.
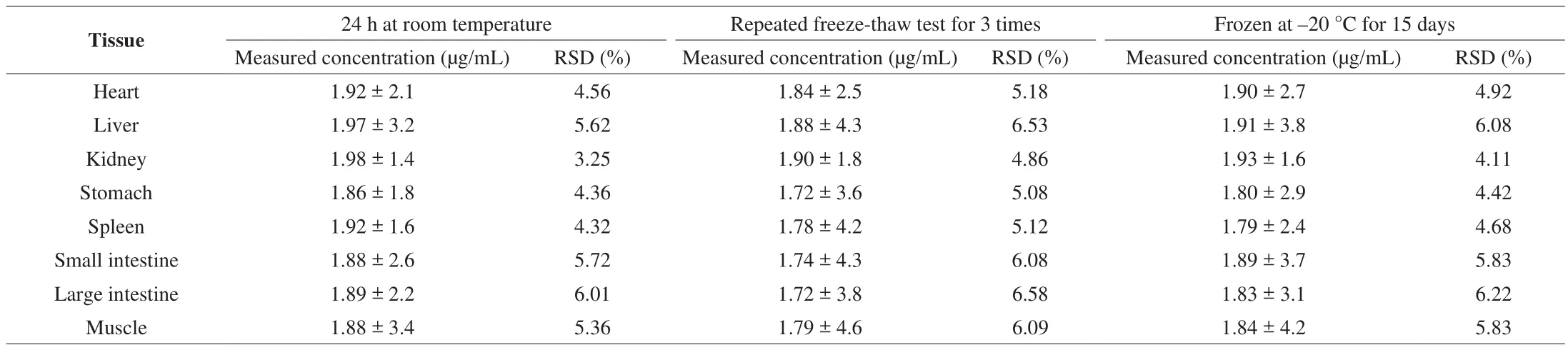
Table 3Stability of LBP-FITC in rat tissues (n = 5).
3.1.4 Recovery and matrix effect
The extraction recovery of LBP-FITC from rat tissue samples was assessed using QC tissue samples spiked with LBP-FITC at various concentrations, specifically low (0.5 μg/mL), medium (5.0 μg/mL),and high (25.0 μg/mL). The results of sample recovery were shown in Table 4. The recoveries of rat tissue samples were between 94.4% and 102.4%, and the RSD were between 1.48% and 9.58%, indicating that the extraction recovery method and instrument measurement met the requirements for biological sample analysis.

Table 4Recovery of LBP-FITC in rat tissues (n = 5).
3.2 Tissue distribution study
The rats were given 100 mg/kg LBP-FITC by gavage, and the rats were sacrificed at 1, 6 and 24 h, respectively. The concentrations of LBP-FITC in tissues of rats were determined. The concentrations of LBP-FITC in plasma and tissues at 1, 6 and 24 h after administration were shown in Table 5.

Table 5The tissue concentration of LBP-FITC in rat at 1, 6 and 24 h after oral administration of LBP-FITC at 100 mg/kg (μg/g or μg/mL).
3.2.1 Tissue distribution at 1 h
As shown in Table 5 and Fig. 1, the LBP-FITC was mainly concentrated in the stomach and small intestine after 1 h of administration, with the highest concentration in the small intestine.The concentrations of LBP-FITC in small intestine, stomach, liver,large intestine, heart and kidney were higher than the concentration in plasma, but the concentrations in spleen and muscle were lower than that in plasma. The ratios of LBP-FITC concentration in the above organs and plasma were 17.0, 10.7, 3.6, 3.1, 2.3, 1.5, respectively;the sequence of LBP-FITC contents in the tissues was as follows:small intestine > stomach > liver > large intestine > heart > kidney >blood > spleen > muscle.
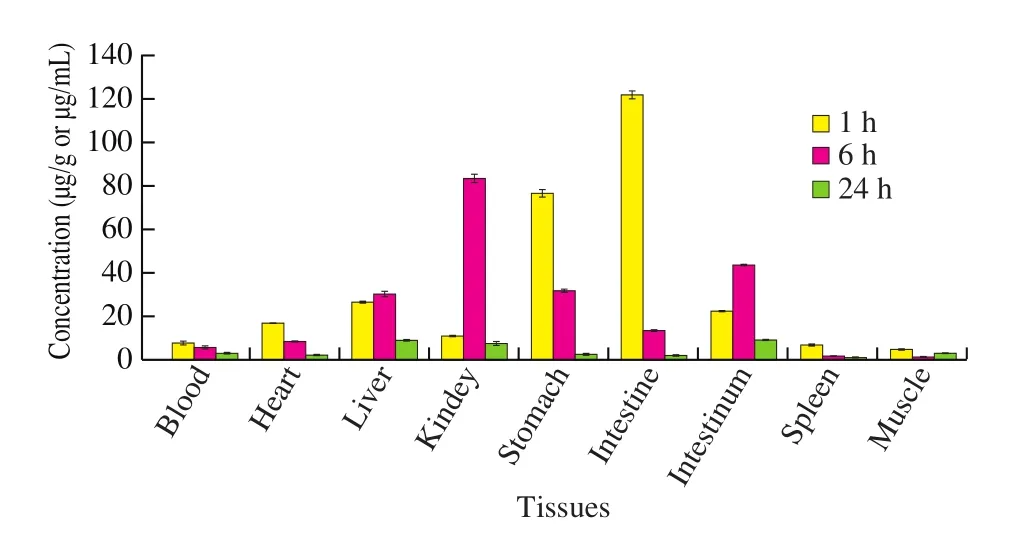
Fig. 1 Distribution of LBP-FITC in rat tissues and blood at 1, 6 and 24 h after oral administration of LBP-FITC 100 mg/kg to rat.
3.2.2 Tissue distribution at 6 h
The content of LBP-FITC in kidney was the highest after 6 h of administration. The concentrations of LBP-FITC in kidney, large intestine, stomach, liver, small intestine and heart were higher than the concentration in plasma, while the concentrations of LBP-FITC in spleen and muscle were still lower than that in plasma. The ratio of LBP-FITC concentration in the above organs and plasma was 16.2,8.5, 6.2, 5.9, 2.6, 1.6, respectively; the order of LBP-FITC content in tissues was as follows: kidney > large intestine > stomach > liver >small intestine > heart. Compared with the concentration at 1 h after administration, the concentrations of LBP-FITC in plasma, stomach,small intestine, heart, spleen and muscle decreased by 28.5%, 58.8%,89.2%, 51.7%, 79.0%, 82.1%, respectively, while the concentrations in kidney, liver and large intestine were 7.9, 1.2, 2.0 times higher than those at 1 h after administration.
3.2.3 Tissue distribution at 24 h
After 24 h of administration, the content of LBP-FITC was the highest in the large intestine, followed by the liver. The concentration in the large intestine, liver, and kidney was higher than the concentration in plasma. The ratio of the concentration of LBP-FITC in the above-mentioned organs and plasma was: 3.2, 3.1 and 2.6; the order of the content of the tissues was as follows: large intestine >liver > kidney > muscle > stomach > heart > small intestine > spleen.Compared with the concentration of LBP-FITC in the tissues after 6 h of administration, the concentration of LBP-FITC in the muscle increased, and the concentration of LBP-FITC in the other tissues decreased.
4. Discussion
The pharmacokinetics and tissue distribution are crucial in understanding of biological activity. The determination of LBPs distribution is one of the key problems in its pharmacokinetic studies.Natural polysaccharides contain very few chromophores and reactive groups. The analysis of LBPs in biological samples is complicated because of its metabolites and its low tissue concentrationin vivoafter oral administration. In our study, the FITC fluorescence method for the detection of LBPs was successful in the characterization of tissue distribution of LBPs in rat tissues. Fluorescent labeling could not only quantify the polysaccharides content, but also determine whether the polysaccharides were degraded by HPGPC fluorescence peak, which is the advantage offluorescent labeling of polysaccharides [17,19].
FITC is a yellow-orange dye with a small molecular weight of 389.4 D and wide range of biological applications [38]. Our previous study has found that the molecular weight of LBP did not change significantly before and after FITC labeling (as shown in Supplemental Fig. 1). This might be the reason why many studies on fluorescent labeling of polysaccharides did not discuss the fluorescent substrate as the control group in detail [17,28,38]. Therefore, we design the study of LBP-FITC without free-FTIC as control. In this study, LBPs at the dose of 100 mg/kg was detected in most of rat tissue 1 h after the intragastric administration. As shown in Fig. 1,LBPs was mainly distributed in both the small intestine and stomach.The results showed that LBPs had strong affinity with intestinal cells.Consistent with our study, Chae et al. [39] revealed middle- and low-Mw (≤ 22 kDa) water-soluble chitosans (WSCs) showed Mw- and time-dependent transport phenomena through the Caco-2 cell layer and low-Mw WSCs (WSC 3.8K and WSC 7.5K) might have the ability to open tight junctions. The study suggested that different transepithelial electric resistance (TEER) variation patterns of low-Mw WSCs might indicate a possibility of penetration phenomenon.As discussed, the initial increase of the TEER value could be explained by the interaction of chitosan with the cell surface with a negligible tight junction opening effect. Without the tight junction opening effect and cellular uptake, chitosan may increase the TEER value by increasing penetration barrier for ions. Correspondingly,chitosan might have passed through the Caco-2 cell layer, which resulted in the progressive reduction of TEER value. Moreover, the study by Li et al. [40]indicated that LBP improve the disruption of the intestinal mucosal barrier evoked by TNF-α in Caco-2 cells, as proved by the normalization of paracellular permeability and endothelial barrier TER, inhibition of pro-inflammatory cytokines, and regulation of intestinal epithelial TJ protein expression. Nagamine et al. [41]also reported that the uptake of fucoidan by intestinal macrophages was observed in the intestine of rats. Similarly, another study [42]that evaluated the effect of chitosan on the intestinal absorption of acyclovir (ACV) in rats, indicated that intestinal ACV absorption was facilitated by chitosan. This family of natural polymers has an appeal to drug delivery as the polymers are comprised with a large number of derivatizable groups [43]. These results also indirectly suggests that LBP may be absorbed into cells and play an anti-inflammatory role.However, the conjecture that LBP can be absorbed by gastrointestinal cells need be further explored to find more direct evidence.
After 6 h of administration, LBP-FITC concentration in small intestine and stomach tissue decreased significantly (P< 0.01), and LBP-FITC concentration in kidney sample reached the highest level,which was 7.9 times of the concentration after 1 h of administration.The level of LBP-FITC in the kidney tissue reached 83.10 μg/g after 6 h, followed by a decrease in concentration, indicating that the kidney has a strong ability to uptake LBP-FITC. This result indicated that LBP-FITC was mainly absorbed into the blood and excreted out of the body with urine through the kidney, which was similar to the studies’ conclusion of Min et al. [44]and Bai et al. [45]. After 24 h of administration, LBP-FITC concentration in all tissues decreased significantly (P< 0.01), but it was slightly higher in large intestine,liver, muscle and kidney. It is suggested that LBP-FITC is excreted by feces in large intestine, which is also the main way of excretion.
Interestingly, the presence of LBP-FITC was identified in the liver and kidney, with the former exhibiting the highest concentration after 24 h administration. This distinct difference in the tissue distribution of LBPs could be attributed to the size of these LBPs in relation to the pore sizes of the vascular beds in the kidney and liver. A few studies con firmed that the liver accumulation and kidney excretion of polysaccharides were related to Mw [23,25,46-48]; polysaccharide selectivity to heart responding to Mw was rarely reported. The theoretically calculated pore size of the capillary wall of the rat kidney was 2–5 nm [49], while the capillary wall diameter of the rat liver sinusoids was 100 nm [50]. Our previous study demonstrate that LBPs is polymerized by glucose, mannose and galacturonic acid,and the molecular weight of LBPs is about 5 kDa [37]. Therefore,LBP is more likely to accumulate in the liver than in the kidney with large pore size. Similar to our study, Kaneo et al. [46]revealed that the tissue distribution of dextrans with different Mws (T-4, T-10,T-20, T-40, T-70, T-150, T-500) was systematically examined in mice. Dextrans with an Mw lower than 20 kDa showed poor hepatic aggregation (2.1%–3.7%) due to their rapid elimination from the blood. Dextrans with an Mw higher than 40 kDa were appreciably distributed in the liver (18.9%–24.0%) and stayed in the liver over a long period. Moreover, it has been reported that the hepatic uptake approach is determined by the structure of the polysaccharides,and further alters the plasma concentration-time curves of polysaccharides [46]. Many polysaccharides including pullulan,arabinogalactan, mannan and dextran are known to distribute in the liver after intravenous injection [46]. Mehvar and Shepard [51]suggested the similar conclusion that the change in renal clearance of dextrans with different Mws was more significant than that of liver clearance, which might be caused by the difference of capillary wall between kidney and liver.
5. Conclusion
This report is the first study of LBPs by FITC fluorescence labeling to reveal its important fundamental tissue distribution after oral administration to rats. We demonstrated that LBP-FITC was mainly absorbed by the small intestine and stomach, and mainly excreted in the urine through the kidney; this distinct difference in the tissue distribution of LBPs could be attributed to the size of these LBPs in relation to the pore sizes of the vascular beds in the kidney and liver. The outcomes of this study provide additional scientific data for the traditional use of LBPs, and this knowledge will enable us to comprehensively understand the biological effects of LBPs following its oral ingestion.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgement
The authors also thank the support from the National Key Research and Development Program of China (No. 2016YFD400604-02), the National Natural Science Foundation of China(No. 82073551, 82003457, 81273069), the Postgraduate Research& Practice Innovation Program of Jiangsu Province (No.KYCX19_0121), the Scientific Research Foundation of Graduate School of Southeast University (No. YBPY1944), the Fundamental Research Funds for the Central Universities (No. 2242020R10006),and CNS Research Fund for DRI.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.03.004.
- 食品科學(xué)與人類健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study

