Sn Alloy and Graphite Addition to Enhance Initial Coulombic Efficiency and Cycling Stability of SiO Anodes for Li-Ion Batteries
Xingyang Du,Hanying Zhang,Xuexia Lan,Bin Yuan,and Renzong Hu*
Silicon monoxide(SiO)has aroused increased attention as one of the most promising anodes for high-energy density Li-ion batteries.To enhance the initial Coulombic efficiencies(ICE)and cycle stability of SiO-based anodes,a new facile composition and electrode design strategy have been adapted to fabricate a SiO-Sn-Co/graphite(G)anode.It achieves a unique structure where tiny milled SiO-Sn-Co particles are dispersed among two graphite layers.In this hybrid electrode,Sn-Co alloys promoted Li+extraction kinetics,and the holistic reversibility of SiO and graphite enhanced the electrical conductivity.The SiO-Sn-Co/G electrode delivered an average ICE of 77.6% and a reversible capacity of 640 mAh g-1at 800 mA g-1,and the capacity retention was above 98% after 100 cycles,which was much higher than that of the SiO with an ICE of 55.3% and a capacity retention of 50% .These results indicated that this was reliable method to improve the reversibility and cycle ability of the SiO anode.Furthermore,based on its easy and feasible fabrication process,it may provide a suitable choice to combine other alloy anodes with the graphite anode.
Keywords
ball milling,cycle performance,initial Coulombic efficiency,silicon monoxide,tin-cobalt alloy
1.Introduction
Lithium-ion batteries(LIBs),as an essential component of various electronic devices,demand a higher electrochemical performance.However,widely used graphite anodes limit the development of LIBs because of their low theoretical capacity(LiC6:372 mAh g-1)and pose a safety risk because of their low lithiation potentials.[1-4]Based on application,an increasing number of researchers have given Si-suboxide anode materials attention because of their low cost and high capacity.They can buffer large volume variations and inhibit the coarsening of Si nanograins with the help of in situ generated lithium silicate and Li2O during the first lithiation,which results in an improved cycling stability.[5,6]However,the application of SiO-based anodes remains hindered by their undesirable thermodynamically stable irreversible products,non-negligible volume effect,and low intrinsic conductivity.
Many methods have been presented to tackle these drawbacks,such as particle downsizing,[7]changing the O content in SiOx,[8]fabricating a core-shell structure,[9]or compositing SiOxwith other metal/alloy materials.[10,11]For mass production,the high-energy milling method(HEMM)is deemed more acceptable to achieve the above modification.Zhang et al.[12]investigated the performance of SiOxafter treatment through HEMM of different durations.An improved cyclic life and excellent rate capacity were achieved in the milled SiOxbecause of the reduced particle size.Huang et al.[13]probed the effect of particle size through planetary ball milling with a series of milling times.However,milled small particles still do not yield a satisfactory electrochemical performance.Hence,more attention has been given to composite SiOxwith other chemical elements,mostly as some metal materials and active substances.For example,SiO/metal or SiO/metal oxide hybrid anodes are appealing because they are prone to achieve improvements through simple milling without capacity loss.Xu et al.[14]fabricated a carbon-coated SiO/Cu composite,which provided a high capacity of 1140 mAh g-1.Inspired by the amorphous ternary Sn-Co-C composite as anode in the SONY NEXELION battery,[15]Amine and coworkers introduced SnxCoyCzand SnxFeyCzinto SiO through ultrahigh-energy ball milling,which led to an improved cycling performance.[16,17]Based on thermal disproportionation and impurity doping,Jihoon et al.fabricated a boron-doped SiO electrode with an enhanced reversible capacity and electrical conductivity.[18]Zhang et al.[19]boosted the ICE of disproportionated SiO by MgO addition,which consumes SiO2.Yang et al.prepared a B-containing SiOxcomposite by heating a mixture of SiO and LiBH4to 500°C.During heating process,B,B2O3,and Li2SiO3can formed at the surface of the product particles to depress the pulverization and fracture of the Si/SiOxactive materials,leading to great cycling stability.[20]
Most SiO-based anodes are hindered by the volume expansion and inferior intrinsic conductivity and require carbon to improve their cycle life.SiO/C hybrid anode materials have been studied extensively,in which the carbon serves as a conductive and buffering medium.Wu et al.prepared SiO/C composite using SiO and glucose as raw materials,leading to an initial charge capacity of 1259 mAh g-1and a high initial coulombic efficiency of 71.9% .[21]Morita et al.synthesized a Si/SiO/C nanocomposite in which carbon served as a matrix,and achieved a capacity above~700 mAh g-1for 200 cycles.[22]Sohn et al.fabricated a disproportionated SiO/graphite composite through HEMM,which showed an initial discharge capacity of 1002 mAh g-1.Even after 100 cycles,the composite maintained a reversible capacity above 710 mAh g-1.[23]Xu et al.[24]developed a blocky SiOx/C with graphite-like structure with SiOxloaded on graphite by exfoliation,which delivered a reversible capacity of~645 mAh g-1and a good cycling stability.Wang et al.[25]prepared an amorphous-Si@SiOx/C composite by ball milling and in situ carbonization,leading to high capacity of 1450 mAh g-1after 100 cycles.Compositing SiO-based production after HEMM with carbon by reasonable methods tended to provide an efficient way to take advantage of carbon to achieve a better performance.However,compositing methods with graphite usually suffer from complex processes or sacrifice capacity and ICE.In our previous work,insightful research was carried out on SnO2-based anodes,which provided an opportunity to overcome the inferior reversibility of Li2O in SiO anode with transition metals.[26,27]Zhang et al.[28]fabricated Sn2Fe@SiOxanodes with an excellent cycling stability and clarified that multi-phase composites have a complicated phase interface and structure evolution that determine the structural stability and Li+diffusion kinetics,which provide support to manufacture SiO-M-Sn composites.
Based on the above considerations,in this work,to use a simple milling method and balance the edges of each component,we report a new SiO-Co-Sn/G anode with milled SiO-Sn-Co composite and commercial graphite coated on Cu foil like a sandwich structure that provides an enhanced electrochemical performance.The trait of this approach was that it yielded better-integrated graphite and dispersed finer milled SiO-Co-Sn hybrid particles between the graphite particle gaps after drying the electrode coating.Under this condition,the unmilled graphite could serve as an active component and provide a conductive path,which led to a higher coulombic efficiency and stable cycling life.The milled Sn and Co were mostly converted to CoSn2,which contributed to a higher ICE without much sacrifice of the reversible capacity.
2.Results and Discussion
Figure 1a provides an illustration of the fabrication procedure for the SiO-Sn-Co/G hybrid electrode.The SiO-Sn-Co composite was prepared by milling.Because the SiO can serve as a brittle grinding aid,it is more likely to peel intermetallic Sn-M(M:transition metal)from a lump of Sn or M particles and generate tiny particles attached to the SiO particle surface.[29-31]In this study,Sn and Co were more inclined to form CoSn2and over-stoichiometric Co was retained as a metallic phase.As a result,a conductive channel formed that promoted Li+extraction from the Si-O-Li matrix.To induce carbon material,a sandwich structure of G|SiO-Sn-Co|G was designed as shown.The structure was achieved by coating SiO-Sn-Co and graphite slurry in sequence.During vacuum drying,large graphite and tiny SiO-Sn-Co particles moved into each other’s layer at the interface.This process promoted extensive SiOSn-Co particle loading onto graphite particles.At the same time,a random porous structure was achieved to accommodate volume variations,which could serve as a conductive matrix.
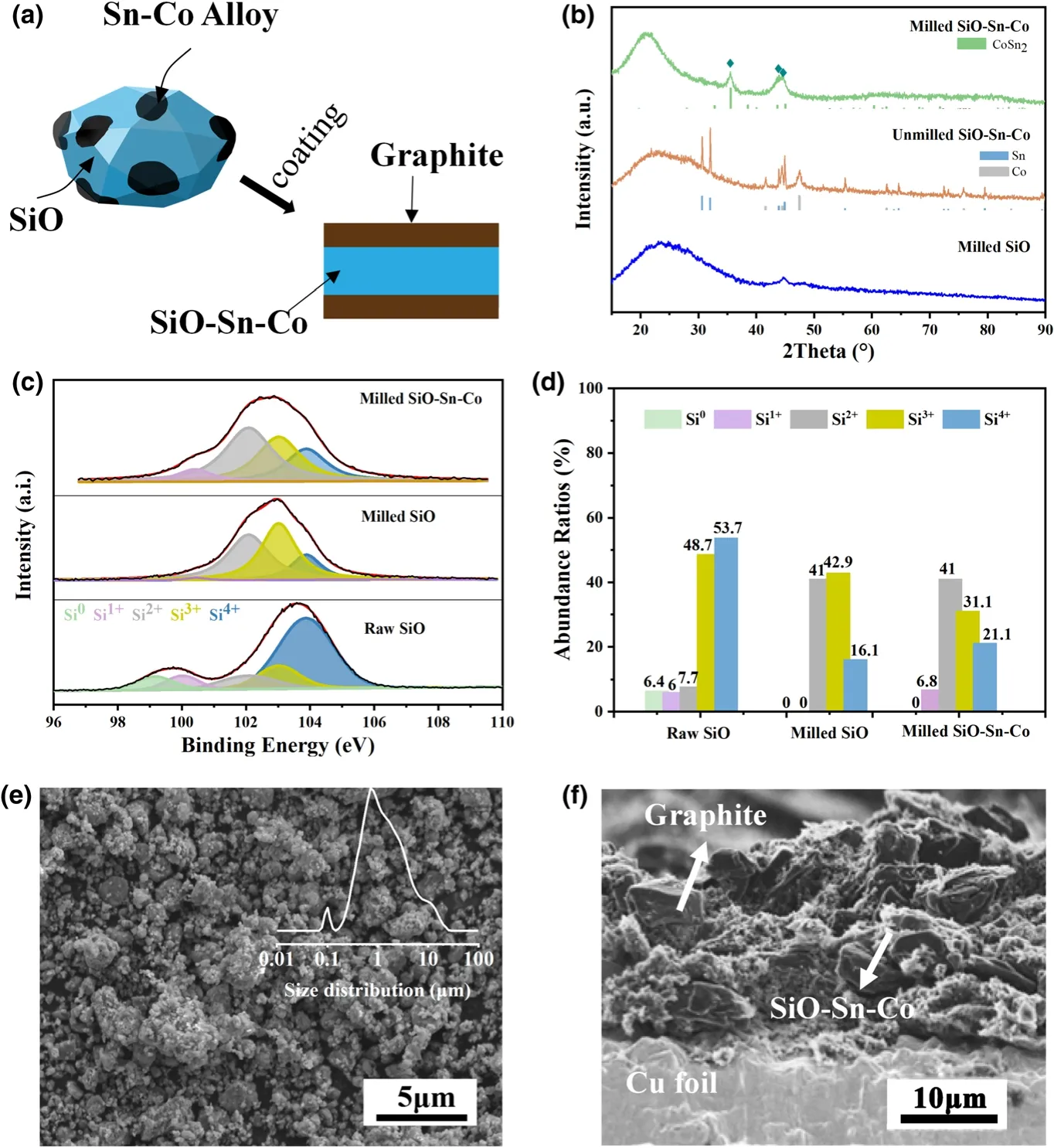
Figure 1.a)Brief illustration of the preparation of SiO-Sn-Co and SiO-Sn-Co/G hybrid electrodes;b)XRD patterns of milled SiO,milled SiO-Sn-Co composite,and unmilled SiO-Sn-Co mixture;c)Si2p peak in XPS spectra of raw SiO,milled SiO,and milled SiO-Sn-Co and d)corresponding abundance ratios;e)SEM image of SiO-Sn-Co composites(particle size distribution as inset);f)SEM image for cross section of SiO-Sn-Co/G electrode.
To determine the phase composition of the milled SiO-Sn-Co particles.XRD patterns of unmilled SiO-Sn-Co,milled SiO,and SiO-Sn-Co are provided in Figure 1b.Similar to the reported results,after milling,SiO had a typical amorphous nature with an obvious broad peak between 15°and 30°(2θ),and no characteristic peak for Si could be indexed in this XRD pattern.For the SiO-Sn-Co composite,three strong peaks existed at 35.6°,43.7°,and 45.0°,which could be indexed to the(2 1 1),(2 0 2),and(3 1 0)planes of CoSn2phase,respectively.The broadened peaks of CoSn2indicated the small grain size.No obvious diffraction of Co or cobalt oxide indicated that most Co reacted with Sn to form CoSn2under the high-speed impact of the grinding balls.XPS analysis was conducted to determine the valence states of Si in the raw SiO,milled SiO,and milled SiO-Sn-Co.As shown in Figure 1c,the Si2p spectra of raw SiO showed two major peaks at 103.58 and 99.73 eV,which could be translated to five peaks that represented Si4+,Si3+,Si2+,Si+,and Si0.[32]Figure 1d shows the Si oxidation state abundance ratio in the Si2p spectra for the raw SiO,milled SiO,and milled SiO-Sn-Co.It was concluded that an obvious trend existed in that Si0and Si4+changed to intermediate valence during milling.
Figure 1e shows the secondary electron SEM image of the as-milled SiO-Sn-Co composite powders.During milling,SiO particles were broken into numerous tiny particles.A summary of the average size of milled SiO-Sn-Co particles was obtained.The inset image in Figure 1e shows that the sizes of most particles ranged from 0.2 to 10 μm.A few particles reached 100 nm,which are likely Sn-Co alloy particles,according to the previous experience that hard SiO can re fine Sn-Co alloy particles as grinding aids.Compared with the raw powder(>325 mesh),after milling the sizes of the major SiO particles showed a remarkable reduction.As reported,the particle size can affect the electrochemical performance significantly.A nanoscale particle tends to mean a shorter Li+diffusion path and more stable structure but a lower tap density and more SEI formation in the initial discharge than at the microscale.[33]According to our design,a structure in which many nanoscale Sn-Co alloy particles attach to large particles may translate into a synergy that leads to an improved electrochemical performance.The milled SiO-Sn-Co powders were compounded with graphite during anode slurry preparation as described above.Figure 1f shows the cross-sectional SEM image of the SiO-Sn-Co/G electrode.The graphite and SiO-Sn-Co particles were partly marked in the SEM image.The jagged surface formed from uneven collapse during drying.Large graphite particles with an irregular shape were distributed mainly in the top and bottom layers.Many graphite particles were wrapped by tiny SiO-Sn-Co particles and formed a conductive skeleton.
Figure 2a shows a typical backscatter SEM image of the as-milled SiO-Co-Sn composite,where the bright zones are Co-Sn alloys and the dark zones are SiO,respectively.Most tiny Co-Sn alloy particles attached to large SiO particles.For more detailed information,the Si,Sn,and Co element mappings for SiO-Sn-Co nanoparticles were provided by HAADF-STEM.As shown in Figure 2b,the Sn and Co dispersion were close.Because the Sn-Co alloy was softer than SiO,it tended to attach tightly to SiO particles.Figure 2c shows the TEM image of some SiO-Sn-Co particles.The darker zones are ascribed to the Sn-Co alloy.A similar dispersion and morphology existed compared with the above results.Figure 2d shows the HRTEM image of the SiO-Sn-Co composite.The CoSn2phase was distinguished and calibrated according to lattice fringes,which confirms the XRD result.These data match our expectation that Sn-Co particles exist as tiny particles and attach to or insert into the SiO surface and act as conductive paths in charge/discharge,which leads to better kinetics for Li-ion reaction with SiO.

Figure 2.a)Back-scattered SEM image,b)HAADF-STEM images of SiO-Sn-Co composite with elemental EDS mappings of Sn,Si,and Co;c)TEM image;and d)high-resolution TEM image of SiO-Sn-Co composite.
The electrochemical performance was investigated in half cells.To illustrate the consistency of the SiO-Sn-Co/G electrodes,we prepared different cells and tested them at a current density of 50 mA g-1.Figure 3a shows that the discharge/charge curves of the four cells nearly overlapped for 0.01-1.5 V.The initial discharge/charge capacity of cell 1 reached 833.4/642.3 mAh g-1,and its ICE was 77.07% .Figure 3b shows that all cells achieved an ICE above 75% .As a comparison,a top result in those reported without pre-lithiation was 78.3% .[19]A narrow gap exists between the cells in our work and the outstanding cells.With a consideration of the complex components,the discharge/charge curves and corresponding differential capacity(dQ/dV)plots were plotted to explain the lithiation/delithiation behaviors.As shown in Figure 3c,three peaks were visible at 0.1,0.22,and 0.64 V during discharge.The weak peak at 0.64 V was attributed to the formation of lithium silicate and Li2O.The peak at 0.22 V represented an overlap of the alloy process of Si and Sn,and the low potential range(below 0.13 V)could be attributed to the formation of LixC6and amorphous LixSi.The charge curves for the SiO-Sn-Co/G electrode in Figure 3d showed independent reaction peaks of graphite,Si,and Sn.Graphite delithiation contributed most to the charge capacity below 0.21 V.The peaks at 0.28 and 0.46 V represented two steps in the dealloying of LixSi.The peak at 0.52 V is attributed to Li+extraction from the Sn-Co alloy whose location was related to the ratio of Sn and Co.[16]
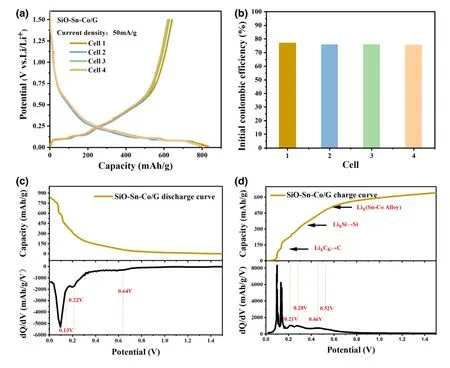
Figure 3.a)Discharge/charge curves at initial cycle of SiO-Sn-Co/G electrode in four cells,b)ICE of different cells,c,d)selected discharge/charge curves and corresponding differential capacity curves.
Multi-phase composites may cause a complex structure evolution and interface phase transformation.[26]To certify the stability of the multi-layer structure at different current rates,the SiO-Sn-Co/G electrode was cycled at a gradually increased current density of 50,100,200,400,800,and 1000 mA g-1.As shown in Figure 4a,the SiO-Sn-Co/G electrode exhibited a high reversible capacity of 758 mAh g-1at 50 mA g-1.No capacity decay occurred,except for the activation process during the initial several cycles.As the current density was enhanced,the main falloff occurred at 800 mA g-1,and when the current rate returned to 400 mA g-1,the capacity recovered as well.In comparison,the rate ability of SiO,SiO-Sn-Co,and SiO-Sn-Co/G electrodes at different current densities is provided under the rate curve of the SiO-Sn-Co/G electrode,which was calculated with a ratio of capacity of each last cycle at a different current density to that of the cycle at 50 mA g-1.SiO-Sn-Co and SiO-Sn-Co/G electrodes show similar performances that surpass the ability of the SiO electrode.These results show that the electrode can maintain good kinetics even with additional interference between large graphite and tiny SiO-Sn-Co particles.To probe the changes in electrochemical behaviors at different current densities,the charge/discharge plot of the last cycle at each rate condition is shown in Figure 4b.The lithiation potentials did not change visibly with a low polarization,as the current rate increased from 50 mA g-1to 1 A g-1,which suggested the superior lithiation kinetics of the SiO-Co-Sn/G electrodes.Because of the multi-phase reaction in a low potential range,the corresponding dQ/dV curves were plotted as shown in Figure 4c.Some tangle peaks were observed below 0.03 V(parts in red box)especially at low current rates of 50 and 100 mA g-1as the inset,which may result from large graphite particles being squeezed.When the current density changed to 1000 mA g-1from 400 mA g-1,the anodic peak at 0.15 V moved to a higher potential,whereas other parts remained almost unchanged.This result may have arisen because of the unstable SiO-Sn-Co/G interface structure.To determine whether the structure affects the cycling life,the charge capacities in each cycle for the SiO-Sn-Co/G anode were separated into three parts,namely 0.01-0.3,0.3-0.75,and 0.75-1.5 V,according to the different delithiation processes.As shown in Figure 4d,the main capacity loss occurred for the restructure of the Li-Si-O matrix at a high potential during the initial cycles.An increase of 0.01-0.3 V is ascribed to destroyed particles that provide a new diffusion path for trapped Li+.[28]The interfaces tend to achieve stability instead of leading to destruction.A long-term cycle life at 800 mA g-1for 0.01-1.5 V is shown in Figure 4e.A capacity retention of 70.45% was achieved even after 400 cycles,which is deemed suitable.The significant volume change of SiO resulted in most SiO-based anodes undergoing obvious and sustained capacity decay from the beginning and a tremendous de-activating phenomenon within a short cycle life.[34]In contrast,for the SiO-Sn-Co/G hybrid electrode,no such falloff resulted,which indicated the effectiveness of this method in buffering the volume expansion of the active SiO and Sn-Co alloy.
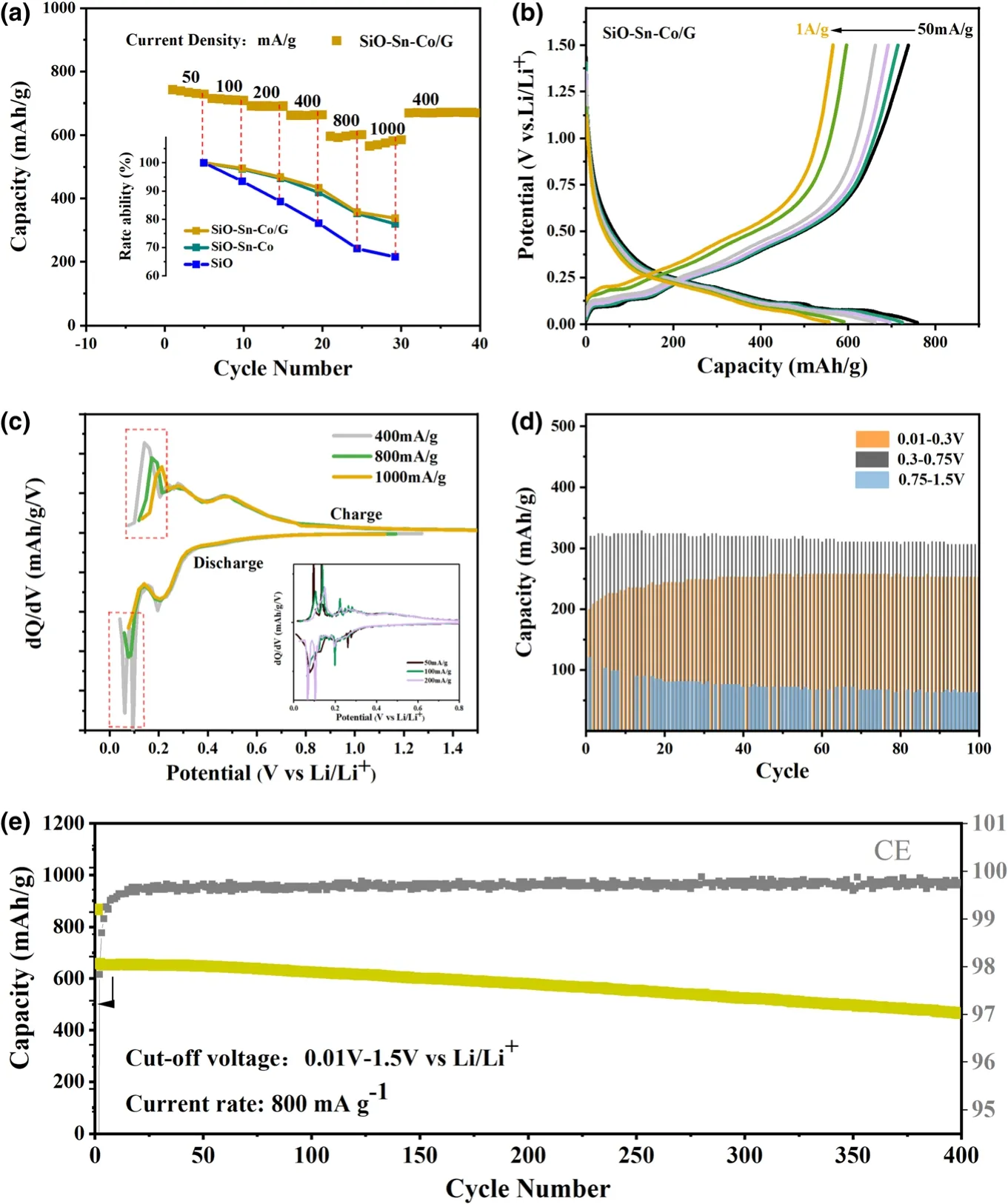
Figure 4.Cycle performance of SiO-Sn-Co/G electrode.a)Rate performance,b)discharge/charge curves,c)differential capacity curves of SiO-Sn-Co/G electrode at different current densities,d)reversible capacity at 800 mA g-1within three potential ranges,and e)long-term cycle performance at 800 mA g-1between 0.01 and 1.5 V.
To clarify the use of Sn,Co,and graphite,the SiO,SiO-Sn-Co,and SiO-Sn-Co/G electrodes were compared.Figure 5a exhibits the elevated ICE after each processing step.Along with Sn and Co,the electrode ICE was promoted by~15% compared with the milled SiO.Graphite or other carbon materials are used extensively in various anodes for a better stability,but in general,they decrease the ICE.This composite method with commercial graphite increased the ICE to above 77% .To understand the mechanism of increase in ICE,potential profiles of SiO,SiO-Sn-Co,and SiOSn-Co/G electrodes as a function of normalized capacity are given in Figure 5b.The lithiation curves were divided into three steps.The first step was termed the surface step of conversion,in which initial lithium ions gather in the surface layers of active particles and form an SEI film with electrolyte decomposition.[35]As a certain amount of lithium ions entered the active material,the conversion reaction will be triggered.[36]In this step,SiO shows a lower trigger potential at 0.5 V,whereas SiOSn-Co and SiO-Sn-Co/G were triggered at 0.7 V,which revealed better kinetics for the SiO-Sn-Co and SiO-Sn-Co/G electrodes.The third step was the alloying process,which provided the main reversible capacity of SiO.Because of the sluggish kinetics of Si,trapped Li ions in Si could lead to 30% of the initial discharge storage loss,which partly accounted for the low ICE and accelerated decay in subsequent cycles.[37-39]In the delithiation process,the degree of dealloying and reconversion dominated the ICE and reversibility capacity.The charge curves showed a different delithiation behavior among the three electrodes.In the dealloying region(below 0.75 V),more Li ions were extracted for SiO-Sn-Co and SiO-Sn-Co/G hybrid electrodes, which showed that the Sn-Co alloy contributed to a reduced Li+trapped in the LixSi alloy.In the reconversion region (above 0.75 V),the gentler slope for SiO-Sn-Co indicated Si and Li-Si-O glass rebuilding to SiO,which
contributed to part of the reversible capacity.This result agreed well with our previous work that Sn-M(M=Fe,Mn,Cu,Co)intermetallic promoted the reconversion of Li-Sn-O.[28]
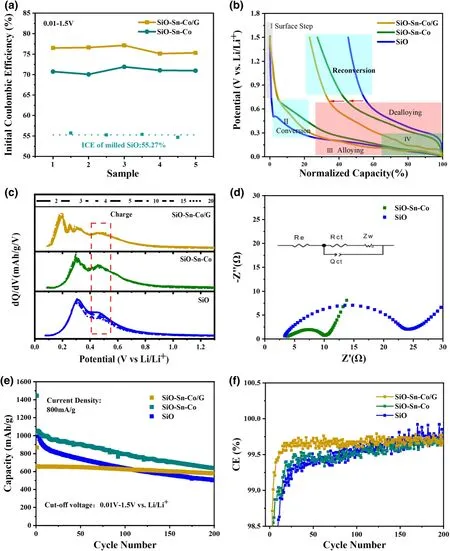
Figure 5.a)Initial columbic efficiency,b)potential profile of each electrode as a function of normalized capacity,and c)differential capacity curves of SiO,SiO-Sn-Co,and SiO-Sn-Co/G electrodes;d)EIS spectrum of SiO electrode and SiO-Sn-Co electrode;e)cyclic performance and f)corresponding CEs of SiO,SiO-Sn-Co,and SiO-Sn-Co/G electrodes.
Figure 5c shows the dQ/dV curves of the SiO,SiO-Sn-Co,and SiOSn-Co/G electrodes.During charging,the SiO peaks weakened gradually,whereas those of the SiO-Sn-Co and SiO-Sn-Co/G almost coincided.This phenomenon was more obvious for 0.4-0.6 V.The existence of a CoSn2phase improved the SiO reversibility and offers more energy storage.Further kinetic characterization was conducted by EIS to explore the effect of the Sn-Co alloy.As shown in Figure 5d,the Rctin the equivalent circuit represented the charge transfer resistance through the electrode/electrolyte interface.A clear enhancement is shown with the Rctof SiO and SiO-Sn-Co being 20.81 Ω and 7.12 Ω,respectively.As mentioned above,close contact between SiO and Sn-Co alloy may be positive.As a result,faster charge transfer kinetics promoted the interaction of Li-Si-Sn-O,which led to an improved reversibility.
Besides an improved ICE,the SiO-Sn-Co/G electrodes possessed a more stable reversible capacity,whereas the SiO electrodes were more likely destroyed with the continuous capacity decay.Comparisons were carried out on cycle performance.Figures 5e,f show the reversible capacity and coulombic efficiency(CE)along cycles of the SiO,SiO-Sn-Co,and SiO-Sn-Co/G at 800 mA g-1between 0.01 and 1.5 V.With commercial graphite,the SiO-Sn-Co/G hybrid electrode is adjusted to a low capacity.A much higher capacity retention results for the SiO-Sn-Co/G electrode compared with that for the other two electrodes.For the SiO-Sn-Co/G electrode,even after 200 cycles,the capacity retention remained at 90% ,which is higher than 49% for the SiO and 62% for the SiO-Sn-Co,as well as many reported SiO-based electrodes.[34]As a key index of electrode application in full Li-ion batteries,the CE of the SiOSn-Co/G is higher than the SiO and SiO-Sn-Co electrode.In general,the CE reflected the degree of side reactions,electrical contact between active materials,and stable SEI during lithiation/delithiation.[40,41]The SiO-Sn-Co/G hybrid electrode reached 99% after the third cycle and the average CE was as high as 99.71% from the 10th to the 400th cycle,which reflected the stable SEI and improved electrical connection.The gradually increasing CE of SiO and SiO-Sn-Co indicated an unstable structure.Therefore,graphite enhanced the conductivity,buffered the volume variation of the electrode,and achieved a highly stable electrode/electrolyte interface and superior cycling stability.
3.Conclusions
We have described a facile approach to fabricate SiO-Sn-Co/G anodes with a good electrochemical performance.A rough sandwich structure was achieved in which large graphite particles were wrapped in tiny SiO-Sn-Co particles.This approach helped to keep graphite integrated and a combination of particles with uneven sizes can accommodate the volume effect.An optimized SiO-Sn-Co composite enhanced the kinetics and reduced trapped Li.Graphite and milling promoted the ICE,CE,and provided an outstanding cycling stability for the SiO-Sn-Co/G electrode.This simple and facile method achieved a better comprehensive electrochemical performance for SiO-based anodes,which may allow for the optimization of other high-capacity anode materials.
4.Experimental Section
Material Preparation and Characterization:SiO powder(99.9% pure,~325 mesh,Shanghai Aladdin Bio-Chem Technology Co.,Ltd.,Shanghai,China),Sn(99.5% pure,Sinopharm Chemical Reagent Co.,Ltd.,China),and Co(99.9% pure,Shanghai Aladdin Bio-Chem Technology Co.,Ltd.,Shanghai,China)were used as raw materials without further purification.In a typical experiment,a mixture of three powders with a mass ratio of 70 wt% SiO/20 wt% Sn/10 wt% Co was sealed in a stainless steel jar.We milled~4 g of the mixture powders with a shake mill apparatus(QM-3C,Nanjing NanDa Instrument Plant,Nanjing,China)with a ball-to-powder mass ratio of 25:1 for 4 h with vibration frequency of 1200 rpm,to yield a final product as the SiO-Sn-Co composite.To clarify the usefulness of component addition,SiO was milled under the same condition.The entire milling process was suspended for 30 min to cool in case of an excessively high temperature.
The phase structure of the SiO-Sn-Co composite was identified by X-raydiffractometer (XRD, PANalytical) with Cu-Kα radiation. The sample morphologiesand microstructures were studied by using a Carl Zeiss Supra 40 field emission scanning electron microscope (SEM) and a JEM-2100 transmission electron microscopy(TEM) at 200 kV. Sample mapping was characterized by an energy dispersive X-rayspectrometer (EDS) with the TEM. An Escalab 250 X-ray photoelectron spectrometer (Thermo Fisher Scientific, USA) was used to analyze the electronic states of elemental Si, Sn, and Co elements in the unmilled and milled samples.
Half-Cell Assembly and Electrochemical Measurements:To composite with commercial graphite anode materials(99% pure,Sunwoda Electronic Co.,Ltd.,Shenzhen,China),the slurry of SiO-Sn-Co and graphite was stirred at the same time.Both had the same component mass ratio of 80wt% as-prepared active materials,10wt% carboxymethyl cellulose(CMC)binder,and 10wt% acetylene black(super P)conductive agent.The prepared slurry was coated onto copper foil as a sandwich structure of G|SiO-Sn-Co|G(termed SiO-Sn-Co/G electrode)by using a knife coater and dried at 80°C in a vacuum oven for 12 h.Cointype half cells(CR2016)were assembled in a glovebox filled with argon to measure the electrochemical properties.A galvanostatic charge-discharge test was performed on a battery testing system(LANDCT2001A,China)with a potential range of 0.0 to 1.5 V(vs Li/Li+).The electrochemical impedance spectroscopy(EIS)test was carried out on Gamry Interface 1000 electrochemical working station from 100 kHz to 0.01 Hz at a 5 mV amplitude signal.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.52071144,51822104,51831009,and51621001).
Conflict of Interest
The authors declare no conflict of interest.
 Energy & Environmental Materials2022年1期
Energy & Environmental Materials2022年1期
- Energy & Environmental Materials的其它文章
- Introduction of Frontier Outlook
- Biomass Template Derived Boron/Oxygen Co-Doped Carbon Particles as Advanced Anodes for Potassium-Ion Batteries
- A Stretchable Ionic Conductive Elastomer for High-Areal-Capacity Lithium-Metal Batteries
- Gas Generation Mechanism in Li-Metal Batteries
- Understanding the Coffee ring Effect on Self-discharge Behavior of Printed micro-Supercapacitors
- Directional Oxygen Functionalization by Defect in Different Metamorphic-Grade Coal-Derived Carbon Materials for Sodium Storage
