Directional Oxygen Functionalization by Defect in Different Metamorphic-Grade Coal-Derived Carbon Materials for Sodium Storage
Hanqing Zhao ,Dan Zhao,Jianqi Ye,Pengfei Wang,Maosheng Chai,and Zhong Li*
As the limiting factor for an energy storage technique from lab-scale to industrial-scale,cost means not only the price of raw materials but also the simplicity of processing technics.In this work,the oxygen functionalized carbon materials were obtained from three representative different metamorphic-grade coals,that is,lignite,bitumite,anthracite.Oxygen functional groups like quinones,carboxylic anhydrides,and lactones are easier to form near defects according to the thermogravimetric-mass spectrometry measurements and density functional theory calculation.Considering the highest amount of defects and C=O contained functional groups,the low metamorphic-grade lignite derived carbon exhibits a reversible capacity of 259.7 mA h g-1after 50 cycles at 0.03 A g-1,best among these micron sized coal-based carbons.The surface active sites contribute highly stable and majority of sodium storage capacity evidenced by in situ Raman spectra and cyclic voltammetry curves at different scan rates.The coal-based carbon materials in this work offer options for industrial applications of sodium-ion battery anode materials.
Keywords
anode,coal-based carbon,oxygen functional groups,sodium-ion battery
1.Introduction
Large-scale energy storage techniques hinder the development and effective usage of new energy sources like solar and wind energies.Sodium-ion battery(SIB)is a rising shining star in the field of energy storage and regarded as an alternative to lithium-ion battery to address the above issues,considering the low costs,wide distributions,and high earth abundances of sodium sources(23 000 ppm vs 20 ppm of Li).[1]Carbon has drawn great research interests as SIB anode material in virtue of excellent conductivity,stable,and long electrochemical cycling behaviors.The sodium storage in carbon materials can be concluded mainly as two aspects:[2]1)Na+absorbed by defects and oxygen functional groups(OFGs)on carbon surface;2)intercalation of Na+into carbon layers.The intercalation and deintercalation of Na+suffers from the limited d-spacing of carbon layers and sluggish Na+transfer dynamics,polarization and consequent poor rate capability,and moreover prone to Na metal deposition at high current densities.[3]Comparably,Na+absorption on the surface active sites of carbon materials at electrode/electrolyte interfaces displays remarkable dynamic advantages and hence make great contribution to fast charge/discharge capacities.
The coal with worldwide distribution and low cost,matches well with the large-scale and low-cost sodium storage techniques.The prices per ton of raw coals depend on the grade:~200 RMB/31 USD per ton for lignite,~500 RMB/77 USD per ton for bitumite,and~900 RMB/140 USD per ton for anthracite.Comparably,the price of batterygrade graphite is much higher:~70 000 RMB/~10 000 USD)per ton.Recently,carbon materials derived from coal chemical industry chains have come into the views of SIB anode researchers,owing to the reliable raw material supplies and the feasibility to large-scale energy storage industries.Qiu and coworkers fabricated porous three-dimensional N doped carbon networks using coal pitch as principal raw material under NH3atmosphere,in which pitch supply high conductive graphitic microdomain to enhance electron transfer.These anodes showed reversible capacity of about 200 mA h g-1at 1 A g-1after long cycles.[4,5]Hu and coworkers obtained carbon materials with different oxygen content(0.3-2.9 at.% )and defect concentrations,from coal pitch using different heat treatment temperatures.Lower degree of graphitization and more defects were found in the sample treated at 800°C,which delivered 263 mA h g-1at 0.15 C and 2.5 times higher than that treated at 1550°C.[3]
As the origin of all the carbon materials in coal chemical industry chains,coals show different physical characteristics and chemical compositions according to metamorphic grade,under the joint effect of temperature,pressure,and time during its formation process.It has been confirmed that anthracite after simple heat treatment shows a practical energy density of 100 Wh kg-1when assembled as SIB pouch cells and could endure a series of safety tests such as short-circuit,overcharge,and nail penetration.[6]Our previous work indicated that oxygen functionalized anthracite/graphene based three-dimensional architecture could deliver a reversible capacity of 254 mA h g-1at 0.05 A g-1after 350 cycles and over 100 mA h g-1at 2 A g-1after 2000 cycles.[7]Carbon materials derived from some other different metamorphic-grade coals,may show different structures and sodium storage properties.
In this work,three different representative metamorphic-grade coals(lignite,bitumite,and anthracite)with significant diversities in graphitization and amorphous degrees were selected as raw materials.After removal of mineral elements by acid etching and oxygen functionalization,these coal-based carbons are directly used as SIB anodes.Most content of defect exists in the low metamorphic-grade lignite derived carbon(lignite-C),which is directly observed by Raman mapping.Meanwhile,maximum content of OFGs are formed in the defect-rich lignite-C,which is confirmed by X-ray photoelectron spectroscopy(XPS),Fourier transform infrared spectroscopy(FTIR)and thermogravimetric-mass spectrometry(TG-MS).Surprisingly,the amounts of OFGs like quinones,carboxylic anhydrides,and lactones varies much more violently than those of carboxylic acids,phenols and ketones,and density functional theory(DFT)calculation was employed to detect the OFGs formation mechanism near defects on the carbon surfaces.When assembled as SIB cells,the lignite-C delivers a reversible capacity of 259.7 mA h g-1after 50 cycles at 0.03 A g-1,higher than bitumite-C(217.0 mA h g-1)and anthracite-C(175.0 mA h g-1),because of plentiful defects and C=O contained functional groups.The in situ Raman spectra and cyclic voltammetry(CV)curves at different scan rates demonstrate that surface active sites contribute highly stable and overwhelming majority of sodium storage capacity.The coal-based carbon SIB anodes matches well with declaration of sodium-ion battery in low-cost and large-scale energy storage techniques and provides the possibility of industrial application.
2.Results and Discussion
The joint effort of heat and deep burial pressure for different time,various coefficients of thermal expansion between mineral compounds and carbon phase,would give rise to a complex strain on the carbon phase during the coal-forming process,and then cause the reconstruction of local carbon atoms and subsequently the formation of defects.To obtain a comprehensive situation of defects in these coal-derived carbons,Raman mapping with area of 17 μm × 17 μm was collected.Figure 1a shows the Raman mapping of D/G intensity ratio,where peak D represents the defective or disordered carbon and peak G represents graphitic carbon.It is obvious to see that,more defects exist in the low metamorphic-grade coal-derived lignite-C,while comparably fewer defects in anthracite-C.The three-dimensional Raman spectra(Figure 1b)show significant variations in frequency distributions for the three samples.The distribution of D-band and G-band frequencies in the Raman spectra of lignite-C is 1253-1455 cm-1and 1515-1637 cm-1,respectively.Comparably,the frequency distributions are narrower for the bitumite-C(D-band:1277-1428 cm-1,G-band:1538-1626 cm-1)and anthracite-C(D-band:1303-1408 cm-1,G-band:1552-1623 cm-1).Strains among the various sized aromatic ring systems,[8]higher concentration of electron-rich oxygen atoms[9]and more C/O atoms with complicated combination form,will influence the molecular vibration and rotation,and hence lead to weakened and diffused profiles of Raman peaks for lignite-C.In further analysis of Raman peak fitting(Figure S1 in supporting information),the areal ratio of integrated peak D and G are 1.65,1.56,and 1.49 for lignite-C,bitumite-C,and anthracite-C,respectively,in accordance with observation of Raman mapping.The distance between defects(LD)has been proved to be an impactful reference point to evaluate the defect densities.[10,11]The calculated defect distance in lignite-C(8.8 nm)is smaller than those in bitumite-C(9.0 nm)and anthracite-C(9.2 nm),confirming more intrinsic defects distributed in lower metamorphicgrade coal-derived carbon material.Moreover,the sp2-hybridization domain size was calculated to be 10.2 nm,10.7 nm,11.2 nm in the lignite-C,bitumite-C,and anthracite-C,respectively,in accordance with the Lavalue in XRD.In the defect-rich regions,some carbon atoms bonded with oxygen atoms to form OFGs during the oxygen functionalized process,while some other carbon atoms bonded with hydrogen atoms to reach the structure stability.That is to say,fewer H atoms exist in bitumite-C and anthracite-C with fewer defects.Weak magnetization intensity of H atoms and strong C-C dipole effect in high grade bitumite-C and anthracite-C,would make the13C-1H Cross-Polarization Magic-Angle-Spinning Nuclear Magnetic Resonance(CPMAS NMR)signal difficult to collect.Yet the13C-1H CPMAS NMR spectra was comparable easy to collect for the defect-rich lignite-C(Figure S2 in supporting information).The evidences from NMR are in line with the above tests on the defect concentrations of these coal-based carbons.More defects in carbon material would contribute more sodium storage sites.
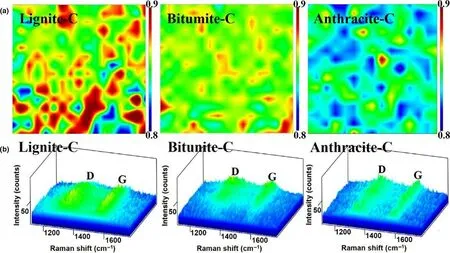
Figure 1.a)Raman mapping of D/G intensity ratio under same range of 0.8-0.9,b)corresponding three-dimensional Raman spectra.
Under high temperature and pressure of deep burial for millions of years,the ordered carbon with sp2hybridization was formed in the coals.[12]More defect-rich amorphous carbon exists in lignite because of comparably shorter deep burial time,while more ordered carbon in anthracite.Additionally,after removal of mineral compound and oxygen functionalization,more nano-sized carbon fragments were observed in the low metamorphic-grade lignite derived carbon(lignite-C,see the scanning electron microscope images in Figure S3).All coal-derived carbons exhibit amorphous structure,confirmed by the broad(002)and(100)peaks in X-ray diffraction patterns(XRD,Figure S4)and high resolution transmission electron microscopy(Figure S5).The Lcand Lavalue(see the inset of Figure S4)imply that graphite domains with smallest size exist in lignite-C,where Lais the average size of graphite crystals along a axis and Lcis the thickness of graphite crystals deposited along c axis.Therefore,there are most pathways for oxygen to enter into the carbon layers of lignite-C during oxygen functionalization process and d-spacing was enlarged to be 3.76 °A,while 3.69 °A for bitumite-C and 3.62°A for anthracite-C.To reveal the variation in the specific surface area of the coal-based carbons,nitrogen adsorption-desorption isotherms were conducted and shown in Figure S6a.The isotherms indicate the three samples show mesoporous structures with H2 hysteresis loops.The lignite-C exhibits higher specific surface area of 306.1 m2g-1compared with bitumite-C(281.0 m2g-1)and anthracite-C(166.4 m2g-1).The reason for this can be mainly summarized as two factors.Firstly,because of the strong compaction effect within coal bulks by deep burial,the number of mesopores and macropores would maintain in the low metamorphic-grade coals,while the pores would decrease gradually during the burial process for bitumite and anthracite.[13,14]Secondly,more organic matters in the lower rank coal were oxidized into water-soluble and gaseous products during the oxidation process,causing formation of more pores and higher surface area.[15]The comparably higher specific surface area of lignite-C would helpful to the penetration of electrolyte and expose more sodium storage active sites at the electrode/electrolyte interfaces.
The theoretical calculation suggests that the reactivity of carbon was enhanced near the defect sites,and oxidation could start and stabilize at these sites.[16]Oxygen concentration on the surface of lignite-C,bitumite-C,and anthracite-C are 28.4,22.6,and 13.6 at.% ,respectively,as reflected by XPS.No obvious impurity elements such as Si(103.7 eV),Al(74.7 eV),P(135-136 eV),S(164-168 eV),and N(398.4-401 eV)were detected in the full spectra(Figure S7),indicating the complete removal of impurity elements.The C 1s spectra(Figure 2a)can be fitted into four subpeaks at about 284.5,285.2,286.5,and 288.5 eV,corresponding to C-C sp2,C-C sp3,C-O,and C=O,respectively.[17]The C-C sp3hybridization implies the three-dimensional carbon atom configuration as well as the presence of defects.Hence the maximum C-C sp3area ratio of 16.4% for lignite-C indicates the highest concentration of defects(Figure 2c),which is constant with the Raman observations.Similar with defects,C=O contained OFGs could store Na+by the reversible reaction,C=O+Na++e-?C-O-Na.The C=O peak area ratio of 17.8% in C 1s spectra for lignite-C is higher than bitumite-C(16.7% )and anthracite-C(12.1% ).For further accurate analysis,O 1s spectra were also collected(Figure 2b)and can be fitted into four subpeaks at about 531.5,532.7,533.3,and 536.7 eV,referring to C=O,C-OH/C-O-C,O=C-OH and chemisorbed oxygen.Among t+hese OFGs,C=O and O=C-OH contain active double bonds to store Na and were added up to evaluate the sodium storage active sites(Figure 2c).High concentration of defects and C=O contained functional groups will play positive roles in the sodium storage behavior of lignite-C.
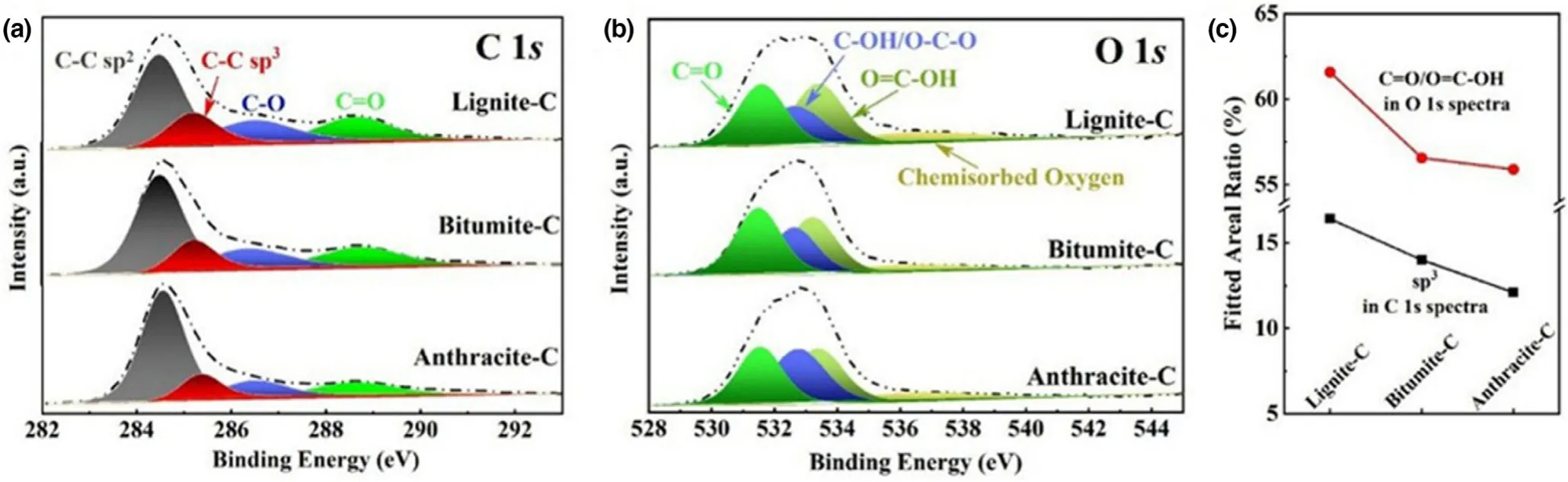
Figure 2.a)C 1s and b)O 1s XPS spectra of the samples,c)corresponding fitted areal ratios of C=O contained functional groups in O 1s spectra and sp3 in C 1s spectra.
FTIR was used to investigate the evolution of OFGs on these coals before and after oxygen functionalization.In the raw coals(see the Figure S8a),vibration peaks of Si-O-Al,Si-O-Si and CaCO3verify existence of mineral elements in the raw coals.[18]The peak at~2972 cm-1might relate to the signal of CO2adsorbed on the surface of samples,based on the blank test of pure KBr(not present here).Additionally,abundant polar functional groups like-OH and C-O have existed,especially for the raw lignite with loose structure and high internal surface area.These groups would be exposed after removal of minerals and parts of them might be maintained after oxidation.Notably,no C=O contained functional groups,the reversible active sites to store Na+,can be observed.Moreover,no oxygen was introduced onto the coal-derived carbons during the mineral removing process treated by mixed solution of HF and HCl.Therefore,it is the oxygen functionalization process that bring the C=O contained OFGs onto the surface of coal-derived carbons.As observed in Figure S8b,all peaks related to the mineral elements disappeared after removal of mineral elements and oxygen functionalization.Most OFGs including C=O groups(at~1724 cm-1),were formed on the surface of the lignite-C(Figure S8b),with smallest particle size and highest defect concentration.Additionally,OFGs would accelerate the infiltration of ester electrolyte into these carbon material.The contact angles between carbon materials and electrolyte(marked by red lines in Figure S8c),were calculated by Young Laplace equation to be 19.2°,20.1°,and 20.9°for lignite-C,bitumite-C,and anthracite-C,respectively.Obviously,lignite-C shows best infiltration of electrolyte.Additionally,the electrolyte drops would be adsorbed in less than 0.04 s,indicating the rapid electrolyte infiltration into the oxygen functionalized coal-based carbons.
Considering limited detection depth(usually<5 nm)of XPS and the qualitative characterization of FTIR,TG-MS analysis is a more powerful technique with higher accuracy to investigate the OFGs distributed on all the surface of porous carbons.[19]Because of various thermal stability,the OFGs on the carbon would be pyrolyzed into CO and CO2under inert atmosphere at different temperatures.The TG-MS CO and CO2evolution spectra were recorded and fitted according to the decomposed temperature of the functional groups(Figure 3a,b for lignite-C,Figure S9 for bitumite-C and anthracite-C).[20,21]The CO signals at different temperatures are originated from ketones(200-400 °C),carboxylic anhydrides(400-650 °C),phenol groups(600-800 °C),and quinones(750-1000 °C).The CO2signals are originated from carboxylic acids(150-450°C),carboxylic anhydrides(300-650 °C)and lactones(600-800 °C).The relative amounts(10-11mg-1)of different OFGs according to the fitted areas were summarized in Figure 3c for the three coal-derived carbons.Constant with XPS and FTIR tests,the defect-rich lignite-C owns the largest total amount of C=O bond contained OFGs.According to our previous DFT calculations,[22]these OFGs with various atomic and electronic environments show diverse bond angles to stabilize the energy when forming C-O-Na bonds.Among these groups,carboxylic anhydrides and quinones possess the stable configurations with two C=O bonds,high adsorption energy to Na+and more importantly,excellent electronic conductivity even better than C=C/C-C bonds.Therefore,most plentiful carboxylic anhydrides and quinones groups would make outstanding sodium adsorption and fast storage contribution to lignite-C among these coal-based carbons.
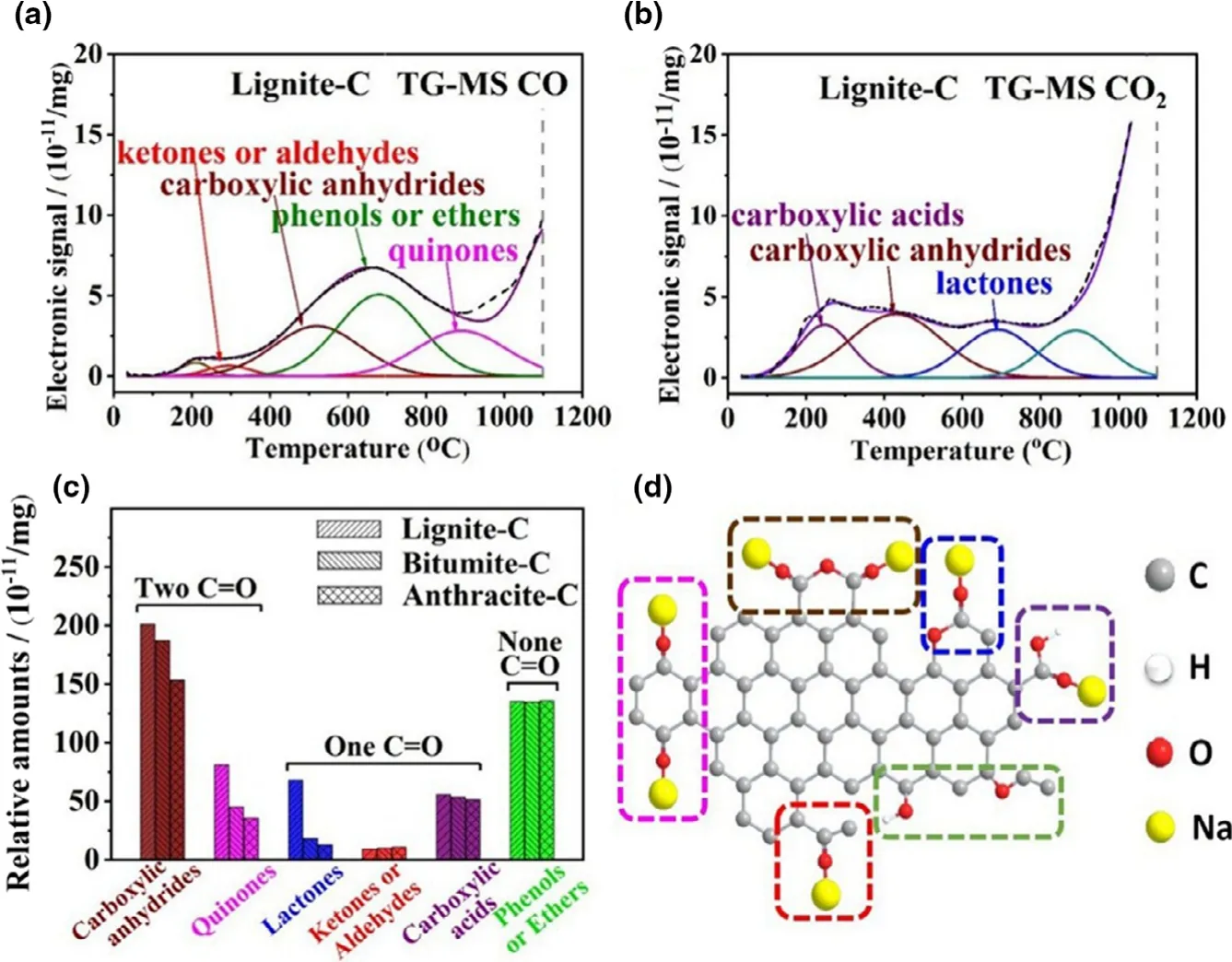
Figure 3.TG-MS a)CO and b)CO2evolution spectra of lignite-C,c)the relative amounts,and d)corresponding sodium storage diagram of different OFGs.
In order to dig the underlying reason why the concentrations of different OFGs varies,among the three coal-based carbons under the same oxidation treatment,we have calculated the formation energy of these OFGs with presence of defect on the carbon layers.As shown in Figure 4,all the formation energies are negative,indicating that all these OFGs can form and stabilize on the carbon surface theoretically.The formation energies for quinones,carboxylic anhydrides,lactones are-1.397,-1.183,and-1.012 eV,respectively.In obvious contract,the formation energies for carboxylic acids(-0.561 eV),phenols(-0.372 eV),ketones(-0125 eV)are much more positive.This demonstrates that quinones,carboxylic anhydrides,lactones groups are much easier to form near defects on the carbon surface,compared with groups like carboxylic acids, phenols, ketones. Differential charge densitycalculations witnessed the different charge transfer behaviors during theOFGs formation process, and the result indicates more electrons are transferred between quinones (carboxylic anhydrides, lactones) groups andgraphene, compared with other groups. The calculation of formationenergies and differential charge density supply evidence in theory forcontent variations of different OFGs on the surface of these coal-derivedcarbons, constant with the result obtained by TG-MS techniques.
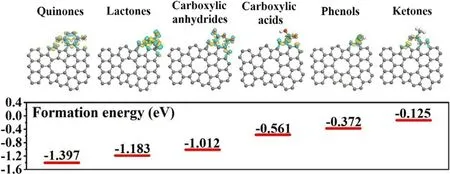
Figure 4.DFT calculation of formation energy of various OFGs near defects.
Since the surface potential can reveal the electron transfer and electronic conductivity of electrode materials,Kelvin Probe Force Microscopy(KPFM)was conducted to evaluate the surface potential for the coal-derived carbons and shown in Figure 5.The average values of surface potential for lignite-C,bitumite-C,and anthracite-C are-0.56,-0.57,-0.56 V,respectively,demonstrating similar electron conductivity.The conductivity of these carbons revealed by KPFM is consistent with electrochemical impedance spectroscopy data(see Figure S10 in supporting information).The reason for this should be that abundant highly conductive carboxylic anhydrides and quinones groups would recoup the electron conductivity losses caused by other poorly conductive OFGs.Further,the defects in the carbon structure are beneficial to improve the electron conductivity,due to higher densities of states(DOS)of defective carbon than the ideal one.[17]

Figure 5.Surface potential maps for the coal-derived carbons(color bar refers to relative intensity of surface potential).
In order to evaluate the sodium storage behaviors,the lignite-C,bitumite-C,and anthracite-C were assembled as half coin-type cells.The solution of 1MNaClO4dissolved in the mixture of ethylene carbonate and propylene carbonate(1:1 v/v)with 5 wt% FEC addition was selected as electrolyte.The sodium storage mechanism of lignite-C was studied by CV at voltage range of 0.01-3 V(Figure 6a).The peaks at around 0.5 and 1.1 V in the profiles of 1st scan correspond to the irreversible reactions such as decomposition of electrolyte,solid-electrolyte interphase formation and irreversible reduction of the OFGs.CV curves of subsequent scans nearly overlapped suggesting good sodiation/desodiation reaction reversibility.The galvanostatic charge-discharge curve of lignite-C at 0.03 A g-1was carried out and shown in Figure 6b.The charge/discharge capacities during 1st cycle are 431.9 and 1240 mA h g-1,and the irreversible reactions are responsible for the fading capacity,similar with observation in the CV curves.Generally,tailoring binders and electrolyte compositions in the SIB cells are beneficial to solve the problem of low initial Coulombic efficiency.[23]Although d-spacing of lignite-C is expanded to be 3.76°A,no apparent voltage plateau were detected below 0.1 V indicates negligible Na+intercalation into the carbon layer,according to Na+“adsorption-insertion”storage mode in carbon materials.[24]Moreover,since the closed pores contribute the plateau capacity[25]and no obvious plateau regions were observed in the charge/discharge curves in this work,it can be deduced that the pores in these coals are mainly “open pores” and rarely “closed pores”.Therefore,it is the surface sodium storage sites,that is,defects and C=O contained functional groups,that supply the overwhelming majority of sodium storage capacity.Identical sodium storage modes exist in the bitumite-C and anthracite-C,evidenced in the similar profiles of CV and charge/discharge curves(Figure S11).Figure 6c shows the cycling performance of the three carbon materials at 0.03 A g-1.The cycling stabilities were gradually realized after 10 cycles with Coulombic efficiency up to nearly 100% .The lignite-C delivers a reversible capacity of 259.7 mA h g-1after 50 cycles,higher than bitumite-C(217 mA h g-1)and anthracite-C(175 mA h g-1),because of plentiful defects and C=O contained functional groups.Meanwhile,best rate performance is also obtained in lignite-C compared with bitumite-C and anthracite-C(Figure 6d).The reversible capacities of 345.4,291.2,252.1,225.8,187.9,and 162.4 mA h g-1are achieved at current densities of 0.03,0.05,0.1,0.2,0.5,and 1 A g-1, respectively. The reversible capacity maintains at 290.9 mA h g-1when the current density goes back to 0.03 A g-1.The best sodium storage behavior for lignite-C among these coalderived carbons should be attributed to three aspects: firstly,abundant defects and OFGs as sodium storage sites,especially the carboxylic anhydrides and quinones groups with two C=O bonds.Secondly,higher surface areas and open pores as the electrode/electrolyte interfaces for fast Na+transport and storage.Thirdly,equivalent electron conductivity compared with other two coal-derived carbons.After activated at 0.03 A g-1for first ten cycles,the long cycling test for lignite-C electrode was carried out at 1 A g-1to estimate the cycling stability(Figure 6e).As is shown,the corresponding Coulombic efficiency climbed sharply to approximately 100% after the activation cycles and displayed a high capability of 151 mA h g-1after 2000 cycles.Comparably,the coals without oxygen functionalizations show inferior the rate performances(Figure S12 in supporting information),confirming the positive role of OFGs on the sodium storage for these coal-based carbons especially the performances at high current densities.Similar experimental phenomenon were observed in the oxygen functionalized anthracite by mechanochemistry using CO2,in which introduction(or removal)of oxygen would increase(or decrease)the sodium storage capacities substantially.[26]The good rate retention of the sample after oxygen functionalizations should be attributed to the excellent electrochemistry dynamics of OFGs at the electrode/electrolyte interface.Of cause,superior electron conductivity is highly needed for the electrode to make OFGs complete the sodium storage reactions.Hence,building a sophisticated conductive networks using graphenes[27]or carbon nanotubes,[28]would be an effective choice to improve the electrochemical performances of the micro sized coal-based carbons further.The stable reaction reversibility of C=O contained OFGs during the initial sodiation/desodiation cycle at current density of 50 mA g-1and even 4000 cycles at current density of 2 A g-1,have been confirmed by ex situ FTIR measurement in our previous work.[22]Here,as another sodium storage sites,the reaction reversibility of defects would be studied by in situ Raman spectra.
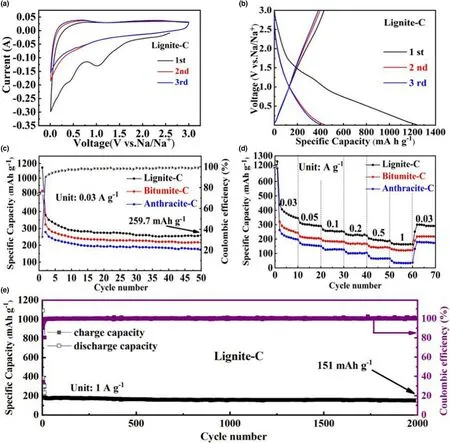
Figure 6.a)CV curves,b)Galvanostatic charge/discharge voltage profiles at 0.03 A g-1for lignite-C,c)cycling performance at 0.03 A g-1,d)rate performances for all samples,e)cycling performance at 1 A g-1for lignite-C.The voltage ranges are 0.01-3 V for all tests at room temperature.
The storage of alkali metal ions onto the surface and into the layers of carbons will pose a strong influence on the positions of D-band and G-band in the Raman spectra.[29-31]In the case of lignite-C in this work,since sodium ions are prone to be absorbed by the defects and OFGs on the surface of carbon,the electron concentration and chemical bonds between carbon atoms near these defects would be under radical change,and therefore the vibration and rotation of defective carbon(D-band)will be influenced in the process of sodiation.As shown in the Figure 7,the peak D shifted from 1354 cm-1at the open-circuit voltage to 1343 cm-1at the state of full sodiation during the discharge process,and then shifted back to 1353 cm-1at state of full desodiation after charge process in the half cells,indicating the good reversible stability of absorption/desorption Na storage mode for lignite-C.Comparably and obviously,the position of peak G stabilized at~1598 cm-1during the whole discharge/charge process.This is attributed to the undamaged sp2hybridized carbon and the stable C/C bonds,confirming the absence of Na+insertion into the carbon layers.The sodium storage mechanism investigated by the in situ Raman technique agrees well with the galvanostatic charge/discharge voltage profiles.
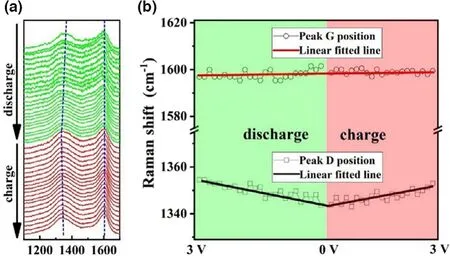
Figure 7.a)In situ Raman spectra at the first cycle of lignite-C with the current density of 0.03 A g-1and b)the corresponding positions of peak D and G.
Generally,there are two main types of sodium storage modes for carbons:1)capacitance process,that is,the Na+absorbed on the active sites like defects and C=O contained functional groups on the surface of carbon;2)the diffusion of Na+during the carbon layers.To investigate the Na+storage contribution in lignite-C,CV curves at different scan rates were carried out(Figure 8a).The correlation between peak current(i)and scan rate(v)can be summarized as the equation i=avb,in which a and b are variable.The b-value of 0.5 implies diffusion-controlled mechanism in the reaction process,and the value closing to 1 indicates the surface capacitance storage mechanism.[32,33]Figure 8b shows the fitted line of the log(i)-log(v)value,and b-value of 0.83 indicates that the Na+storage kinetics for lignite-C is dominated by surface capacitive mechanism.Further,the ratio of capacitive contribution at scan rate up to 2 mV s-1is determined to be 91.1% (Figure 8c),due to abundant Na+storage active sites on the surface of the material.
Conclusion
In conclusion,three representative different metamorphic-grade coals after simple treatment were used as anode materials for sodium-ion battery.It is amazing to see that quinones,carboxylic anhydrides and lactones groups are prone to form near defects compared with other groups,which is confirmed experimentally and theoretically.On account of highest concentration of defects and OFGs,lignite-C shows a reversible capacity of 259.7 mA h g-1after 50 cycles at 0.03 A g-1and 151 mA h g-1even after 2000 cycles at 1 A g-1,best among these micron sized coal-based carbons.The defects and OFGs could contribute dominate and stable sodium storage capacity,evidenced by in situ Raman and CV curves at different scan rates.Higher industrial potentials of coal and simple treatment process in this work,make coalbased carbon materials a reliable and practical choice for sodium-ion battery anode materials.
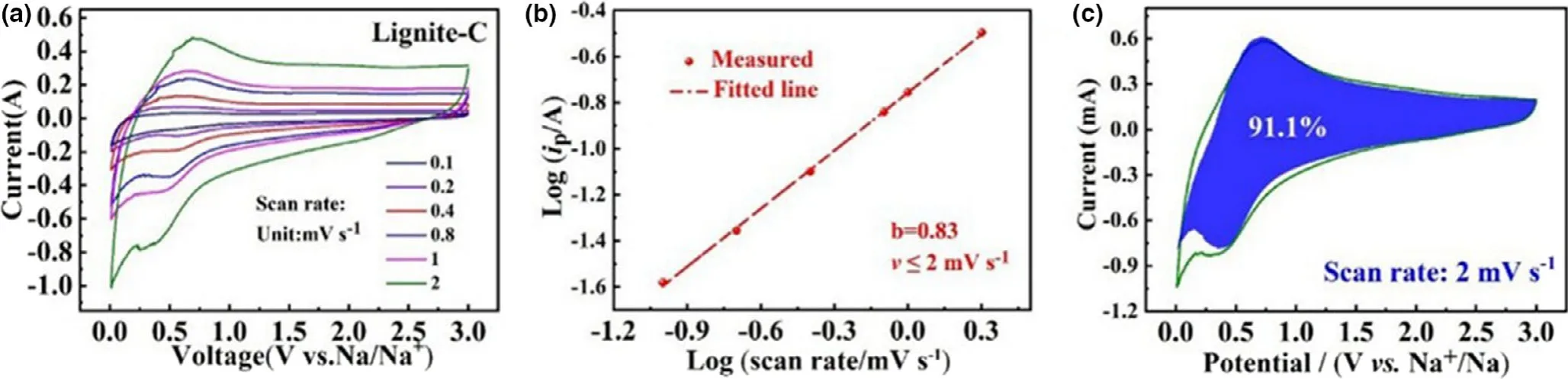
Figure 8.a)CV curves at different scan rates,b)the fitted line of log(i,peak currents)-log(v,scan rates),and c)the ratio of capacitance contribution at the scan rates of 2 mV s-1for the lignite-C.
Acknowledgements
The authors appreciate Dr.Qian Zhang and Dr.Yangang Mei for the fruitful discussions on structure analysis of coals.This work was financially supported the National Natural Science Foundation of China(No.21878207)and Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi Province(2019),Key Research and Development Project(International Science and Technology Cooperation Program)(No.201803D421011).
Conflict of Interest
The authors declare no conflict of interest.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
 Energy & Environmental Materials2022年1期
Energy & Environmental Materials2022年1期
- Energy & Environmental Materials的其它文章
- Introduction of Frontier Outlook
- Sn Alloy and Graphite Addition to Enhance Initial Coulombic Efficiency and Cycling Stability of SiO Anodes for Li-Ion Batteries
- Biomass Template Derived Boron/Oxygen Co-Doped Carbon Particles as Advanced Anodes for Potassium-Ion Batteries
- A Stretchable Ionic Conductive Elastomer for High-Areal-Capacity Lithium-Metal Batteries
- Gas Generation Mechanism in Li-Metal Batteries
- Understanding the Coffee ring Effect on Self-discharge Behavior of Printed micro-Supercapacitors
