Protective effect of Scrophularia striata combined with trehalose and cysteine added to diluents on cryopreservd goat epididymal sperm
Zeinab Maleki,Abbas Farshad,Jalal Rostamzadeh
Laboratory of Reproduction Biology,Department of Animal Sciences,Faculty of Agriculture,University of Kurdistan,Sanandaj,Kurdistan,Iran
ABSTRACT
Objective:To evaluate antioxidant effects of Scrophularia (S.)striata ethanol extract,trehalose and cysteine added to diluents on cryopreserved goat epididymal sperms.
Methods:Motility and standard motion parameters of sperm were assessed by using computer assisted sperm motility analysis system.Sperm viability was evaluated by eosin-nigrosin staining method.Hypo-osmotic swelling test was used to evaluate membrane health.Thiobarbituric acid testing was used to measure malondialdehyde(MDA) concentrations. To assess DNA fragmentation,sperm chromatin dispersion test was used. In Experiment 1,treatments consisting of basal Tris diluent supplemented with 25,50 or 100 μg/mL of S. striata ethanol extract gave the best concentration to the freezing diluents. Experiment 2 was carried out to compare the best concentration of S. striata ethanol extract (50 μg/mL)resulting from the first experiment with 150 mM trehalose and/or 5 mM cysteine alone or in combination.
Results:S. striata ethanol extract (50 μg/mL) significantly increased sperm viability,motility and progressive motility and at the same time decreased MDA concentration and DNA fragmentation compared to other treatments (P<0.05). In addition,all treatment groups resulted in viability,membrane health,total motility,progressive motility,curvilinear velocity,straightline velocity higher and MDA lower compared to the control group (P<0.05). Acrosome integrity was significantly higher in 50 μg/mL of S. striata ethanol extract combined with cysteine,trehalose,or cysteine+trehalose groups than those in the control,trehalose,cysteine,and 50 μg/mL of S. striata ethanol extract groups (P<0.05). Regarding DNA,extenders supplemented with 50 μg/mL of S. striata ethanol extract,50 μg/mL of S. striata ethanol extract+trehalose,and 50 μg/mL of S. striata ethanol extract+trehalose+cysteine were superior to other treatments.
Conclusions:Adding 50 μg/mL of S. striata ethanol extract alone or in combination with trehalose and cysteine can improve the quality of cryopreserved epididymal sperms of goats.
KEYWORDS:Scrophularia striata; Cysteine; Trehalose;Oxidative stress; Epididymal; Sperm; Cryopreservation;Antioxidants
1. Introduction
Sperm cryopreservation is a main part of artificial insemination process which is necessary to conserve local recess and also to prevent destruction of their valuable genetic material[1]. Sperm freeze-thawing process causes some structural and functional damages to sperms,leading to a decrease in post-thawed sperm quality,viability,motility and fertility. Such damages include higher levels of production of free radicals,especially reactive oxygen species (ROS) and peroxidation of phospholipid bilayer cell (sperm)membranes[2,3]. Free radicals may cause cell membrane distraction,sperm motility and other sperm disorders.
The imbalance between free radical production and antioxidant capacity in cells results in oxidative stress. Presence of antioxidants is necessary to prevent lipid peroxidation. Sperms use an enzymatic antioxidant system as well as nonenzymatic substances; however,exogenous antioxidants are essential for a proper cryopreservation[4].Herbal plants contain natural substances which have antioxidant activity,on which many studies were focused to examine their efficacy to control free radicals and lipid peroxidation. Antioxidant activity of herbs is equal to or even more than that of synthetic ones.Phenols are multi-functional substances that may act as an important natural antioxidant,namely it can act as a reducing agent to suppress free radical damages[5]. Phenolic compounds and flavonoids play an important role in the adsorption and neutralization of ROS,especially free radicals,and in the decomposition of peroxides due to their oxidizing and revitalizing properties and hydroxyl group donor[6]. Scrophularia (S.) striata containing phenols,phenol propanidids and flavonoids may exert antioxidant activities and reduce ROS production[7]. In a study performed on rats,it was found that the use of S. striata extract caused significant wound contraction and accelerated healing,and could be recommended to treat various types of wounds in humans and animals[8]. Cell plasma membrane plays many key roles and must be conserved during freezingthawing process. Positive effects of trehalose added to extenders during cryopreservation are repeatedly reported,and it is shown that it may prevent cell dehydration[9]. It is supposed that sucrose and trehalose play key roles in freezing-thawing process[10].
Amino acids may protect cells against cold stress. Amino acids,e.g. cysteine,play an important role to prevent membrane protein sedimentation and also to increase sperm resistance against cold stress in goat[11]. Cysteine is a sulfur-containing amino acid that is normally present in seminal plasma and sperms’ nucleic acid,being necessary to keep DNA healthy. As an intracellular antioxidant,cysteine may protect sperms against damages caused by free radicals[12].
To our knowledge,the present study is the first study to evaluate the S. striata extract as antioxidant agent added to diluents can increase the sperm freezability. Moreover,our literatures review indicated that no study has been performed using these antioxidants alone or in a combination in extenders. Therefore,the aim of experiment 1 was to determine antioxidant effects of optimum level of S. striata extract in goat epididymal sperm freezability and the aim of experiment 2 was to determine any probable synergistic effects of ethanol extracts of S.striata,trehalose and cysteine added to diluents.
2. Materials and methods
2.1. Chemicals
All chemicals used in the experiment were obtained from Sigma-Aldrich (St. Louis,MO,136,USA) and Merck (Darmstadt,Germany).
2.2. Extraction of S. striata
S. striata was provided from a medical shop (where spices and dried herbs were provided) in Kermanshah,Iran. The dried S. striata was powdered by a mortar and extracted by Soxhlet extraction equipment(ethanol 96%). The extract was placed in a rotary evaporator vacuum system at 40 ℃°to concentrate. Concentrated solution was placed in a 35 ℃ oven for 24 h,and then it was stored at 4 ℃ to be used later.
2.3. Preparation of spermatozoa
In the study,we conducted two experiments. For each experiment,a total of 24 testes from 12 goats were used in 6 replicates,in each of which 4 testes were obtained from an indus slaughterhouse and stirred. Spermatozoa was taken by making several incisions on the caudal epididymis,and then they were kept in Tyrode lactate solution at 37 ℃ for 15 min. This medium contained 100 mM NaCl,3.1 mM KCl,25 mM NaHCO3,0.29 mM NaH2PO4H2O,21.6 mM Na Lactate,2.1 mM CaCl22H2O,0.4 mM MgCl2H2O,10 mM HEPES buffer,0.0006 g/mL bovine serum albumin,1 mM sodium pyruvate,25 μg/mL gentamycin,and 10 mg/L phenol red[13].Sperm suspension was centrifuged at 700×g for 6 min; subsequently,spermatozoa were used to continue experiments. Only those sperms with total motility >75%-80% were used in the experiments. Then,they were diluted in the basic extender composed of 3.07 g Tris,1.26 g fructose,3.60 g citric acid with 100 mL of distilled water containing 10% (v/v) egg yolk and 5% (v/v) glycerol[14]. Osmolality and pH of the extender were 320 mOsm and 7.2,respectively.
2.4. Experiments design
According to results obtained in our published research,the goal in experiment 1 was to find the best concentration of S. striata ethanol extract using in freezing diluents. The extenders were prepared as following:basic extender+25 μg/mL of S. striata ethanol extract,basic extender+50 μg/mL of S. striata ethanol extract,and basic extender+100 μg/mL of S. striata ethanol extract. In experiment 2,the treatments included:1) basic extender+150 mM trehalose,2) basic extender+5 mM cysteine,3) basic extender+50 μg/mL of S. striata ethanol extract,4) basic extender+50 μg/mL of S. striata ethanol extract+5 mM cysteine,5) basic extender+50 μg/mL of S.striata ethanol extract+150 mM trehalose,6) basic extender+50 μg/mL of S. striata ethanol extract+5 mM cysteine+150 mM trehalose,7)the control group was supplemented with no additives.
Each treatment consisted of 6 replicates. Diluted semen samples were loaded into straws (each 0.25 mL). After blocking the end of straws with polyvinyl chloride powder,they were kept at 4 ℃for 3 h. Next,straws were exposed to nitrogen vapor at a distance of 4 cm from liquid nitrogen level. After 15 min,straws were immersed in the liquid nitrogen. In order to evaluate spermatozoa,frozen straws were thawed at 37 ℃ for 30 s[15].
2.5. Sperm motion characteristics and viability
Motility and standard motion parameters of sperm were assessed by using computer assisted sperm motility analysis system (CASA:IVOS version 12; Hamilton-Thorne 188 Biosciences,MA,USA).Following motility parameters were recorded:total motility (%),progressive motility (%),curvilinear velocity (VCL) (μm/s),straightline velocity (VSL) (μm/s),average path velocity (VAP) (μm/s),linearity (LIN%=VSL/VCL),straightness (STR%=VSL/VAP),amplitude of lateral head displacement (ALH) (μm),and beat/cross frequency (BCF) (Hz). For each evaluation,10 μL of samples was put on a lam and 10 points were evaluated per lam. Sperm viability was evaluated by eosin-nigrosine staining method. 5 μL of diluted semen along with 10 μL of eosin-nigrosine dye was smeared on the lam and left to dry in the air. Sperm cells were then counted using a light microscope (Nikon,Tokyo,Japan) with 40× magnification and the number of non-stained sperms was determined as live spermatozoa[14].
2.6. Sperm membrane and acrosome integrity
Hypo-osmotic swelling test was used to evaluate membrane health.10 μL of diluted semen was mixed with 100 μL of hypoosmotic solution (9.0 g fructose + 4.9 g sodium citrate + 1.0 L distilled water)and kept at 37 ℃ for 60 min. After a drop of the solution was placed on lam and covered with a lam and then 200 sperms were examined according to Revell and Mrode[16].
Formalin-citrate buffer solution (96 mL 2.9% sodium citrate + 4 mL 37.0% formaldehyde) was used to evaluate sperm acrosome integrity. Diluted semen samples were fixed in formalin-citrate buffer solution,a drop of which was placed on the lam and covered by the lamel. Finally,sperms (n=200/slide) were examined using a light microscope (1 000× magnification),and the percentage of spermatozoa with intact acrosome was determined[17].
2.7. Lipid peroxidation
Thiobarbituric acid (TBA) testing was commonly used to measure malondialdehyde (MDA) concentrations. To this end,semen samples were thawed and centrifuged at 1 500×g for 5 min and then the supernatant was separated. After mixing 1 mL of supernatants with 1 mL of ethylene diamine tetraacetic acid (EDTA) (0.037 g EDTA in 10 mL distilled water),1 mL butylated hydroxytoluene (BHT)(0.2 g BHT in 10 mL ethanol) and 2 mL trichloro acetic acid (TCA)(3 g TCA in 30 mL distilled water),we centrifuged the mixture at 1 200 ×g for 15 min. 1 mL of the supernatant of this mixture was incubated with 1 mL of TBA (0.134 g TBA in 20 mL distilled water)in a water bath at 90 ℃ for 20 min. The absorbance was read at 532 nm by a spectrophotometer after the mixture was cooled to the room temperature. MDA concentrations were expressed as nmol/mL[18].
2.8. DNA integrity
To assess DNA fragmentation,sperm chromatin dispersion test was used according to the method of Fernández et al[19]. 150 μL of 65% agarose was placed on a slide and covered by a coverlid. Then,they were kept at 4 ℃ for 5 min,after which the coverlid slid was removed from the slide. A mixture of 30 μL of the thawed sperm sample and 70 μL of 0.7% low melting point agarose was placed on the agarose solid layer of the lam,covered again by the lame and left to dry in the air. The lame was removed and the lam was placed horizontally in acid denaturing solution (0.08 N HCl) at 37 ℃ in darkness. After 7 min,it was transferred to the lysing solution(0.4 M Tris base,0.8 M dithiothreitol,1% sodium dodecyl sulfate,50 mM EDTA,and 2 M NaCl,pH=7.5) for 25 min. Then the slide was washed with distilled water for 5 min and dehydrated in 70%,90% and 100% ethanol,respectively,for 2 min. Having been dried in the air,sperm cells were stained by ethidium bromide staining solution for fluorescence microscopy.
2.9. Statistical analysis
Normality of data and homogeneity of variances were examined using the PROC UNIVARIATE by the SAS v8.0 software. The results showed that data for all investigated traits were normal and homogeneity of variances was assessed. Data were analyzed using Proc GLM of SAS (version 9.1; SAS Institute,2002,Cary,244 NC,USA) in a Completely Randomized Design. The model used is presented as follows:
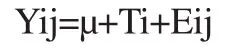
Where Yij=Each individual observation for a given variable,μ=overall mean,Ti=Treatment effect,Eij=Residual error. The differences between the treatments were determined using orthogonal contrasts. Means were compared using Duncan’s new multiple range test and data were expressed as the mean±standard deviation(mean±SD). Values were considered significant when P<0.05.
2.10. Ethics statement
All experimental protocols were in compliance with international guidelines,and approved with the number 961500510 on 5 February 2017 by by Animal Care and Use Committee of University of Kurdistan,Sanandaj,Kurdistan,Iran.
3. Results
3.1. Effect of S. striata ethanol extract on goat post-thawed sperm parameters
Results from the first experiment are presented in Tables 1 and 2.The 50 μg/mL of S. striata ethanol extract significantly increased progressive motility and viability compared to the control,25 and 50 μg/mL of S. striata ethanol extract groups (P<0.05).In comparison with the control and 25 μg/mL of S. striata ethanol extract groups,the 50 μg/mL of S. striata ethanol extract and 100 μg/mL of S. striata group increased total motility significantly(P<0.05). The 50 μg/mL of S. striata ethanol extract significantly increased VAP,VSL,VCL,ALH,STR,and LIN values compared to the control group (P<0.05). In addition,50 μg/mL of S. striata ethanol extract significantly accounted for the highest STR values of all groups (P<0.05). The 100 μg/mL of S. striata ethanol extract significantly increased BCF values compared to the control group(P<0.05) (Table 1).
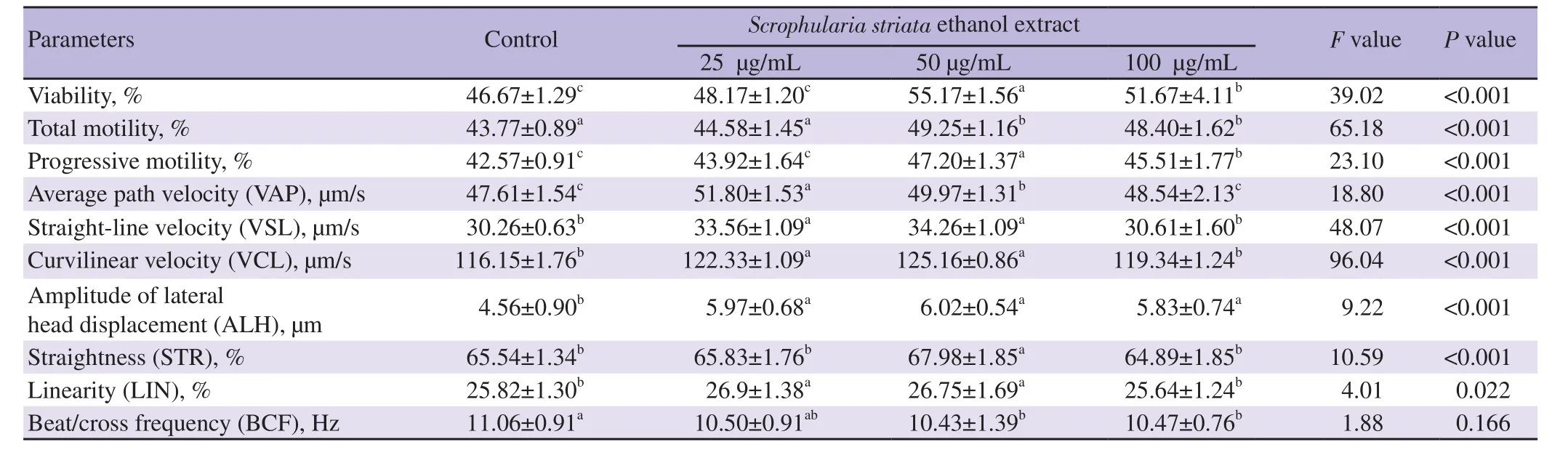
Table 1. Effects of different concentrations of Scrophularia striata ethanol extract on goat post-thawed sperm parameters.
The 50 μg/mL of S. striata ethanol extract resulted in the healthiest membrane compared to the control,25 and 100 μg/mL of S. striata ethanol extract groups (P<0.05). The 50 μg/mL of S. striata ethanol extract significantly increased acrosome integrity in comparison with the 25 and 100 μg/mL of S. striata ethanol extract groups (P<0.05)(Table 2). The 50 μg/mL of S. striata ethanol extract resulted in significantly higher reduction of DNA fragmentation compared to the control group (P<0.05) (Table 2). The 50 μg/mL of S. striata ethanol extract significantly decreased MDA values compared to the control,25 and 100 μg/mL of S. striata ethanol extract groups(P<0.05) (Table 2).
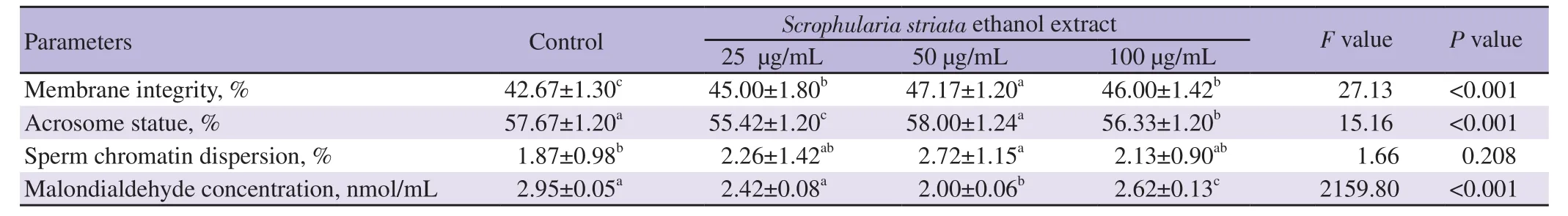
Table 2. Effects of different concentrations of Scrophularia striata ethanol extract on non-motion assessed parameters of goat post-thawed sperm.
3.2. Effects of S. striata ethanol extract,cysteine and trehalose alone or combined on goat post-thawed sperm parameters
Results from the second experiment are presented in Table 3 and Table 4. The 50 μg/mL of S. striata ethanol extract+trehalose significantly increased viability compared to the control,trehalose,cysteine,50 μg/mL of S. striata,and 50 μg/mL of S. striata+cysteine groups (P<0.05). All treatment groups resulted in significantly higher total motility compared to the control group (P<0.05).The 50 μg/mL of S. striata ethanol extract+trehalose group had significantly the highest amount of progressive motility compared to the 50 μg/mL of S. striata ethanol extract+cysteine,cysteine,trehalose,and control groups (P<0.05). Additionally,50 μg/mL of S. striata ethanol extract,50 μg/mL of S. striata ethanol extract+cysteine+trehalose,and trehalose groups significantly increased VAP compared to other groups (P<0.05). The 50 μg/mL of S. striata ethanol extract+cysteine+trehalose,50 μg/mL of S.striata ethanol extract,and trehalose groups had significantly higher VSL values compared to other groups (P<0.05). The trehalose group showed significantly the highest VCL values among all groups (P<0.05). The 50 μg/mL of S. striata ethanol extract+cysteine+trehalose,50 μg/mL of S. striata ethanol extract,and cysteine groups had significantly higher ALH compared to the trehalose and control groups (P<0.05). Besides,50 μg/mL of S. striata ethanol extract+trehalose group caused significantly higher ALH compared to trehalose and control groups(P<0.05). The 50 μg/mL of S. striata ethanol extract+trehalose group had significantly higher STR than cysteine and trehalose groups (P<0.05). The 50 μg/mL of S. striata ethanol extract+cysteine+trehalose group had significantly the highest LIN amount compared to the 50 μg/mL of S. striata ethanol extract,cysteine,trehalose and control groups (P<0.05). In comparison with other groups,the trehalose group showed the highest values of BCF(P<0.05) (Table 3).
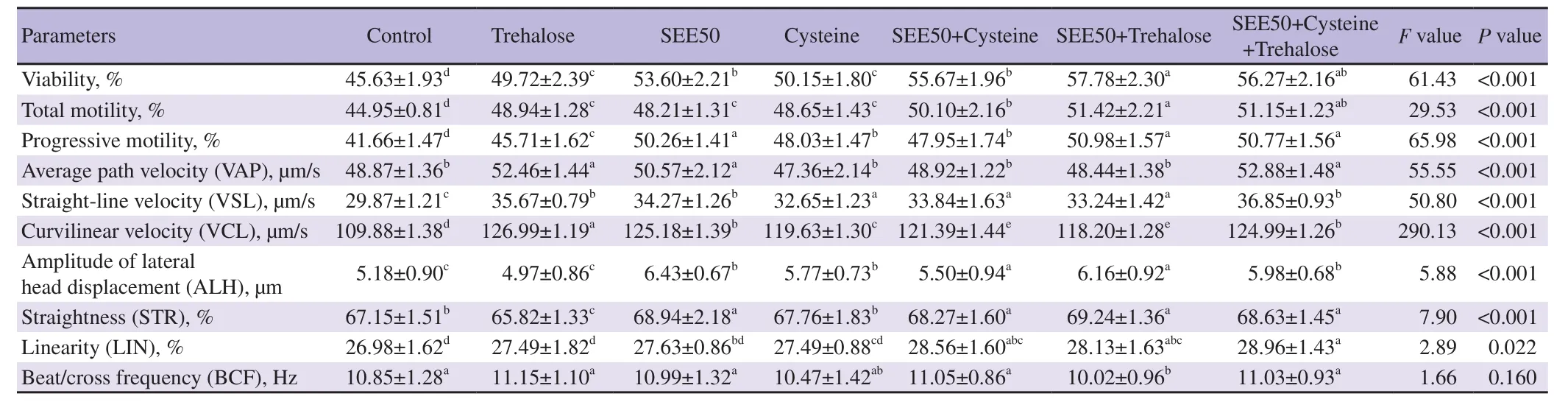
Table 3. Effects of different concentrations of Scrophularia striata ethanol extract,cysteine and trehalose alone or combined on motion parameters of goat post-thawed sperm.
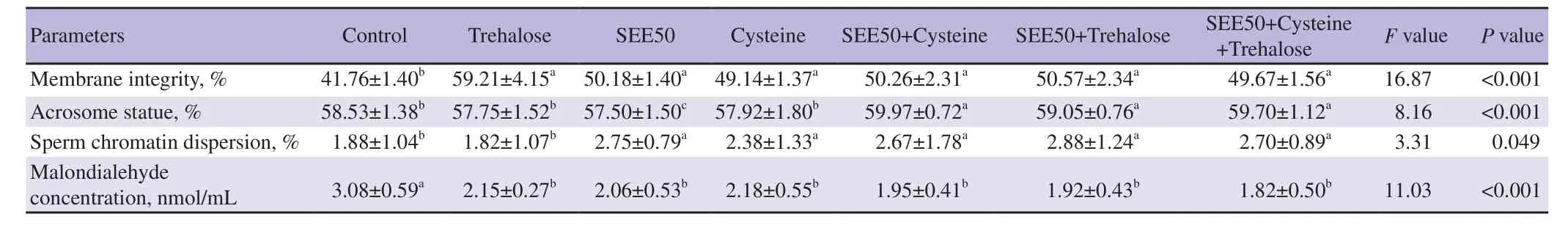
Table 4. Effects of different concentrations of Scrophularia striata ethanol extractcysteine and trehalose alone or combined on non-motion parameters of goat post-thawed sperm.
As shown by Table 4,the significantly highest level of membrane health was detected in all groups compared to the control group(P<0.05). Acrosome integrity was significantly higher in 50 μg/mL of S. striata ethanol extract+trehalose,50 μg/mL of S. striata ethanol extract+cysteine+trehalose,and 50 μg/mL of S.striata ethanol extract+cysteine groups than those in the control,trehalose,cysteine,and 50 μg/mL of S. striata ethanol extract groups(P<0.05). Moreover,all treatments indicated significantly lower MDA concentration compared to the control group (P<0.05) (Table 4). Regarding to the halo to core ratio,compared to the trehalose and control groups,the remaining treatment groups showed significantly higher levels (P<0.05) (Table 4).
4. Discussion
Freezing process produces oxidative stress in the sperm membrane,damages sperm structures,and changes the membrane fluidity and sperm efficiency,leading to reduction of motility,viability and fertility of sperms[19,20]. It was stated that when sperm's environmental temperature reached zero,adenosine triphosphate production stopped,sperm cells hibernated and all other activities came to a halt and/or sperm cells died afterwards. Cell damages occur when the state of sperm cells changes from normal to frozen condition and vice versa,to which the production of ROS contributes[21]. Reproductive male systems,especially the testes and sperms,are highly sensitive to oxidative stress,and ROS are considered as one of the most important causes of infertility in male animals[22,23]. A number of plants have effective antioxidant properties due to having bioactive compounds such as phenols and flavonoids. Therefore,they have the ability to mitigate damages caused by free radicals[24]. Accordingly,in this study antioxidant effect of S. striata ethanolic extract was investigated by adding it alone (Experiment 1) and in combination with trehalose and cysteine (Experiment 2) to the freezing diluent of goat sperms in laboratory. In the present experiment,ethanolic extract of S. striata was added as an antioxidant against cold shock to the freezing diluent of goat sperms,improving their quality. The results obtained from Experiment 1 showed that adding 50 μg/mL of ethanolic extract of S. striata to the diluent caused an increase in the sperm motility,progressive motility and viability compared to the other treatments. Our results from Experiment 1 are consistent with those from previous studies showing that total motility and progressive motility increased significantly by adding 10 mg/L of ginger and echinacea[25].
As a product of lipid peroxidation,MDA,causes the lipid membrane structures to be asymmetric while entering biochemical structure of membranes,thereby reducing their fluidity,which leads to a decrease in sperm motility[26]. According to the results of this experiment,the mean MDA level was significantly lower for goat sperm diluted with 50 g/mL of S. striata compared to the control group. It has been shown that level of MDA production is inversely correlated with sperm motility[27]. The results obtained by this study also showed that increased level of sperm motility was associated with the decreased level of MDA. The results of the present experiment were in line with several experiments[28-30],but they were contrary to the results of the study by Zanganeh et al[31].At different stages of sperm production and freezing,efficiency,viability,and fertility of the sperms will decrease due to formation of ROS,causing physiological and chemical stress on the sperm membrane[32].
Today,the use of natural antioxidants is considered important due to safety problems regarding toxic and carcinogenic compounds in some synthetic antioxidants,and to their economic efficiency[33].Antioxidant property of plants is mainly related to phenolic compounds,flavonoids,phenolic acids,and phenolic diterpenes[34].Roots and aerial parts of S. striata contain flavonoids,saponins,tannins,and terpenoids[35]. Findings showed the ability of S. striata to reduce ROS production and apoptosis[36]. Based on the fact that extract of S. striata contains antioxidant compounds,it can inhibit overproduction of ROS if it functions properly. Therefore,improvement of sperm efficiency in treatments containing extract of S. striata is probably due to the presence of bioactive antioxidant compounds found in the plant extract. Increased level of ROS production due to oxidative stress reduces sperm fertility because of damaged cell membranes,reduced motility,morphological abnormalities such as twisted tail and damaged acrosome,DNA fragmentation,and decreased sperm function. In this study,addition of 50 μg/mL of ethanolic extract of S. striata to cryopreserved diluent of goat sperms significantly reduced the amount of DNA damages compared to other treatments.
Results of the present experiment were consistent with an experiment showing that addition of aqueous and methanolic extracts of Portulaca oleracea to diluents reduced DNA damages,also confirmed by the results of Azimi et al[37]. Based on the results obtained from Experiment 1,among different doses of S. striata extract,50 μg/mL of extract was the most appropriate dose in the diluent to maintain sperm efficiency in the freezing-thawing process.Therefore,it can be said that combining the freezing diluent of goat sperms with ethanolic extract of S. striata can improve the thawing process. Results obtained from Experiment 2 showed a significant increase in parameters of total motility,progressive motility,viability,and health of membrane in all treatments compared to the control group. All treatments in this experiment significantly reduced lipid peroxidation compared to the control group,so that levels of MDA produced in the freezing-thawing process were lower in all treatments than the control group. Also,based on the results,the ratio of halo area to core increased significantly in 50 g/mL of S.striata,50 g/mL of S. striata + cysteine,and 50 g/mL of S. striata +trehalose treatments compared to the control treatment. Therefore,according to the results,it can be said that all treatments reduced DNA damages.
Regarding the 50 g/mL of S. striata treatment,results of our experiments were consistent with an experiment reporting that addition of 1 mM of BHT and butylated hydroxy anisole to goat sperm diluents improved sperm motility,viability and membrane integrity after the freezing-thawing process[38]. Also,in another experiment,it was found that the use of aqueous extract of rosemary had a significant effect on the motility parameters and viability percentage of pig sperms and significantly reduced concentration of produced MDA[39].
Consistent with the results of this experiment on trehalose,results of another experiment showed that addition of trehalose to sperm cryopreserved diluent significantly improved viability and motility of spermatozoa[40]. It has been also shown that addition of 50 mM trehalose to bovine sperm diluent resulted in better performance of parameters of plasma membrane health,motility as well as minimization of sperm DNA fragmentation compared to the control group[41]. Contrary to our results,it was argued that addition of 50 and 100 mM trehalose to the frozen diluent of rabbit significantly reduced motility[42]. Trehalose is one of compounds preventing harmful membrane changes during dehydration which may increase cell dehydration by reducing the negative effects of water passing through the sperm membrane during the freezing process and by minimizing ice crystal formation,thereby improving quality of sperm cell during the freezing-thawing process.
Cysteine is a low molecular weight amino acid containing thiol,which is a precursor to intracellular glutathione. Cysteine can easily pass the membrane,enter the cell,increase intracellular biosynthesis of glutathione (both in-vitro and in-vivo) and retain membrane lipids and proteins due to its indirect neutralizing effect on free radicals. Cysteine has a positive effect on integrity of acrosome and mitochondria against cryopreservation and increases sperm motility after thawing[43]. Consistent with our results,amino acids of cysteine and glycine have been reported to significantly improve progressive sperm motility,viability,and acrosome membrane integrity of ram after thawing process. Among different concentrations of cysteine and glycine (5,10,15,and 20 mmol),10 and 15 mmol of cysteine significantly improved sperm motility,viability,and health of membrane compared to the control group[26]. Also,the results of the studies by Topraggaleh et al[22]and Lotfipour et al[33]were consistent with the results obtained in present experiments.
In addition,our results showed that 50 g/mL of S. striata+trehalose treatment resulted in significantly higher progressive motility and less DNA degradation compared to trehalose treatment.Also,50 g/mL of S. striata+trehalose treatment had a higher mean viability rate than 50 g/mL of S. striata,and trehalose treatments.No mechanism has been reported so far as associated with the protective effect of trehalose in combination with S. striata in sperm cryopreservation. However,according to the results obtained in this experiment,it can be stated that there may be a synergistic effect between trehalose and active ingredients found in S. striata.
For the study’s limitation,it is to note that the artificial insemination as an important limitation factor,was no possible to carry out. In that context,the observed data of fertilization rate could be interpretation of results.
In conclusion,it is found that 50 μg/mL of S. striata is the most desirable level of this extract to be added to diluents. Use of cysteine,trehalose and S. striata as an antioxidant can be useful for the freezing-thawing process of epididymal goat sperms. Moreover,there is probably a synergistic effect between trehalose and the compounds found in extract of S. striata.
Conflict of interest statement
The authors declare no conflict of interest prejudging the impartiality of this scientific work.
Authors' contributions
Zeinab Maleki prepared the research work,made data curation,wrote the original draft,and carried out the implementation. Abbas Farshad conceived the original idea and supervised the project. Jalal Rostamzadeh helped to supervise the project.
 Asian Pacific Journal of Reproduction2022年2期
Asian Pacific Journal of Reproduction2022年2期
- Asian Pacific Journal of Reproduction的其它文章
- INRA82 extender enhances semen quality in ram under cooled and cryopreserved stages
- Profiling of seminal antioxidant indices and sperm quality in Plasmodium bergheiinduced malarial mice treated with Phyllanthus amarus
- An ethnopharmacological survey of medicinal plants used in the traditional treatment of human infertility in eastern Algeria
- Spousal communication,fertility preference and other factors affecting contraceptive use among married couples in Ekiti State,Nigeria
- Determinants of emergency contraceptive pill use in Bangladesh:An analysis of national survey data
- A scoping review of SARS-CoV-2 and male infertility:Concerns and future prospects
