Profiling of seminal antioxidant indices and sperm quality in Plasmodium bergheiinduced malarial mice treated with Phyllanthus amarus
Williams Oshiegbu,Chinwendu Obogheneophruhe Elu,Innocent Onyesom?
1Department of Biochemistry,Delta State University,Abraka,Nigeria
2Department of Medical Biochemistry,Delta State University,Abraka,Nigeria
ABSTRACT
Objective:To evaluate the antiplasmodial activity of Phyllanthus(P.) amarus crude ethanol leaf extract and its effects on semen quality in male BALB/c mice.
Methods:A total of 36 adult mice were divided into six groups,with 6 mice each. Five groups were infected with Plasmodium (P.)berghei,and one group was left uninfeceted. Of the five infected groups,one group was left untreated,three groups were treated with varying doses (100,250 and 400 mg/kg) of P. amarus crude ethanol leaf extract orally for 4 days,and another group was treated with standard drug,artemether and lumefantrine (Lonart?DS).Antiplasmodial activity,seminal quality,some biochemical indices(neutralα-glucosidase,fructose,and citric acid) in seminal plasma and seminal antioxidant markers (catalase,glutathione peroxidase,reduced glutathione,malondialdehyde,total antioxidant capacity,and acid phosphates) were determined. The mice were euthanized 3 days post treatment and semen was collected from the caudal epididymis and processed for analysis using documented methods and procedures.
Results:Malarial infection led to oxidative stress,causing a significant decline in seminal quality (P<0.05). However,treatment with P. amarus crude ethanol leaf extract alleviated oxidative stress and significantly improved seminal quality. The improvement was dose-dependent and compared well with the standard drug,artemether and lumefantrine (Lonart?DS) treatment.
Conclusions:The ethanol leaf extracts of P. amarus alleviate male reproductive capacity during malaria infection in murine model by enhancing antioxidant activities.
KEYWORDS:Malaria; Sperm quality; Phyllanthus amarus; Seminal oxidative stress; Seminal antioxidant markers;Antioxidants; Ethanol extracts; Antimalarials
Significance
Malaria-induced oxidative stress has been associated with several complications including decreased reproductive parameters in infected males. Some studies associated decreased seminal quality to the adverse effects of antimalarials. Our study demonstrates that malaria infection reduced semen quality via free radical-induced oxidative stress. Thus,the significance of this study is that Phyllanthus amarus ameliorated the parasites-induced oxidative stress on semen quality in the course of its antimalarial activity.
1. Introduction
Malaria still remains one of the most important parasitic disease,affecting many regions of the world and accounts for a large number of deaths annually,with the African region carrying the highest share of the global malarial burden. The World Health Organization(WHO)[1]reported that in 2019 alone,229 million cases of malaria and 409 000 deaths occurred globally. Nigeria has 27% morbidity and 23% mortality in malaria[1].
Malarial infection has been reported severally to be associated with the generation of large amounts of reactive oxygen species (ROS),and hence oxidative stress,triggered by the parasite[2]. The parasiteinduced oxidative stress has been linked to the symptoms (headache,muscle/joint pain,stress,nausea,vomiting,fatigue,weakness and in extreme cases; cerebral malaria) of malaria in human[3]. Prolonged malarial infection could,therefore,result in systemic complications,if not treated,including male infertility which has been associated with malarial infection[4]. Recently,Ekhoye et al[5]stated that testes of mice infected with Plasmodium (P.) berghei showed distortion of the seminiferous tubules and interstitial cells of Leydig,causing arrest of spermatogenic development. This may be the reason for the decreased seminal quality in infected mice.
Oxidative stress has been commonly seen in almost half of all infertile men[6]. ROS have been reported to cause infertility in male subjects by either damaging the sperm membrane,thus reducing the sperm’s motility and ability to fuse with oocyte or by directly damaging sperm DNA[7]. Several studies have suggested oxidative stress to be the main underlying pathology that connects varicocele with male infertility[8]. Spermatozoa were the first type of cells reported to show susceptibility to ROS[6]. This susceptibility is basically due to the high concentration of polyunsaturated fatty acids in their cell membranes which could enhance lipid peroxidation.Lipid peroxidation has been reported to cause axonemal damage,decreased sperm viability and increased mid-piece morphological defect,which contributes to decreased sperm motility[9]. It has also been observed that antimalarial agents interfere with the process of sperm DNA synthesis[10]. However,the impact of treating malarial infection with medicinal plants on semen quality has remained scarce.
Phyllanthus (P.) amarus,a medicinal plant used in the Nigerian tradomedicine practice,has been consistently reported to possess antimalarial[11]and antioxidant[12]properties. Karuna et al[13]and Onyesom et al[14]reported that aqueous extract of P. amarus reduces renal oxidative stress. Also,Opajobi et al[15]observed that the blood schizonticidal activity of P. amarus enhanced testicular antioxidant defense capacity in P. berghei infected mice. Nevertheless,the impact of P. amarus antimalarial activity and associated seminal antioxidant defense against the assaults of malarial parasite-induced oxidative stress,is not well known. In this present study,therefore,attempts were made to profile the seminal antioxidant indices and sperm quality in malarial infected mice treated with graded doses of P. amarus crude ethanol leaf extract.
2. Materials and methods
2.1. Collection of plant materials and preparation of crude extracts
Full grown whole plants of P. amarus were harvested from their natural habitat in Abraka community,Ethiope East Local Government Area of Delta State,Nigeria. The plant was identified by a taxonomist at the Forestry Research Institute of Nigeria,Ibadan,Oyo State,Nigeria (vouch No. FHI 109728). Whole plants were collected and the leaves were plucked,washed and then air dried for two weeks at laboratory room temperature (28 ℃-32 ℃) to a constant weight. The dried leaves were,therefore,powdered using laboratory blender (Kenwood,Japan). Then,the powder (300 g) was extracted with 70% ethanol using a soxhlet apparatus. The extract was concentrated to dryness using a rotary evaporator (Buchi R-210,China) under reduced pressure. The percentage yield was 3.8%.The dried extract obtained was dissolved in distilled water and kept refrigerated at 4 ℃ for use. The volumes administered,which were equivalent to doses studied,were calculated as follows.

D=Dose used (g/kg body weight),P=Body weight (kg),C=Concentration of the extract (g/mL),V=Volume of extract (mL)administered[11].
2.2. Parasites
P. berghei parasites (Strain NK65) already passaged into donor mice were obtained from the Department of Parasitology,Nigerian Institute of Medical Research,NIMR,Yaba,Lagos State,Nigeria.
2.3. Experimental animals
Adult Swiss male mice (BALB/c albino strain),about eight weeks old,weighing between 22-28 g were obtained from Laboratory Animal Centre,LAC,Faculty of Basic Medical Sciences,FBMS,Delta State University,DELSU,Abraka,Nigeria,where they were kept under storage at standard room temperature and pressure for two weeks. Animals were fed with growers’ mash (Top Feeds Flour Mill,Sapele,Delta State) and water ad libitum.
2.4. Grouping and inoculation of experimental animals
Thirty-six male mice were used for this study. They were separated into six groups (6 mice per group) and dosage was based on previous study[15]. Group 1 was neither infected nor treated; Group 2 was infected,but not treated; Group 3 was infected with P. berghei and treated with 100 mg/kg of the P. amarus crude ethanol leaf extract;Group 4 was infected with P. berghei and treated with 250 mg/kg of the P. amarus crude ethanol leaf extract; Group 5 was infected with P. berghei and treated with 400 mg/kg of the P. amarus crude ethanol leaf extract; Group 6 (the standard group) was infected with P. berghei and treated with 20 mg/kg of standard drug,artemether and lumefantrine (Lonart?DS).
The experimental mice were infected by obtaining parasitized blood from the cut tail tip of the infected (donor) mice. The inoculum was prepared using phosphate buffered saline. Then,0.1 mL of infected blood was diluted in 0.9 mL of phosphate buffered saline,pH 7.2.
2.5. Antimalarial activity and determination of parasitaemia
The mice were inoculated with 0.1 mL parasitized suspension containing about 12 000 parasites. The P. amarus crude ethanol leaf extract and standard drug (Lonart?DS) doses were administered once daily as designed,using intragastric cannula for a period of four days. Treatment commenced after 72 h of inoculation and infection confirmation. Parasitaemia was assessed at day 0,3,6 and 9 by thick blood smears made by collecting blood from the cut tail tip of only the infected mice and stained with Giemsa stain which was later viewed under the microscope (TH-9845,Serico,China) at×40 magnification[14]. Percentage parasitaemia and chemosupression were calculated using the following formula:

2.6. Determination of mean survival time
Mortality of experimental mice was monitored daily for thirty days from the time of infection up to the incidence of death of each mouse of the treated and control groups. Mean survival time of the mice was calculated with the formula:
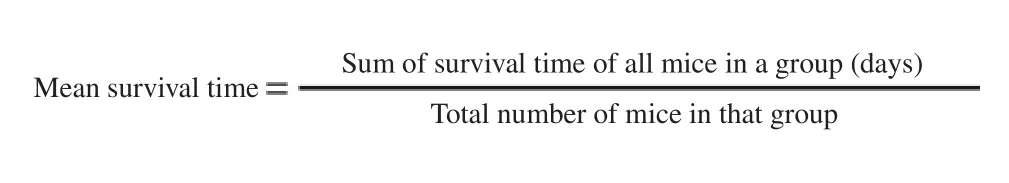
2.7. Animal sacrifice and semen collection
On the 9th day of post infection time (or the 3rd day of post treatment time),the mice were euthanized in a chamber saturated with chloroform (AnalaR Grade,BDH Chemicals,Poole,England).Then,both right and left caudal epididymis were dissected and incisions (about 1 mm) were made in the caudal epididymis and semen was collected for various analyses.
2.8. Semen processing and analysis
2.8.1. Analysis of semen quality
Following liquefaction of the collected semen from the right caudal epididymis within 60 min,semen samples were analyzed for sperm concentration,volume,motility and morphology according to WHO guideline[16]. Volume was measured using small standard graduated cylinder and pH was determined by adding a drop of the liquefied semen unto a pH paper and reading was recorded after about 30 s. Then,sperm motility was assessed using microscope(B240-5,Toledo,Switzerland). A drop of semen was placed on a pre-warmed (37 ℃) slide and covered with a slip. Thereafter,the motile spermatozoa were observed and classified by the standard grading system (progressive motility,non-progressive motility and immotility) using phase-contrast microscope (B240-5,Toledo,Switzerland) at 100× magnification. To determine the live spermatozoa count,the slide with semen drop was stained with eosin-nigrosin and counted by using phase-contrast microscope(B240-5,Toledo,Switzerland) under 40× magnification. However,to evaluate sperm concentration,semen was diluted with phosphate buffer (pH 7.0 at ratio 1:2) and then a drop was placed on Makler counting chamber. The number of cells was counted using phasecontrast microscope (B240-5,Toledo,Switzerland) under 10×magnification. Sperm morphology was assessed by the Papanocolaou staining method.
2.8.2. Preparation of seminal plasma
Fresh semen collected from the left caudal epididymis was separated immediately by centrifuging at 5 000×g for 10 min at 4 ℃. The supernatant was decanted into 2 mL centrifuge and centrifuged again at 11 000×g for 20 min at 4 ℃ to remove the remaining spermatozoa. The seminal plasma (supernatant) was kept frozen for about a month before being used for the determination of biochemical parameters and oxidative stress markers.
2.8.3. Determination of biochemical parameters
Commercially available kits were used to determine neutral α α-glucosidase (NAG) activity,fructose and citric acid concentrations. An improved NAG assay was performed,using glucose as an enzyme inhibitor for background correction[16,17].The yellow colour of 4-nitophenol for both sample reaction and sample inhibitor was measured at a wavelength of 405 nm using a spectrophotometer. Enzyme activity was calculated for both reaction and inhibition and the results were expressed as mIU/ejaculate[15].
2.8.4. Measurement of fructose concentration
Measurement was based on the reaction of fructose with indole in the presence of HCl at 37 ℃. The absorbance of the coloured complex formed,was measured at a wavelength of 405 nm in a spectrophotometer and fructose content was calculated by extrapolating the absorbance from a standard curve. Result was expressed as mg/ejaculate[16].
2.8.5. Determination of citric acid concentration
The assay principle was based on the formation of complex citrate and Fe3+ions complex,whose yellow colour intensity was measured at a wavelength of 405 nm in a spectrophotometer. Results were determined using absorbance of standard and expressed as mg/ejaculate[18].
2.9. Determination of oxidative stress parameters
2.9.1. Total antioxidant capacity measurement
Seminal plasma was diluted (1:9) with total antioxidant capacity assay buffer in the commercial kit (Cayman Chemical,Ann Arbor,MI,USA). The principle of the assay was based on ability of antioxidants to inhibit oxidation of the -2’2-azinodisulfinate-3-ethylbenztiazoline (ABTS?) to ABTS?+radical cation,which was compared with a water soluble tocopherol analogue. Inhibition of the blue-green ABTS?+colour absorbance was estimated at 405 nm using a spectrophotometer and results were expressed asμM of Trolex equivalent[19].
2.9.2. Assay of superoxide dismutase (SOD) activity
Seminal plasma was diluted (1:2) with SOD assay kit sample buffer and SOD activity was then determined based on the reaction between xanthine and xanthine oxidase to yield superoxide anion(O2-) which reacted with tetrazolium salts to produce red formazan dye. The degree of inhibition of this reaction was measured by a spectrophotometer at a wavelength of 450 nm. Results were expressed as U/mL[20].
2.9.3. Determination of catalase activity
During the assay,catalase catalyzed the reaction between H2O2and methanol to form formaldehyde which in turn reacts with a chromogen (Purpald; 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole) to produce a purple colour whose intensity was determined at 540 nm in a spectrophotometer. Then,the formaldehyde concentration was estimated using standard regression curve and data were expressed in nM/min/mL[21].
2.9.4. Estimation of malondialdehyde (MDA) level
In this determination,thiobarbituric acid reacted with lipid peroxidation products at high temperature and acidic environment to form a colour complex that was then extracted with n-butanol(1:1 v/v). Thereafter,the absorbance of the butanol fraction was measured with a spectrophotometer at 532 nm and the MDA content was extrapolated from the standard curve and expressed as μM/mL[22].
2.9.5. Gluthathione peroxidase assay (GPx)
Commercial kit (Cayman Chemical,MI,USA) was used. The measurement was conducted in accordance with the manufacturer’s manual instructions as already described by Martinor et al[23].20 μL of the sample was mixed with 50 μL co-substrate,reduced nicotinamide adenine dinucleotide phosphate and the reaction commenced with 20 μL cumene hydroperoxide. The absorbance was measured five times per minute at 340 nm using Mithras LB 943 Multimode Microplate Reader. Each sample was tested twice,and the mean activity of the glutathione peroxidase (nmoL/min/mL) was calculated as the function of the absorption change (?A) per minute.
2.9.6. Reduced glutathione (GSH) assay
Reduced GSH was determined by the principles based on the method of Shete and Hamid[24]. 0.5 mL of seminal plasma was dispensed into a test tube and 2.0 mL of distilled water was added and mixed thoroughly. Then,the mixture was centrifuged at 2 000×g for 5 min and 0.5 mL of the supernatant was taken,to which 0.5 mL of trichloroacetic acid (5%) was added,and then centrifuged again at 8 000×g for 10 min. Thereafter,0.5 mL of the supernatant was taken and 2.5 mL of phosphate buffer (pH 8) and 1.0 mL of 5,5’-dithiobis (2-nitrobenzoic acid) were added. The resulting solution was inverted three times to mix. The absorbance was read at 412 nm in a spectrophotometer within 4 min. Reduced GSH concentration in seminal plasma was then estimated using a standard curve.
2.9.7. Acid phosphatase assay
To determine the activity of acid phosphatase in the seminal plasma samples,the automated biochemical analyzer BS-3000 P (Synova Medical Science & Technology,Co. Ltd,Nanjing,China) was used based on the principle of the Acid Phosphatase Colorimetric Humazym Test,which involved the use of Orthophosphoric-Monoester Phosphohydrolase enzyme (Human Gesells chaft fur Biochemica und Diagnostica mbH) in accordance with the method of Hillman described by Mitevia et al[25].
2.10. Statistical analysis
Data in normal distribution according to Kolmogorov–Smirnov normality test were expressed as mean±standard deviation (mean±SD) and analyzed with analysis of variance (ANOVA) and Turkey HSD post hoc using GraphPad prism (GraphPad software Inc,LLC,San Diego CA,USA,version 6).
2.11. Ethics statement
This study was approved by the Research and Bioethics Committee,Faculty of Basic Medical Sciences,Delta State University,Abraka,Nigeria (Ethics approval number:REC/FBMS/DELSU/20/67).
3. Results
3.1. Antiplasmodial activity of P. amarus crude ethanol leaf extract
The in vivo antiplasmodial activity of P. amarus crude ethanol leaf extract was evaluated in this study by assessing the antimalarial activity during entrenched infection in rodent model.
Daily progression of parasitaemia in experimental mice is illustrated in Figure 1. This figure shows that at day 9 (i.e. 3 days after the end of the 4-day treatment with P. amarus crude ethanol leaf extract or Lonart?DS),parasitaemia in experimental mice had declined significantly in a dose dependent manner with P. amarus crude ethanol leaf extract treated groups,showing significant differences when parasitaemia on day 9 were compared with those on day 3 (all P<0.05). At day 9,there were significant decreases in parasitaemia for all test groups (Groups 3,4 and 5 mice),but parasitaemia in Group 2 increased. Among the test groups,decrease in parasitaemia was least for Group 3 mice (100 mg/kg P. amarus crude ethanol extract) and highest for Group 5 mice (400 mg/kg P.amarus crude ethanol extract). Parasitaemia reduction for Group 5 mice showed similar trend with Group 6 mice (the standard drug group).
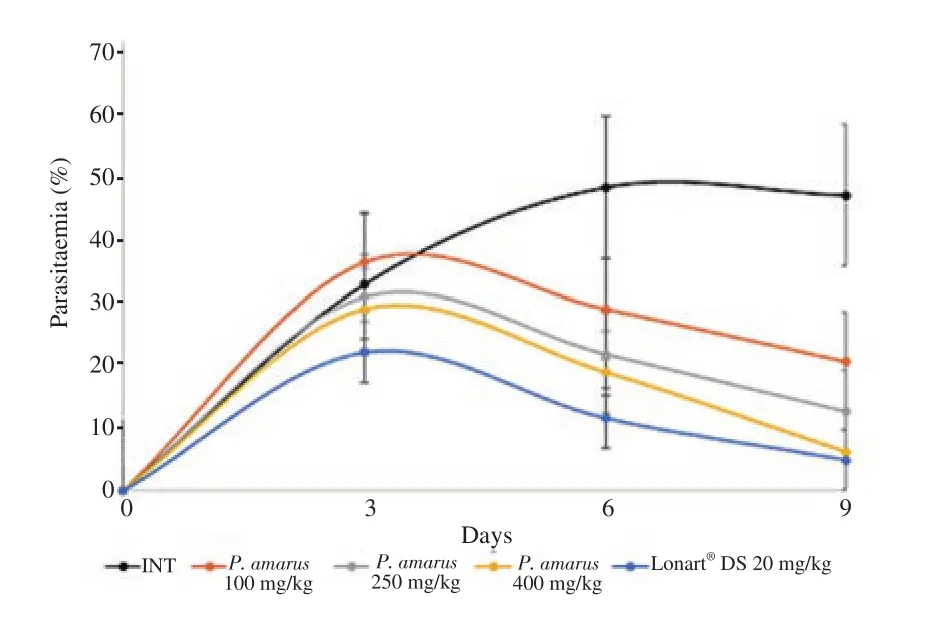
Figure 1. Curative action of Phyllanthus (P.) amarus crude ethanol leaf extract on parasitaemia in BALB/c experimental mice infected with Plasmodium berghei malarial parasite. Values are expressed as mean±SD for n=6 mice per group. Day 3 (72 h after inoculation of mice with Plasmodium berghei) is parasite confirmation and commencement of the 4-day treatment period. Day 6 is the last day of treatment. Day 9 is the third day post treatment. Changes in parasitaemia from day 3 to day 9 are analyzed using analysis of variance. INT:Infected,not treated. Lonart?DS is the standard drug.
As shown in Table 1,all doses of P. amarus extract (100,250 and 400 mg/kg) showed statistically significant decrease in parasitemia when compared with Group 2 (all P<0.05). Hence,there was a general dose dependent increase in chemosupression and a significant improvement in mean survival time (P<0.05).
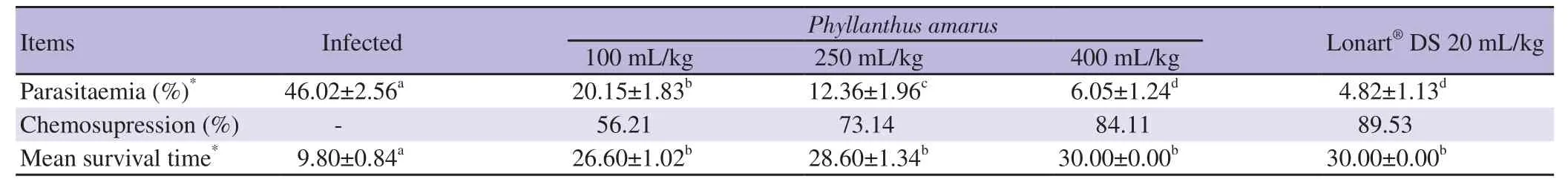
Table 1. Parasitaemia level,chemosupression and mean survival time induced by Phyllanthus amarus crude ethanol leaf extract after the curative test.
Parasitaemia and mean survival time values achieved by the highest dose of P. amarus extract and standard drug (Lonart?DS),were similar,showing no significant difference (P=0.12 and 1,for parasitaemia and mean survival time,respectively; P>0.05),and this indicated that P. amarus extract possesses significant antimalarial activity.
3.2. Effect of P. amarus and Lonart?DS on semen quality in P.berghei infected mice
As shown Table 2,compared to Group 1 mice,Group 2 mice had significantly reduced semen volume,count,motility,normal morphology,concentration,viability,progressive motility,and pH (all P<0.05). P. amarus crude ethanol leaf extract and the standard drug Lonart?DS treatments significantly improved these semen quality indicators (semen volume,count,motility,normal morphology,concentration,viability,progressive motility,and pH) in a dosedependent manner when compared with the Group 2 mice (P<0.05).Viscosity were also improved.
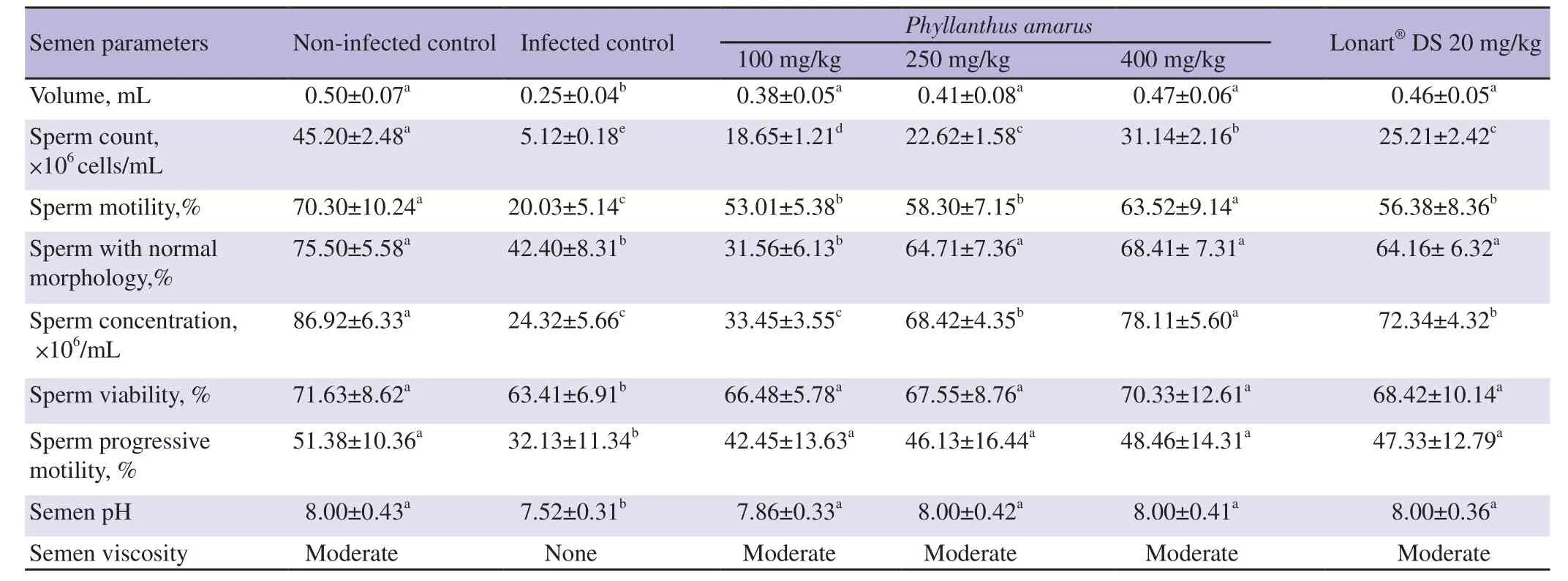
Table 2. Changes in semen quality induced by the treatment of Plasmodium berghei malaria parasite infection with graded doses of Phyllanthus amarus crude ethanol leaf extract.
The ameloriating effect of the extracts of P. amarus was consistent for semen volume,viability,progressive motility,viscosity and pH.There was a dose-depenedent increase among the treatment groups,but all showed no significant difference when compared with each other,Group 1 or the standard drug group (Group 6) (P>0.05).
Increase in sperm count in P. amarus crude ethanol extract treated groups (Groups 3,4 and 5) was also significant (P<0.05) and dose dependent when compared to Group 2,but did not completely reverse the damaging effects of malaria infection as significant difference existed between treated groups and Group 1. However,the sperm count for Group 5 mice showed significant improvement than the standard drug group (Group 6) (P<0.05).
Groups 4,5 and 6 mice showed significant improvement in semen morphology when compared with Group 2 mice (all P<0.05) and this improvement was similar between these groups and Group 1(P>0.05).
Amelioration of sperm concentration was greatest in Group 5 mice and compared well with the Group 1 (P>0.05). Improvement of semen concentration was also observed in Group 4,although to a lower degree than that of Group 1 but it showed similar activity as Group 6. Group 3 mice showed no significant improvement in sperm concentration when compared with the Group 2 mice (P>0.05).
As shown in Table 2,malaria infection caused the production of non-viscous semen,but administration of ethanol extracts of P.amarus reversed this,producing a moderately viscous semen even at the lowest dose (100 mg/kg).
Table 3 indicates the changes in seminal plasma antioxidant indicators in P. berghei malarial-infected mice treated with P.amarus crude ethanol leaf extract or the standard artemisinin-based combination therapy (ACT) drug (Lonart?DS). P. berghei malarial parasite infection in experimental mice significantly increased seminal plasma SOD (P=0.020),GPx (P=0.003),GSH (P=0.030),MDA (P=0.010),and decreased catalase (P=0.010) in Group 2 comparing with Group 1 mice.

Table 3. Changes in seminal antioxidant markers in Plasmodium berghei malaria infected mice treated with crude ethanol leaf extract of Phyllanthus amarus.
However,treatment of the malarial induced infection with the graded doses of P. amarus crude ethanol leaf extract ameliorated the disturbances in a dose-dependent manner comparing with Group 2.The 250 and 400 mg/kg extract groups showed similar activity as Group 6 mice (the standard drug group).
Improvement of antioxidants enzymes,SOD,CAT,GPx,GSH and acid phosphatase,followed the same trend. Groups 4 and 5 showed a significant dose-dependent improvement in these parameters when compared with Group 2 (P<0.05); these groups also showed similar activity with the standard drug group and Group 1 (P>0.05).However,Group 3 (lowest dose of P. amarus) showed no significant improvement when compared with Group 2 (P>0.05) .
Reduction of MDA following administration of doses of P. amarus was dose dependent with all groups showing significant differences when compared with Group 2 (P<0.05). However,activities of Groups 4,5 showed similar activity as the standard drug group(Group 6) and Group 1 (both P>0.05).
No significant changes in concentration of the total antioxidant capacity were observed among the 6 groups (P>0.05).
3.3. Effect of P. amarus crude ethanol leaf extract and Lonart?DS on some biochemical markers in seminal plasma in P. berghei infected mice
Changes in certain biochemical markers that indicated proper sperm synthesis,semen quality and functions are shown in Figure 2.P. berghei malarial infection of experimental mice in Group 2 showed reduced activity of seminal plasma neutralα-glucosidase,concentrations of citric acid and fructose when compared with Group 1 ( P<0.05). But,treatment with 250 and 400 mg/kg of P.amarus crude ethanol leaf extract or Lonart?DS increased the values of these biochemical markers in the seminal plasma when compared with Group 2,with the highest dose (400 mg/kg) of P. amarus crude ethanol leaf extract and Lonart?DS having similar measures.
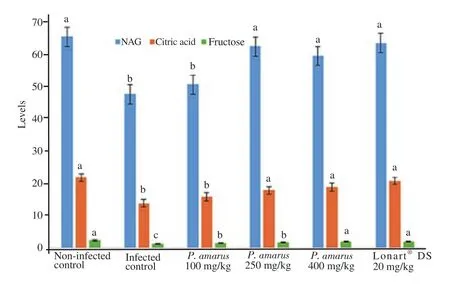
Figure 2. Changes in some biochemical markers [neutralα-glucosidase(NAG,mIU/ejaculate),citric acid (mg/ejaculate),and fructose (mg/ejaculate)]in seminal plasma of Plasmodium berghei malarial infected mice treated with doses of Phyllanthus amarus crude ethanol leaf extract. Values are expressed as mean±SD for n=6 mice per group. Data bearing another superscript differ significantly (P<0.05) in comparison to each group. Lonart?DS is the standard drug.
4. Discussion
Oxidative stress is a condition that results in increased rate of cell damage. The importance of oxidative stress in damaging mammalian spermatozoa has been reported[9]. Malaria chemotherapy has been shown severally to induce a reversible antifertility in mammalian spermatozoa by causing production of ROS. Antimalarials are said to be efficient due to their oxidant effect[26]. Antiplasmodial drugs quinine[27],chloroquine[28],pyrimethamine[29],artemisinin-based combination treatments[30],have been well documented. P. amarus,the plant used in our present study,which presented significant antimalarial activity,has also been reported to induce antifertility.Etta et al[31],Ogbomade[32],Ataman and Ikedashi[33],and Ekpo et al[34]reported a decrease in the reproductive parameters of mice,not infected with malaria,but treated with P. amarus,the plant studied in our present study. However,Percario et al[35]reported that the effective mechanism of action of antimalarials should cause minimal adverse effects to the host.
The back and forth arguments of the antifertility action of antimalarials led us to this study. Our study suggests that male antifertility associated with malaria infection is caused by oxidative stress induced by the parasite rather than antimalarials. The difference in values obtained from the seminal quality of untreated P. berghei-infected mice are glaring. There is a great significant reduction in semen quality in malarial infected mice when compared with the quality in uninfected mice and mice treated with extracts of P. amarus. Evidence from the results indicates that malarial infection in experimental mice produced non viscous semen with reduced fructose,neutralα-glucosidase and citrate content,having significantly reduced sperm count,motility and morphology,suggesting oxidative stress. The results complement other studies that reported decreased motility and abnormal morphology of spermatozoa from animals exposed to ROS[36]. Significantly decreased fructose level with reduced sperm count and motility in untreated P. berghei infected mice,suggests that malarial infection affects the production or utilization of seminal fructose which affects motility,as seminal fructose is the main source of energy for spermatozoa[16]. This implies that malarial infection may affect production or utilization of seminal fructose which in turn affects motility of spermatozoa,and this may cause temporary infertility. In addition,low fructose level observed in the semen of infected mice is a characteristic of ejaculatory duct obstruction,bilateral congenital absence of the vas deference,partial retrograde ejaculation and androgen deficiency[16]. This finding also agrees with previous report that demonstrated P. berghei induced reduction in sperm motility,morphology and count[37].
Lipid peroxidation indicated by the increased levels of MDA,a biomarker of oxidative stress,is greatly elevated in untreated P.berghei infected mice[38]. So,evidence suggests that sperm cell membrane went through rapid lipid peroxidation and this observation is in agreement with other studies that show elevated level of MDA in sperm membrane which results in defective sperm function,motility,reduced sperm quality and infertility[39]. SOD protects catalase against inhibition by superoxide anion. Thus,the balance of this enzyme system may be essential to get rid of superoxide anion and peroxides generated in subcellular compartments of the testis.The antioxidant enzymes,SOD and catalase constitute a mutually supportive team of defense against ROS. The reduced production of catalase and increased production of SOD in infected mice observed in this study is a clear indication of oxidation by malaria infection[40]. The increase in semen acid phosphatase activity level in mice infected with P. berghei may be due to oxidative stress and could be used as additional investigation in the diagnosis of malaria.However,administration of ethanol extracts of P. amarus overrode the oxidant effects of P. berghei infection in mice. Administration of leaf ethanol extracts of P. amarus significantly improved semen quality in a dose dependent manner,even quite greater than our standard drug,Lonart?DS. Our study suggests that P. berghei infection rather than antimalarials plays a noteworthy role in reduction in reproductive quality. Ekhoye et al[5]in a similar study reported that administration of artemether/lumefantrine reversed the antifertility effect of the parasite infection. Results from our study is consistent with studies reported by other researchers. Ojezele et al[37]conducted a study akin with ours on the effect of 300 mg/kg P. amarus seed extracts on reproductive indices in malaria infected mice,recorded a significant increase in semen motility,morphology,viability and count in mice treated with extracts compared with values presented for the infected,untreated mice. This suggests that seminal oxidative stress demonstrated in P. berghei infected mice can be reversed with ethanol leaf extracts of P. amarus.
Although there are reports of extracts of P. amarus producing reduced seminal activity,other researchers who conducted studies of extracts of P. amarus in healthy experimental animals showed ample improvement of semen quality. Azubuike et al[40]recorded significant increase in sperm count,motility and testosterone levels of healthy mice treated with crude methanol and aqueous extracts of P. amarus. Bankole et al[41]also demonstrated that the ethanol extract of P. amarus exerted a positive effect on penile erection properties in male guinea pigs. Opajobi et al[15]reported that the schizonticidal activity of P. amarus ethanol leaf extract enhanced testicular and ovarian antioxidant defense capacity. Results demonstrated by other researchers that were inconsistent with our study may be as a result of the presence of no Plasmodium infection,because,antimalarials,as pro-oxidants,act generally by the production of free radicals in contact with iron within erythrocytes,which facilitates the destruction of parasites[35]. Moreover,increase in ROS and decrease in antioxidants have been reported in malaria patients[6]. With no parasite infection,antimalarials will produce a large number of ROS and this may produce oxidative stress,destroying the body cells,spermatozoa inclusive.
There are some limitations. The preventive and in vitro scavenging antioxidant activity of the P. amarus ethanol leaf extract were not determined. Therefore,the antioxidant compounds were not identified and their antioxidant defense indices could not be established.
In conclusion,the results show that administration of P. amarus crude ethanol leaf extract to P. berghei infected mice reverses the induced alteration in semen quality,sperm viability and the capacity to produce fructose and citric acid,and failure in defense against oxidative damage. Further studies are required to isolate compounds in P. amarus extracts responsible for these observed biological activity.
Conflict of interest statement
The authors declare that there is no conflict.
Authors' contributions
Williams Oshiegbu conducted the laboratory research experiment.Chinwendu Obogheneophruhe Elu analysed and interpreted data and prepared the draft manuscript. Innocent Onyesom concieved the research,wrote the proposal,supervised stages of the research and vetted the manuscript which was read by all authors and approved for submission.
 Asian Pacific Journal of Reproduction2022年2期
Asian Pacific Journal of Reproduction2022年2期
- Asian Pacific Journal of Reproduction的其它文章
- INRA82 extender enhances semen quality in ram under cooled and cryopreserved stages
- Protective effect of Scrophularia striata combined with trehalose and cysteine added to diluents on cryopreservd goat epididymal sperm
- An ethnopharmacological survey of medicinal plants used in the traditional treatment of human infertility in eastern Algeria
- Spousal communication,fertility preference and other factors affecting contraceptive use among married couples in Ekiti State,Nigeria
- Determinants of emergency contraceptive pill use in Bangladesh:An analysis of national survey data
- A scoping review of SARS-CoV-2 and male infertility:Concerns and future prospects
