Effect of structural vacancies on lattice vibration,mechanical,electronic,and thermodynamic properties of Cr5BSi3
Tian-Hui Dong(董天慧) Xu-Dong Zhang(張旭東) Lin-Mei Yang(楊林梅) and Feng Wang(王峰)
1School of Science,Shenyang University of Technology,Shenyang 110870,China2School of Materials Science and Engineering,Shenyang University of Technology,Shenyang 110870,China
In recent years,transition metal silicides have become the potential high temperature materials. The ternary silicide has attracted the attention of scientists and researchers. But their inherent brittle behaviors hinder their wide applications.In this work, we use the first-principles method to design four vacancy defects and discuss the effects of vacancy defects on the structural stability,mechanical properties,electronic and thermodynamic properties of hexagonal Cr5BSi3 silicide.The data of lattice vibration and thermodynamic parameters indicate that the Cr5BSi3 with different atomic vacancies can possess the structural stabilities.The different atomic vacancies change the mechanical properties and induce the Cr5BSi3 to implement the brittle-to-ductile transition. The shear deformation resistance and volume deformation resistance of Cr5BSi3 are weakened by different vacancy defects. But the brittleness behavior is remarkably improved. The structural stability and brittle-to-ductile transition of Cr5BSi3 with different vacancies are explored by the electronic structures. Moreover,the thermal parameters indicate that the Cr5BSi3 with vacancies exhibit different thermodynamic properties with temperature rising.
Keywords: vacancies in Cr5BSi3,brittle-to-ductile transition,electronic properties,thermodynamic properties
1. Introduction
With the development of science and technology, many researchers have discovered that the transition metal silicides can be used as the sharp leading edges materials and high temperature reusable surface materials of hypersonic vehicles and surface coatings, and so on.[1—4]They have the excellent chemical and physical properties, such as high oxidation resistance, high creep resistance, high strength and high melting point.[5—10]Some ternary metal silicides were found and their physical properties were widely investigated.[11—15]Among these ternary silicides,Nowotnyet al.synthesized the Cr5BSi3experimentally and found that it possesses the hexagonal structure withP63/mcmgroup.[15]Zhouet al.predicted the anisotropic properties and band structure of Cr5BSi3by using the first-principles calculations.[16]The theoretical results indicated that the Cr5BSi3silicide shows the brittle behavior.Generally speaking, vacancy defects, doping elements, dislocation slip,etc. can improve the brittle behavior for solid materials.[17—19]For the heat activation effect in high temperature environment, the vacancy has a significant effect on the mechanical properties of high temperature materials. It is necessary to explore the effects of vacancies on the physical properties for Cr5BSi3. In the present work, we design four vacancy models and investigated the influences of these vacancies on the physical properties for Cr5BSi3. The structural stabilities, mechanical properties, electronic structures and thermodynamic properties of Cr5BSi3with various vacancies are comparatively studied by using the first-principles calculations.
2. Theoretical methods
The parent Cr5Si3B and the Cr5Si3B with various vacancies were all investigated by using the CASTEP code.[20]The electron—ion interaction was described by the ultrasoft pseudopotential.[21,22]The Perdew-Burke—Ernzerhof (PBE) functional under the generalized gradient approximation (GGA)approximation was used to treat the exchange—correlation functional.[23]The electronic configurations of Cr,B,Si atoms are 3p63d54s2, 2s22p1, and 3s23p2, respectively. The cutoff energy of 560 eV was set such that the convergence for each of the present models was ensured. The Brillouin-zone was sampled by using an 8×8×10k-points for integrations in reciprocal space. The Broyden—Fletcher—Goldfarb—Shanno (BFGS)minimization scheme,ionic Hellmann—Feynman force,maximum ionic displacement and stress were used for optimizing the structure.[24—26]The tolerance for geometry optimization is different: within?5×10?3eV/atom for the total energy,within 0.04 eV/for maximum ionic Hellmann—Feynman force,within 6×10?3for maximum ionic displacement,and within 0.05 GPa for maximum stress. The current settings ensured that the total energy converged to 1 meV/atom. Convergence tests indicated that the cutoff energy andkmesh in the present work were sufficient to guarantee the convergence of total energy and provide a proper prediction of the latticeparameters. The unit cell sizes and atomic positions were allowed to be fully relaxed without any constraints during geometry optimization.
According to the available literature,[15,16]the Cr5BSi3crystallizes into a hexagonal Cr5CuSn3-type structure withP63/mcmspace group.[27]In the crystal structures of Cr5BSi3,Cr1, Cr2, B, and Si atoms occupy the locations at the Wyckoff sites 6g(0.27, 0, 0.25), 4d(0.33, 0.67, 0), 2b(0, 0, 0),and 6g(0.62,0,0.25),respectively. There are 18 atoms inside the unit cell. According to the four different atomic positions,we designed four vacancy models: Cr?Va1,Cr?Va2,B?Va,and Si?Va. And the four different structural models of Cr5BSi3are shown in Fig. 1. The structural stability depends on the thermodynamical and dynamical condition.Thus,the vacancy formation energy (Ef) for each of the four models was calculated to estimate the thermodynamical stability in Cr5BSi3with the following expression:[28,29]
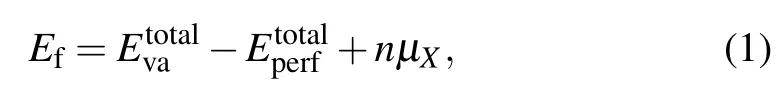
whererepresents the total energy of perfect Cr5BSi3anddenotes the total energy of Cr5BSi3with vacancy defects, andμX(X= Cr, B and Si) is the chemical potential of each species. For the niobium-rich region,μCr=ECr(bulk),μB=EB(bulk), while for the silicon-rich region,μSi=ESi(bulk). Note thatECr(bulk),EB(bulk),andESi(bulk)are the total energy of a Cr atom,a B atom,and an Si atom in a bulk Cr, B, and Si system, respectively.[30—35]Futhermore,the thermodynamic stability for each of the four models was determined by the value of formationenthalpy. The formation enthalpy of perfect Cr5BSi3was calculated from Eq. (2) and the values of formation enthalpy of four vacancy models were calculated from Eq.(3):[35,36]
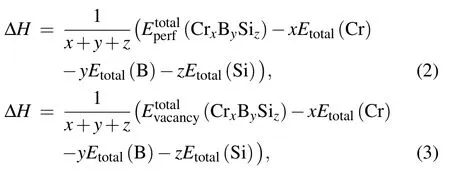
where ΔHis the formation enthalpy,represents the total energy of perfect Cr5BSi3,denotes the total energy of Cr5BSi3with vacancy defects,andEtotal(Cr),Etotal(B),Etotal(Si) refer to the total energy of the crystal of Cr, B, and Si elements, respectively. Moreover, the structural stability is also determined by the dynamical condition. The dynamical stability depends on the phonon frequency. If the solid material possesses the positive phonon frequency,it will possess the dynamical stability. In this work, the phonon dispersions were investigated using the finite displacement method with Phonon code.Moreover,some phonon-related thermodynamic properties were calculated in a quasi-harmonic approximation within the Phonon code.[37—41]The mechanical properties were determined by the elastic constants(Cij),and elastic moduli.[42—44]The strain—stress method and Voigt—Reuss—Hill(VRH)method were used to calculate the elastic constants and elastic moduli.[45,46]According to the Hill’s bulk modulusBHand shear modulusGH,we calculated the elastic hardness(HV),Poisson’s ratio(v),and Young’s modulusE.[47,48]

Fig.1. Crystal structures of Cr5BSi3 with different vacancies.
3. Results and discussion
4. Structural stability of vacancy models
Table 1 shows the lattice parameters and thermodynamic parameters of four vacancy models and perfect Cr5BSi3crystal. It is found that lattice constants of Cr5BSi3approach to the experimental values,[15]which indicates that our computational method is reliable and feasible. As is well known,the positive vacancy formation energy (Ef) suggests that the vacancy model is thermodynamically stable.[35]As shown in Table 1, the values of vacancy formation energyEffor four vacancy models are all positive, indicating that the Cr5BSi3with four vacancies are thermodynamically stable. In Table 1,the values of formation enthalpy ΔHof perfect Cr5BSi3and Cr5BSi3with four vacancies are both negative,confirming that the Cr5BSi3with various vacancies are thermodynamically stable.The Cr?Va1vacancy has the best thermodynamic stability in these vacancies due to the most negative values of formationenthalpy. Furthermore,figure 2 displays the lattice vibration curves of parent Cr5BSi3and four vacancy models. The parent Cr5BSi3and Cr5BSi3with various vacancies exhibit the dynamical stabilities because the parent Cr5BSi3and Cr5BSi3with various vacancies have no imaginary phonon frequencies in the whole regions.[49]It can be found from Table 1 that lattice constants of Cr5BSi3with vacancies are smaller than those of the perfect Cr5BSi3compound. In Fig. 1, each B atom is surrounded by six Cr1 atoms. And Cr2 atom occupies the center formed by three adjacent BCr16octahedra. Additionally, the coplanar BCr16octahedra are interleaved by Si layers along thecaxis. The structural stability of Cr5BSi3stems from the bond state formed by the hybridization among Cr,B,and Si atoms within the stacked layered structure. The removal of Cr, B, and Si atoms can cause lattice to shrink,which indicates that the removal of atoms enhances the atomic interaction among the nearby atoms.
Table 1. Calculated values of lattice parameters a, b, and c (in unit ), vacancy formation energy Ef (in unit eV), and formation enthalpy ΔH (in unit eV)of Cr5BSi3 with four different vacancies.

Table 1. Calculated values of lattice parameters a, b, and c (in unit ), vacancy formation energy Ef (in unit eV), and formation enthalpy ΔH (in unit eV)of Cr5BSi3 with four different vacancies.
aRef.[15], bRef.[16].
Compound Method a(images/BZ_589_1417_292_1450_340.png) b(images/BZ_589_1417_292_1450_340.png) c(images/BZ_589_1417_292_1450_340.png) Ef (eV) ΔH (eV)Cr5Si3B GGA 7.034 7.034 4.740 0.902 Exp.a 7.060 7.060 4.730 Theor.b 7.0306 7.0306 4.7326 Cr?Va1 GGA 6.966 6.967 4.688 0.779 0.894 Cr?Va2 GGA 6.934 6.933 4.732 0.761 0.889 B?Va GGA 6.981 6.981 4.717 0.736 0.878 Si?Va GGA 7.020 7.022 4.679 0.741 0.881

Fig.2. Phonon dispersion curves of(a)parent Cr5BSi3 and Cr5BSi3 with different vacancies: (b)Cr?Va1,(c)Cr?Va2,(d)B?Va,and(e)Si?Va.
4.1. Mechanical properties
Table 2 presents the elastic constantsCi js, elastic moduli and the related mechanical quantities of four models, together with the corresponding parameters of perfect Cr5BSi3.In Table 2, the calculated results are close to the available results.[16]The calculated elastic constants of Cr5BSi3with various vacancies satisfy the Born criteria, indicating that they are mechanicallystable.[50,51]The deformation resistance along theaaxis and thecaxis are determined by the elastic constantsC11andC33, respectively. A larger elastic constantC11orC33corresponds to a better deformation resistance. In the present work,the elastic constantC11of the parent Cr5BSi3is larger than the values of corresponding elastic constantC11of two Cr?Vavacancies and Si?Vavacancy,confirming that the removal of these atoms can weaken the deformation resistance along theaaxis. Meanwhile, elastic constantC33of Cr?Va2vacancy is smaller than that of the parent Cr5BSi3, illustrating that the presence of Cr?Va2vacancy can enhance the deformation resistance along thecaxis of parent Cr5BSi3. Furthermore, a larger elastic constantC44implies a higher shear resistance.[52]As listed in Table 2, the elastic constantC44of Si?Vamodel is 100.7 GPa, which is smaller than those of parent Cr5BSi3and Cr5BSi3with other vacancies. Namely, the removal of Si atom makes the shear resistance of Cr5BSi3lower than those of the parent Cr5BSi3and Cr5BSi3with other vacancies models. Therefore,the removal of atoms can weaken the charge interaction and the local hybridization among atoms in the ground state.
From Table 2 it follows that the bulk modulusBand shear modulusGof the perfect Cr5BSi3are 197.9 GPa and 128.1 GPa, respectively. The values ofBandGof Cr?Vamodel and B?Vamodel are smaller than those of the parent Cr5BSi3,indicating that the presence of Cr?Vamodel and B?Vamodel can weaken the compression resistance and deformation resistance of Cr5BSi3. In addition,when Si atom is removed,the values ofBandGfor Si?Vamodel are larger than those of the parent Cr5BSi3,confirming that the Cr5BSi3with Si?Vavacancy possesses the better volume deformation. The shear modulus of B?Vamodel is 86.9 GPa and it is smaller than those of other vacancy models, illustrating that B?Vamodel has a weaker deformation resistance than other vacancy models. Generally speaking, the smaller Young’s modulus means the lower elastic stiffness. In Table 2, the Young’s moduliof Cr5BSi3and Cr5BSi3with four various models follow the order: Si?Va>Cr5BSi3>Cr?Va2>Cr?Va1>B?Va, which indicates that the Si?Vamodel possesses the best elastic stiffness in the four vacancy models. Additionally,it can be concluded that the presence of Cr atom and B atom vacancies can weaken the elastic stiffness of the parent Cr5BSi3.

Table 2. Values of elastic constants Cij (in unit GPa),bulk modulus B(in unit GPa),shear modulus G(in unit GPa),Young’s modulus E (in unit GPa),B/G ratio,Poisson’s ratio v,and Vickers hardness HV (in unit GPa)of Cr5BSi3 with four different vacancies.
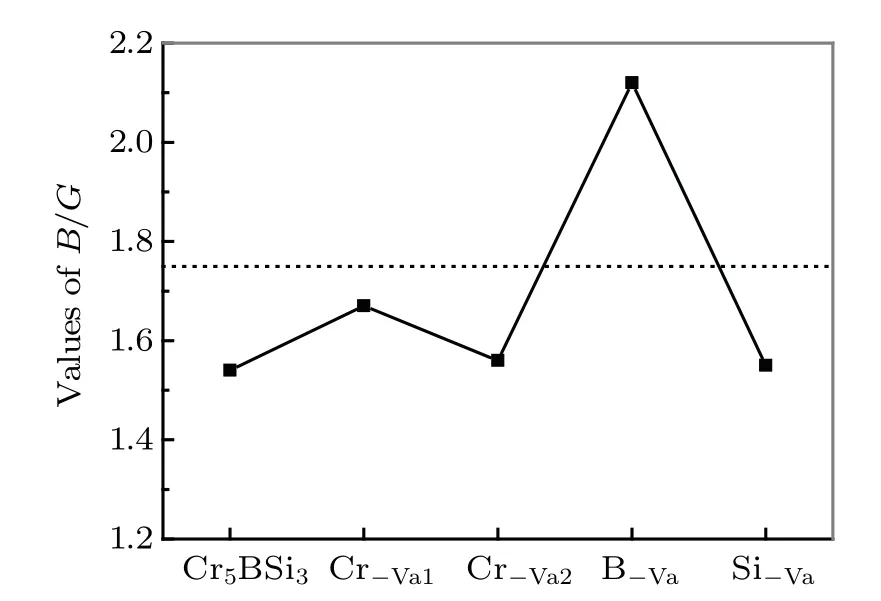
Fig. 3. Curves of B/G ratio of parent Cr5BSi3 and Cr5BSi3 with different vacancies.
The values of hardnessHVof Cr5BSi3and Cr5BSi3with different vacancies are listed in Table 2. The intrinsic hardness of the parent Cr5BSi3is 17.6 GPa. The hardnessHVof the Cr?Vamodel and Si?Vamodel are slightly different from that of Cr5BSi3. The hardnessHVof B?Vamodel is obviously smaller than that of Cr5BSi3. This result suggests that the removal of B atom strongly reduces the hardness of Cr5BSi3.The brittle/ductile behavior is a significant parameter of solid material. TheB/Gratio and Poisson’s ratiovcan be used to assess the brittle or ductile behavior according to the Pugh rule.[53,54]The critical value ofB/Gand Poisson’s ratiovare 1.75 and 0.26,respectively. If the values are less than 1.75 and 0.26, the solid material will be a brittle material. It is obviously found thatB/Gratio of the parent Cr5BSi3is less than 1.75 in Fig.3, indicating that the parent Cr5BSi3exhibits the brittle behavior.[16]The Cr?Vamodel and Si?Vamodel exhibit a slightly largerB/Gratio than the parent Cr5BSi3. However,theB/Gratio of B?Vamodel is larger than 1.75. Namely,the Cr5BSi3with B?Vais a ductile material and the other vacancies improve the brittleness of Cr5BSi3,the variation of which is the same tendency as the variation of hardness. In Table 2,the Poisson’s ratiovof the parent Cr5BSi3is 0.234 GPa,which is less than 0.26,indicating that the Cr5BSi3is a brittle material. Obviously, the Cr5BSi3with B?Vavacancy is ductile,and the Cr?Vamodel and Si?Vamodel are less brittle than the Cr5BSi3, which corresponds to the same variation withB/Gratio. The results indicate that the vacancy models improve the brittle behaviors of Cr5BSi3and the B?Vavacancy gives rise to the brittle-to-ductile behavior transition.
4.2. Electronic properties
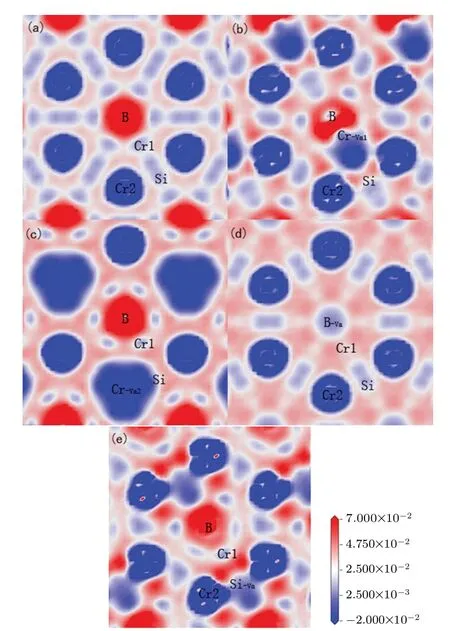
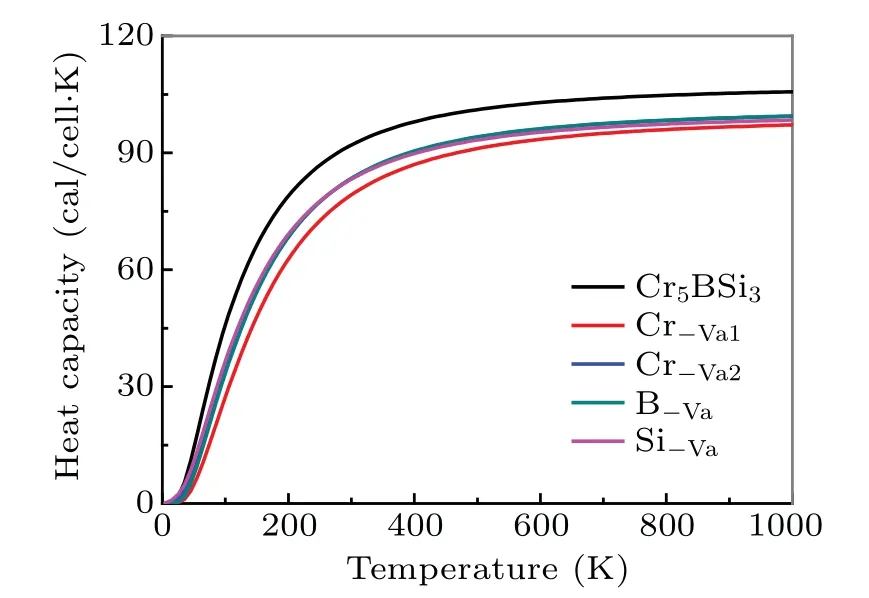
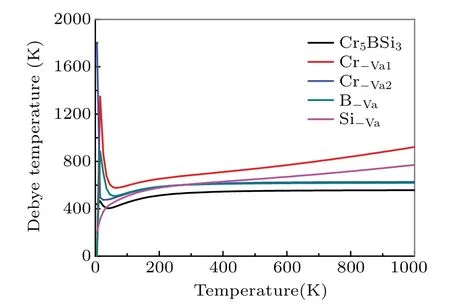
To further investigate the mechanism of electronic properties, the total and partial density of states for the Cr5BSi3with various vacancies are shown in Fig. 4. The black vertical dotted lines in the graphs denote the Fermi levelEF.It is found that there are no energy gaps appearing near the Fermi levelEF, which indicates that the Cr5BSi3exhibits the metallic behavior. As shown in Fig.4(a),the density of states(DOS)of Cr5BSi3is mainly composed of the Cr-3d,B-2s,2p,and Si-3s, 3p orbital states. For the different contributions of Cr,B,and Si electronic states,the localized hybridization between electronic states forms the Cr—B bond,Cr—Si bond,and B—Si bond. The B-2p state penetrates into the B-2s state at?6.35 eV, implying that the electrons transfer from B-2s to B-2p state. The Cr-3d state and Cr-4s state cross the Cr-3p for?8.59 eV and?6.38 eV,respectively,which indicates that the electrons transfer between Cr-3d,Cr-4s,and Cr-3p states.The Si-3p state passes through the Si-3s state at?6.82 eV,which means the formation of Si—Si bonds. It can be seen from the figure,the localized hybridization between the Cr-3d state and the B-2p state in Cr5BSi3is strong, which results in the strong electronic interaction between Cr and B atoms.The density of states (DOS) for Cr5BSi3with various vacancies is slightly different from that of the parent Cr5BSi3. The structural stability can be exhibited by the number of bonding electrons at Fermi level, the smaller the value of the number,the more stable the structure is.[55,56]TheN(EF)values of the Cr5BSi3with four vacancies and the parent Cr5BSi3follow the order: Cr?Va2 Fig.4. Total and partial DOSs of(a)parent Cr5BSi3 and Cr5BSi3 with different vacancies: (b)Cr?Va1,(c)Cr?Va2,(d)B?Va,and(e)Si?Va. Fig. 5. Plots of electron density difference for (a) parent Cr5BSi3 and Cr5BSi3 with different vacancies: (b)Cr?Va1, (c)Cr?Va2, (d)B?Va, and(e)Si?Va. The differences in electron density between the parent Cr5BSi3and the Cr5BSi3with four vacancies on (001) plane are plotted in Fig. 5. In eachpanel, the difference in electron density is in a range from?0.02 e/3to 0.07 e/3. And the blue color and the red color denote the maximum and minimum accumulation of electrons, respectively. The light blue regions refer to the neutral regions and there is no charge transfer in these regions. Comparing with the color bar, it can be found from Fig. 5(a) that there are many electrons accumulating between nearby Cr1 and B atoms,illustrating that there exist the Cr1—B—Cr1 bond chains in the parent Cr5BSi3.There is no electron accumulation between Cr1 atom and Si atom appearing in Fig. 5(a), indicating that the electrons are delocalized between Cr atom and Si atom. However, there is no obvious transformation between the inter-layer B atoms,indicating that the weak B—B interaction appears. Furthermore, the electrons between the neighboring Cr2 atoms are delocalized,and so are the nearby Si atoms,which indicates that Cr2—Cr2 bonds and Si—Si bonds are both metallic bonds. The presence of vacancies can influence the electron accumulation, which changes the state of bond and mechanical properties of the parent Cr5BSi3. From Fig. 5(b), the Cr?Va1vacancy reduces the strength of Cr1—B bond and Cr1—Si bond, which reduces the resistance of deformation on the(001)plane. The Cr?Va2vacancy reduces the localized hybridization between nearby Cr2 atoms. Particularly,when B atoms are removed,the electrons between Cr1 atom and B atom become more delocalized, meanwhile the hybridization between Cr1 atom and Si atom becomes stronger. The weak localized hybridization im-proves the brittle behavior of the parent Cr5BSi3. The Cr1—Si bonds are clear away in Fig.5(e),indicating that the presence of Si?Vavacancy reduces the localized hybridization between Cr1 atoms and Si atoms. As is well known, the thermal response of material can be estimated by the thermodynamic properties. To estimate the thermodynamic properties of the Cr5BSi3with four vacancies,we use the Phonon code to explore their thermodynamic properties. Figures 6 and 7 show the thermodynamic parameters of Cr5BSi3with four vacancies at different temperatures.Figure 6 shows the curves of calculated values of heat capacity at constant volumeCVversus temperature for Cr5BSi3with four vacancies and the parent Cr5BSi3. It is found that the values of heat capacityCVof Cr5BSi3with four vacancies and the parent Cr5BSi3follow the DebyeT3law in low temperature area and are close to the Dulong—Petit limit in high temperature area.[58,59]The values ofCVfor Cr5BSi3with various vacancies are smaller than the value ofCVof parent Cr5BSi3,which indicates that the removal of Cr,B,and Si atoms reduce the value ofCVof the parent Cr5BSi3. Fig.6. Temperature-dependent heat capacity(CV)curves of parent Cr5BSi3 and Cr5BSi3 with different vacancies. Fig. 7. Curves of Debye temperature (ΘD) versus temperature of parent Cr5BSi3 and Cr5BSi3 with different vacancies. Figure 7 shows the curves of Debye temperatureversustemperatue of Cr5BSi3with various vacancies. It is obvious that Debye temperatures of Cr5BSi3with various vacancies and the parent Cr5BSi3increase rapidly in low temperature area and are close to the fixed values in high temperature area.The Debye temperatures of Cr5BSi3with various vacancies in high temperature area are much larger than the Debye temperature of the parent Cr5BSi3, indicating that the existence of these vacancies increases the thermal stability of the parent Cr5BSi3. The Debye temperature of the Cr5BSi3with Cr?Va1vacancy is much bigger than those of other compounds,which illustrates that the Cr5BSi3with Cr?Va1vacancy shows the better thermal stability than other compounds in the elevated temperature region. We use the first-principles calculations to explore the effects of vacancies on the structural stability,electronic and mechanical properties of Cr5BSi3. The conclusions are as follows. (i) Theremoval of Cr, B, and Si atoms result in lattice shrinkage. Their vacancy formation enthalpy values are all smaller than zero,indicating that these vacancy structures are thermodynamically stable. The Cr?Va1model is more stable than other vacancy models. The lattice vibration indicates that the Cr5BSi3with various vacancies are dynamically stable. (ii)The elastic modulus of Cr5BSi3with Cr?Vavacancies and B?Vavacancies are smaller than that of Cr5BSi3,demonstrating that these vacancies weaken the hardness and deformation resistance of Cr5BSi3. The Poisson’s ratiovandB/Gindicate that Cr5BSi3with B?Vavacancy induces the brittleto-ductile behavior transition. (iii) The electronic properties explain the mechanism of an effect of structural vacancies on the physical properties of Cr5BSi3. (iv) The values of thermal parameters indicate that the Cr5BSi3with four vacancies present different thermodynamic properties with the increase of temperature. Acknowledgement Project supported by the Natural Science Foundation of Liaoning Province,China(Grant No.2019JH/30100019).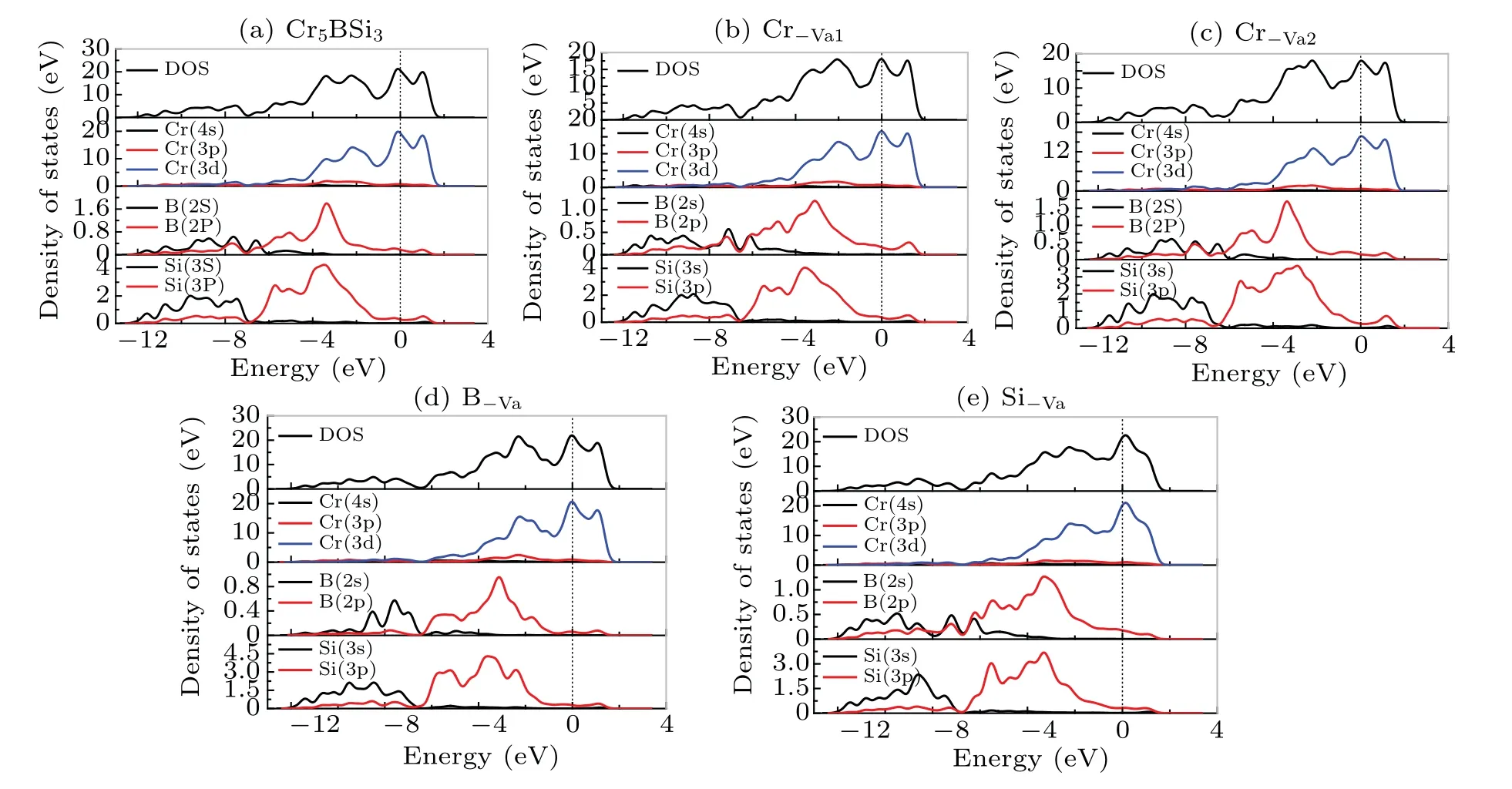
4.3. Thermodynamic properties
5. Conclusions
- Chinese Physics B的其它文章
- A broadband self-powered UV photodetector of a β-Ga2O3/γ-CuI p-n junction
- High-sensitive terahertz detection by parametric up-conversion using nanosecond pulsed laser
- High efficiency,small size,and large bandwidth vertical interlayer waveguide coupler
- High-fidelity resonant tunneling passage in three-waveguide system
- An analytical model for cross-Kerr nonlinearity in a four-level N-type atomic system with Doppler broadening
- Determine the physical mechanism and source region of beat wave modulation by changing the frequency of high-frequency waves