Absorption effect of pure nickel on the corrosion behaviors of the GH3535 alloy in tellurium vapor
Kai Wang?Li Jiang?Xiang-Xi Ye?Jian-Ping Liang?Chao-Wen Li?Fang Liu?Zhi-Jun Li
Abstract In this study,pure Ni was demonstrated to protect the GH3535 alloy from Te vapor corrosion because of its strong absorption capacity.Severe Te corrosion of a single GH3535 alloy sample occurred in Te vapor at 700°C,which manifested as complex surface corrosion products and deep intergranular cracks.However,when pure Ni and the GH3535 alloy were put together in the vessel,the GH3535 alloy was completely protected from Te corrosion at the expense of the pure Ni.Thermodynamic calculations proved that the preferential reaction between pure Ni and Te vapor reduced the activity of Te vapor considerably,preventing the corrosion of the GH3535 alloy.Our study reveals one potential approach for protecting the alloys used in molten-salt reactors from Te corrosion.
Keywords Tellurium corrosion?Molten salt reactor?GH3535 alloy?Tellurides
1 Introduction
Molten salt reactors(MSRs)are a class of fission reactors that use uranium/thorium-containing molten salts(typically fluorides or chlorides)as fuels.The distinctive features of molten salts include a large liquid phase interval,high heat capacity,and low vapor pressure,which afford unique advantages for MSRs,such as high operating temperature,high thermal efficiency,and inherent safety.However,the MSR design also presents a significant challenge for the structural materials used in MSRs,which should meet the harsh requirements of high-temperature strength,sufficient corrosion resistance to molten salts/fission products,and high irradiation resistance.
Hastelloy N alloy,also called GH3535 in China,is a solid-solution-strengthened Ni-based superalloy,which possesses excellent corrosion resistance to molten salt and sufficient high-temperature strength.This alloy has been successfully applied to the world’s first molten-salt reactor experiment(MSRE)[1].However,after 1.5 years of full power operation of the MSRE,surface intergranular cracks were observed in the alloy components in the primary loop,which substantially threatened the service safety of the MSRE.Extensive out-of-pile examinations proved that these intergranular cracks were related to the diffusion and segregation of the fission product,Te,along the grain boundaries in the Hastelloy N alloy[2].Unlike the fixed fuel assemblies in traditional reactors,the fuels of MSRs circle in the primary loop are in the form of a molten salt[3].Thus,the fission product,Te,would pervade the entire primary loop with the circulation of the fuel salts and attack almost all the fuel-contacted metal components[4].In addition,some components above the fuel are attacked by the Te vapor released from the salts.
It is widely known that some active metals can function as absorbers to eliminate the negative effects of corrosive gases(e.g.,O2,S,and N2).One typical example is Ti,which can effectively absorb residual oxygen to create a high-vacuum environment[16,17].Te and O belong to the same group in the periodic table,and the selective tellurization of elements has been widely reported in Hastelloy N alloys and steels[8,18–23].These facts increase our curiosity regarding whether Te can be absorbed by a certain element to protect these vulnerable alloy materials,which could provide a potential path for corrosion prevention for the alloys used in Te corrosion environments.The Gibbs free energies of the tellurides of typical elements were calculated to be in the following order:MnTe<Cr3Te4<Ni3Te2<FeTe0.9<MoTe2[8],which indicates that Cr and Mn are the most effective absorbers.However,these two elements have been proven to be susceptible to molten-salt corrosion.Pure Ni seems to be an appropriate choice because of its excellent molten-salt resistance[24].In fact,several recent studies have proposed pure Ni plating as a protective layer for improving the molten-salt corrosion resistance of alloys in MSRs[25–27].Thus,it is necessary to verify whether the absorption of Te by pure Ni can protect the vulnerable Hastelloy N alloy in a Te corrosion environment.In this study,the corrosion behaviors and mechanical properties of the GH3535 alloy were investigated in two groups of corrosion experiments:one involved pure Ni absorbers,while the other did not.The absorption effect of pure Ni was established to completely protect the GH3535 alloy from Te corrosion and Te embrittlement under our experimental conditions.Additionally,the reaction mechanism is briefly discussed from the viewpoint of chemical equilibrium.
2 Materials and experiments
Table 1 lists the chemical composition of the GH3535 alloy used in this study.The alloy ingots were first fabricated by vacuum-induction melting and vacuum arc remelting,followed by homogenization to relieve element segregation and eliminate casting flaws.The ingots were forged and hot-rolled into alloy plates,which were solidsolution-treated to obtain the final products.To determine the chemical composition of the alloy,the alloy samples were completely dissolved in 20%HNO3solution at 90°C for 4 h,which was examined by inductively coupled plasma optical emission spectrometry(ICP-OES).The carbon concentration was determined using a carbon–sulfur spectrometer(LECO CS844).
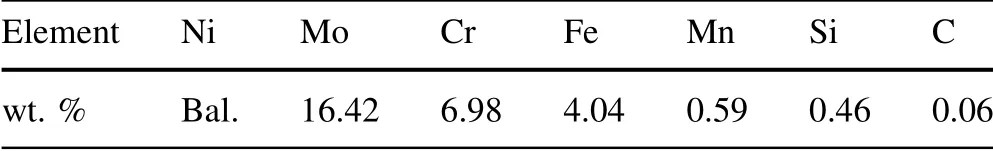
Table 1 Chemical composition of the GH3535 alloy
Tensile test samples of the GH3535 alloy and pure Ni were employed in the Te vapor corrosion experiments.The dimensions of the tensile test samples were 1 mm(thickness)×3 mm(width)×12 mm(length).To obtain a uniform surface finish,all the tensile test samples were ground using abrasive paper with the same mesh numbers.All samples were washed with absolute ethyl alcohol by ultrasonic cleaning and subsequently dried.
Quartz tubes with a dimension of 350 mm(length)×20 mm(inner diameter)were used as the corrosion vessel;all the tubes were dehydroxylated to reduce the influence of the oxidizing hydroxyl radicals during the experiments.The dehydroxylated quartz tubes were examined using the infrared absorbance spectrograms of hydroxyl radicals at~3670 cm-1,using a Fourier transform infrared spectrometer(PE-Frontier,Perkin-Elmer).The concentration of hydroxyl radicals in the quartz tubes was calculated to be~4 ppm.
A schematic diagram of the corrosion experiments is shown in Fig.1.To verify the absorption effect of pure Ni,the tensile test samples of the GH3535 alloy and pure Ni were sealed in a quartz tube for the corrosion experiments.As a control group,only the GH3535 alloy samples were corroded in another quartz tube.The tensile test samples and Te source(pure Te block with a purity of 99.99%)were placed at the two ends of the quartz tubes.Before the vacuum sealing,the quartz tubes were preheated with a flame gun for 30 s to evaporate the moisture on the sample surface and the inner wall of the tube;thereafter,they were evacuated to a vacuum with a pressure of 10-4Pa.After vacuum sealing,the two ends of the sealed quartz tube with the Te block and samples were heated at 325°C and 700°C for 250 h in a gradient furnace.The temperature fluctuation in the furnace did not exceed±2°C.During the corrosion experiment,the Te vapor evaporated from the block,transferred to the end with the samples,and reacted with the GH3535 alloy and pure Ni.After the corrosion experiments,the sealed quartz tubes were air-cooled and subsequently broken to remove the samples.The corroded GH3535 alloy samples from the two sets of experiments are referred to as GH3535-SS(single specimen,only the GH3535 alloy)and GH3535-DS (double specimens,GH3535 alloy with pure Ni as absorber).
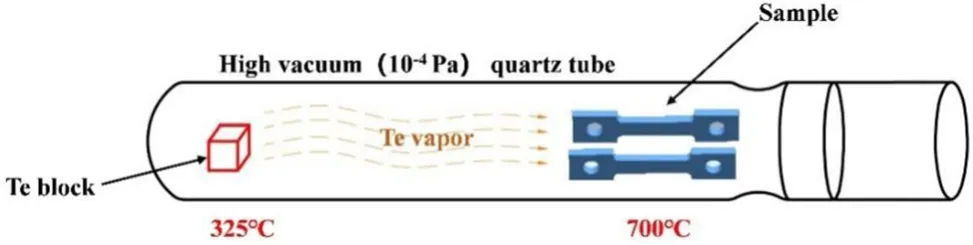
Fig.1 (Color online)Schematic diagram of the corrosion experiments using the quartz tubes as vessels
The phase constituents of the surface-corrosion products were determined using an X-ray diffractometer(Bruker D8 advance)in the angular range of 10°<2θ<80°,with a step size of 0.02°and a scanning time of 1 s/step.The microstructures and elemental distributions of the corrosion products were observed and detected from the surface and cross section by scanning electron microscopy(SEM,Carl Zeiss Merlin Compact)with energy-dispersive X-ray spectroscopy(EDS).An electron probe microanalyzer(EPMA,SHIMADZU-1720 H)was employed to further analyze the distribution of elements at the grain boundaries near the surface,which was operated at a high voltage of 25 kV and a beam current of 100 nA to obtain the minimum beam size.
Steve went home from school, thoughtful, that afternoon. Walking into the house, he took one look around. Both parents were passed out, in various stages of undress, and the stench was overpowering! He, quickly, gathered up his camping gear, a jar of peanut butter, a loaf of bread, a bottle of water, and this time...his schoolbooks. Grim faced and determined7, he headed for the woods.
A Zwick Z100 mechanical tensile testing machine was utilized to test the mechanical properties of the corroded samples at a constant strain rate of 3.5 mm/min at room temperature.The fracture morphologies and surface intergranular cracking on the gage section were observed by SEM and optical microscopy (Axio Imager M2m),respectively.The depth and amount of cracks in the gage section were recorded individually and counted to evaluate the degree of damage.
In this work,the Gibbs free energies of formation of these selected oxides and tellurides were calculated using HSC Chemistry software and embedded databases.The activities of different elements in the GH3535 alloy were calculated using the JMatPro software.
3 Results
3.1 Corrosion phenomenon in the Te vapor
After the corrosion experiments,the gray corrosion products were uniformly attached to the surfaces of GH3535-SS and the pure Ni samples.In contrast,a layer of the light-blue corrosion products was observed on the surfaces of the GH3535-DS samples,which were stripped off in some areas.The XRD patterns of the initial GH3535 alloy and the three corroded samples are shown in Fig.2.According to the characteristic diffraction peaks,the corrosion products consist of Ni3Te2and small amounts of Cr2O3,Cr3Te4,and M6C carbides in the GH3535-SS samples.This result agrees with those of previous studies[7,8,28,29].For the GH3535-DS samples,except for the strong characteristic diffraction peaks of theγmatrix phase,weak peaks indicative of Cr2O3and M6C were observed in the XRD pattern.On the surface of the pure Ni sample,only Ni3Te2was found among the corrosion products,which is extremely thick that the diffraction signals from theγmatrix phase are completely covered.The XRD results indicated that pure Ni samples tend to react with the Te vapor preferentially and protect the GH3535-DS samples.
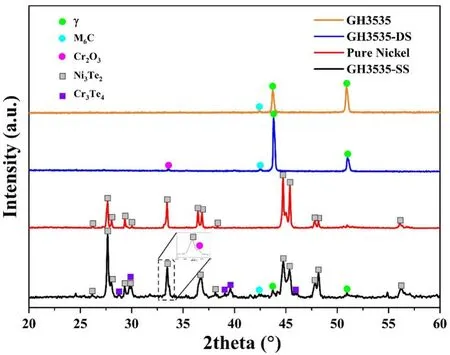
Fig.2 (Color online)XRD patterns for the initial GH3535 alloy and the surface corrosion products of the corroded GH3535-SS,GH3535-DS,and pure Ni samples.To observe the details more clearly,only the patterns in the region of 20°–60°were plotted
The morphologies and chemical compositions of the surface corrosion products in the three corroded samples were detected by SEM and SEM–EDS,respectively(Fig.3).For the GH3535-SS samples,two types of particles exist on the surface of the corrosion products,namely small particles and large surface-faceting irregular particles(Fig.3a).The former mainly comprises Cr and O,while the latter consists of Ni and Te.By combining the XRD(Fig.2)and SEM–EDS(Fig.3a)results,they were identified as Cr2O3and Ni3Te2,respectively.On the surface of the GH3535-DS samples,a continuous and dense Cr-and O-rich layer can be observed(Fig.3b),which can be identified as Cr2O3based on the XRD(Fig.1)and SEM–EDS(Fig.3b)results.The very weak EDS signal of Te in the entire observation area proves that the Cr2O3layer can completely protect the alloy matrix from Te corrosion(Fig.3b).The spallation of the Cr2O3layer in some areas is due to its thermal expansion properties with the matrix during the air-cooling process.On the surface of the pure Ni samples,a mass of surface-faceting irregular particles can be observed(Fig.3c),which was identified as Ni3Te2from the XRD patterns.

Fig.3 (Color online)The morphologies and corresponding EDS mapping images of the surface corrosion products in the GH3535-SS(a),GH3535-DS(b),and pure Ni(c)samples
The morphologies and chemical compositions of the surface corrosion products in the corroded samples can also be observed from the cross-sectional image,shown in Figs.4 and 5.On the surface,the small Cr-and O-rich particles were Cr2O3(Fig.4),which corresponds well with the observations from the surface(Fig.3a).Owing to the metallographic sample preparation,the surface-faceting irregular Ni3Te2particles fell out and could not be observed from the cross-sectional image.The one halfround primary M6C carbide particle in the corrosion product layer indicates that the corrosion products grow inward from the initial surface and that the M6C carbides are more resistant to Te corrosion.The thickness of the corrosion product layer in the GH3535-SS samples was~10μm(Fig.4),and its main body was composed of Ni3Te2according to the diffraction peak intensity(Fig.2)and the elemental distribution observed in the cross-sectional image(Fig.4).Two types of plate-like phases were embedded in the corrosion product layer of Ni3Te2,which were rich in Cr/Te and Mo/Si.The former was identified as Cr3Te4based on the XRD results(Fig.2).The latter was always wrapped in Cr3Te4and extended from the Ni3Te2layer into the matrix interior;the particles also possess the same composition characteristics as the half-round primary M6C carbide particles.In our previous study[8],the Mo-and Si-rich phases were identified as M6C carbides by transmission electron microscopy.This conclusion also applies to the present study.EDS line scanning was also employed to describe the compositional characteristics of Cr2O3,Cr3Te4,and M6C,as shown in Fig.4,lines 1 and 2.
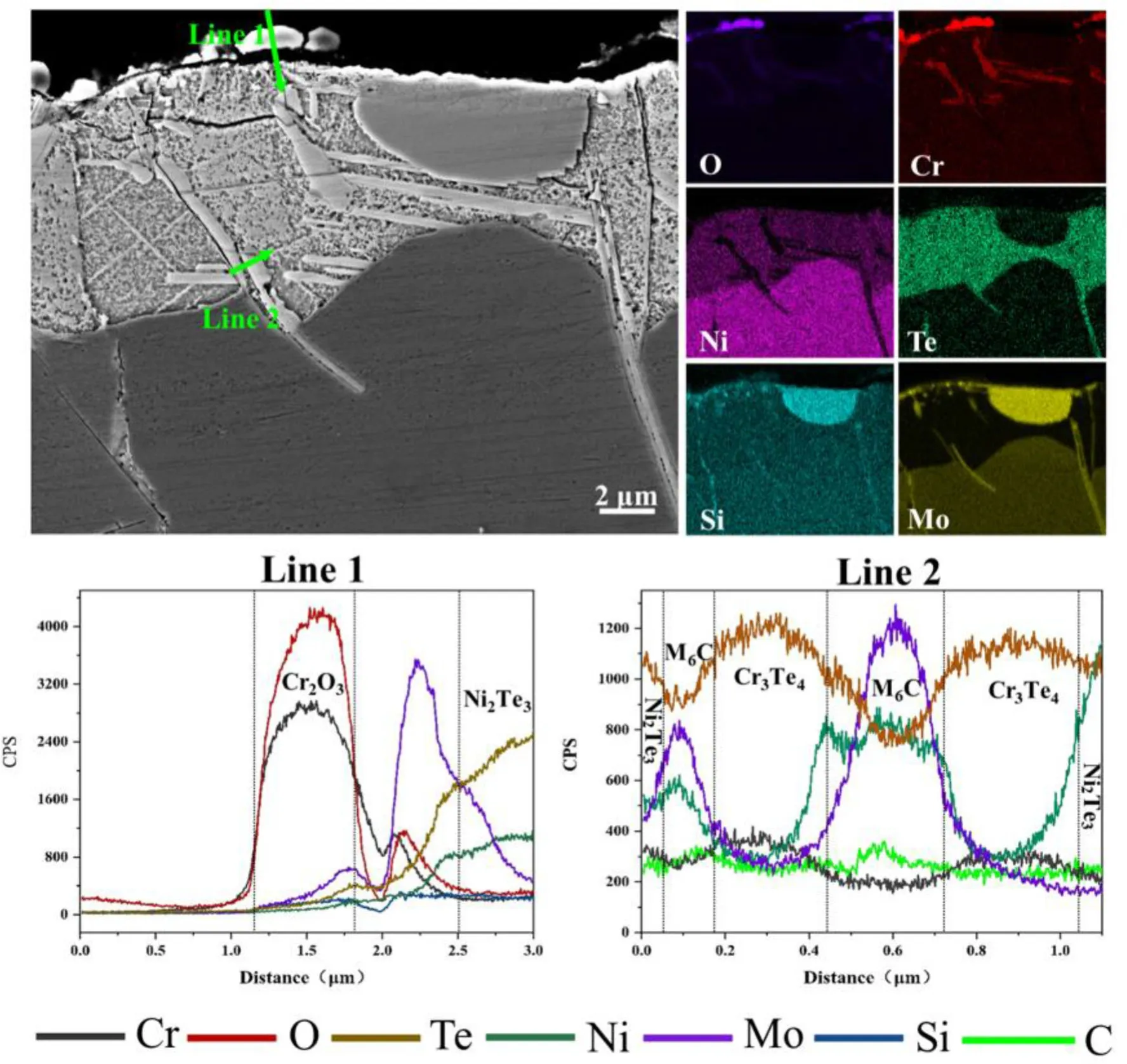
Fig.4(Color online)The morphologies and corresponding EDS mapping images of the corrosion products observed from the cross-section of the GH3535-SS samples.The EDX line scanning results,marked by lines 1 and 2,are also presented
In the GH3535-DS samples,a Cr2O3layer with a thickness of several hundred nanometers can be observed from the cross-sectional image,which detaches from the alloy matrix because of the metallographic sample preparation(Fig.5a).It is reasonable to assume that such a thin Cr2O3layer can only produce a very weak diffraction signal,as shown in the XRD pattern(Fig.1).The bright particles indicate the primary M6C carbides,while the dense small particles at the grain boundaries indicate the secondary M6C particles(Fig.5a).From the cross-sectional image of the corroded pure Ni samples,a discrete outer Ni3Te2layer and a continuous inner Ni2Te3layer with a total thickness of~10μm can be observed(Fig.5b).The discrete outer layer corresponds to the surface-faceting irregular particles observed on the surface(Figs.3c and 5b).
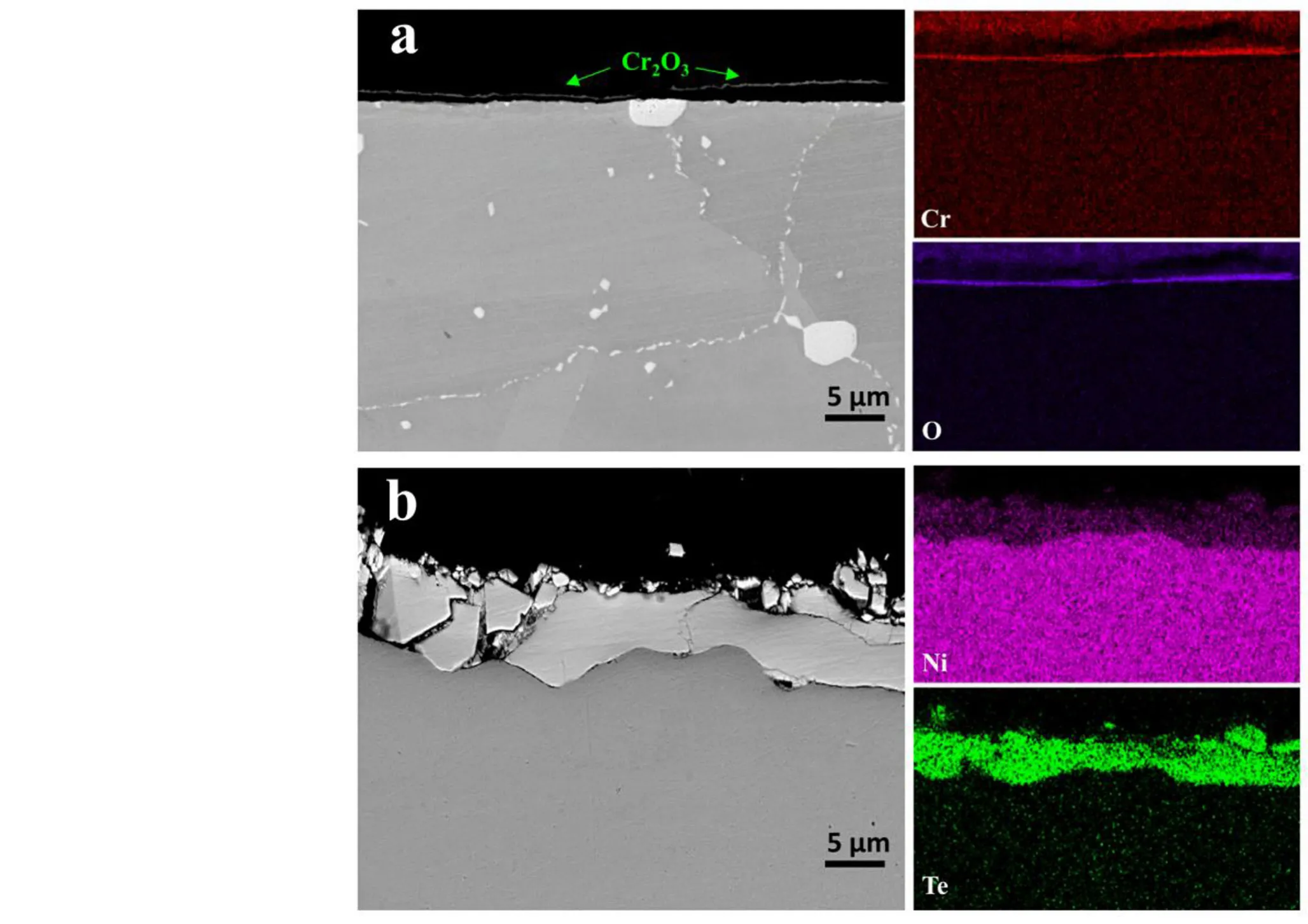
Fig.5 (Color online)The morphologies and the corresponding EDS mapping images of the corrosion products observed from the cross-section of the GH3535-DS and pure Ni samples
The distributions of Te,Ni,and Cr in the GH3535-SS and GH3535-DS samples were determined using the EPMA for comparison.The EPMA samples were cut from the grip ends of the tensile test samples.Few cracks formed in the grip ends,owing to the slight deformation.The enrichment of Te can be observed at the grain boundaries of~178μm in the GH3535-SS samples(Fig.6a),indicating the intergranular diffusion of Te.Correspondingly,the depletion of Cr at the grain boundaries indicates its outward diffusion to the surface for the formation of Cr3-Te4.In contrast,the enrichment of Te and the depletion of Cr at the grain boundaries cannot be observed in the GH3535-DS samples(Figs.6d and f).
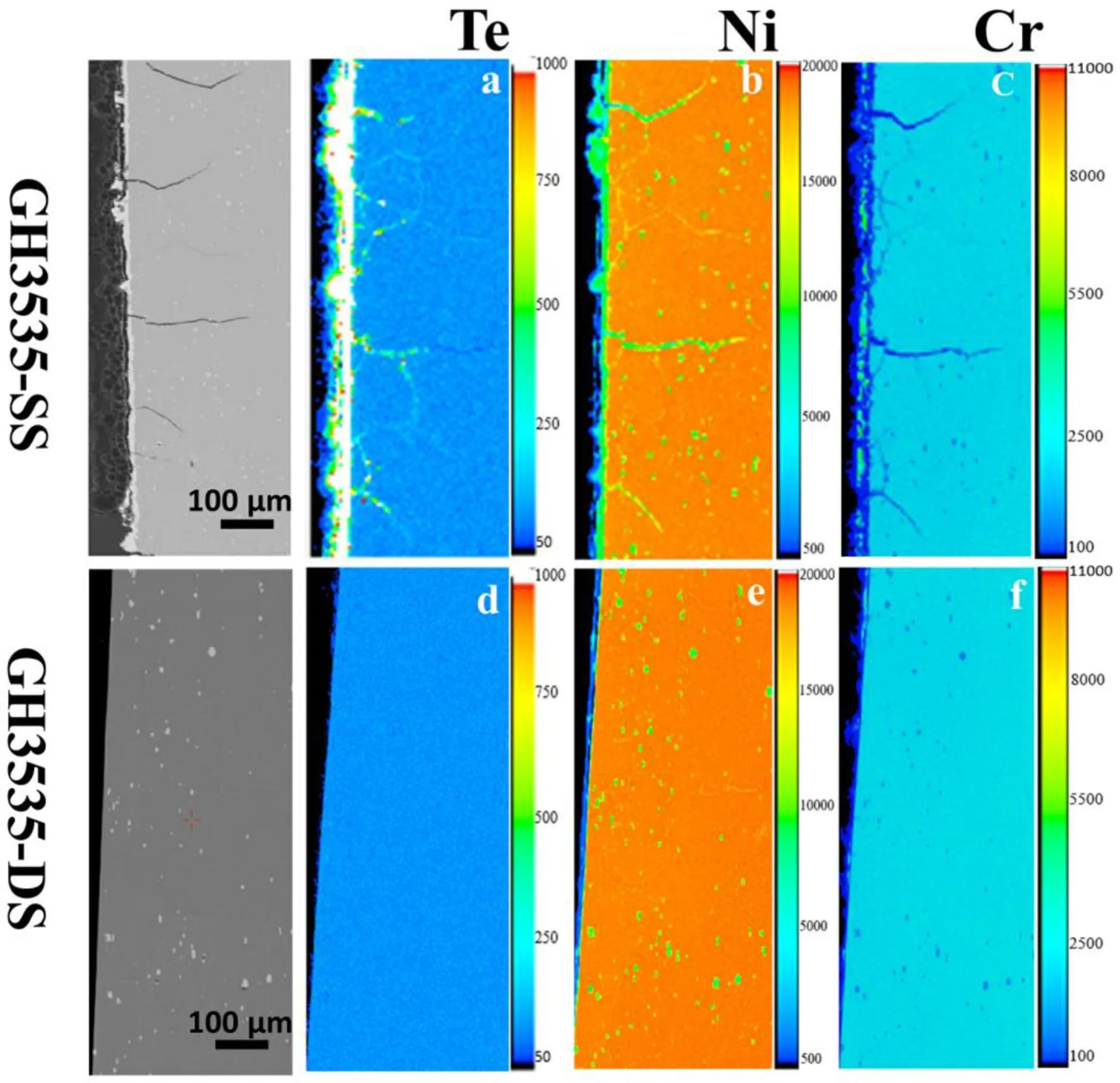
Fig.6 (Color online)The distributions of Te,Ni,and Cr elements on the cross-sections of the GH3535-SS(a–c)and GH3535-DS(d–f)samples.The color is correlated to the concentration in weight percent and is quantitatively indicated by the color scale bar on the right of the image
From the XRD results(Fig.2)and the observations from the surface(Fig.3)and cross-sectional images(Figs.4,5,6),it can be concluded that the GH3535 alloy would be severely corroded by Te vapor to form complex corrosion products,including Ni3Te2and Cr3Te4,as well as small amounts of Cr2O3and M6C carbides.Further,its grain boundaries were profoundly permeated by Te.When corroded with pure Ni,the GH3535 alloy was only slightly oxidized to Cr2O3,and its grain boundaries were immune to Te corrosion,whereas pure Ni was severely corroded by Te vapor to form Ni3Te2.These results prove the effectiveness of pure Ni as an absorber to protect the GH3535 alloy.
3.2 Te embrittlement phenomenon in the Te vapor
To clarify the absorption effect of pure Ni,the GH3535-SS and GH3535-DS samples were subjected to tensile tests at room temperature,after which the morphologies of the cracks and fractures were observed and compared.The stress–strain curves of the GH3535-SS and GH3535-DS samples are plotted in Fig.7.It can be found that the yield strength(YS),ultimate tensile strength(UTS),and elongation(EL)of the GH3535-SS samples are~26%,~46%,and~46%lower than those of the GH3535-DS samples,respectively.Notably,the tensile properties of the GH3535-DS samples are almost identical to those of the as-received samples reported in a previous study[30],which proves that the absorption effect of pure Ni can completely protect the GH3535 alloy from Te embrittlement.
Figure 8 shows the morphologies of the cracks and fractures of the GH3535-SS and GH3535-DS samples tested at room temperature.In the GH3535-SS samples,a noticeable intergranular fracture appeared in the near-surface regions;further,the presence of dimples in the center regions indicates the occurrence of intergranular ductile fracture(Fig.8a).As a typical characteristic of Te embrittlement,intergranular fractures are related to the diffusion and segregation of Te along the grain boundaries.The depth of the intergranular fracture was measured to be~160μm.As shown in Fig.8b,intergranular cracking was observed near the fracture from the cross section of the GH3535-SS samples after the tensile tests.The depth and density of the intergranular cracks were 135μm and 9 number/mm,respectively.
Based on the observations of intergranular cracking and fracture,it can be deduced that the GH3535-SS samples lost 32%of the effective bearing area(EBA)of the asreceived samples in the tensile tests,which is equal to the depth of intergranular cracking(160μm×2)divided by the initial thickness(1000μm)of the tensile test samples.The YS and EBA of the GH3535-SS samples exhibit a similar trend of decreasing amplitude compared with those of the as-received samples,which proves that the relatively low YS mainly results from the loss in the EBA.However,the amplitude of UTS and EL decreased by a high percentage(46%),which cannot be explained only by the loss in the EBA.As shown in Fig.7,the EL of the GH3535-SS samples is noticeably lower than that of the GH3535-DS samples.Thus,it can be deduced that intergranular cracking plays a similar role as the‘‘pre-existing crack’’and prematurely interrupts the plastic deformation process in the tensile tests.Consequently,considerably low EL and UTS were obtained even when the loss in EBA was excluded.In stark contrast,no intergranular fracture or cracking was observed in the tensile test samples of GH3535-DS(Fig.8c and d),which proves that the grain boundaries are free of Te corrosion in this sample.
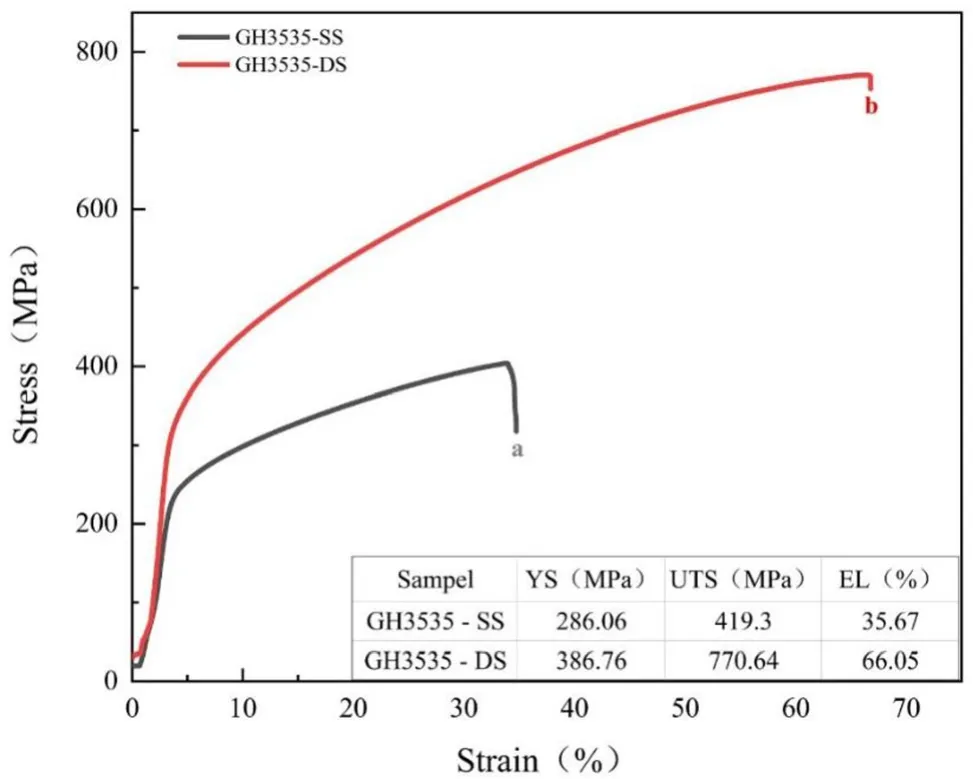
Fig.7 The stress–strain curves of the GH3535-SS and GH3535-DS samples tested at room temperature
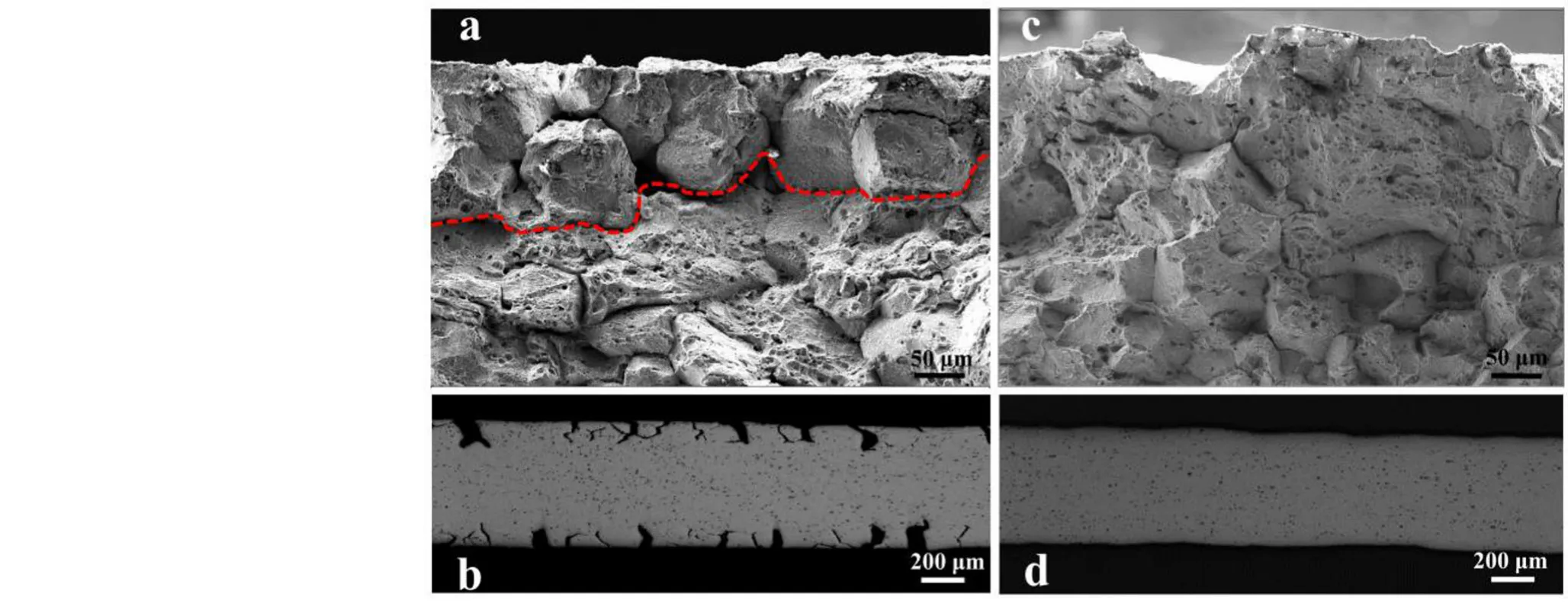
Fig.8 The morphologies of the fractures and cracks of the GH3535-SS(a and b)and GH3535-DS(c and d)samples tested at room temperature
4 Discussion
In the present work,the inhibiting effect of pure Ni on the corrosion and embrittlement of the GH3535 alloy in Te vapor was demonstrated.In this section,the complex evolution of surface corrosion products and the origin of the preferential reaction of pure Ni in Te vapor are elucidated from the viewpoint of chemical equilibrium.
Before the thermodynamic evaluation of different tellurides,it is necessary to clarify the gas components above the heated pure Te and tellurides.Previous mass spectrometric studies have indicated the existence of Tenspecies(n=1–7),and the predominant species was found to be Te2(g)in the saturated vapor of the Te block[31].Using mass spectrometric data,Chattopadhyay et al.[31]calculated the contribution of Te2(g)to the total pressure,which ranged from 0.86 to 1.0,at 327°C.Magapu et al.[32]obtained partial pressure–temperature relationships over various two-phase metal tellurides(Ni,Mo,Cr,Fe,and Mn)by employing Knudsen effusion mass spectrometry,and they found that the detected species mainly consisted of Te(g)and Te2(g).According to these relations,the contribution of Te2(g)to the total pressure was found to be larger than 0.8 at 700°C for all tellurides except CrTe1-x.As a high-temperature phase in the Cr–Te system,CrTe1-xwas not observed as part of the corrosion products,as shown in Fig.2.Thus,it is reasonable to employ Te2(g)as a reference for thermodynamic evaluation in this study.TheM–Te(M=Ni,Mo,and Cr)binary phase diagrams were examined to identify the possible stable phases at 700°C for further analyses.The Ni–Te binary phase diagram was constructed by Lee and Nash[33]and further improved by Arvhult et al.[34]recently.It was established that three types of nickel tellurides(β2,γ2,and δ)can be stable at 700°C,and the Te atomic concentration increases in the order of β2, γ2, and δ(33.33–50<45.95<50–100).Similarly,Cr3Te4,Cr5Te8,Mo3Te4,and MoTe2were selected from the Cr–Te[35]and Mo–Te[36]binary phase diagrams for further thermodynamic evaluation at 700°C.
The chemical potential diagram can be used to depict the stability of various phases as a function of the chemical potentials(or partial pressures)of O2and Te2in the M–Te–O systems.The partial pressure thresholds of O2and Te2for the formation of oxides and tellurides can be calculated from the Gibbs free energy of formation per molecule of O2and Te2.In the diagram,the regions between the threshold lines define the stable phase domains phases,and the lines between two single phases and points represent the two-phase equilibria and three-phase equilibria,respectively.The Gibbs free energies of formation of the selected oxides and tellurides mentioned above were calculated using the HSC Chemistry software and the embedded databases;the results are listed in Table 2.One exception is the energy of Cr5Te8,which was calculated using Sahu’s equation[37].Since the formation of Cr3Te4and Ni3Te2requires a significantly lower Te potential than that required for other tellurides,only the two tellurides and their corresponding oxides(Cr2O3and NiO)were involved in the construction of the chemical potential diagram for simplification.To draw the boundary line between the two solid phases,Cr3Te4–Cr2O3and Ni3Te2–NiO,the reaction equations for No.12 and 13 must be solved as a function of the oxygen partial pressure and Te vapor pressure.Based on the data in Table 2,the Ni–Te–O and Cr–Te–O chemical potential diagrams were drawn and superposed,as shown in Fig.9.In multicomponent alloys,the chemical potential is related to the activity of the metal components in the alloy.The activities of Ni and Cr in the GH3535 alloy were calculated to be 0.58 and 0.092 at 700°C,respectively,by the JMatPro software.The boundary lines for Cr3Te4,Ni3Te2,Cr2O3,and NiO shift toward the high chemical potential region in the diagram,as illustrated by the dashed lines.
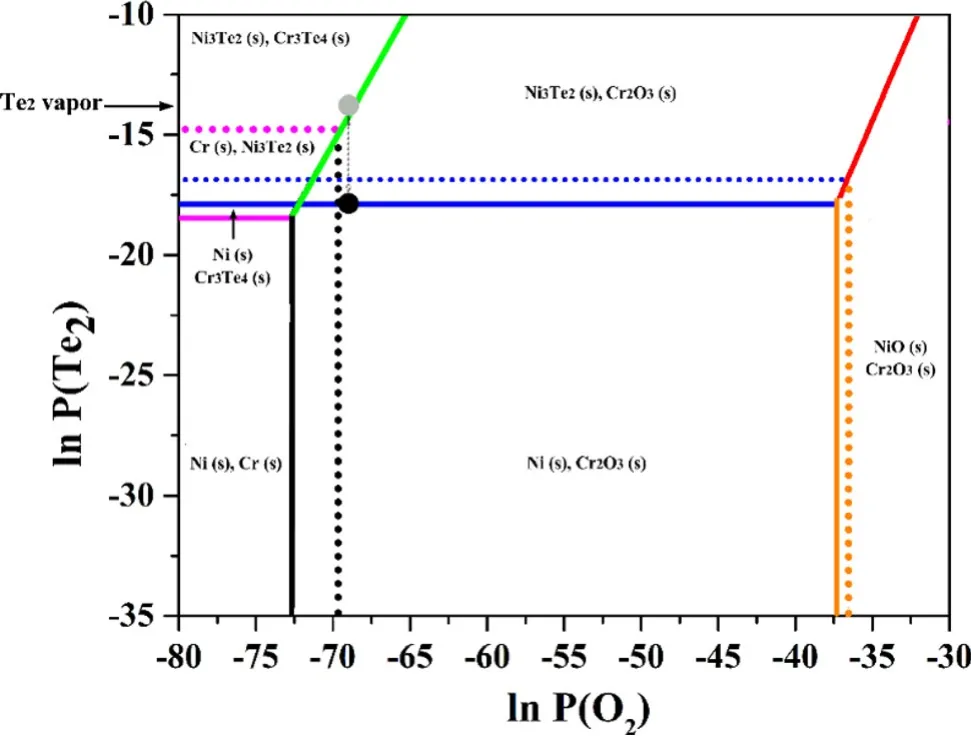
Fig.9 Chemical potential diagram for the Te–Cr–O and Te–Ni–O systems at 700°C.Solid lines are for a Cr=a Ni=1,and the dash lines are for a Cr=0.092 and a Ni=0.58.Gray and black dots stand for the conditions of the GH3535 and GH3535/Ni corrosion experiments,respectively
From Table 2 and Fig.9,it can be deduced that only Ni3Te2and Cr3Te4form on the surface of the GH3535 alloy at 700°C in the Te vapor above the Te block heated at 325°C,which agrees well with the experimental observations.Although the partial pressure threshold of Te2for the formation of Cr3Te4from pure Cr is slightly lower than that for Ni3Te2(solid lines),in the GH3535 alloy,the situation is reversed(dashed lines).This indicates that the Ni atoms in the GH3535 alloy preferentially react with Te vapor to form Ni3Te2at the initial stage of corrosion.The consumption of Ni leads to the depletion of Ni and enrichment of Cr in the residual matrix,which increase the activity of Cr and induce the formation of Cr3Te4.This reaction path can reasonably explain the Ni3Te2/Cr3Te4interlayer structure,as shown in Fig.4.As the corrosion process proceeds,Te atoms diffuse through the preforming Ni3Te2/Cr3Te4to react with the matrix beneath the surface.The consumption of Ni and Cr at the reaction interface affords a supersaturated matrix with Mo,Fe,Si,and C,which would facilitate the nucleation and growth of M6C carbides,as shown in Fig.4.The thermodynamic explanation for the formation of M6C carbides has been elaborated in our previous study[8].Unlike the case for Febased alloys,our data can clarify that the protective inner layer of Cr telluride is difficult to form in Ni-based alloys.As reported by Jia et al.[23],the corrosion products of the Ni-15Cr alloy(wt.%)in Te vapor reveal the Ni3Te2/Cr3Te4interlayer structure;however,the dense inner layer of Cr telluride can be formed in the Fe-5.28Cr alloy(wt.%)[20].Compared with the few thermodynamic merits over Ni3Te2(Fig.9),the chemical potential threshold of Te2for Cr3Te4is considerably lower than that for FeTe0.9,as reported by Arima et al.[22],which explains the preferential formation of a protective Cr3Te4layer in Fe-based alloys[18–21].Our results first explain the formation of the Ni3Te2/Cr3Te4interlayer structure for low-Cr Ni-based alloys and propose that increasing the Cr concentration may not be a very effective solution for the Te-induced intergranular cracking problem in Ni-based alloys from a thermodynamic viewpoint.
In this study,simultaneous oxidation occurs during the corrosion process in Te vapor.The oxygen media mainly result from the hydroxyl radicals in the quartz tubes,as reported in our previous study [38].In the quartz microstructure,hydroxyl radicals were introduced by the reaction between the silicon–oxygen bonds and moisture during the high-temperature fabrication of the quartz tubes.During the corrosion experiments,the hydroxyl radicals tended to diffuse from the inwall of the quartz tubes,facilitated by negative vacuum pressure.Consequently,the self-reaction of hydroxyl radicals would occur in the form of OH+OH→H2O+O.The sequence of the secondary reactions of oxygen and hydrogen atoms promotes the formation of O2and H2O.Since the transformation from hydroxyl radicals to the oxidizing media is very complex,it is difficult to accurately calculate the activity of the oxidizing media.In fact,the hydroxyl radicals in the quartz tubes used in this study were maintained at a very low concentration by the dehydroxylation treatment.In the solely corroded GH3535 alloy,only dispersed Cr2O3particles were observed on the initial surface,which does not disturb the simultaneous Te corrosion.Based on this fact,the chemical potential for the GH3535 corrosion experiment was deduced to be located at the position of the gray dot in the chemical potential diagram,which is the intersection point between the partial pressure line of the heated Te block and the boundary line of Cr3Te4–Cr2O3.At this position,Ni3Te2,Cr3Te4,and Cr2O3can coexist.In the GH3535/Ni corrosion experiment,the formation of Ni3Te2from pure Ni requires a lower Te potential than that for Ni3Te2and Cr3Te4from the GH3535 alloy,as shown in Fig.9.Thus,Te vapor preferentially reacts with pure Ni to form Ni3Te2.At the initial stage,this reaction would reduce the chemical potential of the whole system from the position of the gray dot to that of the black dot.At this chemical potential,only Cr2O3can be formed on the surface of the GH3535 alloy,as shown in Fig.9.The dense Cr2O3would restrain the subsequent Te corrosion of the GH3535 alloy in the GH3535/Ni corrosion experiment,even if the chemical potential recovers because of the sluggish growth of Ni3Te2from pure Ni.The anticorrosion capacity of the Cr2O3layer was also observed in Mg-based alloys corroded in NaCl solutions[39].
In this study,it was determined that the preferential reaction of pure Ni with Te vapor can prevent the corrosion and embrittlement of the GH3535 alloy,which appears to be a good solution for the Te-induced intergranular cracking problem for the components above the liquid level in MSRs.It can be implemented by arranging pure Ni with a high specific surface area in a suitable position in MSRs.There are still some important technical factors that must be considered.For example,the potential effect of the distance between GH3535 and pure Ni on the absorption efficiency should be clarified in future works.As a special case,if the pure Ni coating was directly prepared on the surface of the GH3535 alloy,the absorption and barrier effect could work simultaneously,as reported in other systems[40,41].This is also worth verifying in the future.
The entirely different chemical states of Te-containing fuel salts increase the ambiguity of the absorption effect of pure Ni in such an environment.In the Te-containing fuel salts,Te would exist in the form of ions rather than gas molecules.Furthermore,the fuel salt can also play an important role in Te corrosion.The effectiveness of pure Ni as an absorber in Te-containing fuel salts should be validated in the future.Conversely,our results can provide important indications for out-of-pile corrosion experiments using Te vapor as a corrosion medium.If pure Ni or other materials that react more easily with Te vapor are introduced into the corrosion experiment,the Te activity would decrease dramatically,and the degree of damage would be reduced.
5 Conclusion
(1) In Te vapor,the GH3535 alloy undergoes severe corrosion to form complex products,including Ni3-Te2and Cr3Te4,as well as small amounts of Cr2O3and M6C carbides.Owing to the low activity of Cr in the alloy,Ni3Te2is formed preferentially.The consumption of Ni leads to the depletion of Ni and enrichment of Cr in the residual matrix,which increase the activity of Cr and induce the formation of Cr3Te4,thus promoting the formation of the Ni3-Te2/Cr3Te4interlayer structure.The grain boundaries of the alloy were profoundly permeated by Te.
(2) When corroded with pure Ni,the GH3535 alloy was only slightly oxidized to Cr2O3,and its grain boundaries were immune to Te corrosion.In contrast,the pure Ni was severely corroded by the Te vapor to form Ni3Te2.These results prove the effectiveness of pure Ni as an absorber.
(3) In Te vapor,the tensile properties of the GH3535 alloy,including YS,UTS,and elongation,deteriorate significantly owing to the loss in the EBA and the occurrence of intergranular cracking.As a typical characteristic of Te embrittlement,the intergranular fracture is related to the diffusion and segregation of Te along the grain boundaries.The depth of the intergranular fracture was measured to be~160 μm.When corroded with pure Ni,the tensile properties of the GH3535 alloy were almost identical to those of the as-received alloy.
(4) Ni–Te–O and Cr–Te–O chemical potential diagrams were constructed based on thermodynamic data.It was verified that pure Ni preferentially reacts with Te vapor under the present experimental conditions from a thermodynamic viewpoint,which explains the absorption effect of pure Ni.
Author contributionsAll authors contributed to the study conception and design.Material preparation,data collection and analysis were performed by Kai Wang and Li Jiang.The first draft of the manuscript was written by Kai Wang and Li Jiang and all authors commented on previous versions of the manuscript.All authors read and approved the final manuscript.
 Nuclear Science and Techniques2021年12期
Nuclear Science and Techniques2021年12期
- Nuclear Science and Techniques的其它文章
- Fast nuclide identification based on a sequential Bayesian method
- Screener3D:a gaseous time projection chamber for ultra-low radioactive material screening
- Performance evaluation of ultra-long lithium heat pipe using an improved lumped parameter model
- Verification of SEU resistance in 65 nm high-performance SRAM with dual DICE interleaving and EDAC mitigation strategies
- Design and development of the beamline for a proton therapy system
- Ensuring the possibility of using thorium as a fuel in a pressurized water reactor(PWR)
